Abstract
Stress-sensitive psychopathologies such as post-traumatic stress disorder are characterized by deficits in fear extinction and dysfunction of corticolimbic circuits mediating extinction. Chronic stress facilitates fear conditioning, impairs extinction, and produces dendritic proliferation in the basolateral amygdala (BLA), a critical site of plasticity for extinction. Acute stress impairs extinction, alters plasticity in the medial prefrontal cortex-to-BLA circuit, and causes dendritic retraction in the medial prefrontal cortex. Here, we examined extinction learning and basolateral amygdala pyramidal neuron morphology in adult male rats following a single elevated platform stress. Acute stress impaired extinction acquisition and memory, and produced dendritic retraction and increased mushroom spine density in basolateral amygdala neurons in the right hemisphere. Unexpectedly, irrespective of stress, rats that underwent fear and extinction testing showed basolateral amygdala dendritic retraction and altered spine density relative to non-conditioned rats, particularly in the left hemisphere. Thus, extinction deficits produced by acute stress are associated with increased spine density and dendritic retraction in basolateral amygdala pyramidal neurons. Furthermore, the finding that conditioning and extinction as such was sufficient to alter basolateral amygdala morphology and spine density illustrates the sensitivity of basolateral amygdala morphology to behavioral manipulation. These findings may have implications for elucidating the role of the amygdala in the pathophysiology of stress-related disorders.
Keywords: amygdala, dendritic spine, emotional learning, rat
Introduction
Extinction is an active form of learning in which the expression of a conditioned fear response is reduced following repeated experience of the conditioned stimulus (CS) in the absence of the unconditioned, aversive stimulus (Pavlov, 1927). A reduced ability to extinguish conditioned fear associations might contribute to the persistence of maladaptive fear in stress-sensitive disorders such as post-traumatic stress disorder (PTSD), and may reduce the effectiveness of therapeutic interventions that rely on extinction processes (Akirav & Maroun, 2007; Myers & Davis, 2007; Herry et al., 2010; Holmes & Quirk, 2010).
Interactions between the medial prefrontal cortex (mPFC) and the amygdala are critical for extinction (Quirk & Mueller, 2008; Orsini & Maren, 2012). Acute (Akirav et al., 2009), subchronic and chronic stress (Izquierdo et al., 2006; Miracle et al., 2006; Garcia et al., 2008; Baran et al., 2009; Farrell et al., 2010; Wilber et al., 2011) impair extinction. Stress-induced impairment of extinction and enhanced fear responses may stem from dysfunction in the cortico-amygdalar circuitry. Indeed, the mPFC has been implicated in stress-induced impairments of extinction. For instance, prior stress dampens mPFC in vivo single-unit firing during extinction (Wilber et al., 2011) and impairs the induction of long-term potentiation (LTP) in the mPFC (Maroun & Richter-Levin, 2003; Rocher et al., 2004; Richter-Levin & Maroun, 2010), and mPFC (infralimbic) lesions prevent stress-induced extinction deficits (Farrell et al., 2010; Wilber et al., 2011). However, the basolateral amygdala (BLA) is a critical site of plasticity in extinction (Orsini & Maren, 2012), and therefore stress-induced alterations in BLA function probably also contribute to impaired extinction. This is supported by the observation that acute stress enhances LTP induction in the BLA (Maroun & Richter-Levin, 2003; Maroun, 2006) and increases excitatory synaptic activity in lateral amygdala neurons (Chauveau et al., 2012). In addition, intra-BLA infusion of either D-cycloserine (Akirav et al., 2009; Richter-Levin & Maroun, 2010) or neuropeptide S (Chauveau et al., 2012) rescues the effects of stress on extinction and prefrontal cortex–BLA plasticity or BLA excitability, and alleviates impaired fear extinction in a rat model of PTSD (Yamamoto et al., 2008), further supporting the notion that stress-induced alterations in BLA plasticity contribute to stress-induced impairments in extinction.
Numerous studies have shown that exposure to a variety of stressors of varying durations produces profound changes in mPFC dendritic morphology (Cook & Wellman, 2004; Izquierdo et al., 2006; Radley et al., 2006; reviewed in Holmes & Wellman, 2009; Martin & Wellman, 2011). In the case of acute stress, dendritic retraction of mPFC neurons has been coupled to impaired extinction in the same animals (Izquierdo et al., 2006; reviewed in Holmes & Wellman, 2009). Chronic stress has been shown to produce alterations in dendritic morphology and spine density in the BLA (Vyas et al., 2002, 2004; Govindarajan et al., 2006; Vyas et al., 2006; reviewed in Roozendaal et al., 2009). Although one study has shown delayed increases in BLA spine density after acute immobilization stress (Mitra et al., 2005), the relatively immediate effects of acute stress on BLA neuronal morpohology are unclear. Furthermore, the potential relationship between the effects of acute stress on BLA dendritic morphology and fear extinction has not been clarified. Therefore, the goal of the current study was to examine the effects of an acute (elevated platform) stressor on extinction and the neuronal morphology of BLA pyramidal cells in the same rats, and potential relationships between stress-induced changes in BLA morphology and behavior.
Materials and methods
Subjects and stressor
All procedures were conducted in accordance with NIH Guidelines and the International Guiding Principles for Biomedical Research Involving Animals, and were approved by the University of Haifa Ethics and Animal Care Committee and the Bloomington Animal Care and Use Committee. Young adult male Sprague Dawley rats (age, ~60 days; 250–300 g; Harlan Laboratories, Jerusalem, Israel) weighing 200–280 g were housed in Plexiglas cages (two rats per cage) and maintained on a free-feeding regimen with a 12-h light:12-h dark schedule. See Fig. 1A for the timeline of behavioral testing and stress manipulation. Stress was evoked by placing each rat on an elevated platform (12 × 12 cm) in a brightly lit room for 30 min, as previously described (Xu et al., 1997; Maroun & Richter-Levin, 2003; Maroun, 2006; Richter-Levin & Maroun, 2010). This procedure was previously reported to impair fear extinction (Akirav et al., 2009), as well as LTP in the mPFC (Maroun & Richter-Levin, 2003; Rocher et al., 2004; Richter-Levin & Maroun, 2010).
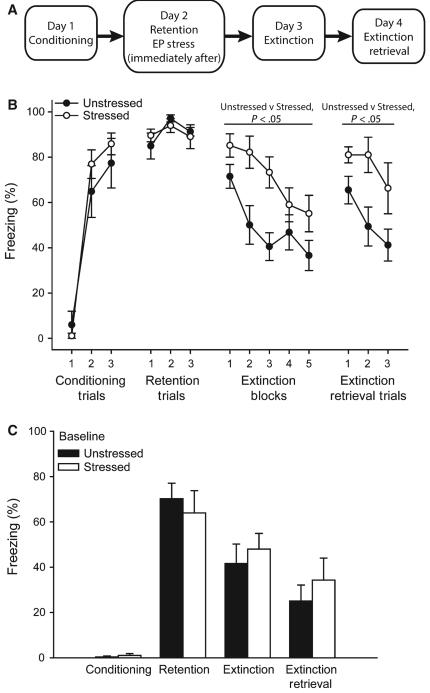
FIG. 1.
Acute stress impairs extinction. (A) Schematic of experimental design. (B) Percentage freezing (mean ± standard error of the mean) in response to a conditioned stimulus during conditioning, fear retention, extinction and extinction retrieval in rats that were either unstressed (n = 8) or stressed (n = 10) or after fear retention testing. (C) Percentage freezing during the 120-s baseline period during conditioning, fear retention, extinction and extinction retrieval in rats that were either stressed or unstressed after fear retention testing.
Fear conditioning and extinction
Fear conditioning was conducted in ‘context A’, a chamber with a grid floor and transparent Plexiglas walls. The conditioning procedure was as previously described (Hikind & Maroun, 2008; Maroun et al., 2012), and comprised three pairings of a conditioned stimulus and unconditioned stimulus (120-s inter-pairing interval) after a 120-s no-stimulus baseline. The CS was a 4-kHz, 80-dB, 30-s tone that co-terminated with delivery of the 0.8-mA, 1-s footshock unconditioned stimulus. On the day after conditioning, rats were placed in ‘context B,’ a chamber with transparent Plexiglas walls and a black Plexiglas floor, and were subjected to fear retention testing via three CS presentations (note that rats had been habituated to context B for 20 min on each of 3 days prior to conditioning).
Immediately after retention testing, stressed rats (n = 10) were exposed to the elevated platform, whereas unstressed rats (n = 8) were returned to their home cages. One day later, rats were given an extinction session composed of 10 CSs in context B. Extinction retrieval was carried out in context B on the following day, by presentation of three CSs. Additional groups of rats (n = 5, unstressed; n = 4, stressed) underwent stress/no-stress, but were not subjected to fear conditioning or extinction testing.
Freezing, i.e. the absence of all movement except for respiration (Blanchard & Blanchard, 1972; Kim et al., 1992), was quantified from video with image-based software (P. Schmid, Behavioral Neurobiology Laboratory, Swiss Federal Institute of Technology, Zurich), and expressed as the percentage of time spent freezing during tone presentation.
Golgi histology and dendritic analyses
Two days after stress (i.e. 1 day after extinction in extinction-tested rats), tissue was processed with Glaser and Van der Loos’ modified Golgi stain, essentially as previously described (Glaser & Van der Loos, 1981; Martin & Wellman, 2011). Rats were overdosed with equithesin, and then perfused with 0.9% saline. Brains were removed and immersed in Golgi–Cox solution for 12 days, after which they were immersed in 30% sucrose in saline. Brains were then dehydrated and embedded in 8% celloidin. Coronal sections were cut at 180 μm on a sliding microtome (AO860; American Optical Company, Buffalo, NY, USA). Free-floating sections were then alkalinized, developed in Dektol (Kodak), fixed in Ilford rapid fixer, dehydrated through a graded series of ethanols, cleared in xylene, mounted, and coverslipped.
Pyramidal neurons in the BLA were drawn (for examples, see Fig. 2A). Analysis was restricted to pyramidal neurons located between 2.0 and 3.8 mm posterior to Bregma. Within this region, the BLA is readily identified in Golgi-stained material, as the external capsule branches into two smaller fiber tracts that define the dorsal, medial and lateral borders of the BLA. Likewise, axon fibers clearly delineate the basal amygdala from the BLA. Four sections evenly spaced through this region were chosen. Within each section, all reconstructable neurons (located in the middle third of the section, did not have truncated branches, unobscured by neighboring neurons and glia, with dendrites that could easily be discriminated by focusing through the depth of the tissue) in the BLA were identified, and, with a random number generator, one neuron per hemisphere was randomly selected for reconstruction. Pyramidal neurons were defined by the presence of at least two basilar dendritic trees with at least third-order branches, a distinct, single apical dendrite, and dendritic spines. For each rat, eight neurons were reconstructed. All neurons were drawn at a final magnification of ×600, and morphology was quantified in three dimensions with a computer-based neuron tracing system (Neurolucida; MBF Biosciences, Williston, VT, USA) with the experimenter blind to condition.
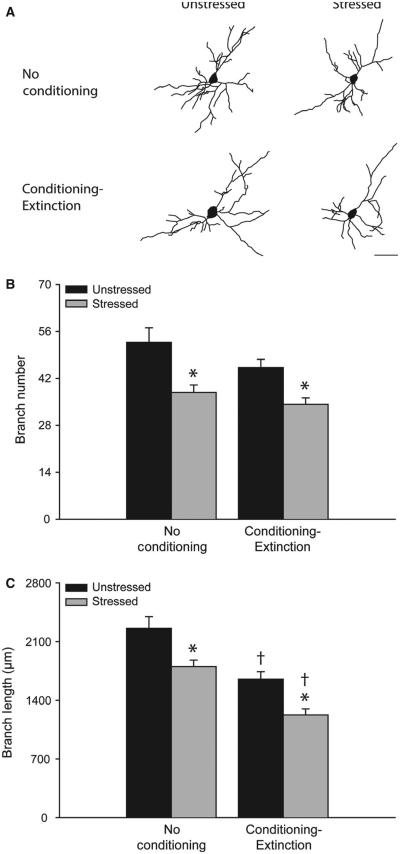
FIG. 2.
Acute stress produces dendritic retraction in basolateral amygdala (BLA) pyramidal neurons. (A) Reconstructions of representative BLA pyramidal neurons from unstressed vs. stressed rats that were either non-conditioned or underwent fear conditioning and extinction. Neurons are at or near the mean for each group. Scale bar: 50 μm. (B) Total branch number [mean ± standard error of the mean (SEM)] for BLA pyramidal neurons in unstressed (black bars) vs. stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. *P < 0.05 relative to unstressed within the behavioral testing condition. †p < 0.05 relative to non-conditioned within the stress condition. (C) Total branch length (mean ± SEM) for BLA pyramidal neurons in unstressed (black bars) vs. stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. *P < 0.05 relative to unstressed within the testing condition. †P < 0.05 relative to untested within the stress condition. Unstressed, no conditioning, n = 5. Stressed, no conditioning, n = 4. Unstressed, conditioning and extinction, n = 8. Stressed, conditioning and extinction, n = 10.
Spines were counted on dendritic branches from eight neurons per rat. Spines were counted on first-order to fourth-order branches, as these make up ~90% of the dendritic arbor of BLA pyramidal neurons in rats. For each neuron, one dendritic tree containing at least one-third-order branch was chosen. One to two branches at each order were drawn, and spines were counted at a final magnification of × 1000 with Neurolucida. The branch lengths sampled averaged 14.15 ± 1.11, 42.71 ± 2.92, 56.03 ± 3.06 and 66.90 ± 5.97 μm for first-order, second-order, third-order and fourth-order dendrites, respectively. Spines were classified as thin, stubby, mushroom, or branched, according to standard morphological criteria (Peters & Kaiserman-Abramof, 1970) (for examples, see Fig. 4A). Because spine density varies with thickness of the dendrite (and therefore branch order), the lengths of dendritic segments were recorded, and spine densities (spines per 10 μm) for each branch order were calculated separately.
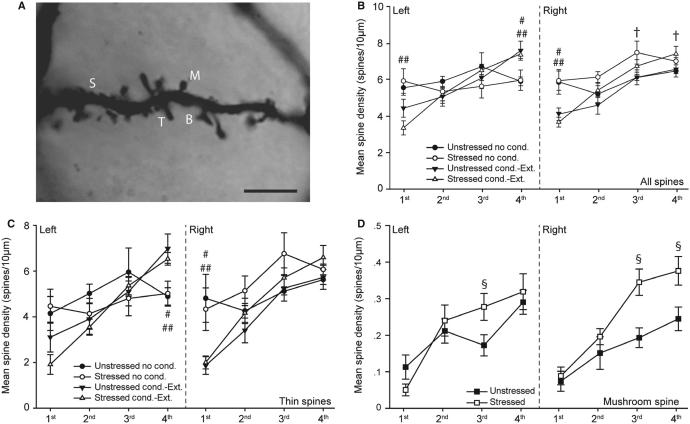
FIG. 4.
Acute stress and fear conditioning and extinction alter spine density on basolateral amygdala (BLA) pyramidal neurons. (A) Digital micrograph demonstrating different spine types on a pyramidal neuron in the BLA. Several photomicrographs were taken at different Z-levels, and merged to increase the number of spines in focus. B, branched; M, mushroom; S, stubby; T, thin. Scale bar: 5 μm. (B) Spine density [mean ± standard error of the mean (SEM)] on first-order to fourth-order branches of BLA pyramidal neurons in the right and left hemispheres in unstressed vs. stressed rats that were either non-conditioned or underwent fear conditioning and extinction. †P < 0.05 for unstressed vs. stressed non-conditioned rats. #P < 0.05 for unstressed non-conditioned rats vs. unstressed rats that underwent conditioning and extinction. ##P < 0.05 for stressed non-conditioned rats vs. stressed rats that underwent conditioning and extinction. (C) Thin spine density (mean ± SEM) on first-order to fourth-order branches of BLA pyramidal neurons in the right and left hemispheres in unstressed vs. stressed rats that were either non-conditioned or underwent fear conditioning and extinction. #P < 0.05 for unstressed non-conditioned rats vs. unstressed rats that underwent conditioning and extinction. ##P < 0.05 for stressed non-conditioned rats vs. stressed rats that underwent conditioning and extinction. (D) Mushroom spine density (mean ± SEM) on first-order to fourth-order branches of BLA pyramidal neurons in the right and left hemispheres in unstressed vs. stressed rats collapsed across learning conditions. §P < 0.05 for unstressed vs. stressed rats. Unstressed, no conditioning, n = 5. Stressed, no conditioning, n = 4. Unstressed, conditioning and extinction, n = 8. Stressed, conditioning and extinction, n = 10.
Statistical analyses
For statistical analyses and graphical presentation, extinction trials were collapsed into blocks of two trials. Percentage freezing during each testing phase was compared between unstressed and stressed groups by the use of two-way repeated measures ANOVAs (for conditioning, retention, and extinction retrieval, stress × trial; for initial extinction, stress × block). When appropriate, subsequent planned comparisons were conducted, consisting of two-group F-tests performed within the overall ANOVA (Hays, 1994; Maxwell & Delaney, 2003). Freezing during the pre-tone baseline periods was compared between groups by the use of a two-way repeated measures ANOVA (stress × testing phase).
Dendritic branch length and number were compared between groups by the use of three-way repeated measures ANOVAS (stress × behavioral testing × hemisphere). Overall spine density and densities of each spine type were compared by the use of four-way repeated measures ANOVAS (stress × behavioral testing × hemi sphere × branch order). When appropriate, subsequent planned comparisons were conducted, consisting of two-group F-tests performed within the context of the overall ANOVA (Hays, 1994; Maxwell & Delaney, 2003).
Results
Acute stress impairs extinction
Acquisition of conditioned fear did not differ across groups [Fig. 1B; effect of trial, F2,36 = 117.24, P < 0.05; effect of stress, F1,18 = 0.44, not significant (NS); stress × trial interaction, F2,36 = 1.31, NS], and retention of the conditioned fear response was also comparable across groups (effect of stress, F1,18 = 0.01, NS; effect of trial, F2,36 = 2.73, NS; stress × trial interaction, F2,36 = 0.50, NS). Subsequent exposure to acute stress impaired extinction, with stressed rats showing significantly more freezing across all extinction blocks than unstressed controls (effect of stress, F1,18 = 7.39, P < 0.05; effect of extinction block, F4,72 = 14.45, P < 0.05; stress × block interaction, F2,72 = 2.26, NS). Likewise, stressed rats showed significantly increased freezing relative to unstressed rats during extinction retrieval trials on the following day (effect of stress, F1,18 = 7.88, P < 0.05; effect of trial, F2,36 = 4.95, P < 0.05; stress × trial interaction, F2,36 = 0.84, NS). Freezing did not differ significantly between groups during the baseline period during any phase of behavioral testing (Fig. 1C; main effect of stress, F1,18 = 0.17, NS; stress × phase interaction, F3,54 = 0.49, NS).
Acute stress and behavioral testing produce BLA dendritic retraction
Acute stress significantly decreased both the number and the length of branches on BLA pyramidal neurons, as compared with non-stressed controls (Fig. 2A–C; branch number, F1,24 = 16.13, P < 0.05; branch length, F1,25 = 15.31, P < 0.05). Fear conditioning and extinction as such also significantly decreased BLA dendritic branch length (F1,24 = 29.66, P < 0.05), but not branch number (F1,24 = 3.98, NS). The effect of stress did not vary across testing conditions for either branch number or branch length (stress × conditioning interaction, F1,24 = 0.83 and F1,24 = 0.07, respectively, both NS). Planned comparisons demonstrated that stress significantly decreased branch number and branch length in both conditioned and non-conditioned rats (Fig. 2B and C; for non-conditioned rats, F1,7 = 7.93 and F1,7 = 7.06 for number and length respectively, P < 0.05; for conditioned rats, F1,17 = 9.96 and F1,17 = 12.48 for number and length respectively, P < 0.05). Branch length was significantly reduced in rats that had undergone conditioning and extinction, regardless of stress condition (Fig. 2C; for unstressed rats, F1,11 = 12.54, P < 0.05; for stressed rats, F1,13 = 18.99, P < 0.05).
The effects of stress and conditioning and extinction varied across hemispheres (Fig. 3A and B; for branch number, stress × hemisphere interaction, F2,24 = 6.31, P < 0.05; conditioning × hemisphere interaction, F2,24 = 7.30, P < 0.05; for branch length, stress × hemisphere interaction, F2,24 = 5.64, P < 0.05; condition ing × hemisphere interaction, F2,24 = 5.57, P < 0.05), although there were no three-way interactions for either branch number or branch length (stress × conditioning × hemisphere interaction, F2,24 ≤ 0.13, NS).
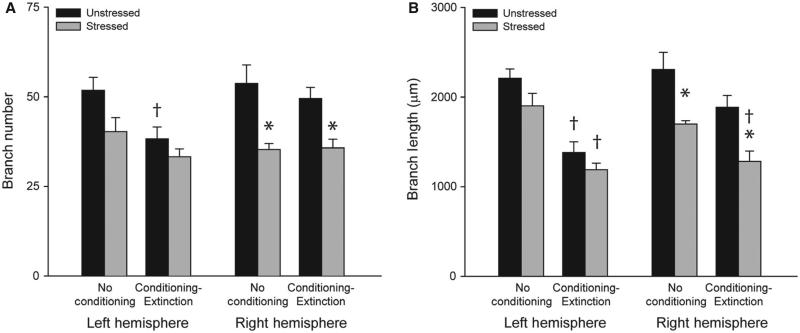
FIG. 3.
Acute stress produces dendritic retraction in the right basolateral amygdala (BLA), whereas fear conditioning and extinction produces dendritic retraction in the left BLA. (A) Total branch number [mean ± standard error of the mean (SEM)] for right and left BLA pyramidal neurons in unstressed (black bars) vs. stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. *P < 0.05 relative to unstressed within the learning condition. †P < 0.05 relative to untested within the stress condition. (B) Total branch length (mean ± SEM) for right and left BLA pyramidal neurons in unstressed (black bars) vs. stressed (gray bars) rats that were either non-conditioned or underwent fear conditioning and extinction. *P < 0.05 relative to unstressed within the learning condition. †P < 0.05 relative to untested within the stress condition. Unstressed, no conditioning, n = 5. Stressed, no conditioning, n = 4. Unstressed, conditioning and extinction, n = 8. Stressed, conditioning and extinction, n = 10.
Planned comparisons revealed significant stress-induced decreases in branch number and branch length that were specific to the right hemisphere and were present regardless of whether or not rats had undergone conditioning and extinction (Fig. 3A and B; for untested rats, right hemisphere, F1,7 ≥ 7.54, P < 0.05; for tested rats, right hemisphere, F1,17 ≥ 11.74, P < 0.05; all left-hemisphere comparisons, NS). In contrast, conditioning resulted in dendritic retraction specific to the left hemisphere, regardless of stress condition (Fig. 3A and B; for unstressed rats, left hemisphere, F1,11 ≥ 7.13, P < 0.05; for stressed rats, left-hemisphere branch length, F1,13 = 22.94, P < 0.05; branch number, F1,13 = 2.54, NS; all right-hemisphere comparisons, NS).
Thus, a single exposure to the elevated platform stressor produced BLA dendritic debranching and retraction in both naṏve and conditioned rats, and this dendritic remodeling was specific to the right hemisphere. In contrast, conditioning and extinction produced dendritic retraction in the left BLA in both unstressed and stressed rats.
Acute stress and prior conditioning alter BLA spine density
Neither stress, conditioning and extinction nor hemisphere had a significant main effect on spine density (main effect of stress, F1,24 = 0.15, NS; main effect of conditioning and extinction, F1,24 = 1.51, NS; main effect of hemisphere, F1,24 = 0.88, NS; stress × conditioning interaction, F2,24 = 0.05, NS). However, stress and conditioning and extinction had effects on spine density that varied as a function of hemisphere and branch order (Fig. 4B; stress × hemisphere interaction, F1,24 = 6.02, P < 0.05; condition ing × branch order interaction, F3,24 = 13.81, P < 0.05; stress × con ditioning × hemisphere × branch order interaction, F3,72 = 3.03, P < 0.05; all other interactions, NS). Planned comparisons revealed that, in non-conditioned rats, stress significantly increased spine density on the third-order and fourth-order branches in the right hemisphere (F1,7 = 5.48 and F1,7 = 5.51, respectively, P < 0.05), whereas in conditioned rats stress did not significantly alter spine density at any branch order in either hemisphere (all F1,17 < 3.36, NS).
Conditioning and extinction as such increased spine density on fourth-order branches in the left hemisphere (unstressed, F1,11 = 5.44; stressed, F1,13 = 7.77, both P < 0.05), and decreased spine density on first-order branches in the right hemisphere (unstressed, F1,11 = 6.87; stressed, F1,13 = 17.39, both P < 0.05), relative to non-conditioned counterparts, in unstressed as well as in stressed rats. However, in the left hemisphere, conditioning and extinction was associated with decreased spine density on first-order branches in stressed rats only (F1,13 = 10.32, P < 0.05). This shows the specificity of combined conditioning and acute stress on altering spine density in the left BLA.
Acute stress and behavioral testing alter densities of specific spine types
Changes in total spine density were driven mainly by alterations in the density of thin and mushroom spines. For thin spines, neither stress nor conditioning and extinction significantly altered density (main effect of stress, F1,24 = 0.05, NS; main effect of conditioning, F1,24 = 2.40, NS; stress × conditioning interaction, F2,24 = 0.003, NS). Although there was no main effect of hemisphere (F1,24 = 0.29, NS), the effects of stress and conditioning and extinction again varied across hemispheres and branch orders (Fig. 4C; stress × hemisphere interaction, F1,24 = 4.71, P < 0.05; stress × conditioning × hemi sphere × branch order interaction, F3,72 = 3.49, P < 0.05; all other effects, NS). Planned comparisons revealed that the density of thin spines was not significantly altered by stress, in either conditioned or non-conditioned rats (all F-values NS). On the other hand, in both stressed and unstressed rats, conditioning and extinction significantly increased thin spine density on fourth-order branches in the left hemisphere (unstressed, F1,11 = 5.68; stressed, F1,13 = 7.36; both P < 0.05), and significantly decreased thin spine density in the right hemisphere (unstressed, F1,11 = 9.55; stressed, F1,13 = 10.39; both P < 0.05). Thus, conditioning and extinction as such produced opposite changes in thin spine density in the left and the right BLA.
For mushroom spines, the main effect of hemisphere was not significant (F1,24 = 0.39, NS). Furthermore, there was no effect of conditioning and extinction (main effect of conditioning, F1,24 = 2.40, NS), and this was consistent across stress conditions, hemispheres, and branch orders (stress × testing interaction, F1,24 = 0.84, NS; test ing × hemisphere interaction, F1,24 = 1.96, NS; testing × branch order interaction, F3,72 = 0.32, NS; testing × stress × hemisphere interaction, F3,72 = 1.30, NS; stress × testing × hemisphere × branch order interaction, F3,72 = 0.18, NS, Fig; 4D). However, stress significantly altered mushroom spine density, and this effect varied across hemispheres and branch orders (stress × hemisphere interaction, F1,24 = 5.51, P < 0.05; stress × branch order interaction, F3,72 = 3.44, P < 0.05). Because conditioning and extinction did not alter mushroom spine densities, data were collapsed across testing conditions for planned comparisons of stress effects. Stress significantly increased mushroom spine density on third-order branches in the left hemisphere (F1,26 = 4.80, P < 0.05). In the right hemisphere, stress dramatically increased mushroom spine density on both third-order and fourth-order branches (F1,26 = 10.81 and F1,26 = 6.42, respectively, both P < 0.05). Thus, stress increased the density of mushroom spines on higher-order branches, and this effect was more pronounced inthe right BLA.
There was no main effect of stress, conditioning and extinction or hemisphere on the density of stubby spines (all F1,24 ≤ 3.74, NS). However, both acute stress and conditioning and extinction significantly altered the density of stubby spines, with effects varying across hemispheres and branch orders (conditioning × branch order interaction, F3,72 = 10.08, P < 0.05; stress × conditioning × branch order interaction, F3,72 = 9.43, P < 0.05; testing × hemisphere × branch order interaction, F3,72 = 2.96; all other F-values NS). Likewise, although there was no main effect of stress, conditioning and extinction or hemisphere on the density of branched spines (all F1,24 ≤ 4.01, NS), the effects of acute stress and conditioning and extinction varied across hemispheres and branch orders (stress × hemisphere interaction, F1,24 = 7.62; conditioning × hemisphere interaction, F1,24 = 6.52; conditioning × branch order interaction, F3,72 = 2.99; stress × conditioning × hemisphere interaction, F3,72 = 4.02; stress × hemisphere × branch order interaction, F3,72 = 7.35; condi tioning × hemisphere × branch order interaction, F3,72 = 5.63; stress × conditioning × hemisphere × branch order interaction, F3,72 = 3.38; all P-values <0.05; all other F-values NS). The results of planned comparisons for branched and stubby spines are summarized in Table 1.
TABLE 1.
Mean ± standard error of the mean density (spines/10 μm) of stubby and branched spines on first-order to fourth-order branches of BLA pyramidal neurons in the left and right hemispheres of unstressed rats vs. stressed rats that have either undergone fear conditioning and extinction (behavioral testing) or have not undergone fear conditioning and extinction (non-tested)
| Left |
Right |
|||||||
|---|---|---|---|---|---|---|---|---|
| Non-tested |
Behavioral testing |
Non-tested |
Behavioral testing |
|||||
| Unstressed | Stressed | Unstressed | Stressed | Unstressed | Stressed | Unstressed | Stressed | |
| Stubby | ||||||||
| 1st | 0.81 ± 0.25 | 1.24 ± 0.31 | 0.54 ± 0.22 | 0.30 ± 0.13* | 0.75 ± 0.27 | 1.39 ± 0.27 | 1.41 ± 0.32 | 0.66 ± 0.16*,† |
| 2nd | 0.36 ± 0.17 | 0.48 ±0.11 | 0.40 ± 0.09 | 0.82 ± 0.15† | 0.32 ± 0.05 | 0.61 ± 0.24 | 0.44 ± 0.08 | 0.65 ± 0.13 |
| 3rd | 0.31 ± 0.10 | 0.22 ± 0.04 | 0.49 ±0.12 | 0.71 ± 0.07* | 0.39 ± 0.15 | 0.41 ± 0.08 | 0.58 ± 0.12 | 0.62 ± 0.08 |
| 4th | 0.21 ± 0.03 | 0.22 ± 0.10 | 0.52 ± 0.10‡ | 0.61 ± 0.11* | 0.26 ± 0.04 | 0.22 ± 0.03 | 0.53 ± 0.08‡ | 0.61 ± 0.01* |
| Branched | ||||||||
| 1st | 0.13 ± 0.08 | 0.02 ± 0.02 | 0.01 ± 0.01 | 0.03 ± 0.02 | 0.12 ± 0.05 | 0.02 ± 0.02 | 0.00 ± 0.00‡ | 0.02 ± 0.02 |
| 2nd | 0.15 ± 0.05 | 0.17 ± 0.02 | 0.05 ± 0.03 | 0.12 ± 0.06 | 0.12 ± 0.02 | 0.13 ± 0.05 | 0.02 ± 0.02‡ | 0.09 ± 0.04 |
| 3rd | 0.34 ± 0.10 | 0.18 ± 0.04 | 0.17 ± 0.13 | 0.14 ± 0.07 | 0.32 ± 0.08 | 0.41 ± 0.12 | 0.04 ± 0.03‡ | 0.19 ± 0.09 |
| 4th | 0.36 ± 0.06 | 0.35 ± 0.06 | 0.13 ± 0.09 | 0.20 ± 0.09 | 0.39 ± 0.05 | 0.52 ± 0.13 | 0.14 ± 0.09 | 0.21 ± 0.10 |
BLA, basolateral amygdala.
P < 0.05 relative to stressed rats that have not undergone fear conditioning and extinction.
P < 0.05 relative to unstressed rats that have undergone fear conditioning and extinction.
P < 0.05 relative to unstressed rats that have not undergone fear conditioning and extinction. All comparisons are within a particular hemisphere.
Acute stress effects on extinction and BLA morphology are correlated
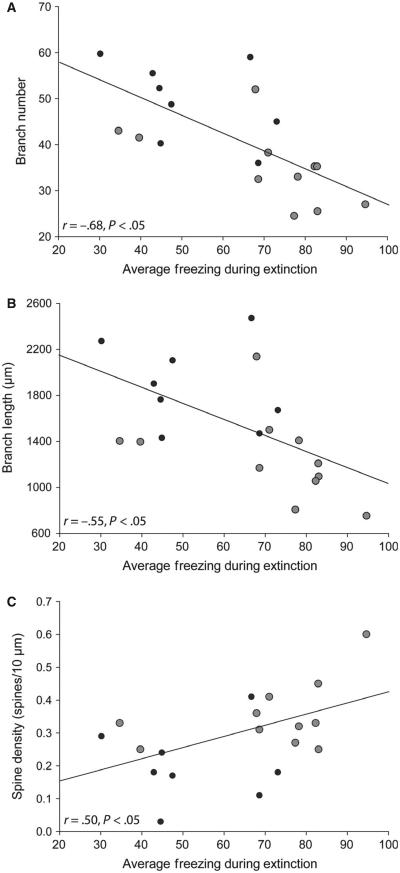
Branch number and length in the right BLA were strongly and negatively correlated with average freezing during extinction (percentage freezing averaged across all extinction trials; r = −0.68 and r = −0.55, respectively, both P <0.05; Fig. 5A and B), whereas neither branch number nor branch length in the left BLA were significantly correlated with extinction learning (r = −0.32 and r = −0.36, respectively, both NS). Average mushroom spine density on higher-order branches in the right but not the left hemisphere was significantly and positively correlated with average freezing during extinction (Fig. 5C; right hemisphere, r = 0.50, P < 0.05; left hemisphere, r = 0.11, NS). Although changes in thin spine density occurred with behavioral testing, these changes were not correlated with freezing during any phase of testing (all r-values <0.26, NS).
FIG. 5.
Stress-induced dendritic remodeling and alterations in mushroom spine density correlate with extinction deficits. (A) Linear regression for average basolateral amygdala (BLA) branch number for unstressed (black dots; n = 8) and stressed (gray dots; n = 10) rats vs. percentage freezing during extinction (averaged across trials). (B) Linear regression for average BLA dendritic length for unstressed (black dots) and stressed (gray dots) rats vs. percentage freezing during extinction (averaged across trials). (C) Linear regression for average mushroom spine density on third-order and fourth-order branches for unstressed (black dots) and stressed (gray dots) rats vs. percentage freezing during extinction (averaged across trials).
Discussion
The main findings of the current study were that acute stress produced significant alterations in the morphology of pyramidal neurons in BLA that occurred in tandem with deficits in fear extinction. Unexpectedly, we found that fear conditioning and extinction as such also had demonstrable effects on dendritic morphology. Furthermore, we provide evidence that the effects of acute stress and conditioning and extinction on BLA neuronal morphology are dissociable and lateralized. Finally, the effects of both stress and conditioning and extinction were rapid and robust.
Hemisphere-specific stress effects on dendritic morphology and spine density
Acute stress produced pronounced dendritic debranching and retraction, which was specific to the right hemisphere, and was present regardless of whether rats had undergone conditioning and extinction. Stress also altered spine density on BLA pyramidal neurons in the right hemisphere, although these cahnges were less pronounced than the changes in dendritic morphology. Consistent with previous reports of chronic stress effects on BLA spine density in rats and mice (Mitra et al., 2005; Govindarajan et al., 2006; Vyas et al., 2006), acute stress produced increased spine density on higher-order branches of BLA neurons. In the present study, this effect varied across spine types, with the increase being most pronounced in mushroom spines. This is consistent with the hypothesis that stress increased the number of mature, stable, ‘memory’ spines (Bourne & Harris, 2007). Thus, the increase in mushroom spines could contribute to the stress-induced BLA hyperexcitability reported previously (Chauveau et al., 2012) and the impaired extinction reported here.
Although this is the first demonstration of a lateralized acute stress effect on BLA morphology, the hemispheric specificity of effects is consistent with neuroimaging studies in human subjects that have indicated lateralization of amygdala function, with activity in the right but not the left amygdala correlating strongly with memory for emotionally arousing stimuli, at least in men (Cahill et al., 1996). Furthermore, the stress-induced alterations in the right BLA concur with earlier evidence of the lateralization of stress effects in the mPFC. For example, chronic daily restraint stress produced retraction of basilar dendrites of layer II–III neurons in the prelimbic cortex in the right hemisphere only, and this was accompanied by right-hemisphere increases in spine density on proximal basilar branches (Perez-Cruz et al., 2009). In addition, there is evidence for lateralization of prefrontal modulation of autonomic and hypothalamic–pituitary–adrenal axis responses to stress. For instance, lesions of the right but not the left mPFC reduced corticosterone release and ulcer formation in response to repeated restraint stress (Sullivan & Gratton, 1999), an effect that may be specific to the right infralimbic cortex, as lesions here also decreased anxiety-like behaviors (Sullivan & Gratton, 2002). Furthermore, in males, increases in dopamine release in response to acute, uncontrollable stress were more pronounced in the right infralimbic cortex (Carlson et al., 1993; Berridge et al., 1999; reviewed in Sullivan & Laplante, 2011).
Our findings in rats parallel neuroimaging data indicating reduced amygdala volume in veterans with PTSD relative to combat-exposed non-case controls (Rogers et al., 2009; Morey et al., 2012). Combat exposure was found to be associated with smaller amygdala volumes, regardless of PTSD diagnosis, with the severity of exposure correlating negatively with amygdala volume (Morey et al., 2012). Providing another parallel with our data for rats, a negative association between severity of trauma and human amygdala volume was stronger in the right hemisphere (Mollica et al., 2009). Our data suggest a potential cellular substrate for these alterations in volume in the form of dendritic retraction, and extend the previous observation that small amygdala volume predicted high fear in mice (Yang et al., 2008).
We found that BLA pyramidal neurons showed robust debranching and dendritic retraction in response to acute stress. This finding conflicts with a previous study that failed to find alterations in either BLA dendritic morphology or spine density 1 day after acute immobilization stress (Mitra et al., 2005), but there are several possible explanations for the apparent discrepancy. First, the use of different stressors (elevated platform vs. immobilization) in the two studies could be a factor if BLA morphology is differentially sensitive to specific acute stressors, as is the case for chronic stressors (Vyas et al., 2002). Another consideration is that we examined dendritic morphology 2 days after the acute stressor, whereas Mitra et al. assessed dendritic morphology either 1 or 10 days post-stress. There may be a dynamic, non-linear time-course of stress-induced changes in the BLA, with neurons undergoing initial dendritic retraction followed by recovery. Such a pattern concurs with the dendritic remodeling seen after experimental manipulations that alter neuronal inputs, such as deafferentation or nerve crush, which typically result in initial dendritic retraction followed by proliferation (Matthews & Powell, 1962; Standler & Bernstein, 1982; Caceres & Steward, 1983). If such a time-course occurs, this might suggest that the smaller amygdala volume in PTSD (Rogers et al., 2009; Morey et al., 2012) reflects a failure of dendritic recovery in vulnerable individuals.
Notwithstanding the above, the present finding clearly contrasts with the effects of chronic stress on BLA dendritic morphology. For instance, 10 days of immobilization stress (2 h/day) increased the length and number of dendritic branches of BLA pyramidal and stellate neurons in the BLA in rats (Vyas et al., 2002, 2004), whereas repeated heterotypic stress exposure over a period of several hours produced dendritic proliferation in pyramidal neurons 7 days later (restraint, forced swim, ether exposure) (Cui et al., 2008). Similar changes in dendritic length are seen after chronic restraint stress in mice (6 h/day for 21 days) (Johnson et al., 2009). Thus, unlike in the mPFC, where retraction is evident after either acute or chronic stress (Holmes & Wellman, 2009), acute stress may have different effects than chronic stress on dendritic morphology in the amygdala.
Stress-induced changes in BLA morphology are associated with impaired extinction
Consistent with previous results (Akirav et al., 2009), one 30-min episode of elevated platform stress following retrieval of a conditioned fear memory impaired subsequent extinction learning and retrieval. Deficient extinction following an acute exposure to this stressor replicates data obtained with acute restraint (Chauveau et al., 2012), as well as the findings of numerous studies examining the effects of subchronic or chronic exposure to a range of different stressors (Izquierdo et al., 2006; Miracle et al., 2006; Garcia et al., 2008; Baran et al., 2009; Farrell et al., 2010; Wilber et al., 2011). It should be noted that the experimental design employed herein, in which stress was applied after a probe for fear retrieval, leaves open the possibility that stress strengthened memory reconsolidation to promote fear during extinction training. We do not discount this possibility, and it would agree with the well-documented facilitating effects of monoaminergic and glucocorticoid stress hormones on consolidation of memory (reviewed in Roozendaal et al., 2009).
Stress-induced deficits in extinction were correlated with the extent of morphological change in the right BLA, such that rats showing relatively fewer and shorter dendritic branches had the highest freezing during extinction. A functional link between the two would not be unexpected, considering that the geometry of the dendritic arbor (e.g. dendritic branching patterns, distribution, and overall shape) determines many functional properties of neurons (Rall et al., 1992; Mainen & Sejnowksi, 1996; Koch & Segev, 2000; Lu et al., 2001; Grudt & Perl, 2002). Although regressive changes in dendritic morphology have been hypothesized to produce a less responsive neuron (Wilber et al., 2011), this is not necessarily the case. In fact, a decrease in neuron size would increase membrane resistance, with a resultant increase in excitability, all other variables being equal. Consistent with this notion, chronic stress-induced dendritic retraction in CA3 hippocampal pyramidal neurons is associated with increased neuronal excitability (Kole et al., 2004). Whereas hippocampal dendritic retraction has typically been interpreted as a neuroprotective response to minimize excitotoxicity, recent data suggest that, in fact, stress-induced CA3 dendritic retraction is associated with increased susceptibility to ibotenic acid-induced excitotoxicity (Conrad et al., 2004; Conrad, 2008). Thus, the dendritic retraction coupled with the increased density of mushroom spines after stress could lead to BLA hyperexcitability, and underlie the deficits in fear extinction. Further studies entailing the examination of intrinsic changes in neuronal physiology in BLA pyramidal neurons and their relationship to morphology after acute elevated platform stress will test this hypothesis, but BLA hyperexcitability has already been reported after acute restraint (Chauveau et al., 2012).
Hemisphere-specific effects of conditioning and extinction on BLA morphology
A surprising observation was that rats subjected to fear conditioning and extinction showed significant BLA dendritic retraction and increased spine density as compared with non-conditioned rats. To our knowledge, this is the first demonstration of an effect of fear conditioning and extinction testing on BLA dendritic morphology. However, other studies have demonstrated that fear conditioning and extinction produce opposing effects on spine elimination in the secondary motor cortex (Lai et al., 2012), whereas consolidation of contextual fear is associated with increased spine density in the anterior cingulate cortex (Restivo et al., 2009; Vetere et al., 2011). In contrast to the effect of stress, conditioning and extinction exerted dendritic changes that were specific to the left, not right, hemisphere. Furthermore, conditioning and extinction produced contrasting changes in the density of thin-type, rather than mushroom, spines, with increased density in the left hemisphere and decreased density in the right hemisphere. The alterations in thin spine density could reflect changes in the turnover of new spines, with turnover being increased in the left hemisphere and decreased in the right hemisphere (Bourne & Harris, 2007). Given the differing patterns of changes in neuronal morphology after stress vs. conditioning and extinction, it seems unlikely that the morphological changes in rats that underwent behavioral testing reflect the stressfulness of the fear conditioning and extinction procedures, but instead may be related to learning. Finally, in the present study, the behaviorally characterized rats underwent both fear conditioning and extinction. Thus, changes in BLA morphology could reflect the effects of either fear conditioning or extinction. Future studies will be required to tease apart these alternatives.
Conclusions
In conclusion, the current data demonstrate that the dendritic and spine morphology of BLA pyramidal neurons is remarkably sensitive to acute stress, as well as behavioral testing. The close association between stress-induced changes in dendritic morphology/spine density and deficient extinction suggests that BLA dendritic and synaptic remodeling could be one mechanism underlying stress-induced impairments in extinction and stress-related disorders such as PTSD.
Acknowledgements
This work was supported by the US-Israel Binational Science Foundation (grant number 2007096 to A. Holmes, M. Maroun, and C. L. Wellman) and the Intramural Research Program of the National Institute on Alcoholism and Alcohol Abuse (Z01-AA000411 to A. Holmes).
Abbreviations
- BLA
basolateral amygdala
- CS
conditioned stimulus
- mPFC
medial prefrontal cortex
- NS
not significant
- PTSD
post-traumatic stress disorder
References
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007;2007:1–11. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Segev A, Motanis H, Maroun M. D-cycloserine into the BLA reverses the impairing effects of exposure to stress on the extinction of contextual fear, but not conditioned taste aversion. Learn. Memory. 2009;16:682–686. doi: 10.1101/lm.1565109. [DOI] [PubMed] [Google Scholar]
- Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol. Learn. Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Mitton E, Clark W, Roth RH. Engagement in a non-escape (displacement) behavior elicits a selective and lateralized suppression of frontal cortical dopaminergic utilization in stress. Synapse. 1999;32:187–197. doi: 10.1002/(SICI)1098-2396(19990601)32:3<187::AID-SYN5>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ. Innate and conditioned reactions to threat in rats with amygdaloid lesions. J. Comp. Physiol. Psych. 1972;81:281–290. doi: 10.1037/h0033521. [DOI] [PubMed] [Google Scholar]
- Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17:381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Caceres A, Steward O. Dendritic reorganization in the denervated dentate gyrus of the rat following entorhinal cortical lesions: a Golgi and electron microscopic analysis. J. Comp. Neurol. 1983;214:387–403. [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc. Natl. Acad. Sci. USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson JN, Fitzgerald LW, Keller RWJ, Glick SD. Lateralized changes in prefrontal cortical dopamine activity induced by controllable and uncontrollable stress in the rat. Brain Res. 1993;630:178–187. doi: 10.1016/0006-8993(93)90655-7. [DOI] [PubMed] [Google Scholar]
- Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape H-C. Prevention of stress-impaired fear extinction through neuropeptide S action in the lateral amygdala. Neuropsychopharmacol. 2012;37:1588–1599. doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD. Chronic stress-induced hippocampal vulnerability: the glucocorticoid vulnerability hypothesis. Rev. Neuroscience. 2008;19:395–411. doi: 10.1515/revneuro.2008.19.6.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad CD, Jackson JL, Wise LS. Chronic stress enhances ibotenic acid-induced damage selectively within the hippocampal CA3 region of male, but not female rats. Neuroscience. 2004;125:759–767. doi: 10.1016/j.neuroscience.2004.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J. Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Cui H, Sakamoto H, Higashi S, Kawata M. Effects of single-prolonged stress on neurons and their afferent inputs in the amygdala. Neuroscience. 2008;152:703–712. doi: 10.1016/j.neuroscience.2007.12.028. [DOI] [PubMed] [Google Scholar]
- Farrell MR, Sayed JA, Underwood AR, Wellman CL. Lesion of infralimbic cortex occludes stress effects on retrieval of extinction but not fear conditioning. Neurobiol. Learn. Mem. 2010;94:240–246. doi: 10.1016/j.nlm.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Garcia R, Spennato G, Nilsson-Todd L, Moreau J-L, Deschaux O. Hippocampal low-frequency stimulation and chronic mild stress similarly disrupt fear extinction memory in rats. Neurobiol. Learn. Mem. 2008;89:560–566. doi: 10.1016/j.nlm.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Glaser EM, Van der Loos H. Analysis of thick brain sections by obverse-reverse computer microscopy: application of a new, high-quality Golgi–Nissl stain. J. Neurosci. Meth. 1981;4:117–125. doi: 10.1016/0165-0270(81)90045-5. [DOI] [PubMed] [Google Scholar]
- Govindarajan A, Rao BS, Nair D, Trinh M, Mawjee N, Tonegawa S, Chattarji S. Transgenic brain-derived neurotrophic factor expression causes both anxiogenic and antidepressant effects. Proc. Natl. Acad. Sci. USA. 2006;103:13208–13213. doi: 10.1073/pnas.0605180103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudt TJ, Perl ER. Correlations between neuronal morphology and electrophysiology features in the rodent superficial dorsal horn. J. Physiol. 2002;540:189–207. doi: 10.1113/jphysiol.2001.012890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hays WL. Statistics. Harcourt Brace; Fort Worth, TX.; 1994. [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Lüthi A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Hikind M, Maroun M. Microinfusion of the D1 receptor antagonist, SCH23390 into the IL but not the BLA impairs consolidation of extinction of auditory fear conditioning. Neurobiol. Learn. Mem. 2008;90:217–222. doi: 10.1016/j.nlm.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Holmes A, Quirk GJ. Pharmacological facilitation of fear extinction and the search for adjunct treatments for anxiety disorders – the case of yohimbine. Trends Pharmacol. Sci. 2010;31:2–7. doi: 10.1016/j.tips.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. R. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Wellman CL, Holmes A. Rapid dendritic retraction in medial prefrontal neurons and impaired fear extinction following exposure to uncontrollable stress. J. Neurosci. 2006;26:5733–5738. doi: 10.1523/JNEUROSCI.0474-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SA, Wang JF, Sun X, McEwen BS, Chattarji S, Young LT. Lithium treatment prevents stress-induced dendritic remodeling in the rodent amygdala. Neuroscience. 2009;163:34–39. doi: 10.1016/j.neuroscience.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Kim J, Fanselow MS, DeCola JP, Landeira-Fernandez J. Selective impairment of long-term but not short-term conditional fear by the N-methyl-D-aspartate antagonist APV. Behav. Neurosci. 1992;106:591–596. doi: 10.1037//0735-7044.106.4.591. [DOI] [PubMed] [Google Scholar]
- Koch C, Segev I. The role of single neurons in information processing. Nat. Neurosci. 2000;3:1171–1177. doi: 10.1038/81444. [DOI] [PubMed] [Google Scholar]
- Kole MHP, Costoli T, Koolhaas JM, Fuchs E. Bidirectional shift in the cornu ammonis 3 pyramidal dendritic organization following brief stress. Neuroscience. 2004;125:337–347. doi: 10.1016/j.neuroscience.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Lai CSW, Franke TF, Gan W-B. Opposite effects of fear condi tioning and extinction on dendritic spine remodelling. Nature. 2012;483:87–91. doi: 10.1038/nature10792. [DOI] [PubMed] [Google Scholar]
- Lu Y, Inokuchi H, McLachlan EM, Li J-S, Higashi H. Correlation between electrophysiology and morphology of three groups of neurons in the dorsal commissural nucleus of lumbosacral spinal cord of mature rats studied in vitro. J. Comp. Neurol. 2001;437:156–169. doi: 10.1002/cne.1276. [DOI] [PubMed] [Google Scholar]
- Mainen ZF, Sejnowksi TJ. Influence of dendritic structure on firing pattern in model neocortical neurons. Nature. 1996;382:363–366. doi: 10.1038/382363a0. [DOI] [PubMed] [Google Scholar]
- Maroun M. Stress reverses plasticity in the pathway projecting from the ventromedial prefrontal cortex to the basolateral amygdala. Eur. J. Neurosci. 2006;24:2917–2922. doi: 10.1111/j.1460-9568.2006.05169.x. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala–prefrontal cortex pathway in vivo. J. Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M, Holmes A, Wellman CL, Motanis H. Enhanced extinction of aversive memories by high-frequency stimulation of the rat infralimbic cortex. PLoS One. 2012;7:e35853. doi: 10.1371/journal.pone.0035853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA Receptor blockade alters stress-induced dendritic remodeling in medial prefrontal cortex. Cereb. Cortex. 2011;21:2366–2373. doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews MR, Powell TPS. Some observations on transneuronal cell degeneration in the olfactory bulb of the rabbit. J. Anat. 1962;96:89–105. [PMC free article] [PubMed] [Google Scholar]
- Maxwell SE, Delaney HD. Designing Experiments and Analyzing Data: A Model Comparison Perspective. Lawrence Erlbaum Associates; Mahwah, NJ.: 2003. [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol. Learn. Mem. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. USA. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollica RF, Lyoo IK, Chernoff MC, Bui HX, Lavelle J, Yoon SJ, Kim JE, Renshaw PF. Brain structural abnormalities and mental health sequelae in South Vietnamese ex-political detainees who survived traumatic head injury and torture. Arch. Gen. Psychiat. 2009;66:1221–1232. doi: 10.1001/archgenpsychiatry.2009.127. [DOI] [PubMed] [Google Scholar]
- Morey RA, Gold AL, LaBar KS, Beall SK, Brown VM, Haswell CC, Nasser JD, Wagner HR, McCarthy G. Mid-Atlantic MIRECC Workgroup (2012) Amygdala volume changes in posttraumatic stress disorder in a large case-controlled veterans group. Arch. Gen. Psychiat. 69:1169–1178. doi: 10.1001/archgenpsychiatry.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol. Psychiatr. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Orsini CA, Maren S. Neural and cellular mechanisms of fear and extinction memory formation. Neurosci. Biobehav. R. 2012;36:1773–1802. doi: 10.1016/j.neubiorev.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Perez-Cruz C, Simon M, Czéh B, Flügge G, Fuchs E. Hemispheric differences in basilar dendrites and spines of pyramidal neurons in the rat prelimbic cortex: activity- and stress-induced changes. Eur. J. Neurosci. 2009;29:738–747. doi: 10.1111/j.1460-9568.2009.06622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Kaiserman-Abramof IR. The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am. J. Anat. 1970;127:321–355. doi: 10.1002/aja.1001270402. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacol. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WGM, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb. Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rall W, Burke RE, Holmes WR, Jack JJ, Redman SJ, Segev I. Matching dendritic neuron models of experimental data. Physiol. Rev. 1992;72:S159–S186. doi: 10.1152/physrev.1992.72.suppl_4.S159. [DOI] [PubMed] [Google Scholar]
- Restivo L, Vetere G, Bontempi B, Ammassari-Teule M. The formation of recent and remote memory is associated with time-dependent formation of dendritic spines in the hippocampus and anterior cingulate cortex. J. Neurosci. 2009;29:8206–8214. doi: 10.1523/JNEUROSCI.0966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Levin G, Maroun M. Stress and amygdala suppression of metaplasticity in the medial prefrontal cortex. Cereb. Cortex. 2010;20:2433–2441. doi: 10.1093/cercor/bhp311. [DOI] [PubMed] [Google Scholar]
- Rocher C, Spedding M, Munoz C, Jay TM. Acute stress-induced changes in hippocampal/prefrontal circuits in rats: effects of antidepressants. Cereb. Cortex. 2004;14:224–229. doi: 10.1093/cercor/bhg122. [DOI] [PubMed] [Google Scholar]
- Rogers MA, Yamasue H, Abe O, Yamada H, Ohtani T, Iwanami A, Aoki S, Kato N, Kasai K. Smaller amygdala volume and reduced anterior cingulate gray matter density associated with history of post-traumatic stress disorder. Psychiat. Res. 2009;174:210–216. doi: 10.1016/j.pscychresns.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat. Rev. Neurosci. 2009;10:423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- Standler NA, Bernstein JJ. Degeneration and regeneration of motoneuron dendrites after ventral root crush: computer reconstruction of dendritic fields. Exp. Neurol. 1982;75:600–615. doi: 10.1016/0014-4886(82)90028-0. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Lateralized effects of medial prefrontal cortex lesions on neuroendocrine and autonomic stress responses in rats. J. Neurosci. 1999;19:2834–2840. doi: 10.1523/JNEUROSCI.19-07-02834.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Behavioral effects of excitotoxic lesions of ventral medial prefrontal cortex in the rat are hemisphere-dependent. Brain Res. 2002;927:69–79. doi: 10.1016/s0006-8993(01)03328-5. [DOI] [PubMed] [Google Scholar]
- Sullivan RM, Laplante F, Conrad CD. The Handbook of Stress: Neuropsychological Effects on the Brain. Blackwell; Chichester, UK: 2011. Stress, prefrontal cortex asymmetry, and depression; pp. 505–523. [Google Scholar]
- Vetere G, Restivo L, Cole CJ, Ross PJ, Ammassari-Teule M, Josselyn SA, Frankland PW. Spine growth in the anterior cingulate cortex is necessary for the consolidation of contextual fear memory. Proc. Natl. Acad. Sci. USA. 2011;108:8456–8460. doi: 10.1073/pnas.1016275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–393. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128:667–673. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Wilber AA, Walker AG, Southwood CJ, Farrell MR, Lin GL, Rebec GV, Wellman CL. Chronic stress alters neural activity in medial prefrontal cortex during retrieval of extinction. Neuroscience. 2011;174:115–131. doi: 10.1016/j.neuroscience.2010.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacol. 2008;33:2108–2116. doi: 10.1038/sj.npp.1301605. [DOI] [PubMed] [Google Scholar]
- Yang RJ, Mozhui K, Karlsson RM, Cameron HA, Williams RW, Holmes A. Variation in mouse basolateral amygdala volume is associated with differences in stress reactivity and fear learning. Neuropsychopharmacol. 2008;33:2595–2604. doi: 10.1038/sj.npp.1301665. [DOI] [PubMed] [Google Scholar]