Summary
Studies using Drosophila have contributed significantly to our understanding of regulatory mechanisms that control stem cell fate choice. The Drosophila blood cell progenitor or prohemocyte shares important characteristics with mammalian hematopoietic stem cells, including quiescence, niche dependence, and the capacity to form all three fly blood cell types. This report extends our understanding of prohemocyte fate choice by showing that the zinc-finger protein Oddskipped promotes multipotency and blocks differentiation. Odd-skipped was expressed in prohemocytes and downregulated in terminally differentiated plasmatocytes. Furthermore, Odd-skipped maintained the prohemocyte population and blocked differentiation of plasmatocytes and lamellocytes but not crystal cells. A previous study showed that Odd-skipped expression is downregulated by Decapentaplegic signaling. This report provides a functional basis for this regulator/target pair by suggesting that Decapentaplegic signaling limits Odd-skipped expression to promote prohemocyte differentiation. Overall, these studies are the basis for a gene regulatory model of prohemocyte cell fate choice.
Keywords: hematopoiesis, stem cells, cell fate choice, multipotency, gene regulatory sub-circuits
INTRODUCTION
Hematopoietic stem cells (HSCs) have the capacity for self-renewal to replenish the stem cell pool and differentiation to sustain blood cell production. The size of the stem cell pool is precisely controlled by regulating the choice between differentiation and maintenance of multipotency. This is accomplished through a complex interplay of intrinsic stem cell factors and extrinsic signals that originate from the surrounding microenvironment or stem cell niche (Akala and Clarke, 2006; Kiel and Morrison, 2008; Moore, 2004; Scadden, 2006; Zon, 2008). Studies using the mouse have identified important biological processes that control stem cell fate choice, including the relationship between stem cell quiescence, proliferation, and differentiation (Orford and Scadden, 2008; Trumpp et al., 2010). The information gained from these studies can be enhanced using invertebrate model systems to rapidly identify gene networks that regulate multipotency and differentiation.
Studies using Drosophila have identified a blood cell progenitor that shares important characteristics with the mammalian HSC, including quiescence, niche dependence, and the capacity to form all three fly blood cell types. This multipotent stem-like cell or prohemocyte develops within a specialized organ called the lymph gland (Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007; Martinez-Agosto et al., 2007). The mature lymph gland consists of one pair of primary lobes and a series of secondary lobes (Dearolf, 1998). Within the primary lobe, three distinct domains or zones have been characterized based on their roles during hematopoiesis. These include the posterior signaling center, which functions as a stem cell niche by maintaining quiescence and multipotency through a number of signal transduction pathways (Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007; Sinenko et al., 2009). The second or medullary zone contains the multipotent prohemocyte pool. During the process of differentiation, these cells migrate to the third domain, called the cortical zone. Here, they continue to develop and give rise to all three blood cell types, specifically, crystal cells, plasmatocytes, and lamellocytes (Jung et al., 2005; Krzemien et al., 2007; Mandal et al., 2007). Plasmatocytes and crystal cells are ubiquitous effector cells. Plasmatocytes function as operational macrophages, whereas crystal cells, named for the crystalline inclusion bodies found within these cells, play a role in wound healing. Lamellocytes are rare under steady-state conditions, but rapidly differentiate in response to particular types of immune challenge (Dearolf, 1998).
The lymph gland begins to develop during midembryogenesis. The zinc-finger transcription factor, Odd-skipped (Odd), is one of earliest markers used to identify the lymph gland primordium (Coulter et al., 1990; Johnson et al., 2007; Jung et al., 2005; Mandal et al., 2004). Odd expression is maintained throughout lymph gland development and continues until the end of the third larval instar when the mature effector cells are released into the hemolymph in preparation for metamorphosis (Jung et al., 2005). The expression pattern of Odd suggests it is an important regulator of Drosophila lymph gland hematopoiesis; however, its function has not been determined. The odd gene was first discovered in a screen designed to identify factors that regulate early embryonic development (Nusslein-Volhard and Wieschaus, 1980). Subsequent studies have shown that Odd regulates eye, leg, and renal development (Bras-Pereira and Casares, 2008; Bras-Pereira et al., 2006; Hao et al., 2003; Tena et al., 2007). Two mammalian homologs, odd-skipped related 1 (Osr1) and oddskipped related 2 (Osr2), have been shown to regulate heart, bone, and urogenital development (James et al., 2006; Kawai et al., 2007; Lan et al., 2001; Tena et al., 2007; Wang et al., 2005).
Here we show that Odd is expressed in prohemocytes and downregulated in terminally differentiated plasmatocytes. During the third larval instar, Odd functions to block both plasmatocyte and lamellocyte differentiation and maintain the prohemocyte pool. Previously, Odd expression was shown to be downregulated by Decapentaplegic (Dpp) signaling (Frandsen et al., 2008). The findings presented in this report provide a functional basis for this regulator/target pair by suggesting that Dpp signaling limits Odd expression to promote prohemocyte differentiation. Finally, identifying a hematopoietic function for Odd may have relevance for studies of mammalian hematopoiesis because Osr1 is regulated by the hematopoietic factor Ikaros (Yamauchi et al., 2008).
MATERIALS AND METHODS
Fly Strains
w1118 served as our wild-type stock. domeless-Gal4 (domeGal4) was a generous gift from M. Crozatier, University Paul Sabatier. dppd6 was a generous gift from S.J. Newfeld, Arizona State University. y1 w; ushvx22/CyO, y+ and y1 w; ushr24/CyO, y+ were generous gifts from R. A. Schulz, and R. P. Sorrentino, University of Notre Dame. We obtained the following strains from the Bloomington stock center: odd5 b1 pr1 c1 px1 sp1/SM6a, odd5/CyO; ftz/lacC, w*; UAS-odd, odd01863, cn1/CyO; ry506, y1 v1; UAS-oddRNAi, y1 v1 hopTum-l/Basc. An additional UAS-oddRNAi stock, transformant number 107257, was obtained from the Vienna Drosophila RNAi Center.
Quantitative Real-Time RT-PCR
The relative levels of the odd transcript were determined in wild-type and u-shaped mutant lymph glands. Larval lymph glands were dissected and stored in RNA Protect Cell Reagent solution (Qiagen) prior to RNA extraction. About 1 µg of RNA was isolated from 100 wild-type or 30 mutant lymph glands. cDNA was prepared using the QuantiTect kit (Qiagen). Quantitative Real-Time RT-PCR (qRT-PCR) was performed with the following odd primers: forward, CTCAACTTAAGCAGA GAACAAGACTTTGC; and reverse, CTCTCCTCAGC GGCTCCTCG using the SYBR Green PCR kit (Applied Biosystems) with three replicates on the ABI 7900 HT thermocycler. Statistical significance was evaluated using the Student’s t test (n =3).
Gene Expression Analyses
The y1 w; ushvx22/ushr24 trans-heterozygote larvae were generated by crossing y1 w; ushvx22/CyO, y+and y1 w; ushr24/CyO, y+ adults and selecting larval progeny with yellow mouth hooks. odd heterozygotes were tested over three complementing chromosomes to verify that the observed phenotype was independent of the balancer chromosome. Gene expression analysis was conducted on lymph glands from mid- to latethird instar larvae as indicated. domeGal4 driven UAS-oddRNAi lymph glands routinely dispersed and UAS-odd animals exhibited reduced viability during the late-third larval instar. For these reasons, lymph glands were dissected from mid-third instar larvae carrying domeGal4 driven transgenes and domeGal4 heterozygous controls. y1 v1 hopTum-l/Basc virgins were crossed to w1118 or y1 w; ushvx22/CyO, y+ or odd5/SM6a males. Adult male progeny from these crosses that were hemizygous for hopTum-l and carried either wild-type, odd, or ush heterozygous second chromosomes were scored for the presence of melanotic tumors. Statistical significance was evaluated using Fisher’s exact test. All animals were cultured at 23°C except domeGal4/+; UAS-odd/+ and the accompanying control domeGal4/+; +/+ which were cultured at 18°C because domeGal4/+; UAS-odd/+ third instar larvae died when shifted to 23°C. Larvae cultured at 18°C were staged as previously described (Krzemien et al., 2010).
Immunofluorescence
Dissection and fixation of larval lymph glands were performed as previously described (Gao et al., 2009). Rabbit anti-U-shaped was used at a 1:1,000 dilution (Fossett et al., 2001). Rabbit anti-Odd was used at a 1:4,000 dilution and was a generous gift from James Skeath, Washington University School of Medicine. The following mouse antibodies directed against specific hemocyte antigens were generous gifts from Istavan Ando, Biological Research Center of the Hungarian Academy of Sciences and were used at the indicated dilutions: P1, 1:50 (Kurucz et al., 2007a) and L1, 1:100 (Kurucz et al., 2007b). Rat anti-DE-cad was diluted 1:20 and was obtained from the Developmental Studies Hybridoma Bank. Alexafluor-555 or −488 conjugated secondary antibodies directed to rabbit, mouse or rat (Invitrogen) were used at a 1:2,000 dilution. Cellular proliferation rates were determined using the mitotic marker, phosphohistone H3 (HP3). Rabbit anti-HP3 (cell signaling) was used at a 1:100 dilution. Lymph glands were counterstained with Dapi and the total number of lymph gland cells was determined as previously described (Krzemien et al., 2010; Minakhina et al., 2007). The mitotic indices were determined by dividing the number of HP3-positive cells by the total number of lymph gland cells. Statistical significance was determined using the Student’s t test. Fluorescence was captured using Zeiss confocal microscopy or Zeiss Axioplan optics. Cell counts, lymph gland size, and densitometric mean values were analyzed using the Zeiss Axiovision 4.6.3 quantitation software as previously described (Gao et al., 2009). Blood cell counts were recorded using the Axiovision Events function. Blood cell counts, lymph gland size, and densitometric means were evaluated for statistical significance using the Student’s t test. At least 10 different lymph glands were analyzed and one measurement was taken from each sample.
RESULTS
Odd Expression is Upregulated in Prohemocytes
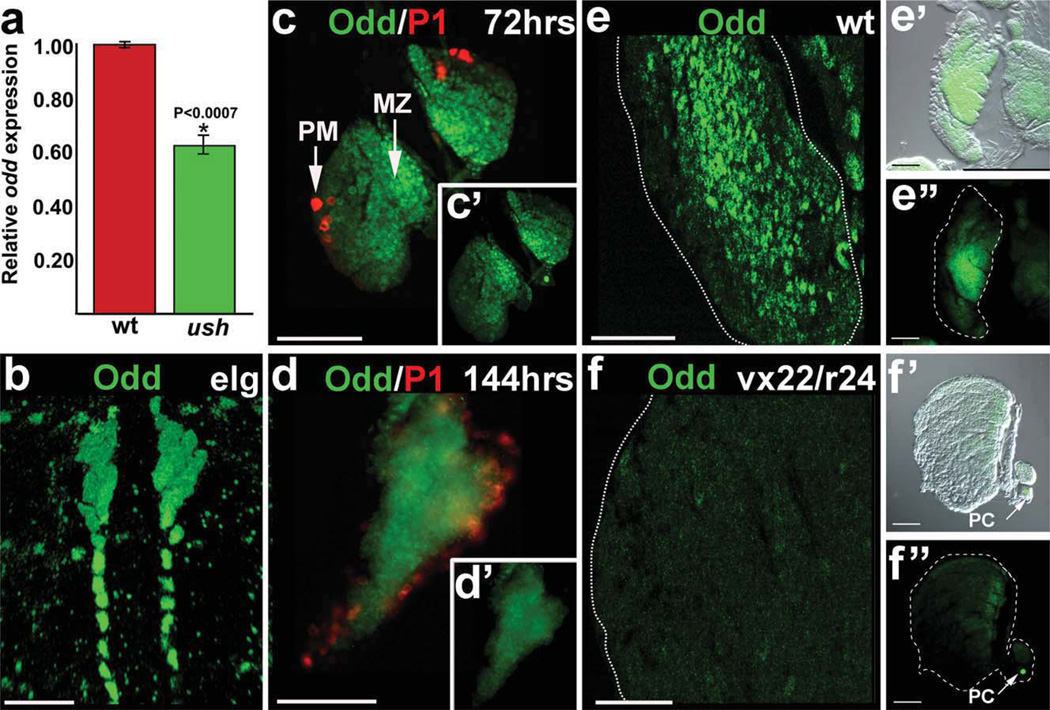
We previously reported that the Friend of GATA ortholog, U-shaped (Ush), is a key component in the process that controls the choice between prohemocyte multipotency and differentiation. These studies showed that Ush is required to maintain the prohemocyte population, whereas downregulation of ush expression promotes differentiation. In ush mutants, the prohemocyte pool is depleted as a result of uncontrolled differentiation (Gao et al., 2009). This phenotype provided an opportunity to identify additional prohemocyte regulators by comparing the transcriptome profiles of ush mutant and wild-type lymph glands. Using this approach, we observed that odd transcript levels were reduced in ush mutants compared to wild-type controls (data not shown), and we verified the transcriptome data using qRT-PCR (Fig. 1a). This result prompted us to determine if the level of odd expression was elevated in prohemocytes compared to differentiated cells and if Odd regulates prohemocyte function.
FIG. 1.
Odd expression during lymph gland development. (a) The odd transcript levels in wild-type (wt) and ushvx22/r24 trans-heterozygous (ush) lymph glands were determined using qRT-PCR. The Rpl32 transcript served as a reference. The relative level of odd expression in ushvx22/r24 trans-heterozygous lymph glands was 0.62 6 ± 0.04 of wild-type (P < 0.0007). (b–d′) Odd expression was elevated in prohemocytes and downregulated in plasmatocytes. (b) Odd expression in a late embryonic lymph gland (elg). (c) Early-third larval instar lymph gland (72 h after egg laying) showing Odd expression in the medullary zone (MZ) and lack of expression in cortical zone plasmatocytes (PM). Plasmatocytes were identified using the specific marker Nimrod (P1). (c’) Inset displays the same lymph gland showing only odd expression. (d) Late-third larval instar lymph gland (144 hr after egg laying) showing Odd expression in green and plasmatocytes in red. (d’) Inset displays the same lymph gland showing only odd expression. (e–f″) Odd was downregulated in ush mutant lymph glands. Wild-type (wt) and ushvx22/ r24 trans-heterozygotes (vx22/r24) lymph glands from late-third instar lavae (144 h after egg laying). (e) wt and (f) vx22/r24 at ×0 magnification. (e’) wt and (f’) vx22/r24 show lymph glands photographed using bright field and fluorescence (merged) at ×40 magnification. (e”) wt and (f”) vx22/r24 show only fluorescence at ×40 magnification. Arrows mark pericardial cells (pc). Abbreviations: odd, odd-skipped; qRT-PCR, Quantitative Real-Time RT-PCR; ush, u-shaped. Scale bars: Panel b, 10 µm; Panels c–f″, 50 µm.
Previous studies have shown that Odd is expressed in the embryonic lymph gland, which contains primarily prohemocytes (Fig. 1b; Jung et al., 2005; Krzemien et al., 2010; Minakhina and Steward, 2010). During the early-third larval instar (72 h after egg laying), the medullary zone prohemocytes are readily distinguishable from the differentiating cells of the cortical zone (Jung et al., 2005; Sinenko et al., 2009). We assayed Odd expression during this developmental period and observed the highest level of expression in medullary zone prohemocytes. Additionally, Odd was downregulated in terminally differentiated plasmatocytes (Fig. 1c). Moreover, this expression pattern was maintained until the late-third larval instar (Fig. 1d). Collectively, these results showed that Odd is expressed in prohemocytes throughout lymph gland development and maturation and that Odd is downregulated as prohemocytes differentiate. Thus, Odd is differentially expressed in lymph gland blood cells, which has not been shown previously.
Prohemocyte numbers are significantly reduced in ush mutant lymph glands, and these lymph glands are comprised primarily of differentiated cells (Gao et al., 2009). If Odd expression is elevated in prohemocytes and downregulated in differentiating cells, then Odd should be downregulated in ush mutant lymph glands. We confirmed that Odd was downregulated (Fig. 1e,f), which provided additional support for our hypothesis that Odd is differentially expressed in lymph gland blood cells. Odd protein expression was dramatically reduced in medullary zone prohemocytes. However, there was only a 38% reduction in odd transcript levels as determined by qRT-PCR (Fig. 1a). This discrepancy may be due in part to the fact that lymph gland dissections routinely contained both lymph gland and pericardial cells and that Odd protein expression in pericardial cells was not reduced in ush mutant animals (Fig. 1f′,f″). These results strongly suggest that the reduction in odd transcript levels in ush mutant lymph glands (Fig. 1a) resulted from loss of the prohemocyte pool. These data prompted us to ask if the prohemocyte pool is maintained by Odd function and if Odd is downregulated to permit prohemocyte differentiation. To address these questions, we began by assessing prohemocyte marker expression in odd mutant lymph glands.
Odd Maintains the Prohemocyte Population
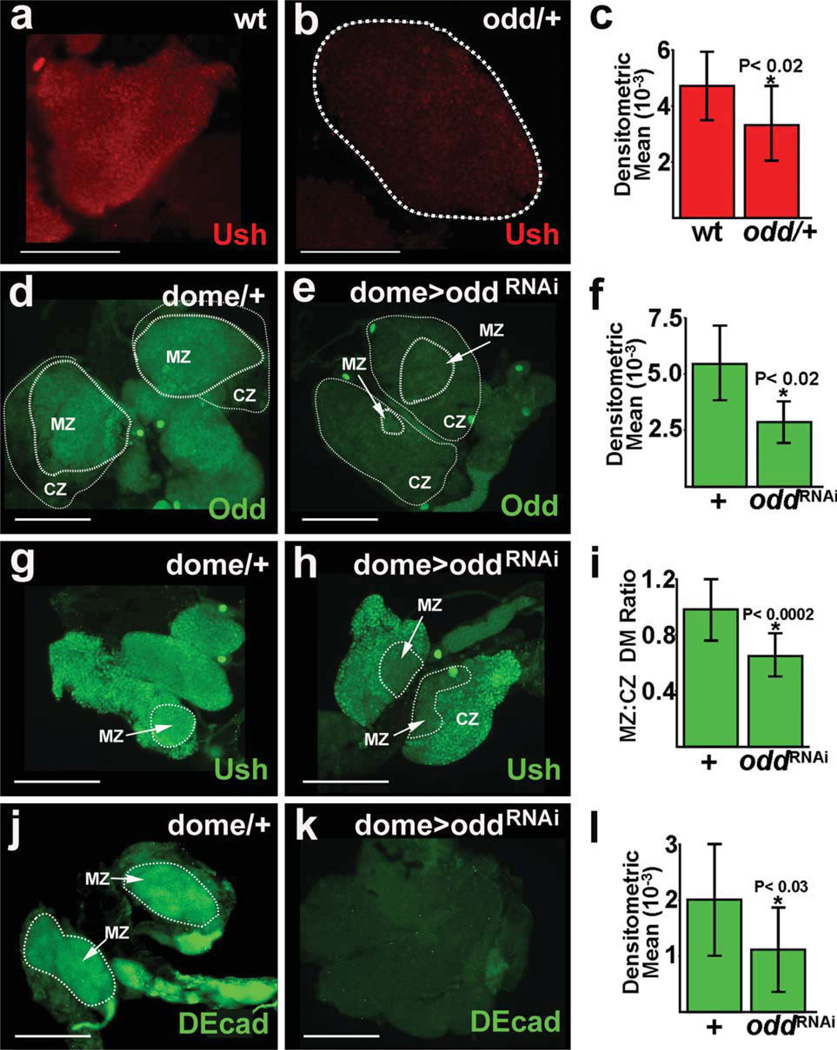
Ush is expressed in a subpopulation of cortical zone cells. However, Ush is also expressed throughout the medullary zone and, as such, serves as a marker for prohemocytes (Gao et al., 2009). To test if Odd maintains the prohemocyte population, we assayed Ush expression in animals with loss of Odd function. odd is an essential gene and null homozygotes die during embryogenesis (Nusslein-Volhard and Wieschaus, 1980) and, for this reason, we assayed the prohemocyte pool in odd heterozygous animals. We observed that Ush was downregulated throughout the lymph gland, including the medullary zone prohemocyte population (Fig. 2a–c). This suggested that Odd functions to maintain the prohemocyte pool.
FIG. 2.
Prohemocyte markers are downregulated in odd mutant lymph glands. (a–c) Ush expression was downregulated in odd heterozygous lymph glands. Lymph glands were from late-third instar larvae (144 h after egg laying). (a) Ush expression in a wild-type (wt) lymph gland. (b) Ush expression in an odd heterozygous (odd/+) lymph gland. (c) Densitometric mean for Ush expression was 4563 ± 1367 in wt and 3307 ± 1264 in odd/+ lymph glands (P < 0.02). (d–l) The prohemocyte pool was assessed in lymph glands with targeted knock-down of odd expression. Lymph glands were from mid-third instar larvae (96 h after egg laying). (d–f) Odd was downregulated in domeGal4 driven UAS-oddRNAi (dome> oddRNAi) lymph glands. (d) Odd expression in a domeGal4 heterozygous control (dome/+). (e) Odd expression in a dome> oddRNAi lymph gland. (f) Densitometric mean for Odd expression was 5590 ± 1825 in domeGal4 heterozygous (+) controls and 2667 ± 993 in dome> oddRNAi (oddRNAi) lymph glands (P < 0.02). (g–i) Ush was downregulated in the medullary zone (MZ) compared to the cortical zone (CZ) of dome> oddRNAi lymph glands. (g) Ush expression in a dome/+ control. (h) Ush expression in a dome> oddRNAi lymph gland. (i) Densitometric means (DM) for Ush expression was determined across the medullary zone and cortical zone for each lymph gland and then expressed as a ratio (MZ:CZ DM Ratio). The ratio was 0.98 ± 0.22 for dome/1 controls (+) and 0.66 ± 0.15 for dome> oddRNAi (oddRNAi) lymph glands (P < 0.0002). (j–l) DE-cadherin (DEcad) was downregulated in dome> oddRNAi lymph glands. (j) DEcad expression in a dome/+ control. (k) DEcad expression in a dome> oddRNAi lymph gland. (+) Densitometric mean for DEcad expression was 1998 ± 1313 in dome/+ controls (+) and 1087 ± 826 in dome> oddRNAi (oddRNAi) lymph glands (P < 0.03). Abbreviations: dome, domeless; odd, odd skipped; ush, u-shaped. Scale bars: 100 µm.
To confirm these results, we used RNAi technology to downregulate odd specifically in prohemocytes and assayed prohemocyte marker expression (Fig. 2d–l). The domeless-Gal4 (domeGal4) driver was used with the UAS/Gal4 binary system to specifically express the UAS-oddRNAi transgene in lymph gland prohemocytes (Brand and Perrimon, 1993; Krzemien et al., 2007). We verified that odd was knocked-down in prohemocytes by showing that Odd protein expression was reduced by 52% compared to genetically matched domeGal4 heterozygous controls (Fig. 2d–f). In contrast, Odd expression was reduced by only 38% in odd heterozygous lymph glands compared to controls (Supporting Information Fig. 1a).
We then determined if Odd maintains the prohemocyte pool by assaying the level of Ush expression in domeGal4 driven UAS-oddRNAi lymph glands. Ush is expressed in both medullary zone prohemocytes and a subpopulation of cortical zone cells (Gao et al., 2009). For this reason, we compared Ush levels across the medullary and cortical zones of domeGal4 driven UAS-oddRNAi lymph glands. Ush expression was significantly reduced in the medullary zone compared to the cortical zone. In contrast, Ush expression was essentially the same across the medullary and cortical zones of dome-Gal4 heterozygous controls (Fig. 2g– i). Thus, targeted knock-down of odd expression lead to a specific loss of the prohemocyte population, but did not reduce the population of differentiating cortical zone cells. This suggests that Odd acts autonomously to preserve the prohemocyte pool. To confirm that reducing odd expression results in prohemocyte loss, we tested an additional prohemocyte marker, DE-cadherin (DEcad); (Jung et al., 2005), in domeGal4 driven UAS-oddRNAi lymph glands and observed that DEcad was downregulated in medullary zone prohemocytes (Fig. 2j– l). In addition, we determined that DEcad was also downregulated in odd heterozygous lymph glands (Supporting Information Fig. 1b). We then determined the number of cells in the medullary and cortical zones of dome-Gal4 driven UAS-oddRNAi lymph glands and compared those results to domeGal4 heterozygous controls. The number of medullary zone cells was significantly reduced in domeGal4 driven UAS-oddRNAi lymph glands compared to controls (Supporting Information Fig. 1c). The loss of medullary zone cells in domeGal4 driven UAS-oddRNAi lymph glands was accompanied by a concomitant increase in the number of cortical zone cells (data not shown). Collectively, these results strongly suggest that Odd is required to maintain the prohemocyte pool.
Odd Blocks Plasmatocyte and Lamellocyte Differentiation
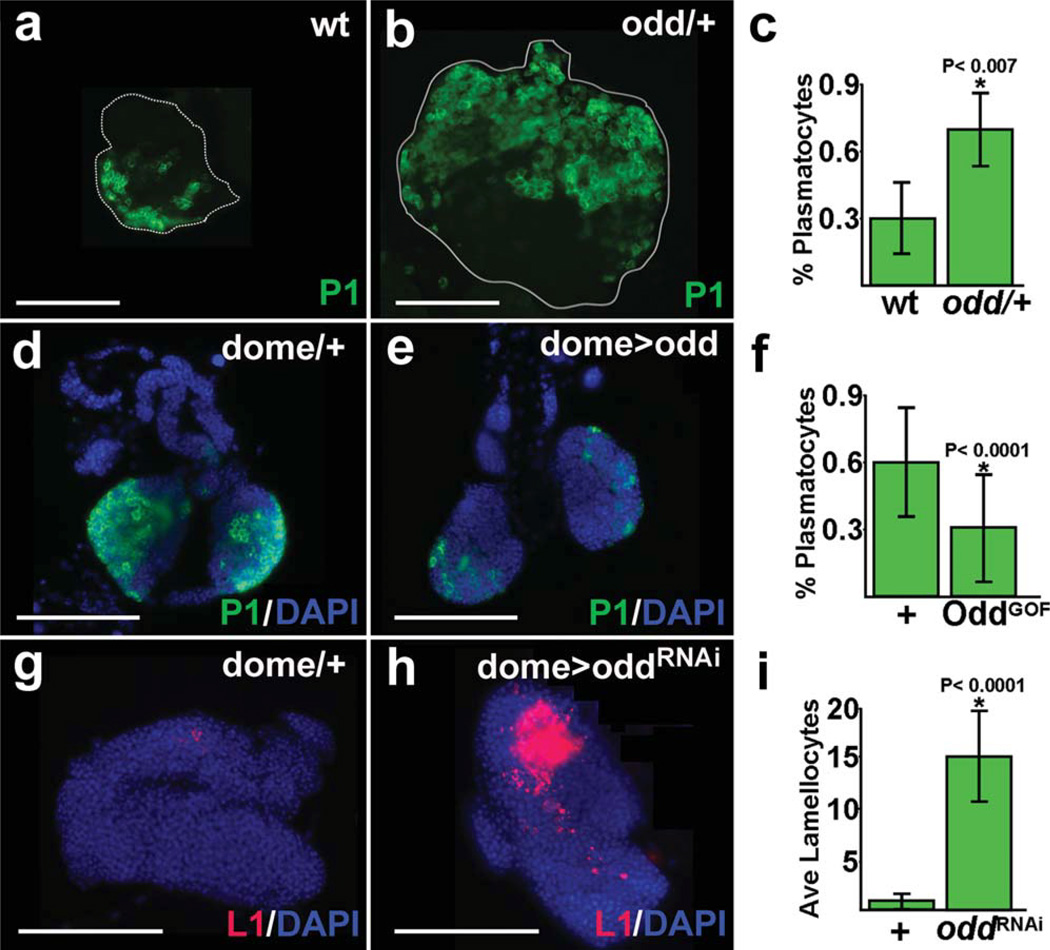
Odd may function to maintain the prohemocyte pool by preventing differentiation. This would predict that loss of Odd function would also result in increased numbers of terminally differentiated blood cells. To test this hypothesis, we assayed blood cell differentiation in odd heterozygous lymph glands. Plasmatocyte numbers increased significantly in odd heterozygous lymph glands compared to wild-type controls. However, odd heterozygous lymph glands were on average two-fold larger than wild-type controls (Fig. 3a,b, data not shown). To determine if the increase in size was the result of increased cellular proliferation, we compared the percent mitotic indices of odd heterozygous lymph glands and wild-type controls. Using this method, we did not observe a significant increase in proliferation rate of odd heterozygous lymph glands. The percent mitotic index for odd heterozygous lymph glands was 1.4 ± 1.7 compared to 1.0 ± 0.8 for wild-type controls (n =12; P > 0.6).
FIG. 3.
Plasmatocyte and lamellocyte differentiation increases with loss of Odd function. (a–c) Plasmatocyte differentiation increased in odd heterozygous (odd/+ lymph glands. Lymph glands were from late-third instar larvae (144 h after egg laying). Differentiation was monitored using the plasmatocyte specific marker, P1. (a) wild-type (wt). (b) odd/+ lymph gland. (c) Plasmatocyte production as a percentage of total lymph gland cells was 30 ± 17 in wt and 68 ± 19 in odd/+ lymph glands (P < 0.007). (d–f) Plasmatocyte differentiation was reduced in domeGal4 driven UAS-odd (dome> odd) lymph glands. Plasmatocyte differentiation was monitored using the specific marker, P1. Lymph glands were from mid-third instar larvae raised at 18°C (190 h, which is equivalent to 96 h after egg laying). (d) domeGal4 heterozygous control (dome/+ and (e) dome> odd lymph gland. (f) Plasmatocyte production as a percentage of total lymph gland cells was 60 ± 17 in dome/+ controls (+ and 30 ± 25 in dome> odd (OddGOF) lymph glands (P < 0.0001). (g–i) Lamellocyte differentiation increased in dome> oddRNAi lymph glands. Lamellocyte differentiation was monitored using the specific marker, L1. Lymph glands were from mid-third instar larvae (96 h after egg laying). (g) dome/+ control and (h) dome> oddRNAi lymph gland. (i) The average number of lamellocytes was 1 ± 0.8 in dome/+ controls (+ and 15 ± 5 in dome> oddRNAi (oddRNAi) lymph glands (P < 0.0001). Abbreviations: dome, domeless; odd, odd skipped. Scale bars: 100 µm.
We then determined if the change in the number of plasmatocytes resulted from increased prohemocyte differentiation or lymph gland size by calculating the percentage of plasmatocytes from among the total number of lymph gland cells. When plasmatocyte counts were adjusted to account for lymph gland size, the number of plasmatocytes increased on average 70% in odd heterozygous lymph glands compared to wildtype controls (Fig. 3a– c). In contrast, the number of crystal cells did not increase significantly (data not shown). These studies indicated that Odd limits plasmatocyte differentiation and predicted that over-expression of odd would block plasmatocyte production. To test this hypothesis, we used domeGal4 to drive UAS-odd in prohemocytes and assayed plasmatocyte production. Over-expression of the odd transgene reduced plasmatocyte number on average 50% compared to domeGal4 heterozygous controls (Fig. 3d– f). However, the number of plasmatocytes in domeGal4 heterozygous controls (Fig. 3d) was greater than that of wild-type controls (Fig. 3a). This is not unusual because lymph gland hematopoietic phenotypes can vary considerably across genotypes (Fossett and Gao, unpublished observations). On the other hand, 7 of 17 (40%) domeGal4 driven UAS-odd lymph glands had plasmatocyte numbers that were significantly less than observed in wild-type controls (P < 0.0001), averaging 5% of the total. Additionally, three of the seven lymph glands were completely devoid of plasmatocytes. This is in stark contrast to wild-type lymph glands in which plasmatocytes averaged 30% of the total (Fig. 3c). Thus, over-expression of odd limits plasmatocyte production when compared to wild-type controls and does not simply suppress the elevated production seen in domeGal4 heterozygous animals. We attribute the higher mean value of the 17 domeGal4 driven UAS-odd samples (Fig. 3f) to incomplete penetrance of the domeGal4 driven UAS-odd genotype. The penetrance of Gal4 driven UAS-transgenes is often incomplete, which is likely due to random fluctuations in gene expression (Fossett et al., 2000, 2001; Raj et al., 2010). Collectively, our results strongly support the notion that Odd blocks plasmatocyte differentiation and preserves the prohemocyte population by blocking their differentiation potential.
To provide additional support for this model, we tested if targeted knock-down of odd produced increased blood cell production. Again, we used dome-Gal4 to drive UAS-oddRNAi in lymph gland prohemocytes. Crystal cell numbers did not increase under these conditions, which was similar to the results obtained using odd heterozygous lymph glands. Additionally, we did not observe a significant increase in the number of plasmatocytes (data not shown). In contrast, we did observe a significant increase in lamellocyte differentiation compared to domeGal4 heterozygous controls (Fig. 3g– i). This was confirmed using an additional UAS-oddRNAi strain (Supporting Information Fig. 1d,e). A number of studies have indicated that lamellocytes are produced from plasmatocytes or at the expense of plasmatocytes (Dearolf, 1998; Gao et al., 2009; Honti et al., 2010; Sorrentino et al., 2007). This may explain why we did not observe a significant increase in plasmatocytes in odd knocked-down lymph glands. The size of the domeGal4 driven UAS-oddRNAi lymph glands was approximately 20% larger than domeGal4 heterozygous controls, but the increase was not statistically significant. The failure to observe a significant difference in size may be due to the fact that domeGal4 driven UAS-oddRNAi lymph glands often dispersed during dissection and processing. This was likely due to excessive lamellocyte production and resulted in a sampling bias that favored smaller domeGal4 driven UAS-oddRNAi lymph glands.
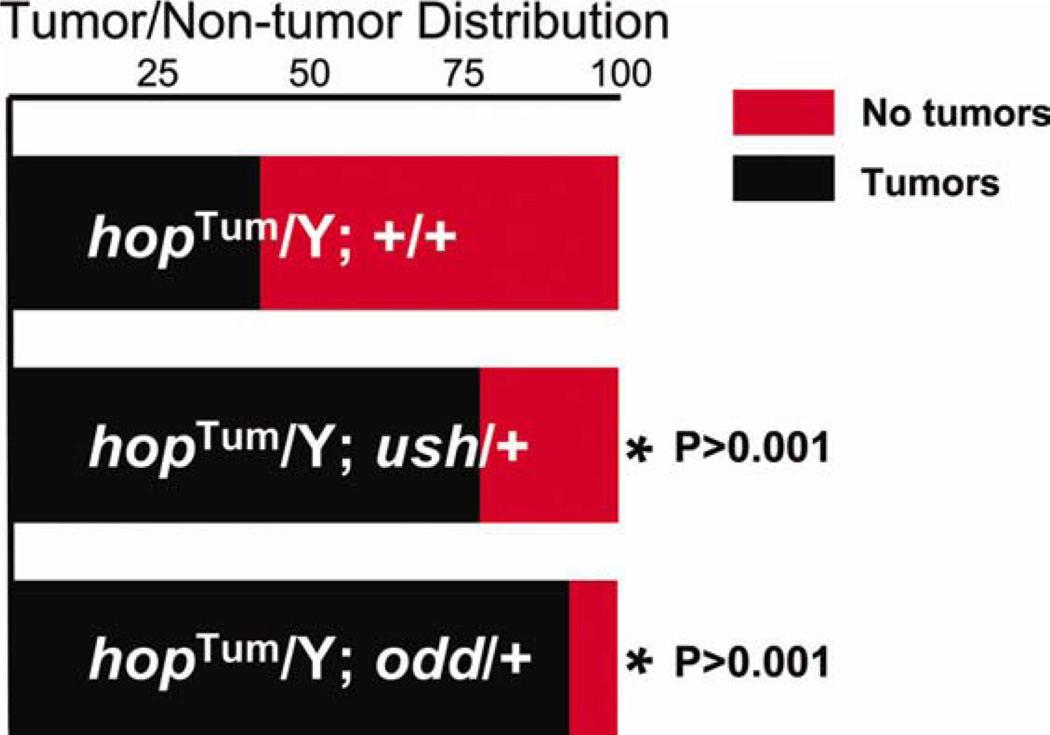
To confirm that Odd blocks lamellocyte production, we determined if reducing the level of odd expression would exacerbate hopscotchTum-1 (hopTum-l)-induced lamellocyte production. hop encodes the Drosophila Janus Kinase (JAK), and the temperature sensitive allele, hopTum-l, produces constitutive activation of the JAK/ STAT pathway (Hanratty and Dearolf, 1993; Harrison et al., 1995; Luo et al., 1995). This leads to aberrant lamellocyte differentiation, resulting in melanotic tumor formation (Hanratty and Dearolf, 1993; Harrison et al., 1995; Luo et al., 1995). We assessed melanotic tumor formation in adult males that carried the hopTum-l mutation and were heterozygous for odd. These results were compared to those obtained with males that carried only hopTum-l (controls). Ush blocks hopTum-l-induced lamellocyte production (Sorrentino et al., 2007) and, as a positive control, we also assessed tumor production in males that carried hopTum-l and were heterozygous for ush. We observed that 92% of adult males that carried both hopTum-l and odd mutations had melanotic tumors. This was significantly greater than the population of males that carried only the hopTum-l mutation, where only 37% of animals had melanotic tumors. Likewise, among the population of males carrying both hopTum-l and ush mutations, there was a significant increase in the number of males with tumors (74%) compared to the population that was mutant for hopTum-l alone (see Fig. 4). Collectively, these results indicated that Odd blocks lamellocyte differentiation.
FIG. 4.
Odd limits hopTum-l-induced melanotic tumor production. (a) Histogram showing the relative distribution of males with and without tumors in hopTum-l/Y; +/+ (controls), hopTum-l/Y; ush/+ and hopTum-l/Y; odd/+ genetic backgrounds. 171/464 (37%) of hopTum-l/Y; +/+ males had tumors; 126/165 (76%) of hopTum-l/Y; ush/+ males had tumors; 85/92 (92%) of hopTum-l/Y; odd/+ males had tumors. Abbreviations: hop, hopscotch; odd odd-skipped. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Blood Cell Differentiation Does Not Increase in odd/ush Transheterozygotes
Both Odd and Ush block blood cell differentiation and preserve the prohemocyte pool. Our analyses showed that Odd is downregulated in ush mutant lymph glands (Fig. 1e–f″. Likewise, Ush is downregulated in odd mutant lymph glands (Fig. 2a–c,g–i). This raised the question as to whether these factors act synergistically, in a mutually reinforcing feedback loop. Alternatively, each factor may act independently of the other. To distinguish between these two possibilities, we investigated if odd and ush act together to limit prohemocyte differentiation. We assayed plasmatocyte, crystal cell and lamellocyte differentiation in the lymph glands of animals that carried one mutant allele for each gene (odd/ush trans-heterozygotes). There was no observed difference in blood cell differentiation between odd/ush trans-heterozygotes and animals that were singularly heterozygous for either odd or ush (Fig. 5 and data not shown). In particular, we did not observe an increase in lamellocyte production (see Fig. 5). This result would have been expected because lamellocyte production increased in both ush and odd hypomorphs: ush trans-heterozygous and domegal4 driven UAS-oddRNAi lymph glands (Figs. 3g –i and 5; Gao et al., 2009; Sorrentino et al., 2007). These results suggest that Odd and Ush act in different pathways to control blood cell development. This is further supported by results that showed Ush blocks crystal cell production (Gao et al., 2009), whereas Odd had no measurable effect on crystal cells (data not shown).
FIG. 5.
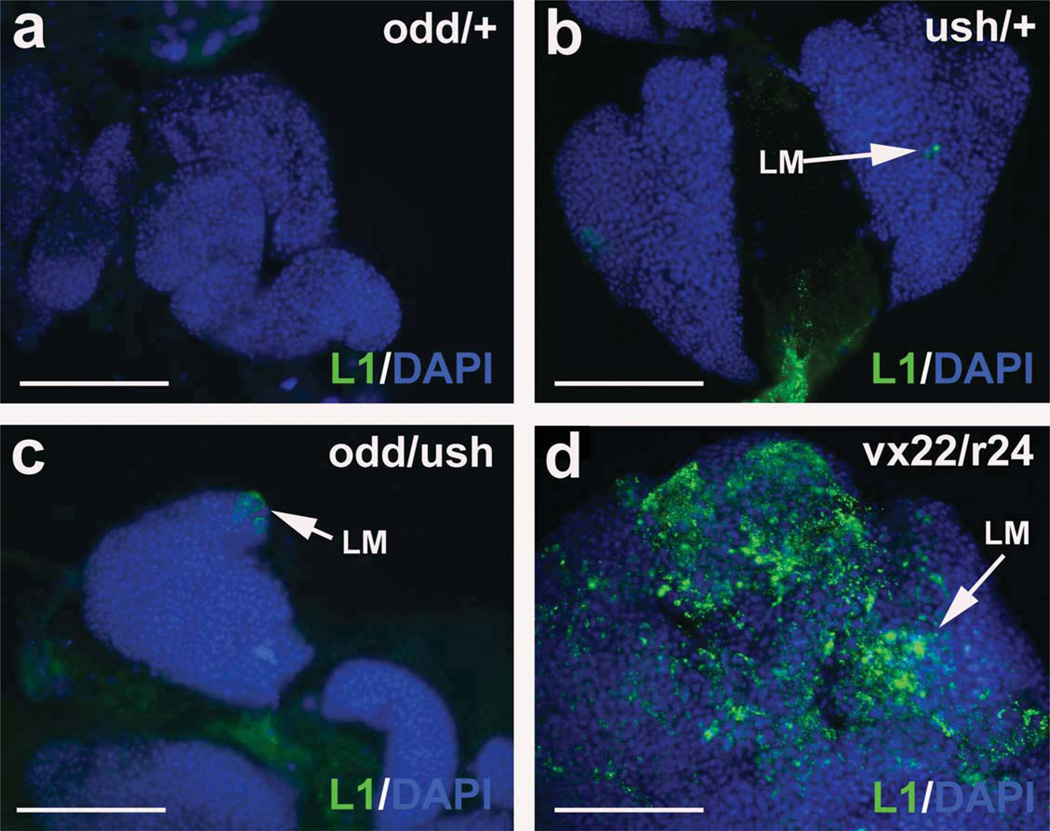
Lamellocyte differentiation does not increase in odd/ush trans-heterozgyotes. Lamellocytes were identified using the specific marker, L1, and are rarely observed in (a) odd heterozygotes (odd/+, (b) ush heterozygotes (ush/+, and (c) odd/ush trans-heterozygotes (odd/ush). The number of lamellocytes was dramatically increased in (d) ushvx22/r24 trans-heterozygotes (vx22/r24). Lymph glands were counterstained with Dapi. Lymph glands were from late-third instar larvae (144 h after egg laying). Abbreviations: odd, odd-skipped; ush, u-shaped. Scale bars: 100 µm.
Dpp Limits Odd Expression and Promotes Plasmatocyte Differentiation
Loss of Dpp signaling results in increased numbers of Odd- and Ush-expressing cells (Frandsen et al., 2008). In this report, we demonstrated that Odd blocks plasmatocyte differentiation. Ush also blocks plasmatocyte differentiation (Gao et al., 2009). These observations predict that loss of Dpp signaling would result in loss of plasmatocyte production due to increased expression of the plasmatocyte antagonists, Odd and Ush. To test this hypothesis, we assayed plasmatocyte production in dpp mutant lymph glands. Although dpp is an essential gene, animals homozygous for the dppd6 allele survive to adulthood (Frandsen et al., 2008). Consistent with the previous study, we observed that Odd expression levels significantly increased in dppd6 mutant lymph glands compared to wild-type controls (Fig. 6a – c). Additionally, plasmatocyte differentiation was blocked in dppd6 lymph glands (Fig. 6a,b), which is most likely a result of increased expression of both Odd (Fig. 3d – f) and Ush (Frandsen et al., 2008; Gao et al., 2009).
FIG. 6.
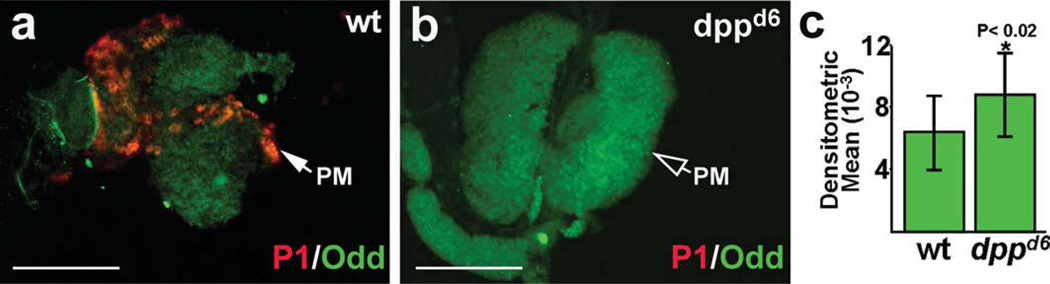
Loss of Dpp signaling blocks plasmatocyte differentiation and upregulates Odd expression. Plasmatocyte differentiation was monitored using the specific marker, P1. (a) Plasmatocyte differentiation and Odd expression in wild-type (wt) lymph glands. (b) Odd expression and lack of plasmatocyte differentiation in dppd6 mutant lymph glands. Closed arrow mark plasmatocytes (PM), open arrow mark lack of plasmatocytes. (c) Densitometric mean of Odd expression was 6332 ± 2363 in wild-type (wt) and 8772 ± 2721in dppd6 lymph glands (P < 0.02). Lymph glands were from late-third instar larvae (144 h after egg laying). Abbreviations: dpp decapentaplegic; odd, odd-skipped. Scale bars: 100 µm.
DISCUSSION
In this report, we showed that Odd is expressed in prohemocytes and downregulated in terminally differentiated plasmatocytes, and we provide strong support for the hypothesis that Odd maintains the prohemocyte pool by blocking both plasmatocyte and lamellocyte differentiation. On the basis of these findings, together with previous results, we purpose the following model for regulation of Drosophila prohemocyte cell fate choice (see Fig. 7). Odd maintains the prohemocyte population and selectively blocks blood cell differentiation, whereas Ush blocks the differentiation of all three blood cell types. Ush expression in prohemocytes is upregulated by the combined actions of the GATA factor Serpent (Srp) and the JAK/STAT signaling pathway (Gao et al., 2009; Muratoglu et al., 2006). In contrast, Srp expression is downregulated by Dpp and thereby limits Ush expression (Frandsen et al., 2008). Dpp also limits Odd expression (Frandsen et al., 2008) and, as a result, promotes plasmatocyte and lamellocyte differentiation (Figs. 3, 4, and 7). Thus, antagonism between Dpp and JAK/STAT signaling may contribute to lineage selection and, importantly, the size of the stem cell-like prohemocyte pool. This model demonstrates how multiple regulatory pathways can interact to maintain the stem cell pool.
FIG. 7.
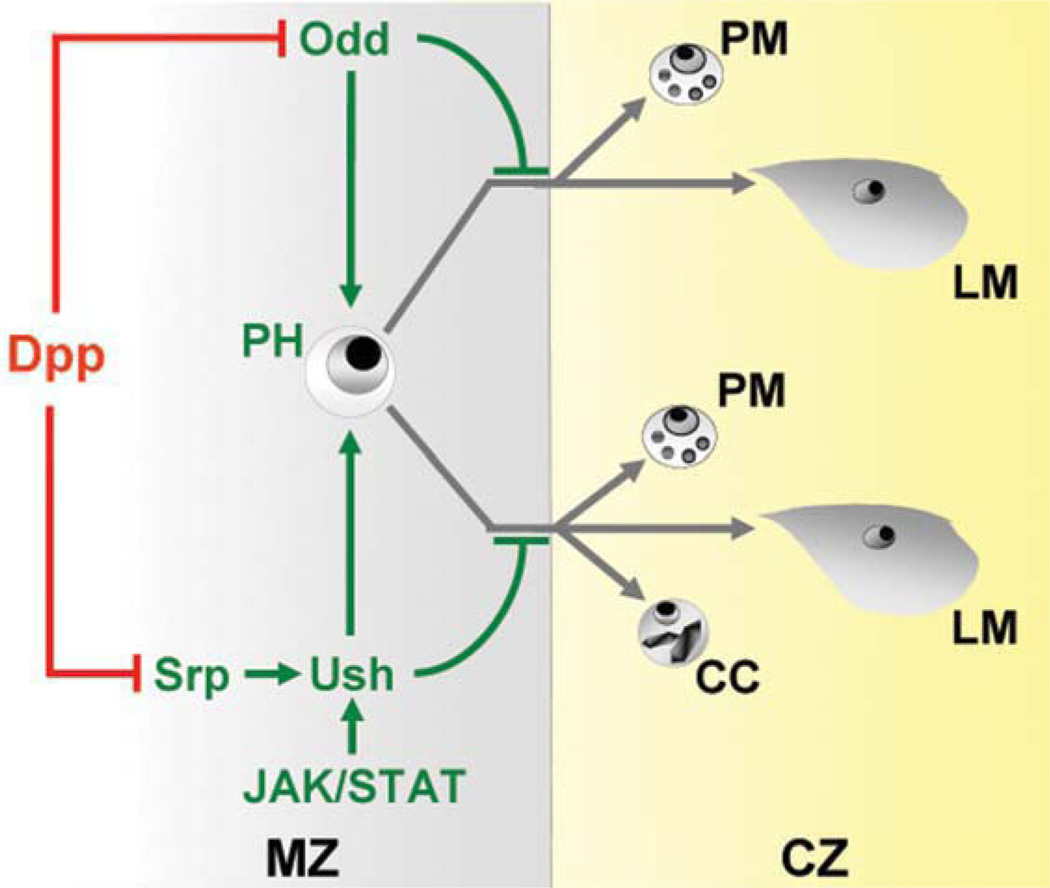
Model depicting Odd and Ush regulation of lymph gland prohemocyte differentiation. Odd and Srp are negatively regulated by Dpp signaling (red blocked line). Dpp inhibition of srp expression leads to loss of ush expression. Conversely, ush expression is maintained by the combined activities of Srp and JAK/STAT signaling. Ush and Odd act in separate pathways to maintain prohemocyte (PH) potency by blocking differentiation. Odd blocks the differentiation of plasmatocytes (PM) and lamellocytes (LM). Ush blocks the differentiation of all three blood cell types, plasmatocytes, crystal cells (CC) and lamellocytes. Green arrows and blocked lines depict pathways that maintain prohemocyte multipotency. Grey lines depict differentiation pathways. The lymph gland medullary zone (MZ) is depicted in grey and cortical zone (CZ) is depicted in yellow. Abbreviations: dpp, decapentaplegic; odd, odd-skipped; srp, serpent; ush, u-shaped.
Odd may control the cell fate switch that determines whether prohemocytes give rise to plasmatocytes or lamellocytes. Our analyses showed that the level of Odd expression influenced lineage choice. Odd expression levels were reduced on average 52% in odd knockeddown lymph glands compared to only 38% in odd heterozygous lymph glands (Fig. 2d – f and Supporting Information Fig. 1a). Plasmatocytes, but not lamellocytes, increased in odd heterozygotes, whereas lamellocytes, but not plasmatocytes, increased in prohemocyte-specific odd knocked-down lymph glands. Thus, a modest reduction in Odd expression leads to increased plasmatocyte production, whereas a further reduction in expression promotes lamellocyte differentiation ostensibly at the expense of plasmatocyte production. This result is reminiscent of our previous work that showed modulation of Ush expression controlled whether the prohemocyte gave rise to the ubiquitous crystal cell and plasmatocyte lineages or the normally rare lamellocyte lineage. Loss of one copy of Ush increased crystal cell and plasmatocyte differentiation, but did not result in increased numbers of lamellocytes. Conversely, loss of greater than one functional copy produced lamellocytes at the expense of both crystal cells and plasmatocytes (Gao et al., 2009). Thus, regulating the relative levels of Odd and Ush may provide a mechanism to control prohemocyte cell fate choice between the ubiquitous plasmatocyte lineage and the specialized immune response lamellocyte lineage. This suggests that Odd and Ush are negative regulators of the lamellocyte response to immune challenge. Additional support for this model comes from recent studies showing that regulators of ush and odd expression are linked to the immune response cascade: Dpp signaling is required for Salmonella-induced lamellocyte differentiation, and JAK/STAT signaling is downregulated to permit lamellocyte differentiation in response to parasitic wasp infection (Frandsen et al., 2008; Makki et al., 2010). These results strongly suggest that Odd and Ush are links between the intrinsic prohemocyte regulatory machinery and extrinsic immune response signaling pathways.
Ush and Odd function to block prohemocyte differentiation to preserve multipotency. Ush blocks plasmatocyte, crystal cell and lamellocyte differentiation, whereas Odd blocks only plasmatocyte and lamellocyte differentiation. One explanation for these differences is that Ush blocks multipotent prohemocyte differentiation, while Odd blocks differentiation of a descendant plasmatocyte/lamellocyte bi-potential precursor. However, downregulating Odd exclusively in prohemocytes increased lamellocyte differentiation (Fig. 3g – i). This provides support for the idea that Odd controls cell fate choice in the more primitive prohemocyte rather than in a descendent bi-potential precursor. The differences between Odd and Ush regulation of prohemocyte cell fate may result from regulatory plasticity within the multipotent prohemocyte pool. In this case, there may be functionally distinct sub-populations of uncommitted multipotent prohemocytes. One subset may have an equal capacity to produce all three blood cell types. In contrast, other subsets may retain the capacity for multipotency but have a propensity towards the production of only one or two of the three blood cell types. This model is consistent with studies in the mouse that identified functional HSC subsets with a bias towards either the myeloid or lymphoid fates (Challen et al., 2010; Dykstra et al., 2007). Additionally, TGF-β signaling was shown to be instrumental in driving differentiation of the myeloid subtype, while limiting differentiation of the lymphoid subtype (Challen et al., 2010). A strikingly similar situation was observed in Drosophila. The TGF-β ortholog, Dpp, appears to promote plasmatocyte (Fig. 6a,b) and lamellocyte differentiation but not crystal cell differentiation (Frandsen et al., 2008). Thus, a conserved property of TGF-β signaling may involve driving functionally distinct HSC subsets towards the production of selected classes of blood cell types. In Drosophila, this likely involves TGF-β repression of Odd and/or Ush expression.
In summary, these studies provide a model for a multicomponent gene regulatory sub-circuit that controls the size of the prohemocyte pool and directs cell fate choice in response to the changing needs of the organism. Overall this work serves to further characterize an important, genetically tractable model of the hematopoietic stem cell/niche system.
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge colleagues for providing fly strains and antibodies. domeless-Gal4 was a generous gift from M. Crozatier, University Paul Sabatier. dppd6 was a generous gift from S.J. Newfeld, Arizona State University. y1 w; ushvx22/CyO, y+ and y1 w; ushr24/CyO, y+ stocks were generous gifts from R.A. Schulz and R.P. Sorrentino. Odd-skipped antibody was a generous gift from James Skeath, Washington University School of Medicine. P1 and L1 antibodies were generous gifts from Istavan Ando, Biological Research Center of the Hungarian Academy of Science.
Contract grant sponsor: NIH, Contract grant number: NIDDK 072229
Footnotes
Additional Supporting Information may be found in the online version of this article.
LITERATURE CITED
- Akala OO, Clarke MF. Hematopoietic stem cell self-renewal. Curr Opin Genet Dev. 2006;16:496–501. doi: 10.1016/j.gde.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Bras-Pereira C, Bessa J, Casares F. Odd-skipped genes specify the signaling center that triggers retinogenesis in Drosophila. Development. 2006;133:4145–4149. doi: 10.1242/dev.02593. [DOI] [PubMed] [Google Scholar]
- Bras-Pereira C, Casares F. An antennal-specific role for bowl in repressing supernumerary appendage development in Drosophila. Mech Dev. 2008;125:809–821. doi: 10.1016/j.mod.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Challen GA, Boles NC, Chambers SM, Goodell MA. Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell. 2010;6:265–278. doi: 10.1016/j.stem.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter DE, Swaykus EA, Beran-Koehn MA, Goldberg D, Wieschaus E, Schedl P. Molecular analysis of odd-skipped, a zinc finger encoding segmentation gene with a novel pair-rule expression pattern. EMBO J. 1990;9:3795–3804. doi: 10.1002/j.1460-2075.1990.tb07593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearolf CR. Fruit fly ‘‘leukemia’’. Biochim Biophys Acta. 1998;1377:M13–M23. doi: 10.1016/s0304-419x(97)00031-0. [DOI] [PubMed] [Google Scholar]
- Dykstra B, Kent D, Bowie M, McCaffrey L, Hamilton M, Lyons K, Lee SJ, Brinkman R, Eaves C. Longterm propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell. 2007;1:218–229. doi: 10.1016/j.stem.2007.05.015. [DOI] [PubMed] [Google Scholar]
- Fossett N, Tevosian SG, Gajewski K, Zhang Q, Orkin SH, Schulz RA. The friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci USA. 2001;98:7342–7347. doi: 10.1073/pnas.131215798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossett N, Zhang Q, Gajewski K, Choi CY, Kim YSchulz RA. The multitype zinc-finger protein U-shaped functions in heart cell specification in the Drosophila embryo. Proc Natl Acad Sci USA. 2000;97:7348–7353. doi: 10.1073/pnas.97.13.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frandsen JL, Gunn B, Muratoglu S, Fossett N, Newfeld SJ. Salmonella pathogenesis reveals that BMP signaling regulates blood cell homeostasis and immune responses in Drosophila. Proc Natl Acad Sci USA. 2008;105:14952–14957. doi: 10.1073/pnas.0808208105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Wu X, Fossett N. Upregulation of the Drosophila friend of GATA gene u-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol Cell Biol. 2009;29:6086–6096. doi: 10.1128/MCB.00244-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanratty WP, Dearolf CR. The Drosophila tumorous-lethal hematopoietic oncogene is a dominant mutation in the hopscotch locus. Mol Gen Genet. 1993;238:33–37. doi: 10.1007/BF00279527. [DOI] [PubMed] [Google Scholar]
- Harrison DA, Binari R, Nahreini TS, Gilman M, Perrimon N. Activation of a Drosophila Janus kinase (JAK) causes hematopoietic neoplasia and developmental defects. EMBO J. 1995;14:2857–2865. doi: 10.1002/j.1460-2075.1995.tb07285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao I, Green RB, Dunaevsky O, Lengyel JA, Rauskolb C. The odd-skipped family of zinc finger genes promotes Drosophila leg segmentation. Dev Biol. 2003;263:282–295. doi: 10.1016/j.ydbio.2003.07.011. [DOI] [PubMed] [Google Scholar]
- Honti V, Csordas G, Markus R, Kurucz E, Jankovics F, Ando I. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster . Mol Immunol. 2010;47:1997–2004. doi: 10.1016/j.molimm.2010.04.017. [DOI] [PubMed] [Google Scholar]
- James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development. 2006;133:2995–3004. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- Johnson AN, Burnett LA, Sellin J, Paululat A, Newfeld SJ. Defective decapentaplegic signaling results in heart overgrowth and reduced cardiac output in Drosophila. Genetics. 2007;176:1609–1624. doi: 10.1534/genetics.107.073569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. doi: 10.1242/dev.01837. [DOI] [PubMed] [Google Scholar]
- Kawai S, Yamauchi M, Wakisaka S, Ooshima T, Amano A. Zinc-finger transcription factor odd-skipped related 2 is one of the regulators in osteoblast proliferation and bone formation. J Bone Miner Res. 2007;22:1362–1372. doi: 10.1359/jbmr.070602. [DOI] [PubMed] [Google Scholar]
- Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- Krzemien J, Oyallon J, Crozatier M, Vincent A. Hematopoietic progenitors and hemocyte lineages in the Drosophila lymph gland. Dev Biol. 2010;346:310–319. doi: 10.1016/j.ydbio.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Markus R, Zsamboki J, Folkl-Medzihradszky K, Darula Z, Vilmos P, Udvardy A, Krausz I, Lukacsovich T, Gateff E, Zettervall CJ, Hultmark D, Ando I. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr Biol. 2007a;17:649–654. doi: 10.1016/j.cub.2007.02.041. [DOI] [PubMed] [Google Scholar]
- Kurucz E, Vaczi B, Markus R, Laurinyecz B, Vilmos P, Zsamboki J, Csorba K, Gateff E, Hultmark D, Ando I. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 2007b;58(Suppl):95–111. doi: 10.1556/ABiol.58.2007.Suppl.8. [DOI] [PubMed] [Google Scholar]
- Lan Y, Kingsley PD, Cho ES, Jiang R. Osr2, a new mouse gene related to Drosophila odd-skipped, exhibits dynamic expression patterns during craniofacial, limb, and kidney development. Mech Dev. 2001;107:175–179. doi: 10.1016/s0925-4773(01)00457-9. [DOI] [PubMed] [Google Scholar]
- Luo H, Hanratty WP, Dearolf CR. An amino acid substitution in the Drosophila hopTum-l Jak kinase causes leukemia-like hematopoietic defects. EMBO J. 1995;14:1412–1420. doi: 10.1002/j.1460-2075.1995.tb07127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makki R, Meister M, Pennetier D, Ubeda JM, Braun A, Daburon V, Krzemien J, Bourbon HM, Zhou R, Vincent A, Crozatier M. A short receptor downregulates JAK/STAT signalling to control the Drosophila cellular immune response. PLoS Biol. 2010;8:e1000441. doi: 10.1371/journal.pbio.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. doi: 10.1038/ng1404. [DOI] [PubMed] [Google Scholar]
- Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapediadependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: A comparative view. Genes Dev. 2007;21:3044–3060. doi: 10.1101/gad.1602607. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Druzhinina M, Steward R. Zfrp8, the Drosophila ortholog of PDCD2, functions in lymph gland development and controls cell proliferation. Development. 2007;134:2387–2396. doi: 10.1242/dev.003616. [DOI] [PubMed] [Google Scholar]
- Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. doi: 10.1242/dev.043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA. Recent advances in defining the hematopoietic stem cell niche. Curr Opin Hematol. 2004;11:107–111. doi: 10.1097/01.moh.0000133652.06863.47. [DOI] [PubMed] [Google Scholar]
- Muratoglu S, Garratt B, Hyman K, Gajewski K, Schulz RA, Fossett N. Regulation of Drosophila friend of GATA gene, u-shaped, during hematopoiesis: A direct role for serpent and lozenge. Dev Biol. 2006;296:561–579. doi: 10.1016/j.ydbio.2006.04.455. [DOI] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Orford KW, Scadden DT. Deconstructing stem cell self-renewal: Genetic insights into cell-cycle regulation. Nat Rev Genet. 2008;9:115–128. doi: 10.1038/nrg2269. [DOI] [PubMed] [Google Scholar]
- Raj A, Rifkin SA, Andersen E, van Oudenaarden A. Variability in gene expression underlies incomplete penetrance. Nature. 2010;463:913–918. doi: 10.1038/nature08781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–1079. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stemlike hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–763. doi: 10.1016/j.devcel.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino RP, Tokusumi T, Schulz RA. The friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev Biol. 2007;311:311–323. doi: 10.1016/j.ydbio.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Tena JJ, Neto A, Calle-Mustienes E, Bras-Pereira C, Casares F, Gomez-Skarmeta JL. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301:518–531. doi: 10.1016/j.ydbio.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Trumpp A, Essers M, Wilson A. Awakening dormant haematopoietic stem cells. Nat Rev Immunol. 2010;10:201–209. doi: 10.1038/nri2726. [DOI] [PubMed] [Google Scholar]
- Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Oddskipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol. 2005;288:582–594. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi M, Kawai S, Kato T, Ooshima T, Amano A. Odd-skipped related 1 gene expression is regulated by Runx2 and Ikzf1 transcription factors. Gene. 2008;426:81–90. doi: 10.1016/j.gene.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Zon LI. Intrinsic and extrinsic control of haematopoietic stem-cell self-renewal. Nature. 2008;453:306–313. doi: 10.1038/nature07038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.