Abstract
Epilepsy, bipolar disorder, and migraines are common disorders that are often associated with disturbances in menstrual function in adolescent girls. Women with untreated epilepsy are more likely to have irregular menstrual cycles than are nonepileptic controls, indicating that the disease itself plays a role in the etiology of these reproductive abnormalities. In addition, many girls with these disorders require chronic maintenance treatment with agents that may perturb the hypothalamic-pituitary-ovarian axis. Valproate is a highly effective antiepileptic drug used widely to treat epilepsy, bipolar disorder, and migraines. Valproate induces features of the polycystic ovary syndrome (PCOS) in approximately 7% of women. Girls with epilepsy, and possibly bipolar disorder, appear particularly susceptible to developing PCOS features on valproate, perhaps on account of the relative immaturity of their hypothalamic-pituitary-ovarian axes. Antipsychotics are highly effective drugs used widely to treat adolescents with bipolar disorder, psychotic disorders, and behavioral disturbances. Some, but not all of the antipsychotic, induce hyperprolactinemia, which may result in oligo- or amenorrhea. Prolonged amenorrhea in association with hyperprolactinemia incurs significant risks for bone health in adolescent girls. Because of the potential reproductive health risks associated with use of specific antiepileptic drugs and selective antipsychotics, these agents are vital treatments for adolescents with severe illnesses. Use of these agents should be considered and weighed against the risk of using alternative agents, which have their own side effects, or not treating these serious neurologic and psychiatric disorders.
Keywords: bipolar disorder, epilepsy, hypothalamic-pituitary-gonadal (HPG) axis, menstrual cycle dysfunction, migraines, polycystic ovarian syndrome (PCOS), valproate
Introduction
Neurologic and psychiatric disorders occur commonly in adolescents. Conditions such as epilepsy, migraines, and bipolar disorder are typically disorders that require chronic medication to treat symptoms and prevent recurrence of episodes. Some of the medications that are frequently used to treat these conditions have the potential to perturb the hypothalamic-pituitary-gonadal (HPG) axis and thus disrupt menstrual cycles. As a result, adolescent girls treated with these medications may present with amenorrhea or oligoamenorrhea.
Medications used to treat these neurologic and psychiatric disorders include: (1) antiepileptic drugs (AEDs) to treat epilepsy, bipolar disorder, migraines, and other psychiatric and behavioral disorders; and (2) antipsychotics to treat bipolar disorder, psychotic disorders, autism, and other psychiatric and behavioral disorders. Two major endocrine disorders presenting with menstrual dysfunction are polycystic ovary syndrome (PCOS), occurring with selected AEDs, and hyperprolactinemia, which may develop on therapy with selected antipsychotic agents.
This review will begin by discussing the prevalence and course of the primary disorders for which specific AEDs and antipsychotics are used in adolescents, as well as the association between menstrual dysfunction and the disorders of epilepsy and bipolar illness. The impact of these agents on the HPG axis and the menstrual cycle, as well as the mechanisms by which these drugs exert their effects will also be reviewed.
Epilepsy, Bipolar, and Migraine Disorders in Adolescents
Epilepsy afflicts 0.4–0.9% of children and adolescents and is more common in boys than girls.1 While in the majority of patients epilepsy develops before age 20, the incidence of epilepsy decreases as children age and become adolescents.2 Chronic maintenance treatment is usually initiated after seizures are recurrent and the specific type of AEDs used depends on the seizure type (i.e., partial, generalized). The majority of seizure disorders in children and adolescents are partial seizure syndromes. First-line AEDs used to treat partial seizures in children and adolescents include: valproate, carbamazepine, topiramate, oxcarbazepine, lamotrigine, phenytoin, and phenobarbital.3
By age 18, the lifetime prevalence of bipolar disorder (also called manic-depression) in the general population is 1%, and another 4–5% have subsyndromal bipolar disorder, a broader spectrum of bipolar illness that also warrants treatment.4,5 The age of onset of bipolar disorder is usually between 15 and 30 years.6 Bipolar disorder type I (manic episodes) occurs equally in men and women, whereas bipolar disorder type II (hypomanic episodes) occurs more commonly in women.7
Treatment of bipolar disorder is indicated for acute episodes of mania and depression, and for prevention of recurrent episodes. Treatment often includes a combination of medications in different classes (i.e., AEDs, lithium, antipsychotics, and antidepressants) that target different symptoms, such as hypo/mania, depression, and psychotic symptoms. Teenagers with bipolar disorder are most likely to receive treatment with an AED (49%) and/or an antipsychotic agent (48%).8 The most common AED used is valproate, used by 31% of bipolar adolescents.8 A wide range of antipsychotics are also used, including the newer atypical antipsychotics (risperidone, olanzapine, quetiapine, aripiprazole, ziprasidone) and the older conventional antipsychotics (e.g., perphenazine, haloperidol). The atypical antipsychotics have therapeutic effects on both psychotic symptoms and mood symptoms.
Migraines are very common in adolescents, particularly in girls. By age 17, approximately 23% of girls and 8% of boys have had a migraine.9,10 In 50% of cases, the disorder begins before age 20, with an average age of onset of 10 years in girls.10–12 Although migraines can be infrequent and not require prophylactic treatment, those with frequent and/or severe migraines require a chronic, maintenance treatment approach. A number of different medication classes can be used for prophylactic treatment in adolescents including AEDs, beta blockers, and tricyclic antidepressants. AEDs that can be used include valproate, gabapentin, carbamazepine, topiramate, and levetiracetam. Migraines can also be linked closely with the premenstrual or menstrual phase of the cycle in some adolescent girls. Menstrual migraines are commonly treated with a “mini prophylaxis” that involves treatment targeted to the specific phase of the menstrual cycle in which the patient is symptomatic.
Epilepsy, Irregular Menstrual Cycles, and Features of PCOS
It is important to review the relationship between menstrual dysfunction and the underlying disorders for which AEDs are indicated before considering the effects of AEDs on the menstrual cycle. Independent of drug effects on the HPG axis, women with epilepsy who are not receiving treatment are more likely to have features of PCOS than are nonepileptic women. The increased prevalence of isolated components of PCOS in untreated epileptic women may result in a greater susceptibility to the development of the full-blown syndrome in the context of valproate use.
Compared with nonepileptic controls, women with epilepsy are more likely to have menstrual cycle irregularities and increased luteinizing hormone (LH) pulse frequency, but no abnormalities in serum testosterone levels.13–15 The basis for these HPG abnormalities in untreated epilepsy is poorly understood. It has been hypothesized that recurrent seizures perturb the HPG axis because paroxysmal epileptic discharges spreading within the hypothalamus might disrupt the gonadotropin-releasing hormone (GnRH) pulse generator.15,16 Thus, central nervous system effects may be the basis for the association between untreated epilepsy with PCOS features.14,17 Unlike menstrual dysfunction and the clinical syndrome of PCOS, polycystic ovarian morphology is not more prevalent in epileptic women according to most,18–21 but not all,22 studies. Regardless, the presence of polycystic ovarian morphology in women with regular menstrual cycles does not appear to predict the development of PCOS.23 Studies of reproductive function in recently postpubescent girls with untreated epilepsy differ from those conducted in adult women. In adolescent girls with epilepsy an association between untreated epilepsy per se and menstrual dysfunction, hyperandrogenemia, or PCOS has not been examined.24 However, these studies are small and additional studies are needed to address this association in adolescent girls.
Some studies suggest that the specific location of the epileptic focus correlates with the likelihood of HPG axis perturbation, which results in specific reproductive endocrine disorders. Left-sided temporal lobe epilepsy (TLE) has been associated with PCOS features, while right-sided TLE has been associated with hypogonadotropic hypogonadism.14,25 However, other evidence suggests that women with generalized epilepsy are more likely to have menstrual cycle dysregulation and PCOS features than those with TLE26,27. In contrast, other studies show no evidence of a relationship between a specific seizure focus location and menstrual dysfunction or PCOS.18,19
Menstrual Cycle Irregularities and PCOS in Women with Bipolar Disorder
Compared with epilepsy, little is known about the prevalence of menstrual dysfunction and PCOS in women with bipolar and migraine disorders. Among women with bipolar disorder, one-third report having had irregular menstrual cycles during adolescence before they were treated for bipolar disorder.28 However, the prevalence of PCOS in women with bipolar disorder independent of psychotropic treatments is similar to that seen in the general population.29 Therefore, like women with epilepsy, those with bipolar disorder may have neuroendocrine dysregulation because of the psychiatric illness, which increases their susceptibility to developing PCOS in the context of other risk factors.
Valproate Use and Polycystic Ovarian Syndrome
In Adult Women with Epilepsy
Isojarvi and colleagues first described an association between valproate and PCOS in 1993.19 In a landmark study, they reported that women with epilepsy were more likely to have menstrual cycle irregularities that those taking carbamazepine or other AEDs (45% on valproate versus 19% on carbamazepine versus 13% on other AEDs). In this study, valproate users were also more likely to have PCO morphology or an elevated testosterone level (56% on valproate versus 20% non-valproate).19 Since that time, another six studies in women with epilepsy have reported that PCOS features are more common in valproate users than those taking other AEDs (carbamazepine is most common comparator),18,22,30–33 while three studies have reported no association between valproate use and PCOS in this population (Table 1).20,26,34 All studies, except for one,22 were conducted in women who were not randomly assigned to AED therapy. In the single randomized clinical trial that has been conducted in this area, 447 women without PCOS features at baseline were randomized to valproate or lamotrigine for one year.22 Those randomized to valproate were significantly more likely to develop hyperandrogenemia or ovulatory dysfunction (indicated by low levels of progesterone) than those randomized to lamotrigine (36% VPA versus 23% LTG), with 7% of women on valproate (versus 1% on lamotrigine) developing new-onset PCOS after one year of treatment.22
TABLE 1.
Studies addressing the association between valproate use and polycystic ovary syndrome (PCOS) features
| Study | Disorder | Association between Valproate Use and Features of PCOSa |
Study Design |
|---|---|---|---|
| Isojarvi et al., 199319 | Epilepsy | + | Cross-sectional |
| Bilo et al., 200118 | Epilepsy | + | Cross-sectional and prospective |
| Morrell et al., 200231 | Epilepsy | + | Cross-sectional |
| Morrell et al., 200332 | Epilepsy | + | Cross-sectional |
| Betts et al., 200330 | Epilepsy | + | Cross-sectional |
| Prabhakar et al., 200733 | Epilepsy | + | Cross-sectional |
| Hayes et al., 200722 | Epilepsy | + | Randomized trial |
| Joffe et al., 200629 | Bipolar | + | Cross-sectionalb |
| Bilo et al., 198826 | Epilepsy | − | Cross-sectional |
| Bauer et al., 200034 | Epilepsy | − | Cross-sectional |
| Luef et al., 200220 | Epilepsy | − | Cross-sectional |
| Rasgon et al., 200538 | Bipolar | − | Cross-sectional |
+ means association between valproate and PCOS features found; − means no association between valproate and PCOS features found.
Excludes women with PCOS that developed prior to diagnosis and treatment of bipolar disorder.
Despite the inconsistencies in the findings across studies of women treated with valproate, the majority did observe an increased prevalence of PCOS features among valproate users. In addition, the observation that 7% of women who are randomly assigned to valproate develop new-onset PCOS adds weight to the evidence that valproate likely induces PCOS in a subset of women with epilepsy who are treated with this AED.22 Other studies indicate that PCOS features occurring in valproate users remit after valproate has been discontinued and lamotrigine initiated.35
There are several possible explanations for the discrepancy in the findings among the 9 studies of epileptic women who were not randomly assigned to AED treatment.36 These include: (1) small sample size in some studies; (2) lack of randomization, which may result in differences in the distribution of characteristics that predispose to the development of PCOS; and (3) differences in the way that PCOS was defined. Key characteristics that can influence the association between valproate and PCOS include the age at which valproate was initiated and the prevalence of obesity in the population being studied.37 Several studies have demonstrated that women are more likely to develop PCOS features on valproate if they are younger when they valproate therapy is started.19,22,27,29 Other studies suggest that obesity may be a risk factor for the development of PCOS on valproate, but few prospective data are available to fully address this possibility.32
In Adult Women with Bipolar Disorder
Compared with epilepsy, fewer studies addressing the association between valproate use and PCOS in adult women with bipolar disorder have been undertaken, and none are randomized trials (Table 1). In addition to the different underlying disease state of bipolar disorder, these studies differ from those conducted in women with epilepsy in that the comparison group of non-valproate users includes women whose primary treatment is lithium, as well as other AEDs. In the largest study conducted to date in this population (n = 230), new-onset PCOS features (oligoamenorrhea and hyperandrogenism) developed in 10% of women taking valproate within one year of its initiation, whereas PCOS features developed in only 1% of non-valproate users.29 However, PCOS morphology was not seen more commonly among valproate users than non-users.29 Other studies in women with bipolar disorder have also found that PCOS occurs more commonly in those on valproate than on non-valproate treatments (6% versus 0%, respectively), although this difference was not statistically significant because of sample size limitations.38
It is notable that the 10% incidence of treatment-emergent PCOS features developing on valproate among women with bipolar disorder29 is consistent with the 7% incidence of new-onset PCOS observed in the randomized trial conducted in women with epilepsy.22 Like the epileptic population,22 women with bipolar disorder also developed PCOS features within one year of treatment, with no women developing new-onset PCOS after they had been taking valproate for more than one year’s duration.29 In addition, similar to the case in women with epilepsy,35 PCOS features developing on valproate remit when other psychotropic agents are substituted for valproate in women with bipolar disorder.39 These data suggest that women with epilepsy and bipolar disorder have a similar level of risk for developing PCOS features on valproate.
In Adolescents with Epilepsy and Bipolar Disorder
Studies conducted in adults have consistently found that PCOS features develop more commonly among those who are younger when valproate treatment is begun.19,22,29 Among adolescents and adult women (13–40 years) with epilepsy who were randomized to valproate, hyperandrogenemia and ovulatory dysfunction developed in 44% of women 13–25 years old, but only in 24% of those who were over 25 years old when they started valproate treatment.22 Other studies in epilepsy have found polycystic ovarian morphology and/or an elevated testosterone level in 80% of women who started taken valproate before age 20.19 Studies in patients with bipolar disorder similarly suggest that PCOS features develop more commonly in those who were younger when they started the AEDs.29
Several studies have been conducted in a group of epileptic girls who are prepubertal, pubertal, or postpubertal. These studies have examined the prevalence of reproductive and metabolic features of PCOS in girls with epilepsy who are taking valproate (Table 2).24,40–44 All have found that serum androgen levels are higher in valproate-treated girls than in girls who are either treated with other AEDs or untreated, or healthy controls. The findings are strongest in postpubescent girls. In addition, obesity and weight gain were associated with valproate use in most,41–44 but not all,40 studies. However, only one study found an association between valproate use and the clinical disorder of PCOS.24 These studies suggest that girls with epilepsy who are undergoing a pubertal transition have PCOS features such as higher androgen levels, and confirm reports in adults that indicate that adolescent girls with epilepsy are susceptible to the development of PCOS features on valproate.
TABLE 2.
Valproate use in girls with epilepsy during the pubertal transition
| No. of Girls with Epilepsy |
Age (yr) | Menstrual Irregularitiesa |
Androgen Levels | Obesity/ Weight Gain |
Insulin | PCOS | |
|---|---|---|---|---|---|---|---|
| Rattya et al., 199944 | 77 | 8–18 | NR | NR | ↑ | ~ | NR |
| Vainionpaa et al., 199943 | 41 | 8–18 | ~ | ↑(all pubertal stages) | ↑ | ~ | NR |
| El-Khayat et al., 200441 | 66 | 8–18 | NR | ↑ (post-pubertal) | ↑ (post-pubertal) | ~ | ~ |
| Mikkonen et al., 200424 | 69 | 8–18 | NR | ↑ | NR | NR | ↑ |
| de Vries et al., 200740 | 88 | 6–20 | ~ | ↑(post-pubertal) | ~ | ~ | ~ |
Among postpubertal girls only. NR = not reported; ~ means no abnormality found.
It is unknown why adolescents and young adults with epilepsy and bipolar disorder are more susceptible to developing PCOS features on valproate. However, given that recently postpubescent girls have a high incidence of anovulation, it is possible that the relative immaturity of the HPG axis at this time period makes it more vulnerable to perturbation.45 Preliminary reports suggest that initiation of valproate during puberty suppresses gonadotropin secretion, but does not influence pubertal development,46 although case reports suggest that pubertal growth and maturation can be arrested.47
In Adolescents with Migraines
Women with migraines provide an important model in which to study the association between valproate and PCOS features, given that women and girls with epilepsy and bipolar disorder may be more susceptible to developing PCOS because of dysregulation of the HPG axis associated with the disorder itself. Data on the association of untreated migraine disorders and menstrual dysfunction are not available, nor is there information on the impact of valproate use on reproductive function in adult women with migraines. However, a recent randomized trial provides some preliminary information about menstrual dysfunction and PCOS features in adolescents 12–17 years old with migraines.48 Participants in this trial were randomized to valproate or placebo for 3 months. A subset of 41 postmenarchal girls who were not taking hormonal contraceptives underwent additional evaluation of reproductive function. After 3 months of treatment, there was no difference in the rates of menstrual disturbances or the change in testosterone levels between adolescents treated with valproate versus placebo.
Mechanisms by Which Valproate May Induce Polycystic Ovary Syndrome
Recent evidence suggests that valproate leads to hyperandrogenemia and PCOS features through direct effects of the AEDs on the ovary. In vitro studies demonstrate that valproate stimulates androgen biosynthesis in human theca cells at doses that represent therapeutic levels in the treatment of epilepsy or bipolar disorder.49 Treatment of theca cell cultures with valproate for 72 hours results in increased levels of dehydroepiandrosterone (DHEA), androstenedione, and 17α-OH-progesterone, and decreased levels of progesterone.49 Valproate-induced androgen biosynthesis has been attributed to its stimulatory effects on gene transcription,49,50 and activity of the enzymes P450scc and P450c17 involved in ovarian steroid production.51 Furthermore, microarray data show common gene expression profiles in PCOS and valproate-treated normal theca cells that are not seen in normal, untreated theca cells,50 suggesting that similarities in theca cell function may explain the features of PCOS that develop in some women treated with valproate.
Other possible mechanisms by which valproate may induce PCOS features include (1) its central nervous system GABA-mediated effects on GnRH and (2) indirectly, by causing weight gain, which in turn leads to insulin resistance.16,36,52
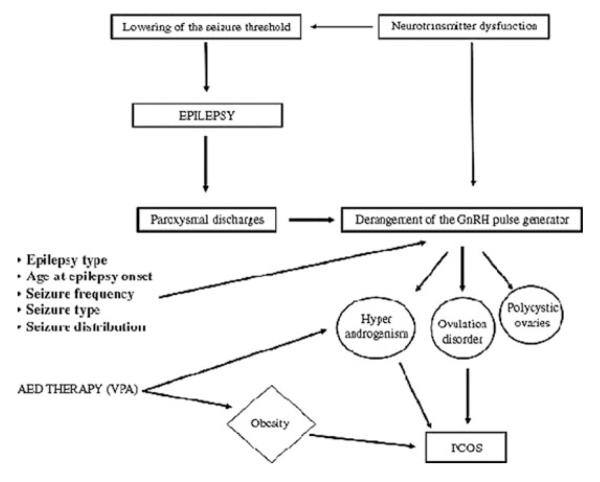
The likelihood of valproate’s resulting in PCOS may also be influenced by the underlying disorder for which the AED is being used (Fig. 1). Other host factors, such as age and proximity to menarche may also influence these pathways.
FIGURE 1.
Pathways to polycystic ovary syndrome (PCOS) in women with epilepsy, including use of valproate (VPA) as an antiepileptic drug (AED). (From Bilo and Meo.16)
Antipsychotics and Hyperprolactinemia
Antipsychotics are used widely in adolescents for treatment of bipolar disorder and psychosis and for behavioral control. There are two types of antipsychotics: the older “conventional” antipsychotics (e.g., haloperidol, thioridazine) and the “atypical” antipsychotics (risperidone, olanzapine, quetiapine, ziprasidone, aripiprazole, clozapine). The conventional antipsychotics work through the D2 receptor antagonism, whereas the atypical antipsychotics have both dopaminergic and serotonergic activity. Most of the widely used atypical antipsychotics do not increase prolactin levels.53,54 Quetiapine, ziprasidone, and aripiprazole are not associated with hyperprolactinemia.53,54 Olanzapine has been associated with mild and transient hyperprolactinemia in some studies.55–58 In contrast, the atypical antipsychotic risperidone and the conventional antipsychotics have been associated with significant elevations in serum prolactin.53,54,59–61 Prolactin levels are increased in 88% of women treated with risperidone and 48% of those treated with conventional antipsychotics.61,62
Prolactin levels increase with selected antipsychotics because they block dopaminergic inhibition of prolactin secretion. Prolactin secretion by lactotrophs in the pituitary is under tonic, physiological inhibition by dopaminergic neurons from the hypothalamus. The extent to which prolactin levels increase depends on the occupancy of D2 receptors, which varies among the antipsychotics.63 With the conventional antipsychotics, prolactin levels increase within several hours of administration and rise 3.8-fold in women within the first few days of administration64 in a dose-dependent fashion.65
Risperidone is an atypical antipsychotic that markedly inhibits the D2 receptor.66 Prolactin levels increase significantly in adults treated with risperidone67–71 in a dose-dependent manner,71,72 with a rapid doubling of levels occurring within the first few hours after treatment initiation.70 Prolactin levels are significantly higher in adult women taking risperidone than in those taking the conventional antipsychotic, haloperidol.72 With prolonged treatment, hyperprolactinemia occurring on conventional antipsychotics and risperidone has been reported to resolve in some, but not all individuals treated with these agents.60,62
Antipsychotic-Induced Hyperprolactinemia Reproductive Effects
As with other causes of hyperprolactinemia, drug-induced hyperprolactinemic states are not universally symptomatic.53,54 Many patients are unaware of hyperprolactinemia as a cause of their reproductive dysfunction as testing for prolactin levels is not routinely done. Hyperprolactinemia results in menstrual-cycle irregularities because prolactin suppresses the HPG axis and ovulation. Menstrual cycle irregularities manifest in 8–48% of women on risperidone and are more likely with higher doses, while galactorrhea occurs in only a small proportion of risperidone-treated women.61,72
Among those treated with conventional antipsychotics, 26–91% have menstrual dysfunction73–76 and galactorrhea has been observed in 19%.77 Analysis of sexual dysfunction in schizophrenics on antipsychotics reveals that it is dose-related and varies with the specific antipsychotic being used, from 18% of those on quetiapine, to 33–38% of those on conventional antipsychotics, to 35% of those on olanzapine, and 43% of those on risperidone.76,78
The clinical effects of prolactin-elevating antipsychotics are reversible, with normalization of menstrual cycles and reduction in prolactin levels to normal when the drug is discontinued or a prolactin-sparing antipsychotic is added.53 These improvements are seen rapidly when patients taking prolactin-elevating antipsychotics are switched to olanzapine,79 quetiapine,80,81 aripiprazole,82 or ziprasidone,83 or when aripiprazole is added for combination antipsychotic therapy.84
Bone Effects
The hypoestrogenism that results when ovulation is suppressed by hyperprolactinemia can result in reduced bone density, although limited data are available to address this issue. Women with hyperprolactinemia induced by conventional antipsychotics had bone mineral density (BMD) in the 90th percentile of age- and weight-matched controls.85 BMD correlated with the vaginal maturation score, an index of estrogen exposure.85 Reduced BMD has been seen with risperidone,67 but not olanzapine,67 on ultrasonography-detected bone speed of sound, but not on conventional dual-energy X-ray absorptiometry (DXA). Decreased BMD correlates inversely with the duration of antipsychotic therapy.86 These findings suggest that estrogen suppression, the specific agent being used, and duration of use may determine the degree of bone loss with antipsychotic use.
Antipsychotics and Hyperprolactinemia in Adolescent Girls
Postpubertal children and adolescents may be more likely than adult women to develop prolactin elevation as a consequence of antipsychotic exposure.59,87,88 This increased susceptibility to a more pronounced prolactin response to antipsychotic exposure may result from a decrease in dopamine receptors that occurs with increasing age.89 Among children and adolescents treated with risperidone, prolactin levels increased 3.8-fold within the first one to two months after treatment and then declined to levels just above normal within one year of continued treatment.62 Another small study suggests that children and adolescents also develop prolactin elevation at a high rate on haloperidol and olanzapine.88 Initiation of prolactin-stimulating antipsychotics at the time of puberty may delay its onset or slow its progression.90 Dopamine agonists may be used to normalize prolactin levels in these circumstances.91
Adolescent girls may be particularly susceptible to the reproductive and bone effects of prolactin-elevating antipsychotics.53 The HPG axis may be more easily perturbed because it is relatively immature in recently postpubertal girls.45 In addition, the ability to achieve peak bone mass may be suppressed because hypoestrogenism associated with hyperprolactinemia will reduce bone anabolism and increase bone resorption.
Conclusion
Epilepsy, bipolar disorder, and migraines are common disorders in adolescent girls that often require chronic maintenance treatment for symptom control and prevention of symptom recurrence. Valproate is a highly effective antiepileptic drug used widely to treat epilepsy, bipolar disorder, and migraines. When used in adolescent girls, its effects on reproductive function should be discussed and monitored as valproate induces PCOS symptoms in approximately 7% of women. Rates of treatment-emergent PCOS features are higher in adolescents beginning this medication. The reproductive effects of valproate manifest within the first year of its use and the risk for new-onset PCOS features does not appear to continue beyond the first year of use. Moreover, these reproductive changes remit within one year of discontinuation of valproate. Girls with epilepsy, and possibly bipolar disorder, may be particularly susceptible to developing PCOS features on valproate because of the relative immaturity of their hypothalamic-pituitary-ovarian axis. Other antiepileptic drugs do not appear to be associated with PCOS features.
Antipsychotics are used widely to treat adolescents with bipolar disorder, psychotic disorders, and behavioral disturbances. They are highly effective drugs that are used chronically in individuals with severe mental disorders. Some, but not all, of the antipsychotics induce hyperprolactinemia, which may be sustained or transient. Hyperprolactinemia occurs in 48% of individuals on conventional antipsychotics and in 88% of those on the atypical antipsychotic, risperidone. Oligo- or amenorrhea results in some, but not all, of women with hyperprolactinemia on antipsychotics, and is reversible upon discontinuation of the responsible medication. Prolonged amenorrhea in association with hyperprolactinemia incurs significant risks for bone health in adolescent girls.
Despite the potential reproductive health risks associated with use of specific antiepileptic drugs and selective antipsychotics, these agents are vital treatments for adolescents with severe illnesses. Alternative treatments for epilepsy, bipolar disorder, and migraines may be important considerations, but have their own side effects and risks that should be weighed in the selection of specific medication treatments. Use of these agents should be considered and weighed against the risk of not treating these serious neurologic and psychiatric disorders.
Acknowledgments
We wish to thank Janet Wozniak, M.D., for scientific guidance, and Eric Pasciullo, B.A., for technical assistance.
Footnotes
Conflicts of Interest Hadine Joffe has had affilations with the following: Research Support: National Institutes of Health, National Alliance for Research on Schizophrenia and Depression, Susan G. Komen Breast Cancer Foundation Award, Harvard Medical School Center of Excellence in Women’s Health Fund Award, Harvard Medical School Kaplan Depression Research Fellowship, Harvard Medical School 50th Anniversary Scholars in Medicine Award, Massachusetts General Hospital Claflin Scholar’s Award, Pfizer/Society for the Advancement of Women’s Health Research Scholars Award, Abbott Laboratories, Astra-Zeneca Pharmaceuticals, Bayer Health-Care Pharmaceuticals, Eli Lilly and Company, Forest Laboratories, Inc., GlaxoSmithKline, Pfizer, Inc., Janssen Pharmaceutical, Organon Biosciences, Sanofi-Aventis, Sepracor, Inc., Wyeth-Ayerst Pharmaceuticals; -Speaking/Honoraria: Eli Lilly and Company, GlaxoSmithKline; Advisory/Consulting: Abbott Laboratories, JDS-Noven Pharmaceuticals, Sepracor. Inc., Wyeth-Ayerst Pharmaceuticals.
Frances Hayes has had affiliations with the following: Research Support: National Institutes of Health, American Diabetes Association, Solvay Pharmaceuticals; Speaking/Honoraria: Solvay Pharmaceuticals; Advisory/Consulting: GlaxoSmithKline.
References
- 1.Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota: 1935–1984. Epilepsia. 1993;34:453–468. doi: 10.1111/j.1528-1157.1993.tb02586.x. [DOI] [PubMed] [Google Scholar]
- 2.Hauser WA, Annegers JF, Kurland LT. Prevalence of epilepsy in Rochester, Minnesota: 1940–1980. Epilepsia. 1991;32:429–445. doi: 10.1111/j.1528-1157.1991.tb04675.x. [DOI] [PubMed] [Google Scholar]
- 3.Tharp BR. Epilepsy in infants and children. In: Rakel RE, editor. Conn’s Current Therapy. W. B. Saunders Co.; Philadelphia, PA: 1998. p. 883. [Google Scholar]
- 4.Lewinsohn PM, Klein DN, Seeley JR. Bipolar disorder during adolescence and young adulthood in a community sample. Bipolar Disord. 2000;2:281–293. doi: 10.1034/j.1399-5618.2000.20309.x. [DOI] [PubMed] [Google Scholar]
- 5.Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch. Gen. Psychiatry. 2007;64:543–552. doi: 10.1001/archpsyc.64.5.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suppes T, Dennehy EB, Gibbons EW. The longitudinal course of bipolar disorder. J. Clin. Psychiatry. 2000;61(Suppl 9):23–30. [PubMed] [Google Scholar]
- 7.Baldassano CF, Marangell L, Nassir Ghaemi S, et al. Gender differences in bipolar disorder: retrospective data from the first 500 STEP-BD participants. Bipolar. Disord. 2005;7(5):465–470. doi: 10.1111/j.1399-5618.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- 8.Moreno C, Laje G, Blanco C, et al. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch. Gen. Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- 9.Dalsgaard-Nielsen T. Some aspects of the epidemiology of migraine in Denmark. Headache. 1970;10:14–23. doi: 10.1111/j.1526-4610.1970.hed1001014.x. [DOI] [PubMed] [Google Scholar]
- 10.Sillanpaa M. Changes in the prevalence of migraine and other headaches during the first seven school years. Headache. 1983;23:15–19. doi: 10.1111/j.1526-4610.1983.hed2301015.x. [DOI] [PubMed] [Google Scholar]
- 11.Vahlquist B. Migraine in children. Int. Arch. Allergy Appl. Immunol. 1955;7:348–355. doi: 10.1159/000228238. [DOI] [PubMed] [Google Scholar]
- 12.Zwart JA, Dyb G, Holmen TL, et al. The prevalence of migraine and tension-type headaches among adolescents in Norway. The Nord-Trondelag Health Study (Head-HUNT-Youth), a large population-based epidemiological study. Cephalalgia. 2004;24:373–379. doi: 10.1111/j.1468-2982.2004.00680.x. [DOI] [PubMed] [Google Scholar]
- 13.Bilo L, Meo R, Valentino R, et al. Abnormal pattern of luteinizing hormone pulsatility in women with epilepsy. Fertil. Steril. 1991;55:705–711. doi: 10.1016/s0015-0282(16)54234-4. [DOI] [PubMed] [Google Scholar]
- 14.Herzog AG, Seibel MM, Schomer DL, et al. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch. Neurol. 1986;43:341–346. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- 15.Nappi C, Meo R, Di Carlo C, et al. Reduced fertility and neuroendocrine dysfunction in women with epilepsy. Gynecol. Endocrinol. 1994;8:133–145. doi: 10.3109/09513599409058035. [DOI] [PubMed] [Google Scholar]
- 16.Bilo L, Meo R. Epilepsy and polycystic ovary syndrome: where is the link? Neurol. Sci. 2006;27:221–230. doi: 10.1007/s10072-006-0675-y. [DOI] [PubMed] [Google Scholar]
- 17.Klein P, Serje A, Pezzullo JC. Premature ovarian failure in women with epilepsy. Epilepsia. 2001;42:1584–1589. doi: 10.1046/j.1528-1157.2001.13701r.x. [DOI] [PubMed] [Google Scholar]
- 18.Bilo L, Meo R, Valentino R, et al. Characterization of the reproductive endocrine disorders in women with epilepsy. J. Clin. Endocrinol. Metab. 2001;86:2950–2956. doi: 10.1210/jcem.86.7.7633. [DOI] [PubMed] [Google Scholar]
- 19.Isojarvi JI, Laatikainen TJ, Pakarinen AJ, et al. Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N. Engl. J. Med. 1993;329:1383–1388. doi: 10.1056/NEJM199311043291904. [DOI] [PubMed] [Google Scholar]
- 20.Luef G, Abraham I, Haslinger M, et al. Polycystic ovaries, obesity and insulin resistance in women with epilepsy: a comparative study of carbamazepine and valproic acid in 105 women. J. Neurol. 2002;249:835–841. doi: 10.1007/s00415-002-0731-3. [DOI] [PubMed] [Google Scholar]
- 21.Murialdo G, Galimberti CA, Magri F, et al. Menstrual cycle and ovary alterations in women with epilepsy on antiepileptic therapy. J. Endocrinol. Invest. 1997;20:519–526. doi: 10.1007/BF03348013. [DOI] [PubMed] [Google Scholar]
- 22.Morrell MJ, Hayes FJ, Sluss PM, et al. Hyperandrogenism, ovulatory dysfunction, and PCOS with valproate vs lamotrigine. Ann. Neurol. 2008 doi: 10.1002/ana.21411. In press. [DOI] [PubMed] [Google Scholar]
- 23.Murphy MK, Hall JE, Adams JM, et al. Polycystic ovarian morphology in normal women does not predict the development of polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2006;91:3878–3884. doi: 10.1210/jc.2006-1085. [DOI] [PubMed] [Google Scholar]
- 24.Mikkonen K, Vainionpaa LK, Pakarinen AJ, et al. Long-term reproductive endocrine health in young women with epilepsy during puberty. Neurology. 2004;62:445–450. doi: 10.1212/01.wnl.0000106942.35533.62. [DOI] [PubMed] [Google Scholar]
- 25.Herzog AG. A relationship between particular reproductive endocrine disorders and the laterality of epileptiform discharges in women with epilepsy. Neurology. 1993;43:1907–1910. doi: 10.1212/wnl.43.10.1907. [DOI] [PubMed] [Google Scholar]
- 26.Bilo L, Meo R, Nappi C, et al. Reproductive endocrine disorders in women with primary generalized epilepsy. Epilepsia. 1988;29:612–619. doi: 10.1111/j.1528-1157.1988.tb03770.x. [DOI] [PubMed] [Google Scholar]
- 27.Lofgren E, Mikkonen K, Tolonen U, et al. Reproductive endocrine function in women with epilepsy: the role of epilepsy type and medication. Epilepsy Behav. 2007;10:77–83. doi: 10.1016/j.yebeh.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 28.Joffe H, Kim DR, Foris JM, et al. Menstrual dysfunction prior to onset of psychiatric illness is reported more commonly by women with bipolar disorder than by women with unipolar depression and healthy controls. J. Clin. Psychiatry. 2006;67:297–304. doi: 10.4088/jcp.v67n0218. [DOI] [PubMed] [Google Scholar]
- 29.Joffe H, Cohen LS, Suppes T, et al. Valproate is associated with new-onset oligoamenorrhea with hyperandrogenism in women with bipolar disorder. Biol. Psychiatry. 2006;59(11):1078–1086. doi: 10.1016/j.biopsych.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Betts T, Yarrow H, Dutton N, et al. A study of anticonvulsant medication on ovarian function in a group of women with epilepsy who have only ever taken one anticonvulsant compared with a group of women without epilepsy. Seizure. 2003;12:323–329. doi: 10.1016/s1059-1311(03)00065-7. [DOI] [PubMed] [Google Scholar]
- 31.Morrell MJ, Giudice L, Flynn KL, et al. Predictors of ovulatory failure in women with epilepsy. Ann. Neurol. 2002;52:704–711. doi: 10.1002/ana.10391. [DOI] [PubMed] [Google Scholar]
- 32.Morrell MJ, Isojarvi J, Taylor AE, et al. Higher androgens and weight gain with valproate compared with lamotrigine for epilepsy. Epilepsy Res. 2003;54:189–199. doi: 10.1016/s0920-1211(03)00085-8. [DOI] [PubMed] [Google Scholar]
- 33.Prabhakar S, Sahota P, Kharbanda PS, et al. Sodium valproate, hyperandrogenism and altered ovarian function in Indian women with epilepsy: a prospective study. Epilepsia. 2007;48:1371–1377. doi: 10.1111/j.1528-1167.2007.01100.x. [DOI] [PubMed] [Google Scholar]
- 34.Bauer J, Jarre A, Klingmuller D, Elger CE. Polycystic ovary syndrome in patients with focal epilepsy: a study in 93 women. Epilepsy Res. 2000;41:163–167. doi: 10.1016/s0920-1211(00)00139-x. [DOI] [PubMed] [Google Scholar]
- 35.Isojarvi JI, Rattya J, Myllyla VV, et al. Valproate, lamotrigine, and insulin-mediated risks in women with epilepsy. Ann. Neurol. 1998;43:446–451. doi: 10.1002/ana.410430406. [DOI] [PubMed] [Google Scholar]
- 36.Joffe H, Taylor AE, Hall JE. Polycystic ovarian syndrome—relationship to epilepsy and antiepileptic drug therapy. J. Clin. Endocrinol. Metab. 2001;86:2946–2949. doi: 10.1210/jcem.86.7.7788. [DOI] [PubMed] [Google Scholar]
- 37.Joffe H, Hall JE, Taylor AE, et al. A putative relationship between valproic acid and polycystic ovarian syndrome: implications for treatment of women with seizure and bipolar disorder. Harv. Rev. Psychiatry. 2003;11(2):99–108. doi: 10.1080/10673220303957. [DOI] [PubMed] [Google Scholar]
- 38.Rasgon NL, Altshuler LL, Fairbanks L, et al. Reproductive function and risk for PCOS in women treated for bipolar disorder. Bipolar Disord. 2005;7:246–259. doi: 10.1111/j.1399-5618.2005.00201.x. [DOI] [PubMed] [Google Scholar]
- 39.Joffe H, Cohen LS, Suppes T, et al. Longitudinal follow-up of reproductive and metabolic features of valproate-associated polycystic ovarian syndrome features: a preliminary report. Biol. Psychiatry. 2006;60:1378–1381. doi: 10.1016/j.biopsych.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 40.de Vries L, Karasik A, Landau Z, et al. Endocrine effects of valproate in adolescent girls with epilepsy. Epilepsia. 2007;48:470–477. doi: 10.1111/j.1528-1167.2006.00953.x. [DOI] [PubMed] [Google Scholar]
- 41.El-Khayat HA, Abd El-Basset FZ, Tomoum HY, et al. Physical growth and endocrinal disorders during pubertal maturation in girls with epilepsy. Epilepsia. 2004;45:1106–1115. doi: 10.1111/j.0013-9580.2004.66303.x. [DOI] [PubMed] [Google Scholar]
- 42.Rattya J, Turkka J, Pakarinen AJ, et al. Reproductive effects of valproate, carbamazepine, and oxcarbazepine in men with epilepsy. Neurology. 2001;56:31–36. doi: 10.1212/wnl.56.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Vainionpaa LK, Rattya J, Knip M, et al. Valproate-induced hyperandrogenism during pubertal maturation in girls with epilepsy. Ann. Neurol. 1999;45:444–450. doi: 10.1002/1531-8249(199904)45:4<444::aid-ana5>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Rattya J, Vainionpaa L, Knip M, et al. The effects of valproate, carbamazepine, and oxcarbazepine on growth and sexual maturation in girls with epilepsy. Pediatrics. 1999;103:588–593. doi: 10.1542/peds.103.3.588. [DOI] [PubMed] [Google Scholar]
- 45.Lemarchand-Beraud T, Zufferey MM, Reymond M, Rey I. Maturation of the hypothalamo-pituitaryovarian axis in adolescent girls. J. Clin. Endocrinol. Metab. 1982;54:241–246. doi: 10.1210/jcem-54-2-241. [DOI] [PubMed] [Google Scholar]
- 46.Lundberg B, Nergardh A, Ritzen EM, Samuels-Son K. Influence of valproic acid on the gonadotropin-releasing hormone test in puberty. Acta Paediatr. Scand. 1986;75:787–792. doi: 10.1111/j.1651-2227.1986.tb10291.x. [DOI] [PubMed] [Google Scholar]
- 47.Cook JS, Bale JF, Hoffman RP. Pubertal arrest associated with valproic acid therapy. Pediatr. Neurol. 1992;8:229–231. doi: 10.1016/0887-8994(92)90075-a. [DOI] [PubMed] [Google Scholar]
- 48.Laforet GA, Apostol G, Robieson WZ, et al. Reproductive endocrine effects of divalproex sodium extended-release in adolescent females. Child Neurology Society, 36th Annual Meeting; Quebec City, Canada. 2007. [Google Scholar]
- 49.Nelson-Degrave VL, Wickenheisser JK, Cockrell JE, et al. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology. 2004;145:799–808. doi: 10.1210/en.2003-0940. [DOI] [PubMed] [Google Scholar]
- 50.Wood JR, Nelson-Degrave VL, Jansen E, et al. Valproate-induced alterations in human theca cell gene expression: clues to the association between valproate use and metabolic side effects. Physiol. Genom. 2005;20:233–243. doi: 10.1152/physiolgenomics.00193.2004. [DOI] [PubMed] [Google Scholar]
- 51.Fluck CE, Yaworsky DC, Miller WL. Effects of anticonvulsants on human p450c17 (17alpha-hydroxylase/17,20 lyase) and 3beta-hydroxysteroid dehydrogenase type 2. Epilepsia. 2005;46:444–448. doi: 10.1111/j.0013-9580.2005.38404.x. [DOI] [PubMed] [Google Scholar]
- 52.Herzog AG. Polycystic ovarian syndrome in women with epilepsy: epileptic or iatrogenic? Ann. Neurol. 1996;39:559–560. doi: 10.1002/ana.410390503. [DOI] [PubMed] [Google Scholar]
- 53.Misra M, Papakostas GI, Klibanski A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J. Clin. Psychiatry. 2004;65:1607–1618. doi: 10.4088/jcp.v65n1205. [DOI] [PubMed] [Google Scholar]
- 54.Joffe H. Reproductive biology and psychotropic treatments in premenopausal women with bipolar disorder. J. Clin. Psychiatry. 2007;68(Suppl 9):10–15. [PubMed] [Google Scholar]
- 55.Crawford AM, Beasley CM, Tollefson GD. The acute and long-term effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr. Res. 1997;26:41–54. doi: 10.1016/S0920-9964(97)00036-4. [DOI] [PubMed] [Google Scholar]
- 56.Tollefson GD, Beasley CM, Tran PV, et al. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am. J. Psychiatry. 1997;154:457–465. doi: 10.1176/ajp.154.4.457. [DOI] [PubMed] [Google Scholar]
- 57.Tran PV, Hamilton SH, Kuntz AJ, et al. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J. Clin. Psychopharmacol. 1997;17:407–418. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- 58.Svestka J, Synek O, Tomanova J, et al. Differences in the effect of second-generation antipsychotics on prolactinaemia: six weeks open-label trial in female inpatients. Neuro Endocrinol. Lett. 2007;28:881–818. [PubMed] [Google Scholar]
- 59.Correll CU, Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J. Am. Acad. Child Adolesc. Psychiatry. 2006;45:771–791. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- 60.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Hyperprolactinemia in response to antipsychotic drugs: characterization across comparative clinical trials. Psychoneuroendocrinology. 2003;28(Suppl 2):69–82. doi: 10.1016/s0306-4530(02)00128-2. [DOI] [PubMed] [Google Scholar]
- 61.Kinon BJ, Gilmore JA, Liu H, Halbreich UM. Prevalence of hyperprolactinemia in schizophrenic patients treated with conventional antipsychotic medications or risperidone. Psychoneuroendocrinology. 2003;28(Suppl 2):55–68. doi: 10.1016/s0306-4530(02)00127-0. [DOI] [PubMed] [Google Scholar]
- 62.Findling RL, Kusumakar V, Daneman D, et al. Prolactin levels during long-term risperidone treatment in children and adolescents. J. Clin. Psychiatry. 2003;64:1362–1369. doi: 10.4088/jcp.v64n1113. [DOI] [PubMed] [Google Scholar]
- 63.Nordstrom AL, Farde L. Plasma prolactin and central D2 receptor occupancy in antipsychotic drugtreated patients. J. Clin. Psychopharmacol. 1998;18:305–310. doi: 10.1097/00004714-199808000-00010. [DOI] [PubMed] [Google Scholar]
- 64.Meltzer HY, Fang VS. The effect of neuroleptics on serum prolactin in schizophrenic patients. Arch. Gen. Psychiatry. 1976;33:279–286. doi: 10.1001/archpsyc.1976.01770030003001. [DOI] [PubMed] [Google Scholar]
- 65.Smith S, Wheeler MJ, Murray R, O’Keane V. The effects of antipsychotic-induced hyperprolactinaemia on the hypothalamic-pituitary-gonadal axis. J. Clin. Psychopharmacol. 2002;22:109–114. doi: 10.1097/00004714-200204000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Richelson E, Souder T. Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci. 2000;68:29–39. doi: 10.1016/s0024-3205(00)00911-5. [DOI] [PubMed] [Google Scholar]
- 67.Becker D, Liver O, Mester R, et al. Risperidone, but not olanzapine, decreases bone mineral density in female premenopausal schizophrenia patients. J. Clin. Psychiatry. 2003;64:761–766. doi: 10.4088/jcp.v64n0704. [DOI] [PubMed] [Google Scholar]
- 68.Henderson DC, Goff DC, Connolly CE, et al. Risperidone added to clozapine: impact on serum prolactin levels. J. Clin. Psychiatry. 2001;62:605–608. doi: 10.4088/jcp.v62n0805. [DOI] [PubMed] [Google Scholar]
- 69.Kearns AE, Goff DC, Hayden DL, Daniels GH. Risperidone-associated hyperprolactinemia. Endocr. Pract. 2000;6:425–429. doi: 10.4158/EP.6.6.425. [DOI] [PubMed] [Google Scholar]
- 70.Turrone P, Kapur S, Seeman MV, Flint AJ. Elevation of prolactin levels by atypical antipsychotics. Am. J. Psychiatry. 2002;159:133–135. doi: 10.1176/appi.ajp.159.1.133. [DOI] [PubMed] [Google Scholar]
- 71.Volavka J, Czobor P, Cooper TB, et al. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. J. Clin. Psychiatry. 2004;65:57–61. doi: 10.4088/jcp.v65n0109. [DOI] [PubMed] [Google Scholar]
- 72.Kleinberg DL, Davis JM, de Coster R, et al. Prolactin levels and adverse events in patients treated with risperidone. J. Clin. Psychopharmacol. 1999;19:57–61. doi: 10.1097/00004714-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Magharious W, Goff DC, Amico E. Relationship of gender and menstrual status to symptoms and medication side effects in patients with schizophrenia. Psychiatry Res. 1998;77:159–166. doi: 10.1016/s0165-1781(97)00137-6. [DOI] [PubMed] [Google Scholar]
- 74.Polishuk WZ, Kulcsar S. Effects of chlorpromazine on pituitary function. J. Clin. Endocrinol. Metab. 1956;16:292–293. doi: 10.1210/jcem-16-2-292. [DOI] [PubMed] [Google Scholar]
- 75.Smith SM, O’Keane V, Murray R. Sexual dysfunction in patients taking conventional antipsychotic medication. Br. J. Psychiatry. 2002;181:49–55. doi: 10.1192/bjp.181.1.49. [DOI] [PubMed] [Google Scholar]
- 76.Ghadirian AM, Chouinard G, Annable L. Sexual dysfunction and plasma prolactin levels in neuroleptic-treated schizophrenic outpatients. J. Nerv. Ment. Dis. 1982;170:463–467. doi: 10.1097/00005053-198208000-00004. [DOI] [PubMed] [Google Scholar]
- 77.Windgassen K, Wesselmann U, Schulze Monking H. Galactorrhea and hyperprolactinemia in schizophrenic patients on neuroleptics: frequency and etiology. Neuropsychobiology. 1996;33:142–146. doi: 10.1159/000119265. [DOI] [PubMed] [Google Scholar]
- 78.Bobes J, Garc APMP, Rejas J, et al. Frequency of sexual dysfunction and other reproductive side-effects in patients with schizophrenia treated with risperidone, olanzapine, quetiapine, or haloperidol: the results of the EIRE study. J. Sex. Marital. Ther. 2003;29:125–147. doi: 10.1080/713847170. [DOI] [PubMed] [Google Scholar]
- 79.Kim KS, Pae CU, Chae JH, et al. Effects of olanzapine on prolactin levels of female patients with schizophrenia treated with risperidone. J. Clin. Psychiatry. 2002;63:408–413. doi: 10.4088/jcp.v63n0506. [DOI] [PubMed] [Google Scholar]
- 80.Keller R, Mongini F. Switch to quetiapine in antipsychotic agent-related hyperprolactinemia. Neurol. Sci. 2002;23:233–235. doi: 10.1007/s100720200047. [DOI] [PubMed] [Google Scholar]
- 81.Takahashi H, Higuchi H, Kamata M, et al. Effectiveness of switching to quetiapine for neuroleptic-induced amenorrhea. J. Neuropsychiatry Clin. Neurosci. 2003;15:375–377. doi: 10.1176/jnp.15.3.375. [DOI] [PubMed] [Google Scholar]
- 82.Lee BH, Kim YK, Park SH. Using aripiprazole to resolve antipsychotic-induced symptomatic hyperprolactinemia: a pilot study. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2006;30:714–717. doi: 10.1016/j.pnpbp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 83.Weiden PJ, Daniel DG, Simpson G, Romano SJ. Improvement in indices of health status in outpatients with schizophrenia switched to ziprasidone. J. Clin. Psychopharmacol. 2003;23:595–600. doi: 10.1097/01.jcp.0000095347.32154.08. [DOI] [PubMed] [Google Scholar]
- 84.Shim JC, Shin JG, Kelly DL, et al. Adjunctive treatment with a dopamine partial agonist, aripiprazole, for antipsychotic-induced hyperprolactinemia: a placebo-controlled trial. Am. J. Psychiatry. 2007;164:1404–1410. doi: 10.1176/appi.ajp.2007.06071075. [DOI] [PubMed] [Google Scholar]
- 85.Ataya K, Mercado A, Kartaginer J, et al. Bone density and reproductive hormones in patients with neuroleptic-induced hyperprolactinemia. Fertil. Steril. 1988;50:876–881. doi: 10.1016/s0015-0282(16)60365-5. [DOI] [PubMed] [Google Scholar]
- 86.Bilici M, Cakirbay H, Guler M, et al. Classical and atypical neuroleptics, and bone mineral density, in patients with schizophrenia. Int. J. Neurosci. 2002;112:817–828. doi: 10.1080/00207450290025833. [DOI] [PubMed] [Google Scholar]
- 87.Woods SW, Martin A, Spector SG, Mc-Glashan TH. Effects of development on olanzapine-associated adverse events. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:1439–1446. doi: 10.1097/00004583-200212000-00015. [DOI] [PubMed] [Google Scholar]
- 88.Wudarsky M, Nicolson R, Hamburger SD, et al. Elevated prolactin in pediatric patients on typical and atypical antipsychotics. J. Child Adolesc. Psychopharmacol. 1999;9:239–245. doi: 10.1089/cap.1999.9.239. [DOI] [PubMed] [Google Scholar]
- 89.Seeman P, Bzowej NH, Guan HC, et al. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- 90.Becker AL, Epperson CN. Female puberty: clinical implications for the use of prolactin-modulating psychotropics. Child Adolesc. Psychiatr. Clin. N. Am. 2006;15:207–220. doi: 10.1016/j.chc.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 91.Cohen LG, Biederman J. Treatment of risperidone-induced hyperprolactinemia with a dopamine agonist in children. J. Child Adolesc. Psychopharmacol. 2001;11:435–440. doi: 10.1089/104454601317261618. [DOI] [PubMed] [Google Scholar]