Abstract
D-serine, an endogenous co-agonist of NMDA receptors in vertebrate retina, may modulate glutamate sensitivity of retinal neurons. This study determined at the functional and molecular level the transport process responsible for D-serine in retinal Müller cells. RT-PCR and immunoblotting showed that serine racemase (SR), the synthesizing enzyme for D-serine, is expressed in the rMC-1 Müller cell line and primary cultures of mouse Müller cells (1°MCs). The relative contributions of different amino acid transport systems to D-serine uptake were determined based on differential substrate specificities and ion dependencies. D-serine uptake was obligatorily dependent on Na+, eliminating Na+-independent transporters (asc-1 and system L) for D-serine in Müller cells. The Na+:substrate stoichiometry for the transport process was 1:1. D-serine transport was inhibited by alanine, serine, cysteine, glutamine, and asparagine, but not anionic amino acids or cationic amino acids, suggesting that D-serine transport in Müller cells occurs via ASCT2 rather than ASCT1 or ATB0,+. The expression of mRNAs specific for ASCT1, ASCT2, and ATB0,+ was analyzed by RT-PCR confirming the expression of ASCT2 (and ASCT1) mRNA, but not ATB0,+, in Müller cells. Immunoblotting detected ASCT2 in neural retina and in 1°MCs; immunohistochemistry confirmed these data in retinal sections and in cultures of 1°MCs. The efflux of D-serine via ASCT2 by ASCT2 substrates was demonstrable using the Xenopus laevis oocyte heterologous expression system. These data provide the first molecular evidence for SR and ASCT2 expression in a Müller cell line and in 1°MCs and suggest that D-serine, synthesized in Müller cells by SR, is effluxed via ASCT2 to regulate NMDA receptors in adjacent neurons.
Keywords: D-serine, retinal transport, retinal Müller cells, serine racemase, neurotransmitter
Introduction
D-serine can act as a neurotransmitter activating the ‘glycine’ site of the N-methyl-D-aspartate (NMDA) subtype of glutamate receptors with a potency three-fold greater than glycine (Mothet et al, 2000). It is synthesized from L-serine by serine racemase (SR), which is localized to regions of the brain that are enriched in NMDA receptors (reviewed in Mustafa et al, 2004), particularly astrocytic glia that ensheath synapses.
Mechanisms for storage and release of D-serine have been studied in astrocytes from the brain. Initial studies of D-serine release performed by Schell and colleagues suggested that AMPA/KA receptors mediate release of D-serine (Schell et al, 1995). Work by Mothet and co-workers, using a sensitive assay to monitor D-serine release, showed that ∼40% of D-serine is stored in small, synaptic-like vesicles, which are VAMP2-positive; release of D-serine from these vesicles is calcium-sensitive (Mothet et al, 2005). Recent studies using C6 glioma cells demonstrated that the binding of SR by glutamate receptor interacting protein (GRIP) augments the activity of the enzyme and D-serine release (Kim et al, 2005).
Once released into the extracellular milieu, neurotransmitters must be removed/recycled, a process that requires an uptake system. Studies using C6 glioma cells or primary astrocyte cultures from rat brain investigated the potential role of various amino acid transport systems for the uptake of D-serine. Using known substrates for a variety of neutral amino acid transporters (ASCT1, ASCT2, system A, system L), functional evidence suggested that D-serine uptake is mediated by the ASC type of transporters (Hayashi et al, 1997; Ribeiro et al, 2002), though no functional studies were performed to distinguish between ASCT1 and ASCT2 transporters, nor were molecular analyses performed to determine which transporter mediated D-serine transport.
Recently, the retina has been analyzed for the presence of D-serine and SR and both have been localized, in several species, to the major glial cell type in the retina, the Müller cells, as well as to astrocytes (Stevens et al, 2003). In whole retina preparations from the larval tiger salamander, O'Brien and colleagues explored the possibility that neutral amino acid transporters mediate D-serine uptake, postulating that an amino acid exchanger that can take up and release D-serine would offer a mechanism for regulation of D-serine levels in retina (O'Brien et al, 2005). These studies used a novel capillary electrophoresis method and demonstrated that D-serine uptake was Na+-dependent, thus ruling out the Na+-independent asc-1 transporter (SLC7A10), which mediates transport of small neutral L- and D- amino acids, as a transporter for D-serine in retina. Their findings that the Na+-dependent uptake of D-serine was inhibited by L-alanine, L-serine and L-cysteine were consistent with transport mediated by the ASC types of neutral amino acid transporters. The ASCT1 (SLC1A4, alias: SATT) and ASCT2 (SLC1A5, alias: ATB0) transport systems are Na+-dependent and have high affinity for alanine, serine, and cysteine. They exhibit distinct substrate selectivity; in addition to the common substrates of ASCT transporters, ASCT2 also accepts glutamine and asparagine as high affinity substrates, whereas ASCT1 does not (Kanai and Hediger, 2003). The studies by O'Brien et al (2005) did not distinguish between the ASCT1 or the ASCT2 transporters, nor did they rule out transport by a unique amino acid transporter ATB0,+, which is energized by Na+- and Cl-- gradients and membrane potential. ATB0,+ (SLC6A14) has broad substrate specificity and concentrative ability and recognizes neutral as well as cationic amino acids. ASCT2 as well as ATB0,+ have been shown to mediate D-serine uptake (Thongsong et al, 2005; Hatanaka et al, 2002). Immunohistochemical analysis of ocular tissues using an antibody specific for ATB0,+ provided evidence that this transporter is present in mammalian retina (Hatanaka et al, 2004).
Given that in retina, D-serine has been shown to be abundant in Müller cells (O'Brien et al, 2005), we sought to analyze the mechanism(s) of D-serine uptake in these cells using a rat Müller cell line (rMC-1) (Sarthy et al, 1998) as well as primary cultures of Müller cells isolated from mouse retina (Umapathy et al, 2005; Jiang et al, In Press). Our approach used functional methods to distinguish among the three likely transporter candidates (ASCT1, ASCT2, ATB0,+), and used molecular methods to establish the identity of the transporter likely to be responsible for the uptake and/or efflux of D-serine in Müller cells.
Materials and Methods
Reagents
Reagents used in this study were obtained from the following vendors: Dulbecco's modified Eagle's medium F12 (DMEM: F12) medium, TRIzol reagent, and penicillin-streptomycin (Gibco-Life Technologies, Rockville, MD), fetal bovine serum, L-glutamine, L-alanine, L-glutamate, D-serine, L-lysine, N-methyl-D-glucamine, D-gluconic acid sodium salt, D-gluconic acid calcium salt and D-gluconic acid potassium salt (Sigma Chemical Company, St. Louis, MO), collagenase type 2 (Worthington, Lakewood, NJ), [3H] D-serine (Moravek Biochemicals, Brea, CA), purified mouse anti-serine racemase monoclonal antibody (BD Transduction Laboratories, San Diego, CA), anti-ASCT2 polyclonal antibody (Chemicon International, Temecula, CA), Alexa Fluor 488-conjugated anti-goat IgG (In Vitrogen, Carlsbad, CA); goat anti-mouse IgG-HRP and goat anti-rabbit IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA), ECL detection kit (Pierce Biotechnology, Rockford, IL), RNA PCR kit (Applied Biosystems, Branchburg, NJ), PCR kit (TaKaRa Bio, Otsu, Shiga, Japan), sense and antisense primers for ASCT1, ASCT2 and SR (Integrated DNA Technologies, Coralville, IA). The rat retinal Müller cell line (rMC-1) was a kind gift of Dr. V. Sarthy (Northwestern University, Evanston, IL).
Cell culture
The development of the rat Müller cell line (rMC-1) has been described (Sarthy et al, 1998). Primary cultures of Müller cells (hereafter referred to as 1°MCs) were isolated from 7-10 day old C57Bl/6 mice following our published method (Umapathy et al, 2005; Jiang et al, In Press), which was adapted from that of Hicks and Courtois (1990). Mice were maintained following institutional guidelines for humane treatment of animals. rMC-1 and 1°MCs were maintained at 37°C in a humidified chamber of 5% CO2 in DMEM/F12, supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin. The culture medium was replaced with fresh medium every other day. Upon confluence, cultures were passaged by dissociation in 0.05% (w/v) trypsin in 0.01M phosphate-buffered saline (PBS), pH 7.4.
Uptake experiment in cultured cells
rMC-1 cells and 1°MCs were seeded in 24-well culture plates at an initial density of 0.1×106 cells/well and were grown to confluence in 24-well plates. At the time of uptake experiments, the culture medium was removed and cells were washed twice with uptake buffer. To determine whether the uptake of [3H] D-serine in Müller cells was sodium and chloride-dependent or -independent, uptake was examined using different buffers. The composition of the sodium-containing uptake buffer (NaCl buffer) was 25 mM 4-(2-hydroxyethyl)-1-piperazineethansulfonic acid (HEPES)/Tris, 140 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgSO4, and 5 mM glucose, pH 7.5. For the buffer lacking sodium (NMDG buffer), 140 mM NaCl was replaced by N-methyl-D-glucamine chloride (NMDG-Cl); for the chloride-free buffer (Na-gluconate buffer), the NaCl, CaCl2, and KCl were replaced by D-gluconic acid sodium salt, D-gluconic acid calcium salt and D-gluconic acid potassium salt, respectively. Uptake was initiated by adding 250 μl uptake medium containing [3H] D-serine. Uptake measurements were done with a 30 min incubation at 37°C, which was in the linear range for uptake. Uptake was terminated by removal of the medium by aspiration followed by two washes with ice-cold uptake buffer without the radiolabeled substrates. Cells were solubilized in 0.5 ml of 0.1% sodium dodecyl sulfate (SDS) in 0.2 N NaOH and transferred to scintillation vials for quantitation of radioactivity. This protocol is the same as described previously from our laboratory for the measurement of amino acid uptake in mammalian cells (Bridges et al, 2001).
Analysis of D-serine efflux using the X. laevis oocyte heterologous expression system
Human ASCT2 cRNA was prepared using previously described methods (Torres-Zamorano et al, 1998). Briefly, the pSPORT-hASCT2 cDNA construct was linearized with NotI and the cDNA insert was transcribed in vitro using T7 RNA polymerase in the presence of RNase inhibitor and RNA cap analog. The Ambion MEGA script kit was used for this purpose. The resultant cRNA was extracted with phenol/chloroform and precipitated with ethanol. Concentration of RNA was determined by UV spectrophotometry and integrity of RNA was verified by 1% denaturing formaldehyde-agarose gel electrophoresis and visualized using ethidium bromide fluorescence.
Oocytes isolated from X. laevis were digested with collagenase A (1.6 mg/ml) in a Ca2+ -free buffer (82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2 and 5 mM Hepes/Tris, pH 7.5) for 1 h at room temperature and then manually defolliculated under a stereomicroscope. Mature (stage V-VI), defolliculated oocytes were selected and stored overnight at 18°C in modified Barth's medium (88 mM NaCl, 1 mM KCl, 2.4 mM NaHCO3, 0.82 mM MgSO4, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, and 20 mM Hepes/Tris, pH 7.5) with 10 mg/ml gentamycin sulfate. Oocytes were injected 1 day after isolation with 50 nl aqueous solution of cRNA (1 μg/μl) or 50 nl of water. The injected oocytes were stored in modified Barth's medium at 18°C for 5 days prior to uptake or efflux measurements. Uptake of D-serine in water-injected and hASCT2 cRNA-injected oocytes was determined as described previously (Torres-Zamorano et al, 1998) by incubating the oocytes with 5μM [3H] D-serine for 1 h in a NaCl-containing medium. For efflux experiments, cRNA-injected oocytes were incubated for 1 h at room temperature with 5 μM [3H] D-serine in NaCl-containing uptake buffer. Following this incubation, oocytes were washed rapidly with uptake buffer and then transferred into 1 ml NaCl-containing uptake buffer with or without unlabeled amino acids. The efflux of [3H] D-serine was measured by determining the amount of radiolabel retained in the oocytes after 30 min incubation.
RT-PCR analysis of ASCT1, ASCT2, ATB0,+ and SR mRNA in Müller cells
RNA was prepared from rMC-1 cells and 1°MCs using TRIzol reagent. RT-PCR was carried out using primer pairs specific for rat and mouse ASCT1, ASCT2, ATB0,+ and SR (see Table I). For reverse transcriptase (RT)-PCR analysis, 1 μg total RNA was reverse transcribed using the Gene-Amp RNA-PCR kit according to the manufacturer's protocol. PCR was then performed using 0.4 μmoles each of sense and antisense primers and 2.5 units of AmpliTaq DNA polymerase and the following cycling conditions: 95°C 5 min, 35 cycles of 94°C 1 min, 60°C 1 min, and 72°C 2 min, 1 cycle of 72°C 30 min. The products were size-fractionated on an agarose gel and stained by ethidium bromide.
Table I.
Primer pairs used for RT-PCR analysis of transporter proteins.
| Transporter | Sense primer | Anti-sense primer | Product size (bp) and accession number |
|---|---|---|---|
| rat ASCT1 | 5′-GTTTGCGACGGCTTTTGCGACCTG-3′ | 5′-GCATCCCCTTCCACGTTCACCACA-3′ | 399 bp; AB103401 |
| mouse ASCT1 | 5′-GTCTGCAACCGATTACACA-3′ | 5′-ACCTGCTGATCCTCTTGTCTA-3′ | 536 bp; U75215 |
| rat ASCT2 | 5′-GCGCCTGGGCCCTGCTCTTTTT-3′ | 5′-ACAATCTTGCCGGCCACCAGGAAC-3′ | 478 bp; AJ132846 |
| mouse ASCT2 | 5′-CGCAGTGCACCAACCAAA-3′ | 5′-CGGGTGAAGAGGAAGTAGATG-3′ | 506 bp; D85044 |
| rat ATB0,+ | 5′-CTGCCATGGGCTAATTGTTC-3′ | 5′-CCGGATATATGAGCCATGTG-3′ | 635 bp, cloned sequence, unpublished |
| mouse ATB0,+ | 5′-TGGACAGATTGAAGTGCCCGAACT-3′ | 5′-TCCTGGC CGAAAAACTTCACTACC-3′ | 617 bp, BC054765 |
| rat serine racemase | 5′-CCCAAAGCCGTTGTTACTCACA-3′ | 5′-CATTGGAAGGTTCAGCAGCGTACA-3′ | 415 bp; NM_198757 |
Immunodetection of SR and ASCT2
Western blot analysis was performed to determine whether SR was detectable in the Müller cell line rMC-1 and in 1°MCs. Cells were grown to confluence and subjected to SDS-PAGE following our published methods (Dunn et al, 2006). Protein concentrations were estimated according to the method of Lowry et al (1951). After transferring the separated proteins onto nitrocellulose membranes, the membranes were blocked for 1.5 h at room temperature with Tris-buffered saline–0.1% Tween-20 containing 5% non-fat milk. The membranes were incubated for 2 h at room temperature with mouse monoclonal antibody against SR (1:200). The membranes were probed with a secondary HRP-conjugated goat anti-mouse IgG antibody (1:3000) for 1.5 h, washed and the proteins visualized using the ECL Western detection system. The membranes were washed and reprobed with an antibody against β-actin as an internal control.
Additional experiments were performed to detect ASCT2 in neural retina and in 1°MCs. Neural retina was isolated from mouse eye, and because ASCT2 is expressed in lung, brain and kidney (Utsunomiya-Tate et al, 1996), these tissues were used as positive controls. Protein was extracted as described (Dunn et al, 2006) and membranes were incubated for 2 h at room temperature with the polyclonal antibody against ASCT2 (1:500). The membranes were probed with a secondary HRP-conjugated goat anti-rabbit IgG antibody (1:2000) for 1.5 h. For immunohistochemical analysis of ASCT2, intact retinas and 1°MCs were used. Frozen sections of mouse retina, obtained as described in Dunn et al, 2006, were incubated with the polyclonal antibody against ASCT2 (1:50) and detected using Alexa Fluor 488-conjugated anti-goat IgG, 1°MCs were isolated as described above, plated on coverslips and subjected to immunocytochemistry with the ASCT2 antibody (1:25) followed by the Alexa Fluor 488 IgG antibody. ASCT2 was detected by epifluorescence using a Zeiss Axioplan-2 microscope (Carl Zeiss, Göttingen, Germany) and the axiovision program.
Data analysis
Each uptake experiment was performed in duplicate or triplicate and was repeated two to four times. Data analysis (analysis of variance) was performed using the SPSS statistical software package (p<0.05was considered significant). For data in which variances were equivalent the LSD post-hoc test was used and for tests in which they were not equivalent Dunnett's test was used. Kinetic analysis was done using Fig. P (version 6.0, Biosoft, Cambridge, UK). Data are presented as means ± SE.
Results
RT-PCR was used to analyze the expression of SR in rMC-1 cells. As shown in Fig. 1A, an expected 415 bp cDNA was amplified by RT-PCR with rMC-1 cell mRNA and primers specific for rat SR. Extraction of proteins from rMC-1 and 1°MCs and subsequent SDS-PAGE and immunoblotting with a mouse monoclonal antibody for SR detected only one band with the expected molecular size of ∼38 kD (Fig. 1B). The membrane was stripped and reprobed with antibody against β-actin as a loading control and a single band of molecular size ∼45kD was detected. Thus, the enzyme responsible for conversion of L- to D-serine is present in isolated Müller cells.
Figure 1. Expression of serine racemase (SR) mRNA and protein in retinal Müller cells.
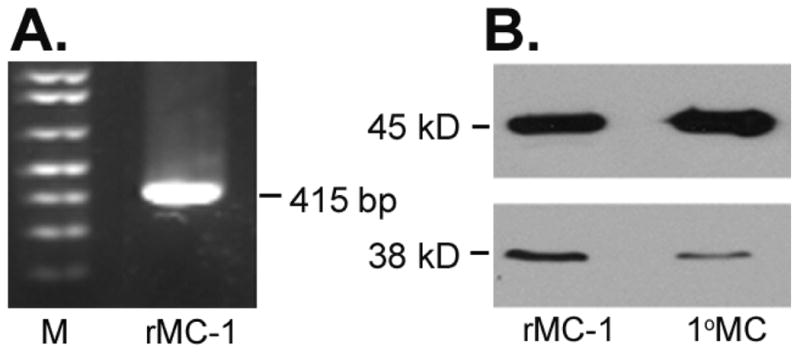
(A) Total RNA (1 ug) isolated from rMC-1 cells was reverse-transcribed into cDNA and subjected to PCR using rat-specific SR primers (415 bp) (right lane). Molecular size markers were loaded in the left lane (M). The RT-PCR products were analyzed by 1% agarose gel electrophoresis. (B) Western blot analysis of SR (Mr ∼38 kD) and β-actin (Mr ∼45 kD) with proteins extracted from the rat retinal Müller cell line (rMC-1) and primary mouse Müller cells (1°MC).
The role of Na+ as well as Cl- in the uptake of D-serine was examined in Müller cells. As shown in Fig. 2A, there was robust uptake of D-serine in rMC-1 cells when NaCl was present in the medium. The uptake decreased significantly when Cl- was omitted, but the presence of this anion was not obligatory for the uptake process because there was still substantial D-serine uptake even in the absence of Cl-. In contrast, the uptake was drastically reduced when Na+ was absent. These data show that D-serine uptake in rMC-1 cells is absolutely dependent on Na+ and that Cl- is not obligatory for the process. The uptake in the presence of NaCl was linear for at least 30 min. Therefore, subsequent uptake measurements were done with this time period to reflect linear uptake rates. The Na+-activation kinetics were analyzed to determine the Na+:D-serine stoichiometry. The dependence of D-serine uptake on Na+ concentration displayed a hyperbolic relationship and the analysis of the data by the Hill equation yielded a value of 0.8 ± 0.03 for the Hill coefficient (Fig. 2B), indicating a Na+:D-serine stoichiometry of 1:1. We confirmed the ion-dependence of D-serine uptake in 1°MCs (Fig. 2C). The uptake in these cells exhibited a similar ion-dependence as in rMC-1 cells.
Figure 2. Ion dependence of D-serine uptake in retinal Muller cells.
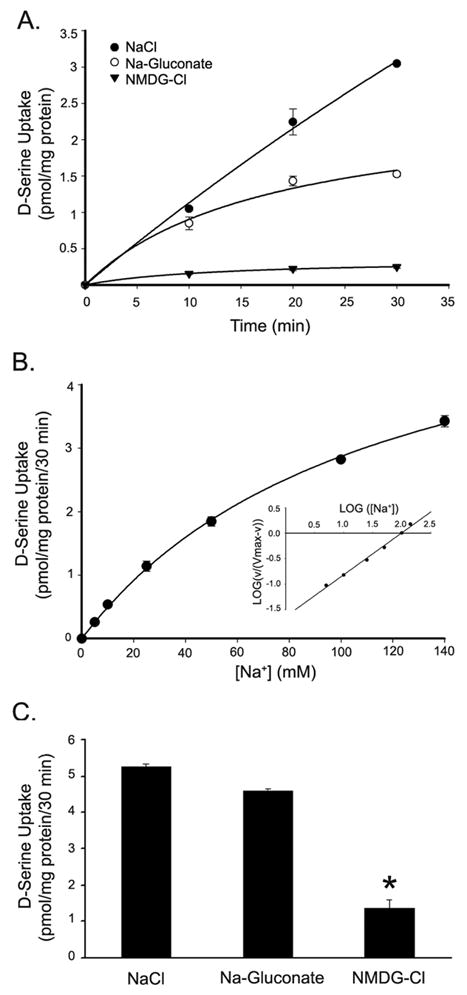
(A) Time course and ion dependence of D-serine uptake in rMC-1 cells. Uptake of [3H] D-serine (0.1 μM) in rMC-1 cells incubated at various times in NaCl-, Na-gluconate- and NMDG-Cl-containing uptake buffers. (B) Na+-activation kinetics of D-serine uptake in rMC-1 cells. Uptake of [3H] D-serine (0.1 μM) was determined following a 30 min incubation in uptake medium containing increasing concentrations of Na+ (0-140 mM). The concentration of Cl- was kept constant at 140 mM. Inset, Hill plot. (C) Ion dependence of D-serine uptake in primary Müller cells; uptake of [3H] D-serine (0.1 μM) was determined following a 30 min incubation in NaCl-, Na-gluconate- and NMDG-Cl-containing uptake medium. Values are means ± SE for three determinations from two independent experiments. (* Significantly different from NaCl value, p<0.05.)
The substrate specificity of the transport system responsible for D-serine uptake in Müller cells was examined by assessing the abilities of various amino acids to compete with D-serine uptake in the presence of NaCl. In rMC-1 cells, the uptake of D-serine was inhibited markedly by L-alanine, L-serine, L-cysteine, L-asparagine, and L-glutamine (Fig. 3A). Unlabeled D-serine competed with [3H] D-serine as expected. However, the anionic amino acids L-aspartate and L-glutamate, and the cationic amino acids L-lysine and L-arginine failed to inhibit the uptake of D-serine. Similar results were obtained for D-serine uptake in 1°MCs (Fig. 3B). These data show that the transport process that mediates the uptake of D-serine in Müller cells is specific for neutral amino acids and excludes anionic and cationic amino acids.
Figure 3. Substrate specificity of the transport system responsible for D-serine uptake in Müller cells.
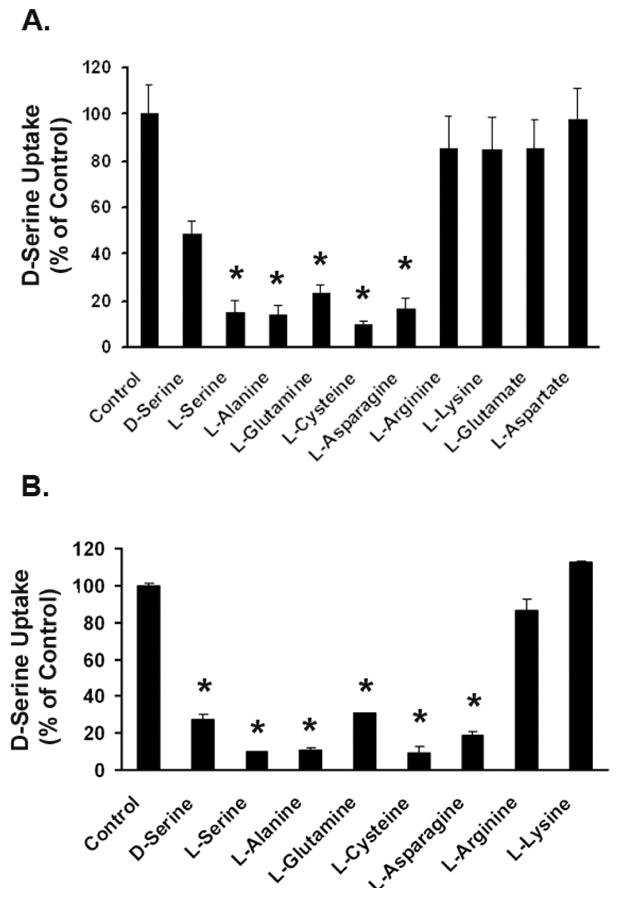
Uptake of 0.1 μM [3H] D-serine was measured in the absence or presence of various unlabeled amino acids (5 mM) in (A) rMC-1 cells and (B) 1°MCs. Uptake was measured for 30 min in the presence of NaCl. Data are presented as the percentage of control uptake (100%) measured in the absence of competing amino acids. Values are means ± SE for three determinations from two independent experiments. (* Significantly different from control value, p<0.05.)
The data in Fig. 3 indicated that even though the transport system recognized the D- and L-isomers of serine, the affinity for D-serine was lower than that for L-serine as the magnitude of inhibition with L-serine was significantly greater than with D-serine. Therefore, to compare the affinities of these two isomers more accurately, we performed a dose-response study for the inhibition of [3H] D-serine uptake by L-serine and unlabeled D-serine in rMC-1 cells and in 1°MCs (Fig. 4). In both cases, L-serine was more potent in inhibiting [3H] D-serine uptake than D-serine. The IC50 values (i.e., the concentration of serine which produces 50% of the maximum inhibitory response) for L-serine and D-serine in rMC-1 cells were 0.34 ± 0.05 and 3.4 ± 0.4 mM, respectively. The corresponding values in 1°MCs were 0.24 ± 0.05 and 1.6 ± 0.5 mM. Thus, even though D-serine is a transportable substrate for the transport system, the affinity for this isomer is approximately 10 times lower than for the L-isomer.
Figure 4. Relative affinities of D-serine and L-serine for the Na+-dependent D-serine uptake process in Müller cells.
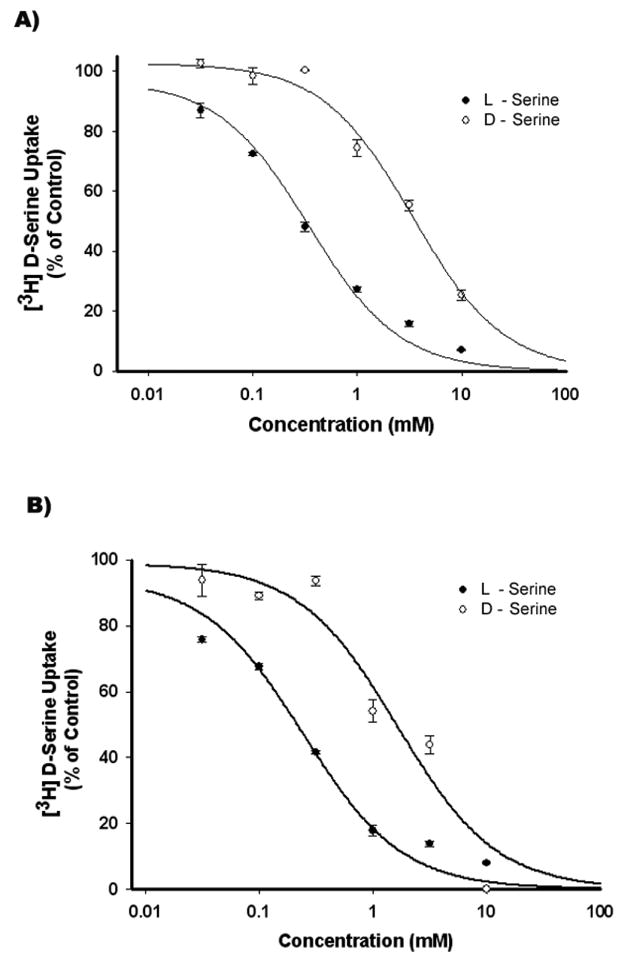
The Na+-dependent uptake of [3H] D-serine (0.1 μM) was measured for 30 min in the presence of increasing concentrations of unlabeled D-serine (○) and L-serine (●) in rMC-1 cells (A) and 1°MCs (B). Data are presented as the percentage of control uptake (100%) measured in the absence of competing amino acids.
We then addressed the molecular identity of the transporter responsible for the uptake of D-serine in these cells. The analysis focused on the Na+-dependent transporters ASCT1, ASCT2 and ATB0,+, and did not include Na+-independent transporters (system L and system asc). With RNA prepared from rMC-1 cells and 1°MCs, RT-PCR was performed with species-specific primers unique for each of these three transporters (Fig. 5). ASCT1 and ASCT2 are expressed in both cell types (Fig. 5A and 5B). ATB0,+ was not expressed in either rMC-1 or 1°MCs, though was expressed in mouse lung, used as a positive control (Fig. 5C).
Figure 5. RT-PCR analysis of the expression of ASCT1, ASCT2 and ATB0,+in rMC-1 and 1°MCs.
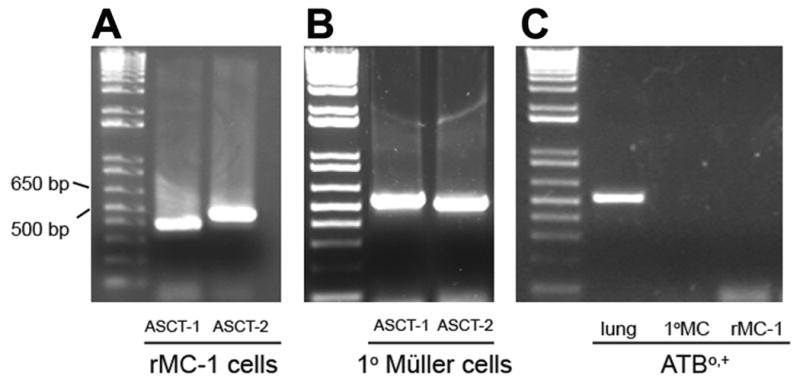
(A) Total RNA (1 μg) isolated from rMC-1 cells was reverse-transcribed into cDNA and subjected to PCR using rat-specific ASCT1 and ASCT2 primers yielding products with the expected band sizes of 399 and 478 bp, respectively. (B) Total RNA (1 ug) isolated from 1°MCs reverse-transcribed into cDNA and subjected to PCR using mouse-specific ASCT1 and ASCT2 primers yielded products with the expected sizes 536 and 506, respectively. (C) Total RNA (1μg) was isolated from mouse lung (positive control), rMC-1 and 1°MCs, reverse-transcribed into cDNA, and subjected to PCR using primers specific for mouse or rat ATB0,+. The mouse lung yielded a product with the expected band size of 617 bp; however, neither rMC-1 nor 1°MCs yielded any PCR product for ATB0,+. In each panel, the left lane represents DNA markers and the band sizes of 500 bp and 650 bp are labeled.
To confirm the presence of ASCT2 in the retina and in 1°MCs, we obtained a commercially available antibody specific for the ASCT2 transporter and used it in western blot analysis and immunohistochemistry. As shown in Fig. 6A, ASCT2 was detected by western blotting in retina isolated from mouse. Tissues known to express ASCT2 (lung, brain and kidney) were used as positive controls. In all four tissues a single band was detected that was consistent with the molecular size of ∼38-45 kDa, which is slightly less than that reported for ASCT2 in lens. Differences in the molecular weights for ASCT2 in various tissues are thought to result from differential glycosylation and/or phosphorylation of ASCT2 (Avissar et al., 2001). Similar results were obtained when 1°MCs were subjected to immunoblotting (Fig. 6B). We then used retinal cryosections obtained from mouse and cultures of 1°MC in immunohistochemical studies and found robust ASCT2 expression in the cells of the inner nuclear layer (Fig. 6C) consistent with its presence in Müller cells, which have their cell body in this retinal layer. ASCT2 was detected also in the 1°MCs (Fig. 6D). The distribution of the protein was largely cytoplasmic as shown in the inset (Fig. 6D). This is consistent with reports of ASCT2 localization in the lens by Lim et al, 2006. These data provide strong evidence for the presence of ASCT2 in Müller cells.
Figure 6. Immunodetection of ASCT2 in retina and primary mouse Müller cells (1°MCs).
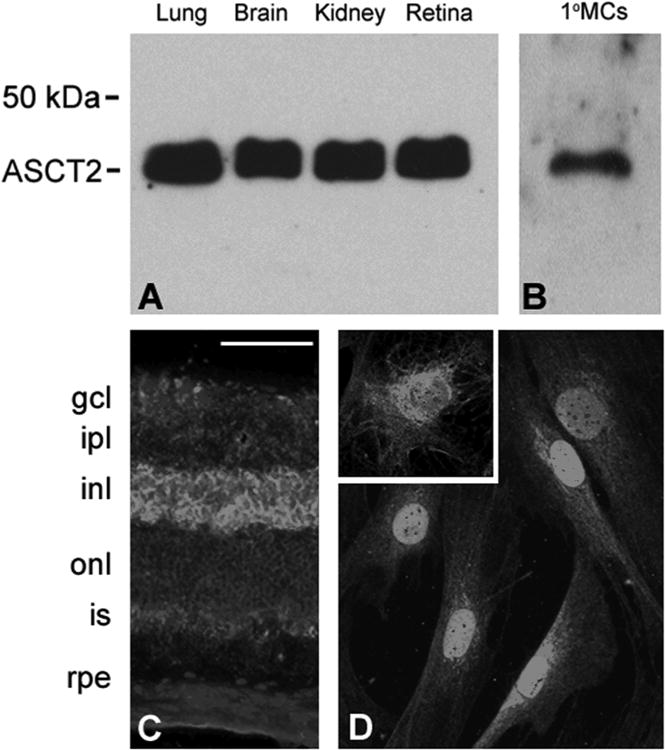
(A) Immunoblotting of lung, brain, kidney and neural retina showing detection of ASCT2 (Mr ∼36-45 kD). (B) Immunoblotting of 1°MCs to detect ASCT2. (C) Immunohistochemical detection of ASCT2 in intact retina. Cyrosections were prepared from mouse retina and subjected to immunohistochemistry as described to detect ASCT2 followed by the Alexafluor 488 conjugated secondary antibody (green staining); DAPI was used to label nuclei (blue). Calibration bar = 50 μm. (D) ASCT2 was detected in 1° MCs, and as shown in the inset, the labeling in these primary cells was largely cytoplasmic.
A key feature of ASC-type transporters is that they do not function as unidirectional transporters. Instead, they function as amino acid exchangers in a Na+-coupled manner (Kanai and Hediger, 2003). The Na+-coupled entry of amino acids in exchange for Na+-coupled exit of amino acids is obligatory for the transport function of ASCT1 and ASCT2. Our functional studies point to ASCT2 as the transporter responsible for D-serine uptake in Müller cells. Even though we studied the transport function of this transporter only by monitoring the Na+-coupled entry of D-serine into cells, the transporter should also be able to mediate the efflux of D-serine when ASCT2 substrates are present in the external medium. Previously, we characterized the cloned human ASCT2 for its amino acid exchange characteristic using the X. oocyte heterologous expression system (Torres-Zamorano et al, 1998). However, the ability of ASCT2 to mediate the efflux of D-serine in the presence of amino acid substrates on the trans-side has not been studied. Therefore, the X. laevis oocyte expression system was used to examine the ability of the cloned human ASCT2 to mediate the efflux of D-serine coupled to the entry of ASCT2 substrates. The transporter was expressed in oocytes by injection of ASCT2 cRNA. Water-injected oocytes were used as control. The uptake of [3H] D-serine (5 μM) in water-injected oocytes was compared with that of cRNA-injected oocytes in the presence of NaCl (Fig. 7A). The uptake in cRNA-injected oocytes was ∼3.5-fold higher than in water-injected oocytes, demonstrating the functional expression of the transporter. Additionally, ASCT2-expressing oocytes were preincubated with 5 μM [3H] D-serine and then monitored for the efflux of radiolabel in the presence of 2.5 mM alanine, glutamine, or glutamate in a NaCl-containing buffer. Following the 30 min incubation, the radiolabel retained in the oocytes was determined. We found that the presence of alanine and glutamine on the trans-side decreased the amount of radiolabel retained in the oocytes, suggesting that these two amino acids, both of which are substrates for ASCT2, stimulated the efflux of D-serine (Fig. 7B). In contrast, glutamate, which is not a substrate for ASCT2, had little or no effect on D-serine efflux. These data show that ASCT2 can mediate the efflux of D-serine in response to the presence of ASCT2 substrates on the trans-side.
Fig. 7. [3H]-D-serine efflux in hASCT2-expressing oocytes.
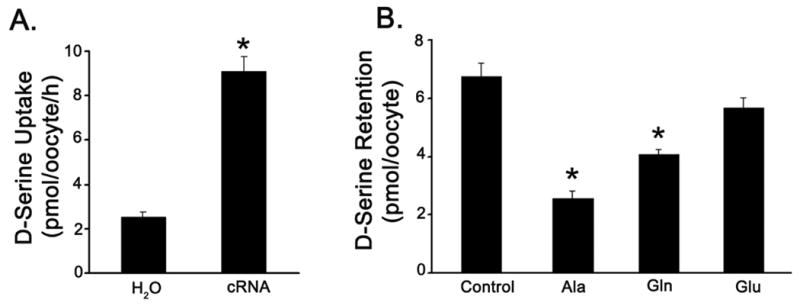
(A) Oocytes injected with water (H2O) or hASCT2 cRNA were incubated with [3H] D-serine (5μM) for 1 h in a Na+-containing medium. At the end of the incubation, oocytes were washed and the radioactivity associated with the oocytes was determined to calculate D-serine uptake. (B) Oocytes injected with hASCT2 cRNA were preloaded with [3H] D-serine by incubation for 1 h in a Na+-containing medium with 5 μM [3H] D-serine. Oocytes were then washed and used for efflux measurements. Efflux of radiolabel was measured for 30 min by incubating the oocytes in a medium containing either no amino acid or 2.5 mM unlabeled L-alanine (Ala), L-glutamine (Gln) or L-glutamate (Glu) in the presence of NaCl. Data are expressed as mean ± SE. (* Significantly different from control value p<0.05.)
Discussion
The discovery that D-serine is a co-agonist of NMDA receptors has significant implications for its role in modulating the activities of this abundantly-expressed glutamate receptor in the nervous system including the retina (Miller, 2004). The initial studies of D-serine were performed in brain; more recently D-serine and SR have been shown to be present in retina. Convincing evidence was presented in intact tissue by Stevens and co-workers that D-serine and SR are present in retinal Müller cells (Stevens et al, 2003). Studies of the uptake of D-serine were performed in retina utilizing whole salamander retina preparations by O'Brien et al (2005). The authors suggested that a member of the Na+-dependent ASC transporter family mediated D-serine uptake. The actual molecular identity of the specific transporter responsible for D-serine uptake was not determined. Given the intriguing possibility that glial cells may modulate the NMDA receptor via D-serine, we were interested in determining the identity of the transport system for D-serine specifically present in retinal Müller cells. To perform these studies we exploited a rat Müller cell line, rMC-1 (Sarthy et al, 1998) as well as Müller cells isolated from mouse retina to investigate this question comprehensively.
Our studies of D-serine transport in Müller cells began with analysis of the absolute requirements for Na+ and Cl-. It was clear that Na+ was essential for the uptake process, while Cl- was not. The requirement for Na+ ruled out the Na+-independent transporters system asc (asc-1 and asc-2) and system L (LAT1 and LAT2) (reviewed in Verrey et al, 2004) as mediators of D-serine uptake in Müller cells. It was clear from our studies that the stoichiometry of D-serine uptake in the presence of Na+ was 1:1. The requirement for Na+ in the uptake process narrowed the likely transport systems for D-serine in Müller cells to the ASC family of transporters as well as the ATB0,+ system. It was essential to consider this latter transporter system for D-serine uptake because in other cell types (e.g., colonocytes) this transporter has been shown to mediate the uptake of D-serine (Hatanaka et al, 2002). Given that ATB0,+ has been detected in mammalian retina (Hatanaka et al, 2004), it was a possible candidate as the mediator of D-serine uptake in Müller cells. ATB0,+, however, requires Cl- for its function. In addition, lysine and arginine are transported by ATB0,+. In our studies, D-serine uptake was reduced when Cl- was absent from the uptake buffer, but it was not eliminated. Moreover, the uptake of D-serine was not inhibited by lysine or arginine. An additional difference is the Na+:amino acid stoichiometry. The Na+:D-serine stoichiometry for ATB0,+ is 2:1 (Hatanaka et al, 2002), whereas the corresponding value for D-serine uptake in Müller cells is 1:1. These functional differences made ATB0,+ unlikely to be responsible for D-serine uptake in Müller cells. Furthermore, molecular analysis revealed that the mRNA encoding ATB0,+ was not expressed in Müller cells. These data, taken collectively, rule out ATB0,+ as a mediator of D-serine uptake in Müller cells, despite its role in this process in cells of other tissues.
We were then left with the two family members of the ASC transporter group as likely mediators of D-serine uptake in Müller cells. The mRNAs encoding ASCT1 and ASCT2 were both expressed in the rMC-1 and primary Müller cells. To distinguish between the two isoforms, we used a battery of known substrates for ASCT1 and ASCT2. Owing to the finding that uptake of D-serine was inhibited not only by alanine, serine and cysteine, but also by asparagine and glutamine, substrates specific for ASCT2, ASCT2 emerged as the mediator of D-serine transport in Müller cells. Our immunodetection experiments confirmed the presence of ASCT2 in neural retinal and in 1°MCs. Our studies with the X. laevis oocyte heterologous expression system and the cloned human ASCT2 showed that ASCT2 was able to mediate not only the uptake, but also the efflux, of D-serine, a distinct characteristic of ASC type transporters.
This study represents the first comprehensive analysis of the transport mechanisms for D-serine in a purified population of the retinal Müller cells. While earlier studies had established the likely requirement for Na+ in the process in brain (Hayashi et al, 1997; Ribeiro et al, 2002) and in whole retinal preparations (O'Brien et al, 2005) and had speculated that one of the family members of the ASC system was responsible for uptake, the molecular identify of the transporter had not been determined. The present study fills that void. Whether alterations in the function of ASCT2 in retinal Müller cells could have a negative impact on NMDA receptor function in neuronal cell types is not known, but is worthy of investigation.
Another interesting issue raised by the present studies is the mode of function of ASCT2 in D-serine transport in Müller cells under physiologic conditions. The transporter is an obligatory exchanger capable of hetero-exchange (i.e., Na+-coupled entry of one amino acid in exchange of Na+-coupled exit of a different amino acid) (Torres-Zamorano et al, 1998). Therefore, whether the transporter functions in Müller cells in the release of D-serine into the extracellular medium or in the uptake of D-serine from the extracellular medium will be dependent upon the relative concentrations of D-serine and other ASCT2 substrates as well as Na+ in the cytoplasm of the cell versus the extracellular medium. Since Müller cells synthesize D-serine using serine racemase, we suggest that the primary function of the transporter may be to mediate the release of this neuromodulator.
Acknowledgments
This work was supported by the National Eye Institute, National Institutes of Health (R01 EY014560).
References
- Avissar NE, Ryan CK, Ganapathy V, Sax HC. Na(+)-dependent neutral amino acid transporter ATB(0) is a rabbit epithelial cell brush-border protein. Am J Physiol Cell Physiol. 2001;281:C963–C971. doi: 10.1152/ajpcell.2001.281.3.C963. [DOI] [PubMed] [Google Scholar]
- Bridges CC, Kekuda R, Wang H, Prasad PD, Mehta P, Huang W, Smith SB, Ganapathy V. Structure, function, and regulation of human cystine/glutamate transporter in retinal pigment epithelial cells. Invest Ophthalmo l Vis Sci. 2001;42:47–54. [PubMed] [Google Scholar]
- Dun Y, Mysona B, Van Ells T, Amarnath L, Shamsul Ola M, Ganapathy V, Smith SB. Expression of the cystine-glutamate exchanger (x(c) (-)) in retinal ganglion cells and regulation by nitric oxide and oxidative stress. Cell Tissue Res. 2006;324:189–202. doi: 10.1007/s00441-005-0116-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy ME, Ganapathy V. Amino Acid Transporter ATB0,+ as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:357–364. doi: 10.2174/156800805774912953. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Haramura M, Fei YJ, Miyauchi S, Bridges CC, Ganapathy PS, Smith SB, Ganapathy V, Ganapathy ME. Transport of amino acid-based prodrugs by the Na+- and Cl(-) -coupled amino acid transporter ATB0,+ and expression of the transporter in tissues amenable for drug delivery. J Pharmacol Exp Ther. 2002;308:1138–1147. doi: 10.1124/jpet.103.057109. [DOI] [PubMed] [Google Scholar]
- Hatanaka T, Huang W, Nakanishi T, Bridges CC, Smith SB, Prasad PD, Ganapathy ME, Ganapathy V. Transport of D-serine via the amino acid transporter ATB(0,+) expressed in the colon. Biochem Biophys Res Commun. 2002;291:291–295. doi: 10.1006/bbrc.2002.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F, Takahashi K, Nishikawa T. Uptake of D- and L-serine in C6 glioma cells. Neurosci Lett. 1997;239:85–88. doi: 10.1016/s0304-3940(97)00892-6. [DOI] [PubMed] [Google Scholar]
- Hicks D, Courtois Y. The growth and behaviour of rat retinal Müller cells in vitro. 1 An improved method for isolation and culture Exp Eye Res. 1990;51:119–129. doi: 10.1016/0014-4835(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization and regulation of sigma receptor in retinal Müller cells. Invest Ophthamol Vis Sci; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol. 2003;479:237–247. doi: 10.1016/j.ejphar.2003.08.073. [DOI] [PubMed] [Google Scholar]
- Kekuda R, Prasad PD, Fei YJ, Torres-Zamorano V, Sinha S, Yang-Feng TL, Leibach FH, Ganapathy V. Cloning of the sodium-dependent, broad-scope, neutral amino acid transporter Bo from a human placental choriocarcinoma cell line. J Biol Chem. 1996;271:18657–18661. doi: 10.1074/jbc.271.31.18657. [DOI] [PubMed] [Google Scholar]
- Kim PM, Aizawa H, Kim PS, Huang AS, Wickramasinghe SR, Kashani AH, Barrow RK, Huganir RL, Ghosh A, Snyder SH. Serine racemase: activation by glutamate neurotransmission via glutamate receptor interacting protein and mediation of neuronal migration. Proc Natl Acad Sci U S A. 2005;102:2105–2110. doi: 10.1073/pnas.0409723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lorentzen KA, Kistler J, Donaldson PJ. Molecular identification and characterization of the glycine transporter (GLYT1) and the glutamine/glutamate transporter (ASCT2) in the rat lens. Exp Eye Res. 2006;83:447–455. doi: 10.1016/j.exer.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NH, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Miller RF. D-Serine as a glial modulator of nerve cells. Glia. 2004;47:275–283. doi: 10.1002/glia.20073. [DOI] [PubMed] [Google Scholar]
- Mothet JP, Parent AT, Wolosker H, Brady RO, Jr, Linden DJ, Ferris CD, Rogawski MA, Snyder SH. D-serine is an endogenous ligand for the glycine site of the N-methyl-D-aspartate receptor. Proc Natl Acad Sci U S A. 2000;97:4926–4931. doi: 10.1073/pnas.97.9.4926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothet JP, Pollegioni L, Ouanounou G, Martineau M, Fossier P, Baux G. Glutamate receptor activation triggers a calcium-dependent and SNARE protein-dependent release of the gliotransmitter D-serine. Proc Natl Acad Sci U S A. 2005;102:5606–5611. doi: 10.1073/pnas.0408483102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa AK, Kim PM, Snyder SH. D-Serine as a putative glial neurotransmitter. Neuron Glia Biol. 2004;1:275–281. doi: 10.1017/S1740925X05000141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien KB, Miller RF, Bowser MT. D-Serine uptake by isolated retinas is consistent with ASCT-mediated transport. Neurosci Lett. 2005;385:58–63. doi: 10.1016/j.neulet.2005.05.009. [DOI] [PubMed] [Google Scholar]
- Ribeiro CS, Reis M, Panizzutti R, de Miranda J, Wolosker H. Glial transport of the neuromodulator D-serine. Brain Res. 2002;929:202–209. doi: 10.1016/s0006-8993(01)03390-x. [DOI] [PubMed] [Google Scholar]
- Sarthy VP, Brodjian SJ, Dutt K, Kennedy BN, French RP, Crabb JW. Establishment and characterization of a retinal Muller cell line. Invest Ophthalmol Vis Sci. 1998;39:212–216. [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci U S A. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens ER, Esguerra M, Kim PM, Newman EA, Snyder SH, Zahs KR, Miller RF. D-serine and serine racemase are present in the vertebrate retina and contribute to the physiological activation of NMDA receptors. Proc Natl Acad Sci U S A. 2003;100:6789–6794. doi: 10.1073/pnas.1237052100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongsong B, Subramanian RK, Ganapathy V, Prasad PD. Inhibition of amino acid transport system a by interleukin-1beta in trophoblasts. J Soc Gynecol Investig. 2005;12:495–503. doi: 10.1016/j.jsgi.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Torres-Zamorano V, Leibach FH, Ganapathy V. Sodium-dependent homo- and hetero-exchange of neutral amino acids mediated by the amino acid transporter ATB degree. Biochem Biophys Res Commun. 1998;245:824–829. doi: 10.1006/bbrc.1998.8434. [DOI] [PubMed] [Google Scholar]
- Umapathy NS, Li W, Mysona BA, Smith SB, Ganapathy V. Expression and function of glutamine transporters SN1 (SNAT3) and SN2 (SNAT5) in retinal Müller cells. Invest Ophthalmol Vis Sci. 2005;46:3980–3987. doi: 10.1167/iovs.05-0488. [DOI] [PubMed] [Google Scholar]
- Utsunomiya-Tate N, Endou H, Kanai Y. Cloning and functional characterization of a system ASC-like Na+-dependent neutral amino acid transporter. J Biol Chem. 1996;271:14883–14890. doi: 10.1074/jbc.271.25.14883. [DOI] [PubMed] [Google Scholar]
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]


