Abstract
Objective
Antipsychotic (AP) utilization has grown significantly in long-term care (LTC) settings. Although a growing literature associates AP use with higher mortality in elderly with dementia, the association of APs with hospital events is unclear. The authors examine prevalence and trends in AP use by Medicare beneficiaries residing in LTC and the association of APs and other drug use variables with hospital events and mortality.
Design
Retrospective analysis using sequential multivariate Cox proportional hazards models.
Setting
Medicare Current Beneficiary Survey linked to Institutional Drug Administration and Minimum Data Set files.
Participants
A total of 2,363 LTC Medicare beneficiaries, 1999–2002.
Measurements
Trends in LTC AP use overall and by type and duplicative use; association of AP utilization and two outcomes: hospital events and all-cause mortality.
Results
AP use rose markedly from 1999 to 2002 (26.4%–35.9%), predominantly due to increased use of atypical agents. After controlling for sociodemographic and clinical factors, AP use is not related to hospital events (hazard ratio [HR] = 0.98, 95% confidence interval [CI] = 0.82–1.63 p = 0.7951). AP use is associated with reduced mortality in unadjusted and intermediate models, but loss of significance in the final model (HR = 0.83, 95% CI = 0.69–1.00, p = 0.0537) suggests that disease and drug burden factors may confound the AP-mortality relationship.
Conclusion
This study provides no evidence of increased hospital events or mortality in LTC residents who use AP medications. Findings contribute to a growing body of evidence that APs, particularly atypical agents, may be associated with reduced mortality in LTC residents.
Keywords: Antipsychotic medications, long-term care, medicare beneficiaries, drug trends
Use of antipsychotic (AP) medications in long-term care (LTC) has grown significantly over the past decade.1,2 A primary driver of increased use is the shift from older first generation agents, such as haloperidol and perphenazine, to the newer atypical agents (risperidone, olanzapine, aripiprazole, ziprasidone, and quetiapine).2,3 Despite the marked rise in the use of this therapeutic class, there is little information on the prevalence, appropriateness, or outcomes associated with AP prescribing in LTC residents. Further, the evidence that is available is contradictory or inconclusive, leaving patients and their providers unable to make sound medication choices.
In the spring of 2005, concerns about the safety of atypical APs led the U.S. Food and Drug Administration to issue a black box warning against their use in treating elderly patients with dementia.4 It is estimated that approximately half of newly admitted nursing home residents have dementia, and estimates of dementia prevalence among all residents range from 40% to 75%.5 APs are frequently used in this setting for behavioral disturbances that may accompany dementia, such as aggression and agitation6; however, such use is generally considered outside the U.S. Food and Drug Administration-approved indications.7
In a large study of Pennsylvania retirees using administrative claims data conducted by Wang et al.,8 AP use by nursing home residents was associated with increased risk of mortality, although the risk was slightly lower than that of community-dwelling elders. Moreover, another population-based, retrospective cohort study, conducted by Rochon et al.,9 compared short-term serious events (acute care hospital admission or death) after initial AP use between older adults with dementia in community-dwelling and nursing home in Canada. Results from this study showed that patients who received conventional AP therapy were a little more likely to develop any serious event during the 30 days of follow-up. The pattern of serious events was similar but less pronounced among older adults living in a nursing home. However, other research evaluating the impact of APs on adverse outcomes in the LTC-residing older adults have produced mixed results due to differences in data source, methodological approach, sample size, and geographic variation in treatment practices. For instance, although some studies8,10 discerned a positive association between AP use and mortality, especially in the use of conventional agents, other studies report that APs seem to have either no effect—or even a protective effect—on mortality.11
A similar mix of findings is reported when examining hospitalization associated with AP use.11,12 In a sample of geriatric patients admitted to a psychiatric center, Barak et al.12 found no association between hospitalization and class of AP, whereas Raivio et al.11 found a lower mean number of hospital admissions among elderly institutionalized dementia using APs relative to non-AP users, regardless of AP class. Yet another study found increased risk for hospitalization for adverse cardiac effects among conventional AP users only.13 Finally, the somewhat larger literature examining the association of AP use and adverse outcomes in the community-dwelling older adult population also fail to discern clear-cut findings of risk.8,12,14–19
Conclusions from these studies are further complicated by their focus on individuals with dementia and on specific APs or AP class (i.e., conventional versus atypical). Although several studies have examined the association between AP use and older adults with dementia9,17,18,20,21 and without,8,14,19 findings were not definitive. Furthermore, there is no clear association between mortality and type of AP, regardless of dementia status. Although some studies have found higher mortality associated with conventional APs,9,14,15 others have found statistically and clinically important associations with atypical agents.8,10,15,18
Despite the prevalence of AP use in LTC residents, there is a paucity of studies in this population. Such studies are necessary as LTC residents typically reflect radically different care and medication prescribing patterns than their community-dwelling counterparts. These few studies are limited in that they generally utilize small sample sizes, reflecting single or a small number of homes, and are conducted in other countries, thereby limiting generalizability to the U.S. population.9,11,20,21 Although three studies that include a subset of LTC residents use large databases,8,9,13 both also are limited geographically.
This study examines the utilization of APs in LTC facilities in the United States. Using a national sample of Medicare beneficiaries residing in LTC facilities, we provide national estimates of the prevalence of and trends in use of APs from 1999 to 2002. We further investigate the association of AP use with hospital events and mortality, controlling for sociodemographics, clinical indicators, and other key covariates.
METHODS
Data Source
This study is a retrospective analysis of the 1999–2002 Medicare Current Beneficiary Survey (MCBS) Cost and Use Surveys. The MCBS is a continuous sample of U.S. Medicare beneficiaries that employs a panel design in which beneficiaries are surveyed three times annually for a maximum of 4 years.22 Beneficiaries may live in the community or in LTC facilities. For the purposes of this study, the designation “LTC” includes both skilled nursing facilities and other congregate care settings, such as “high end” assisted living facilities, which receive Medicare reimbursement, provide round-the-clock skilled nursing, and have centralized medication distribution. For the purposes of this analysis, we excluded beneficiaries residing in psychiatric facilities (n = 20).
The MCBS contains information on sociodemographics and medical services used. These files were augmented with information from Institutional Drug Administration files and extracts from the Minimum Data Sets for LTC residents. The Institutional Drug Administration file contains information abstracted monthly Medication Administration Records and includes names and frequency of administration of all medications. Only drugs actually administered are included in analysis.
To maximize sample size, data are arranged as annual crosssections for all 4 years and concatenated. For individuals included in multiple data years, one observation year was randomly chosen. Additional inclusion criteria include maintaining individuals with at least one Medication Administration Record and one Minimum Data Set record. The final analytic sample consists of 2,363 unique individuals identified as staying in an LTC facility at least 1 day during the study year.
Measures
We created a binary variable to measure administration of any AP medication in the first 6 months of the study year. We further differentiated AP use by atypical (i.e., clozapine, risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole) and conventional status. We also flagged duplicative AP use, operationalized as the use of two or more unique APs used concomitantly for 2 or more months. This measure excludes occasional use of APs (i.e., acute pro re nata use), as well as minimizes duplicative use likely due to titration and/or switching APs. For those patients using both atypical and conventional APs, we grouped them into atypical users.
Outcomes in this investigation are acute hospital events and all-cause mortality. Time to outcome event is defined as days from the first day of recorded AP exposure to the first outcome event occurring within the calendar year. This “exposure to event” time period has been used in other studies.8,10 “Hospital event” is defined as transfer from a LTC facility to an inpatient hospital stay or emergency department visit. We consider only the first hospital event in analysis. Mortality is determined from Medicare enrollment eligibility files.
Sociodemographic variables include age, sex, race, marital status, geographic region of residence, income, and education. Health-related variables include count of reported physical conditions, mental health disorders, anxiety, mood disorders, schizophrenia/psychotic disorders, signs and symptoms (delusions and delirium), medical and prescription drug insurance status, self-rated health, limitations in activities of daily living (ADL), body mass index, and physical restraint use. We also constructed a variable measuring psychoactive drug burden, operationalized as a count of all unique, non-AP psychotherapeutic drug classes administered within the study year.
Data Analysis
We use Wald χ2 tests and t-tests to compare AP users and nonusers, as well as atypical versus conventional AP users. Multiple Cox proportional hazards models are applied to estimate time from AP exposure to death or hospital event, controlling for covariates. Starting with an unadjusted proportional hazards model, we use a model building approach that sequentially adds groups of related variables. Next, we add demographic variables, socioeconomic characteristics, and observation year, followed by a third model adding general health-related variables and use of physical restraints. The final model further includes disease and drug burden variables. Assumptions of proportional hazards were tested and verified by plotting the log-log survivor functions for AP users and nonusers for each outcome, with resulting curves being roughly parallel for both AP users and nonusers.
We report both p values and associated 95% confidence intervals. For categorical explanatory variables with two or more categories, we will report group χ2 in text and individual category χ2 in the tables. For trend analyses, we report the Cochrane-Armitage test for trend. All analyses relied on SAS statistical software, version 9.1 (SAS, Cary, NC).
Institutional Review Board’s Approval and Funding
This investigation was deemed exempt by the University of Maryland, Baltimore Institutional Review Board, as the analysis relied on a limited dataset. This study was funded by a contract with the Centers for Medicare and Medicaid Services (CMS). The funding source collaborated in forming the research questions but not in study design or analysis. Preliminary analyses upon which this publication is based were performed under Contract Number HHSP23320044302EC entitled “Impact of Antipsychotic Use on Hospital Event and Mortality in Medicare Beneficiaries Residing in Long-Term Care Facilities,” sponsored by the CMS, Department of Health and Human Services. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The authors assume full responsibility for the accuracy and completeness of the ideas presented.
RESULTS
Characteristics of the Sample
Table 1 summarizes the characteristics of AP users and nonusers. During the study period (6 months of follow-up during the years 1999–2002), almost one third of residents received at least one AP medication. Nearly one third of residents experienced at least one hospital event and more than a quarter died within the follow-up period. In bivariate analysis, the use of APs was not significantly associated with hospital events; however, mortality was significantly lower among AP users than AP nonusers. AP users were younger, more often male, and less often married than nonusers. AP users also had fewer ADL limitations, and reported greater prevalence of anxiety, schizophrenia, mood disorders, delirium, and delusion than nonusers. AP users also more often used other psychotherapeutic medication classes, including antidepressants, mood stabilizers, and anxiolytics.
TABLE 1.
Characteristics of Antipsychotic Users and Nonusers
| Characteristic | Total (n = 2,363) n (%) | Antipsychotic Users (n = 742) n (%) | Nonusers (n = 1,621) n (%) | χ2 Statistic | p Value |
|---|---|---|---|---|---|
| Hospital event | 719 (30.4) | 231 (31.1) | 488 (30.1) | 0.25 | 0.6145 |
| Death | 682 (28.9) | 170 (22.9) | 512 (31.6) | 18.65 | <0.0001 |
| Age group | |||||
| Less than 65 years | 409 (17.3) | 186 (25.1) | 223 (13.8) | ||
| 65–74 years | 193 (8.2) | 82 (11.1) | 111 (6.9) | ||
| 75–84 years | 672 (28.4) | 230 (31.0) | 442 (27.3) | ||
| 85 years or older | 1,089 (46.1) | 244 (32.9) | 845 (52.1) | 92.03a | <0.0001 |
| Gender, male | 740 (31.3) | 271 (36.5) | 469 (28.9) | 13.63 | 0.0002 |
| Race, white | 2,016 (85.3) | 631 (85.0) | 1,385 (85.4) | 0.07 | 0.7984 |
| Currently married | 1,425 (60.3) | 402 (54.2) | 1,023 (63.1) | 16.96 | <0.0001 |
| High school graduate | 907 (38.4) | 270 (36.4) | 637 (39.3) | 1.82 | 0.1772 |
| Income at or below federal poverty level | 951 (40.3) | 317 (42.7) | 634 (39.1) | 2.76 | 0.0967 |
| Urban residence | 1,737 (73.5) | 562 (75.7) | 1,175 (72.5) | 2.77 | 0.0961 |
| Supplemental health insurance | 1,989 (84.2) | 635 (85.6) | 1,354 (83.5) | 1.61 | 0.2049 |
| Prescription drug insurance | 582 (24.6) | 187 (25.2) | 395 (24.4) | 0.19 | 0.6621 |
| Fair or poor self-rated health | 1,336 (56.5) | 427 (57.6) | 909 (56.1) | 0.45 | 0.5033 |
| Three or fewer ADL limitations | 1,666 (70.5) | 494 (66.6) | 1,172 (72.3) | 8.02 | 0.0046 |
| BMIb group | |||||
| <18.5 (underweight) | 438 (18.5) | 123 (16.6) | 315 (19.4) | ||
| 18.5–24.9 (desirable weight) | 1,062 (44.9) | 327 (44.1) | 735 (45.3) | ||
| 25.0–29.9 (overweight) | 542 (22.9) | 172 (23.2) | 370 (22.8) | ||
| >30.0 (obese) | 321 (13.6) | 120 (16.2) | 201 (12.4) | 7.78a | 0.0507 |
| Number of reported comorbid physical conditions | |||||
| 0–3 | 660 (27.9) | 185 (24.9) | 475 (29.3) | ||
| 4–6 | 650 (27.5) | 197 (26.6) | 453 (28.0) | ||
| 7–9 | 534 (22.6) | 192 (25.9) | 342 (21.0) | ||
| 10 or more | 519 (22.0) | 168 (22.6) | 351 (21.7) | 9.21a | 0.0266 |
| Anxiety | 193 (8.2) | 91 (12.3) | 102 (6.3) | 24.20 | <0.0001 |
| Mood disorders | 634 (26.8) | 219 (29.5) | 415 (25.6) | 3.97 | 0.0463 |
| Schizophrenia or psychotic disorders | 138 (5.8) | 114 (15.4) | 24 (1.5) | 178.41 | <0.0001 |
| Delusion | 135 (5.7) | 88 (11.9) | 47 (2.9) | 75.87 | <0.0001 |
| Delirium | 726 (30.7) | 268 (36.1) | 458 (28.3) | 14.79 | 0.0001 |
| Physical restraint order | 185 (7.8) | 55 (7.4) | 130 (8.0) | 0.26 | 0.6100 |
| Other classes of psychoactive drugs | |||||
| Antidepressant user | 960 (40.6) | 388 (52.3) | 572 (35.3) | 61.02 | <0.0001 |
| Mood stabilizer user | 340 (14.4) | 188 (25.3) | 152 (9.4) | 105.25 | <0.0001 |
| Hypnotic user | 207 (8.8) | 76 (10.2) | 131 (8.1) | 2.97 | 0.0846 |
| Anxiolytic user | 847 (35.8) | 352 (47.4) | 495 (30.5) | 63.24 | <0.0001 |
| Central nervous system | |||||
| Stimulant user | 33 (1.4) | 15 (2.0) | 18 (1.1) | 3.07 | 0.0798 |
| Year (group) | |||||
| 1999 | 678 (28.7) | 179 (24.1) | 499 (30.8) | ||
| 2000 | 543 (23.0) | 162 (21.8) | 381 (23.5) | ||
| 2001 | 496 (21.0) | 169 (22.8) | 327 (20.2) | ||
| 2002 | 646 (27.3) | 232 (31.3) | 414 (25.5) | 16.24a | 0.0010 |
Degrees of freedom is equal to 3 for variables with two or more categories. Degrees of freedom for all other variables is equal to 1.
Body mass index (kg/m2).
Although not reported in tabular form, subanalyses found that of the 742 residents who received APs, 456 (61.5%) were administered only atypicals, 194 (26.2%) received only conventional medications, and 92 (12.4%) used both AP classes. Relative to conventional AP users, atypical AP users were more often white (86.9% versus 79.9%, χ2 = 5.46, df = 1, p = 0.0194), more often used mood stabilizers (28.7% versus 16.0%, χ2 = 12.16, df = 1, p = 0.0005) and anxiolytics (50.2% versus 39.7%, χ2 = 6.33, df = 1, p = 0.0119), and reported more comorbid conditions (6.8 [SD = 4.14] versus 6.0 [SD = 4.14], t = −2.39, df = 740, p = 0.0172).
Prevalence and Trends in AP Use
AP use by Medicare beneficiaries residing in LTC facilities rose markedly during the 4-year period spanning 1999–2002 (Fig. 1). The use of any AP increased from 26.4% in 1999 to 35.9% in 2002 (Cochrane-Armitage test for trend, z = −3.99, p <0.0001), indicating increasing proportions of beneficiaries with any AP use. Increased use of the atypical agents dominated much of this trend, with atypical use more than doubling from 13.9% in 1999 to 31.6% in 2002 (Cochrane-Armitage test for trend, z = −7.98, p <0.0001). Conventional AP use decreased by about half, from 15.9% to 8.4% (Cochrane-Armitage test for trend, z =4.33, p <0.0001). Duplicative AP use increased from 2.1% of AP users in 1999 to 4.5% by 2002 (Cochrane-Armitage test for trend, z = −2.41, p = 0.0159).
FIGURE 1.
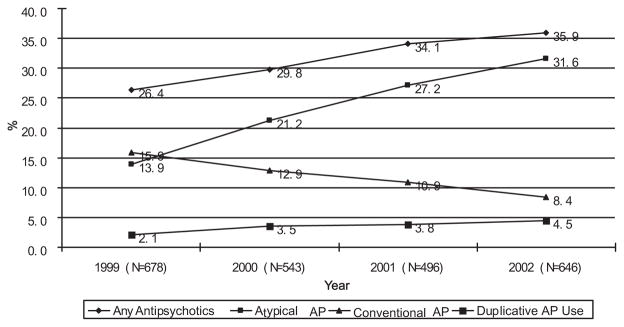
Antipsychotic Use by Medicare Beneficiaries During the 4-Year Period Spanning 1999–2002
Association of AP Use on Hospital Events and Mortality
Table 2 shows findings from the Cox proportional hazards models of the association between any AP use and hospital events and mortality. Although AP use showed an inverse relationship with mortality in unadjusted and partially adjusted models, the association failed to maintain statistical significance in the final, fully adjusted model. Use of any AP did not reach statistical significance for hospital events in any model.
TABLE 2.
Cox Proportional Hazards Modelsa for Associations Between Antipsychotic Use and Hospital Events and Mortality Among Medicare Beneficiaries, 1999–2002 (n = 2,363)
| Variable | Model I (Unadjusted) | Model II (Model I + Demographics, Socioeconomic Status) | Model III (Model II + Health Variables) | Model IV (Model III + Disease and Drug Burden) |
|---|---|---|---|---|
| I. Hospital Events HR (95% CI) p | ||||
| Antipsychotic use | 1.03 (0.88–1.21) 0.6693 | 1.06 (0.90–1.24) 0.4921 | 1.04 (0.89–1.22) 0.6436 | 0.98 (0.82–1.63) 0.7951 |
| Gender, male | 1.09 (0.92–1.29) 0.3052 | 1.11 (0.94–1.31) 0.2217 | 1.07 (0.90–1.27) 0.4300 | |
| Age groupb | ||||
| <65 years | 0.53 (0.38–0.74) 0.0001 | 0.59 (0.42–0.82) 0.2217 | 0.94 (0.68–1.31) 0.7137 | |
| 75–84 years | 0.87 (0.66–1.15) 0.3373 | 0.89 (0.67–1.17) 0.0015 | 0.85 (0.64–1.13) 0.2630 | |
| 85 and older | 0.91 (0.69–1.12) 0.4868 | 0.95 (0.72–1.25) 0.6917 | 0.94 (0.71–1.25) 0.6753 | |
| Race, white | 0.91 (0.75–1.12) 0.3847 | 0.91 (0.74–1.12) 0.3631 | 1.00 (0.81–1.23) 0.9954 | |
| Poverty statusc | 1.18 (1.01–1.38) 0.0386 | 1.18 (1.01–1.39) 0.0386 | 1.18 (1.00–1.38) 0.0501 | |
| Currently married | 1.10 (0.91–1.34) 0.3043 | 1.04 (0.88–1.29) 0.5309 | 1.04 (0.86–1.26) 0.6834 | |
| High school education | 0.93 (0.80–1.09) 0.3657 | 0.94 (0.80–1.09) 0.3990 | 0.94 (0.80–1.10) 0.4316 | |
| Urban residence | 1.02 (0.86–1.21) 0.8211 | 1.03 (0.87–1.22) 0.6960 | 0.84 (0.71–0.99) 0.0478 | |
| Yeard | ||||
| 2000 | 1.18 (0.95–1.47) 0.1312 | 1.17 (0.94–1.46) 0.1536 | 1.09 (0.88–1.37) 0.4323 | |
| 2001 | 1.19 (0.99–1.56) 0.0521 | 1.28 (1.02–1.60) 0.0315 | 1.16 (0.93–1.46) 0.1935 | |
| 2002 | 1.19 (0.96–1.47) 0.1072 | 1.21 (0.97–1.50) 0.0806 | 0.94 (0.76–1.17) 0.5913 | |
| BMI groupe | ||||
| Underweight | 1.10 (0.90–1.34) 0.3629 | 1.20 (0.98–1.47) 0.0816 | ||
| Overweight | 0.94 (0.77–1.14) 0.5108 | 0.88 (0.73–1.08) 0.2164 | ||
| Obese | 1.20 (0.96–1.51) 0.1103 | 1.06 (0.84–1.33) 0.2164 | ||
| ADL impairment | 1.04 (0.86–1.26) 0.6873 | 1.23 (1.01–1.49) 0.0425 | ||
| Supplemental health insurance | 0.90 (0.73–1.10) 0.2936 | 0.66 (0.54–0.82) 0.0001 | ||
| Supplemental drug coverage | 0.90 (0.75–1.08) 0.2415 | 0.86 (0.71–1.03) 0.0941 | ||
| Fair or poor self-rated healthd | 1.47 (1.24–1.74) <0.0001 | 1.14 (0.96–1.35) 0.1235 | ||
| Physical restraint order | 0.90 (0.86–1.18) 0.4342 | 1.08 (0.82–1.43) 0.5871 | ||
| Number of reported physical conditions | 1.24 (1.22–1.26) <0.0001 | |||
| Reported psychiatric diagnoses | ||||
| Anxiety disorder | 1.00 (0.77–1.33) 0.9588 | |||
| Mood disorder | 0.78 (0.65–0.93) 0.0070 | |||
| Schizophrenia | 0.98 (0.69–1.41) 0.0079 | |||
| Signs and symptoms | ||||
| Delirium | 1.14 (0.82–1.60) 0.0468 | |||
| Delusions | 0.98 (0.83–1.16) 0.6066 | |||
| Number administered psychotherapeutic drug classesf | 1.03 (0.96–1.11) 0.4436 | |||
| Duplicative antipsychotic use | 1.17 (0.75–1.83) 0.4918 | |||
| II. Mortality | ||||
| Antipsychotic use | 0.67 (0.57–0.80) <0.0001 | 0.83 (0.69–0.98) 0.0328 | 0.78 (0.65–0.93) 0.0058 | 0.83 (0.69–1.00) 0.0537 |
| Gender–male | 1.36 (1.15–1.62) 0.0004 | 1.36 (1.15–1.62) 0.0004 | 1.34 (1.13–1.59) 0.0010 | |
| Age groupb | ||||
| <65 years | 0.18 (0.10–0.31) <0.0001 | 0.21 (0.12–0.36) <0.0001 | 0.23 (0.13–0.40) <0.0001 | |
| 75–84 years | 1.38 (0.99–1.93) 0.0568 | 1.38 (0.99–1.93) 0.0563 | 1.33 (0.95–1.86) 0.0978 | |
| 85 and older | 1.89 (1.36–2.61) 0.0001 | 1.82 (1.32–2.53) 0.0003 | 1.70 (1.22–2.36) 0.0016 | |
| Race, white | 1.09 (0.87–1.37) 0.4510 | 1.04 (0.82–1.30) 0.7594 | 1.07 (0.85–1.35) 0.5707 | |
| Poverty status | 0.93 (0.79–1.10) 0.3858 | 1.06 (0.89–1.26) 0.5020 | 1.04 (0.87–1.23) 0.6805 | |
| Currently married | 1.07 (0.89–1.30) 0.4580 | 1.04 (0.86–1.26) 0.6739 | 1.02 (0.84–1.23) 0.8610 | |
| High school education | 1.15 (0.98–1.34) 0.0846 | 1.11 (0.94–1.29) 0.2143 | 1.08 (0.92–1.26) 0.3652 | |
| Urban residencec | 0.94 (0.80–1.11) 0.4487 | 0.98 (0.83–1.15) 0.7919 | 0.97 (0.83–1.1) 0.75465 | |
| Yeard | ||||
| 2000 | 1.11 (0.91–1.37) 0.3094 | 1.13 (0.91–1.39) 0.2765 | 1.17 (0.95–1.44) 0.1520 | |
| 2001 | 0.91 (0.73–1.14) 0.4295 | 0.82 (0.77–1.23) 0.2765 | 1.00 (0.80–1.26) 0.9771 | |
| 2002 | 0.64 (0.51–0.80) <0.0001 | 0.69 (0.55–0.87) 0.0016 | 0.72 (0.57–0.9) 0.0062 | |
| BMI groupe | ||||
| Underweight | 1.61 (1.34–1.93) <0.0001 | 1.62 (1.35–1.94) <0.0001 | ||
| Overweight | 0.82 (0.66–1.01) 0.0628 | 0.83 (0.67–1.03) 0.0888 | ||
| Obese | 0.86 (0.64–1.15) 0.2983 | 0.99 (0.74–1.31) 0.4684 | ||
| ADL impairment | 1.06 (0.87–1.29) 0.5953 | 0.97 (0.79–1.20) 0.8045 | ||
| Supplemental health insurance | 0.37 (0.31–0.44) <0.0001 | 0.36 (0.30–0.44) <0.0001 | ||
| Supplemental drug coverage | 1.06 (0.87–1.30) 0.5523 | 1.06 (0.87–1.29) 0.5859 | ||
| Poor/fair self-rated healthd | 1.42 (1.19–1.69) <0.0001 | 1.43 (1.20–1.70) <0.0001 | ||
| Physical restraint order | 1.23 (0.96–1.59) 0.0996 | 1.18 (0.92–1.53) 0.3100 | ||
| Number of reported physical conditions | 1.00 (1.00–1.02) 0.7941 | |||
| Reported psychiatric conditions | ||||
| Anxiety disorder | 0.87 (0.65–1.17) 0.3535 | |||
| Mood disorder | 0.96 (0.79–1.16) 0.6435 | |||
| Schizophrenia | 0.38 (0.18–0.81) 0.0121 | |||
| Signs and symptoms | ||||
| Delirium | 1.39 (1.17–1.64) 0.0001 | |||
| Delusions | 1.02 (0.71–1.47) 0.9315 | |||
| Number administered psychotherapeutic drug classesg | 0.95 (0.87–1.03) 0.2392 | |||
| Duplicative antipsychotic use | 0.44 (0.18–1.09) 0.0760 | |||
For this study, we used a survival analysis (Cox proportional hazard model). This method of analysis estimates and associated p values are based upon a df of 1 for all parameters in the model.
65–74 years is the reference group.
100% of federal poverty level (FPL) versus those greater than 100% FPL.
Good, very good, or excellent health is the reference.
Desirable weight category is the reference group.
1999 is the reference year.
Antidepressant, mood stabilizer, sedative/hypnotic, anxiolytic, stimulant, and skeletal muscle relaxant. Excludes antipsychotic medications and prn orders.
Other explanatory variables are associated with our outcomes of interest. We only comment on those factors found to be statistically significant in the final adjusted models. Additional factors positively associated with hospital events included ADL impairment and increasing numbers of comorbidities, whereas other positive correlates of mortality included male sex, reporting fair or poor health, presence of delirium, 85 and older age group, being underweight, and observation year.
Possession of supplemental health insurance seemed to have a protective effect on the risk of incurring a hospital event. Diagnosis of a mood disorder and urban residence also shortened the time to a hospital event, whereas age less than 65 and a diagnosis of schizophrenia were associated with lower mortality.
We performed analyses stratified by AP type, age group (<65 versus those 65 and older), type of LTC residence (skilled nursing facility versus other site types), and dementia status (Table 3). We found no statistically significant differences in hospital events for any of these stratified analyses. Mortality, however, was inversely associated with the use of atypical APs only. Reduced mortality also was associated with use of any AP among residents living in nursing homes (relative to other types of LTC), beneficiaries with dementia, and beneficiaries aged 65 years and older.
TABLE 3.
Cox Proportional Hazards Modelsa for Associations Between Antipsychotic Use and Hospital Events and Mortality Among Medicare Beneficiaries, 1999–2002, Stratified by Antipsychotic Type, Nursing Home Residence, Dementia Status, and Age
| Hospital Events
|
Mortality
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted
|
Adjustedb
|
Unadjusted
|
Adjustedb
|
|||||||||
| Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value | Coefficient | 95% CI | p Value | |
| I. By antipsychotic type | ||||||||||||
| Only atypical antipsychotics (n = 456) | 0.93 | 0.77–1.13 | 0.4762 | 0.86 | 0.71–1.06 | 0.1544 | 0.66 | 0.53–0.81 | 0.0001 | 0.77 | 0.62–0.96 | 0.0227 |
| Only conventional antipsychotics (n = 194) | 0.90 | 0.68–1.18 | 0.4399 | 0.97 | 0.73–1.29 | 0.8396 | 0.84 | 0.63–1.11 | 0.2217 | 0.89 | 0.67–1.19 | 0.4245 |
| II. By nursing home residence | ||||||||||||
| Any antipsychotics, nursing home residents (n = 1610) | 1.04 | 0.87–1.24 | 0.6869 | 0.96 | 0.79–1.17 | 0.6596 | 0.66 | 0.55–0.81 | <.0001 | 0.78 | 0.63–0.96 | 0.0192 |
| Any antipsychotics, nonnursing home LTC residents (n = 753) | 1.02 | 0.74–1.40 | 0.9229 | 1.06 | 0.71–1.57 | 0.7862 | 0.70 | 0.48–1.02 | 0.0654 | 0.98 | 0.63–1.52 | 0.9137 |
| III. By dementia status | ||||||||||||
| Any antipsychotics, those with dementia diagnosis (n = 995) | 1.19 | 0.86–1.38 | 0.4634 | 0.91 | 0.70–1.17 | 0.4488 | 0.72 | 0.57–0.90 | 0.0036 | 0.77 | 0.60–0.98 | 0.0345 |
| Any antipsychotics, those without dementia diagnosis (n = 1368) | 1.00 | 0.81–1.23 | 0.9763 | 1.01 | 0.79–1.32 | 0.9203 | 0.54 | 0.41–0.72 | <0.0001 | 0.93 | 0.69–1.26 | 0.6287 |
| IV. By age | ||||||||||||
| Any antipsychotics, those aged <65 years (n = 409) | 1.33 | 0.80–2.02 | 0.1795 | 1.02 | 0.57–1.81 | 0.9604 | 0.76 | 0.29–1.96 | 0.5693 | 1.61 | 0.54–4.85 | 0.3963 |
| Any antipsychotics, those aged >65 years (n = 1954) | 1.04 | 0.88–1.24 | 0.6293 | 0.99 | 0.82–1.19 | 0.9073 | 0.76 | 0.64–0.91 | 0.0024 | 0.78 | 0.65–0.94 | 0.0091 |
For this study, we used a survival analysis (Cox proportional hazard) model. Associated p values are based upon a df of 1 for each parameter in the model.
Adjusted for sex, age, marital status, education, poverty status, urban residence, BMI, physical function, self-reported health, supplemental insurance status, prescription drug insurance, year, number of reported physical conditions, physical restraint use, psychiatric diagnoses, delusion, delirium, number of psychiatric drug classes, and duplicative antipsychotic use.
CONCLUSIONS
This is the first study to document the association of AP use, as well as the differential influence of atypical AP use, with mortality and hospital events in a national LTC population while controlling for important sociodemographic, economic, and clinical characteristics. It furthers previous work examining the quality of AP prescribing conducted by the researchers by updating national estimates and patterns of AP use.23 We find AP use highly prevalent in LTC facilities, with 35.9% of LTC Medicare beneficiaries administered at least one AP in 2002. Although this estimate is somewhat higher than that estimated in a recent study by Kamble et al.2; however, that study used a 1-day cross-sectional capture of medications and was restricted to a nursing home population.
Contrary to research that finds a positive association between AP use and mortality (relative to no AP use),8,10,17,18 our study suggests that use of APs, regardless of AP class, appears not to increase mortality risk among Medicare beneficiaries residing in LTC facilities. This possibility is consistent with findings from other studies of institutionalized patients with dementia.11,21 Indeed, our study suggests that use of any AP is associated with reduced hazard of mortality in unadjusted and partially adjusted models that do not control for disease and drug burden (Table 2). However, we do find significant, inverse associations between AP use and mortality in our fully adjusted stratified analyses of beneficiaries residing in nursing homes relative to other facilities, beneficiaries diagnosed with dementia relative to nondementia, and beneficiaries aged 65 years and older (Table 3).
We suspect that this study’s inclusion of appropriate diagnostic information, as well as controlling for drug burden, health status, and markers of functional status (e.g., inclusion of ADLs), and other patient-level information not commonly available in administrative claims data eliminates much of the heterogeneity between AP users versus nonusers. In other words, it explains differences in both medical conditions and the severity of these conditions that might otherwise be attributable to the APs and/or the psychotic disorders themselves.
Prior investigations of LTC populations have used various strategies to control for the important potential confounders of comorbidity and severity-of-illness, such as behavior disturbance, drug burden, functional limitations, and use of physical restraints.9–11,13,24 No previously conducted study has successfully controlled for all of these factors. For example, a study by Hollis et al.24 inferred comorbidity from prescription drug claims data and was, therefore, unable to measure factors beyond overall morbidity and drug burden. Gill et al.10 and Rochon et al.9 used propensity scores for comorbidity and drug burden, whereas Liperoti et al.13 controlled for several health-related factors (behavior problems, functional status, body mass index, concomitant medications, and comorbidity) using information from the Systematic Assessment of Geriatric Drug Use via Epidemiology database. In another secondary analysis,11 a range of health-related covariates, including restraint use, ADL dependence, number of medications used, and comorbidity (measured using the Charlson comorbidity index, which assesses risk of 1-year mortality) were directly assessed, finding (as does our study) that neither atypical nor conventional AP use leads to increased hospitalization or mortality.
When contrasting the use of atypical and conventional APs (Table 3), we find no significant differences for hospital events. However, our findings do suggest that atypical agents may reduce the risk of mortality in this population. The three studies that note a positive association of conventional AP use to adverse outcomes also find a positive and clinically important association with atypical agents.8–10 Consonant with our results, at least one study of elderly institutionalized dementia patients finds lower mortality among atypical users compared with nonusers.11
Our study results may differ from previous studies for several reasons, including those noted above. For one, we explicitly control for many sociodemographic, health-related, comorbidity, and drug burden characteristics that other studies, because of data availability issues, do not. This difference is bolstered by our finding that although unadjusted analyses find an association between mortality and AP use (but not hospital events); the relationship becomes attenuated with adjustment for disease and drug covariates. Indeed, in the study most closely resembling ours,10 sensitivity analysis suggests that unmeasured confounders (such as general health status, physical functioning, and total medication burden) may independently increase the risk for death. Although some potential confounders were included in the propensity score adjustment component of the analyses by Gill et al.10 and Rochon et al.,9 they differ from the ones we employed and are used differently in analysis.
Another potential limitation is that we examine prevalent, rather than incident, AP use. If this is the case, examining prevalent use would tend to bias results toward the null. As well, prior work has found the deleterious effects of AP use strongest shortly after initiation of treatment.8,9,16,25 For example, Rochon et al.9 defined serious event as within 30 days of initiating AP therapy, which is different from our outcome definition, the first day of recorded AP exposure to the first outcome event occurring within the calendar year. Although it is possible that those residents most susceptible to AP-related mortality could have died before they could be observed in our analysis (thus weakening the relationship between AP use and mortality), our finding of decreased mortality risk with AP use suggests a possible, longer-term protective effect of these agents that requires further investigation.
It is important to note our study focuses on Medicare beneficiaries of all ages; indeed, 17.3% of our population is composed of individuals less than 65 years of age who qualify for Medicare on the basis of disability. To address the issue of age, we repeated analysis for those younger than age 65 and those aged 65 and older and found that although AP use is not related to hospital events among either age group, there is a significant inverse association of mortality and AP use among those aged 65 and older. We also were concerned that AP use in nursing home residents might differ from use by those residing in other settings, such as assisted living or congregate care facilities. In our study, even in final adjusted models, mortality remains lower among those nursing home patients using APs, the group in which we would expect the greatest disease and drug burden. Finally, because many previous investigations include only patients with dementia,8,9,14,15 we find a positive significant relationship between AP use and decreased mortality in demented residents. In this dataset of Medicare beneficiaries residing in LTC, proportionally more people with dementia live in nursing homes than other types of LTC, so similar results for the two stratifications are not surprising.
Our dataset has a high representation of skilled nursing facilities and nursing facilities certified by CMS; therefore our findings may not be fully generalizable to the entire LTC population. Confidence intervals for our results are moderately wide, which indicates that our ability to find differences is limited. Diagnoses are not necessarily confirmed through clinical assessment and therefore may be underreported. Finally, data limitations preclude the ability to follow subjects farther than the end of a calendar year.
Despite these limitations, this study has notable strengths: it employs a national sample, we directly control for important sociodemographic and health-related covariates, and it addresses AP use in a particularly vulnerable population. Further studies examining incident AP use with longer term follow-up and more current data are needed to determine whether and which older population groups are at risk for negative health outcomes associated with AP administration.
Acknowledgments
This study was funded by contract number HHSP23320044302EC with the Centers for Medicare and Medicaid Services (CMS) (to Dr. LS-W). Dr. Zuckerman was supported by a K1 award from the National Institute of Aging, number K01AG22011. Dr. Ryder was supported by a T32 training grant in the epidemiology of aging from the National Institute of Aging, number T32AG000262.
References
- 1.Shorr RI, Fought RL, Ray WA. Changes in antipsychotic drug use in nursing homes during implementation of the OBRA-87 regulations. JAMA. 1994;271:358–362. [PubMed] [Google Scholar]
- 2.Kamble P, Chen H, Sherer J, et al. Antipsychotic drug use among elderly nursing home residents in the United States. Am J Geriatr Pharmacother. 2008;6:187–197. doi: 10.1016/j.amjopharm.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Dewa CS, Remington G, Herrmann N, et al. How much are atypical antipsychotic agents being used, and do they reach the populations who need them? A Canadian experience. Clin Ther. 2002;24:1466–1476. doi: 10.1016/s0149-2918(02)80050-9. [DOI] [PubMed] [Google Scholar]
- 4.Food and Drug Administration. FDA Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioral Disturbances. Washington, DC: Department of Health and Human Services; 2005 pp. [Google Scholar]
- 5.Zuckerman IH, Langenberg P, Baumgarten M, et al. Inappropriate drug use and risk of transition to nursing homes among community-dwelling older adults. Med Care. 2006;44:722–730. doi: 10.1097/01.mlr.0000215849.15769.be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Selbaek G, Kirkevold O, Engedal K. The course of psychiatric and behavioral symptoms and the use of psychotropic medication in patients with dementia in Norwegian nursing homes—a 12-month follow-up study. Am J Geriatr Psychiatry. 2008;16:528–536. doi: 10.1097/JGP.0b013e318167ae76. [DOI] [PubMed] [Google Scholar]
- 7.Wang PS, Brookhart MA, Setoguchi S, et al. Psychotropic medication use for behavioral symptoms of dementia. Curr Neurol Neurosci Rep. 2006;6:490–495. doi: 10.1007/s11910-006-0051-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang PS, Schneeweiss S, Avorn J, et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med. 2005;353:2335–2341. doi: 10.1056/NEJMoa052827. [DOI] [PubMed] [Google Scholar]
- 9.Rochon PA, Normand SL, Gomes T, et al. Antipsychotic therapy and short-term serious events in older adults with dementia. Arch Intern Med. 2008;168:1090–1096. doi: 10.1001/archinte.168.10.1090. [DOI] [PubMed] [Google Scholar]
- 10.Gill SS, Bronskill SE, Normand SL, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146:775–786. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 11.Raivio MM, Laurila JV, Strandberg TE, et al. Neither atypical nor conventional antipsychotics increase mortality or hospital admissions among elderly patients with dementia: a two-year prospective study. Am J Geriatr Psychiatry. 2007;15:416–424. doi: 10.1097/JGP.0b013e31802d0b00. [DOI] [PubMed] [Google Scholar]
- 12.Barak Y, Baruch Y, Mazeh D, et al. Cardiac and cerebrovascular morbidity and mortality associated with antipsychotic medications in elderly psychiatric inpatients. Am J Geriatr Psychiatry. 2007;15:354–356. doi: 10.1097/JGP.0b013e318030253a. [DOI] [PubMed] [Google Scholar]
- 13.Liperoti R, Gambassi G, Lapane KL, et al. Conventional and atypical antipsychotics and the risk of hospitalization for ventricular arrhythmias or cardiac arrest. Arch Intern Med. 2005;165:696–701. doi: 10.1001/archinte.165.6.696. [DOI] [PubMed] [Google Scholar]
- 14.Barnett MJ, Perry PJ, Alexander B, et al. Risk of mortality associated with antipsychotic and other neuropsychiatric drugs in pneumonia patients. J Clin Psychopharmacol. 2006;26:182–187. doi: 10.1097/01.jcp.0000203598.43314.34. [DOI] [PubMed] [Google Scholar]
- 15.Schneeweiss S, Setoguchi S, Brookhart A, et al. Risk of death associated with the use of conventional versus atypical antipsychotic drugs among elderly patients. CMAJ. 2007;176:627–632. doi: 10.1503/cmaj.061250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollis J, Grayson D, Forrester L, et al. Antipsychotic medication dispensing and risk of death in veterans and war widows 65 years and older. Am J Geriatr Psychiatry. 2007;15:932–941. doi: 10.1097/JGP.0b013e31813547ca. [DOI] [PubMed] [Google Scholar]
- 17.Trifiro G, Verhamme KM, Ziere G, et al. All-cause mortality associated with atypical and typical antipsychotics in demented outpatients. Pharmacoepidemiol Drug Saf. 2007;16:538–544. doi: 10.1002/pds.1334. [DOI] [PubMed] [Google Scholar]
- 18.Schneider LS, Dagerman KS, Insel P. Risk of death with atypical antipsychotic drug treatment for dementia: meta-analysis of randomized placebo-controlled trials. JAMA. 2005;294:1934–1943. doi: 10.1001/jama.294.15.1934. [DOI] [PubMed] [Google Scholar]
- 19.Aparasu RR, Jano E, Johnson MM, et al. Hospitalization risk associated with typical and atypical antipsychotic use in community-dwelling elderly patients. Am J Geriatr Pharmacother. 2008;6:198–204. doi: 10.1016/j.amjopharm.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Nonino F, De Girolamo G, Gamberini L, et al. Survival among elderly Italian patients with dementia treated with atypical antipsychotics: observational study. Neurol Sci. 2006;27:375–380. doi: 10.1007/s10072-006-0716-6. [DOI] [PubMed] [Google Scholar]
- 21.Suh GH, Shah A. Effect of antipsychotics on mortality in elderly patients with dementia: a 1-year prospective study in a nursing home. Int Psychogeriatr. 2005;17:429–441. doi: 10.1017/s1041610205002243. [DOI] [PubMed] [Google Scholar]
- 22.Adler GS. A profile of the Medicare Current Beneficiary Survey. Health Care Financ Rev. 1994;15:153–163. [PMC free article] [PubMed] [Google Scholar]
- 23.Briesacher BA, Limcangco MR, Simoni-Wastila L, et al. The quality of antipsychotic drug prescribing in nursing homes. Arch Intern Med. 2005;165:1280–1285. doi: 10.1001/archinte.165.11.1280. [DOI] [PubMed] [Google Scholar]
- 24.Hollis J, Forrester L, Brodaty H, et al. Risk of death associated with antipsychotic drug dispensing in residential aged care facilities. Aust N Z J Psychiatry. 2007;41:751–758. doi: 10.1080/00048670701519864. [DOI] [PubMed] [Google Scholar]
- 25.Schneider LS, Dagerman K, Insel PS. Efficacy and adverse effects of atypical antipsychotics for dementia: meta-analysis of randomized, placebo-controlled trials. Am J Geriatr Psychiatry. 2006;14:191–210. doi: 10.1097/01.JGP.0000200589.01396.6d. [DOI] [PubMed] [Google Scholar]


