Abstract
The ventral tegmental area (VTA), and in particular dopamine (DA) neurons in this region of midbrain, has been shown to play an important role in motivation (goal-directed behavior), reward, and drug addiction. Most evidence that implicates VTA DA neurons in these functions are based on widely accepted but indirect electrophysiological characterization, including the hyperpolarization activated non-specific cation current (Ih), spike frequency, and inhibition by D2 receptor agonists. In this study, we used a known neuronal dopamine transporter (DAT) fluorescent substrate [4-(4- (dimethylamino) styryl)-N-methylpyridinium iodide] (ASP+) to visualize DAT-containing cell bodies of DA neurons in VTA region in rat brain slices. Uptake of 100 nM of ASP+ in brain slices of rat VTA region marked 38% of visible neurons, while other neurons from this region and 100% neurons from hippocampus slices were not fluorescent. Using patch-clamp techniques, we have found that pronounced Ih current was present in all fluorescent neurons from VTA area, also spike frequency was similar to the widely accepted values for DA neurons. Furthermore, additional study has shown that there are 84% coincidence of ASP+ fluorescence in neuronal cell bodies and Falck-Hillarp labeling of DA cells. Electrophysiological recordings during ASP+ application have confirmed that low concentrations (100 nM) of ASP+ have no visible effect on neuronal activity during 1–2 hours after staining. Thus, uptake of fluorescent monoamine analog ASP+ by DAT can be an additional criterion for identification of DAT-containing neurons in slices.
Keywords: ventral tegmental area, dopamine neurons, DAT fluorescent substrate
Introduction
Ventral tegmental area (VTA) neurons are a source of dopaminergic (DA) projections to the cortical structures and ventral forebrain, and they have been implicated in reward and goal-directed behavior [1]. The conclusion that neurons in question are dopaminergic is critical to hypotheses about the role of dopamine signaling in these functions.
The criteria for identifying midbrain DA neurons were initially developed in the substantia nigra pars compacta (SNc). Cytochemical methods have shown that DA neurons in SNc possessed action potentials of relatively long duration, slow rate pacemaker firing in vitro or burst firing in vivo, and DA agonist-induced inhibitions; while neurons with action potentials of short duration and higher firing rates were not dopaminergic [2;3]. Electrophysiogicaly characterized DA-cells were also shown to express a hyperpolarization-activated inwardly rectifying non-specific cation current (Ih), whereas supposed non-DA neurons did not [4].
VTA is a midbrain region adjoining to the SNc, and because of this proximity, the same electrophysiological criteria were extended to distinguish neurons in VTA. Processing for hydroxylase (TH) immunoreactivity had shown that neurons filled with marker and by their electrophysiological properties that were presumed to be DA neurons are appearing to be non-DA-containing. For example, while the absence of an Ih in a VTA neuron is a reliable predictor that the cell is not DA-containing (Margolis et al. 2003, 2006), the presence of an Ih does not reliably predict TH co-labelling [5;6;7].
Introduction of two major neuronal classes in VTA, (i) principal DA neurons that are inhibited by DA agonists and (ii) secondary GABA-ergic neurons that are inhibited by μ-opioid receptor (MOP-R) agonists [8], as additional criteria has not solved the DA-neurons identification problem either. Even the original report has shown that 38% of neurons inhibited with DA-agonist were TH(−) [8]. Moreover, VTA neurons referred to as tertiary cells are inhibited by both DA and MOP-R agonists. Approximately one-third of tertiary neurons are TH(+) [5]. Collectively, the data suggest that an additional simple identification method to clarify whether a neuron is dopaminergic would be appreciated, especially if the neuron has a prominent Ih-current.
Recently, Schwartz et al. (2003) introduced the fluorescent substrate for monoamine transporters, 4-(4-(dimethylamino) styryl)-N-methylpyridinium iodide, or ASP+, which accumulates in cytoplasm allowing to visualize transporter-containing cells. ASP+ as a substrate for DAT transporter is structurally similar to the well known MPP+ (1-methyl-4-phenylpyridinium), but is not toxic [9]. It was successively used to distinguish DA-neurons in culture [10]. Here we report ASP+ accumulation in midbrain cells in VTA in slice, allowing making patch-clamp recording from fluorescent cells and getting electrophysiological characterization.
Methods
Animals and slice preparation
All experimental procedures were performed accordingly to the US Public Health Service publication Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee at the Universidad Central del Caribe. Sprague-Dawley rats of either sex between 20 and 30 days postnatal were decapitated. After rapid removal of the brain, cerebral horizontal slices (350 µM) containing the midbrain VTA area were prepared using the vibratome (VT1000S, Leica, Germany). Determination of VTA boundary was done according to [11] as we described previously [12]. Slices were cut in an ice-cold oxygenated artificial cerebrospinal fluid (ACSF) containing (in mM): 127 NaCl; 2,5 KCl; 1,25 NaH2PO4; 25 NaHCO3; 2 CaCl2; 1 MgCl2; and 25 glucose (d). The solution was previously saturated with a 95%O2 – 5%CO2 gas mixture to a pH=7,4. Slices were transferred to an intermediate chamber and were incubated at 35° C in the same solution for 0.5 h before being relocated to the recording chamber (0.1 ml/sec). In experimental slices, fluorescent substrate ASP+ (100 nM) was added to ACSF in the incubation camera to visualize fluorescence of neurons that accumulated the stain.
Histochemistry
We fist tried to use immunostaining of DA cells using antibody against Tyrosine Hydroxylase (TH). Due to the constant problems with the double staining [ImG TH-ASP+], we decided to use Falck–Hillarp method to reveal DA neurons. In order to visualize TH inside the cells, application of membrane perforation Triton-like agents is required. However, soluble ASP+ must be retained inside the intact cell while perforating membrane and constant wash-out during immunostaining usually lead to the ASP+ loss from neurons. Slice thickness also became a setback. On the contrary, the glyoxylic acid condensation reaction is commonly used for visual detection of biogenic amines, and it doesn’t need any special membrane perforating agents. This method is a refinement of the techniques for fluorescence histochemistry of monoamines (Falck-Hillarp method), all based on the cyclization of ethylamine side chains by highly reactive aldehydes, either formaldehyde or glyoxylic acid, producing compounds which fluoresce under suitable excitation. Historically, Falck-Hillarp method was the first method used to map midbrain DA neurons in VTA (A10 nuclei) [13]. This method directly reveals dopamine inside the neurons.
Thus, this study used a modified Falck-Hillarp (F-H) method to visualize DA – containing neurons in slices [14]. Briefly, slices were incubated in freshly made glyoxylate-containing solution at 4°C for 2 h, placed on a glass slide, dried with air blower at room temperature for 1.5–2 h, heated in an oven at 85°C for 9 min, covered with paraffin oil, coverslipped, and viewed using a Fluoview FV1000 fluorescence confocal microscope (Olympus, Japan) with UV-GFP filter set (excitation 400±30nm, emission 508±20nm). The incubating solution contained (in mM) 500 sodium glyoxylate, 40 N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), and 100 sucrose, dissolved in deionized water, pH 7.0. The final pH of the solution was adjusted to 7.0 with either glyoxylic acid or sodium bicarbonate crystals. For double staining with ASP+, slices were maintained 30 min in oxygenated ACSF with ASP+ (100 nM) added. A CY3 filter set (excitation 520±30nm, splitter 565nm, barrier filter >580nm) was used to reveal ASP+ staining.
Brain slices were then analyzed in the VTA region for ASP(+)/DA double labeling using an Olympus FV1000 Confocal Laser Scanning Microscope with the appropriate filter sets. Confocal images were captured using ×10 and ×40 PlanApochromat objective. Series of images at 1.2 µm intervals throughout the VTA-defined regions were collected for analysis. Images are presented as projections of image stacks or individual optical slices. The images were adjusted using NIH ImageJ (http://rsbweb.nih.gov/ij/download.html) with Loci_tools plug-in, allowing to open Olimpus files in this program. We considered a neuron to be fluorescent if its luminance exceeded the background by at least 20%.
Whole cell recordings from fluorescent and non-fluorescent neurons in slices
Membrane currents were measured with the single electrode whole-cell patch-clamp technique. Cells in the VTA area were visualized using an Olympus infrared microscope equipped with DIC (BX51WI Olympus, Japan. Fluorescent cells accumulating ASP+ were identified using CY3 excitation–emission filter with DP30BW epifluorescence system and 40× LUMPlan FL/IR water-immersion objective (Fig.1 B). Piezoelectric micromanipulators (MX7500 with MC-1000 drive, Siskiyou, OR) were used for voltage-clamp and current-clamp recording.
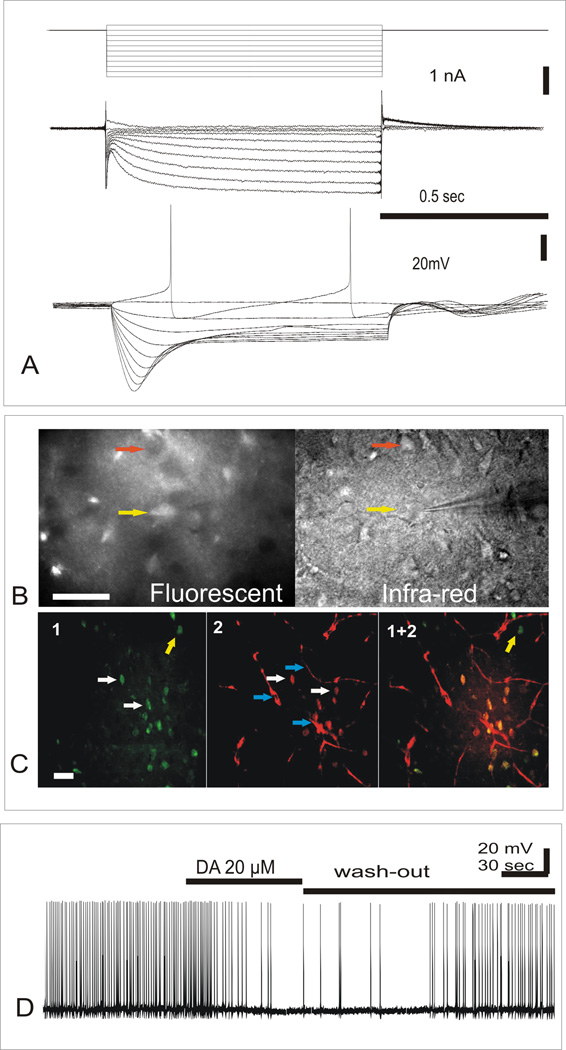
Fig.1.
A. Whole cell patch-clamp recording from fluorescent cells after slice being stained with 100 nM ASP+. Upper trace: Stimulation step protocol (see. Methods), Middle trace: Recording of fluorescent DA neuron currents as a response to series of 10 (1 sec) 10 mV voltage step stimulations, from −30 to −120 mV. Lower trace: Recording of DA neuron voltage responses to series of 10 (1 sec duration) 200 pA current step stimulations, from +200 pA from holding level to −1400 pA (−45 mV).
B. Fluorescent (left) and infra-red (IR) image (right) of path-clamp recording from fluorescent neuron in VTA. Electrode is positioned to record from fluorescent neuron (yellow arrow) that coincides with infra-red image of neuronal cell body, while another neuronal cell body(red arrow) that have no florescence can be seen in IR image as well. Scale:100 µm.
C. Double staining of VTA neurons with Falck-Hillarp method and ASP+. From left to right: (1) F-H fluorescence (green); (2) ASP+ fluorescence (red); and (1+2) their colocalization (see Methods). White arrows point to fluorescent neuronal cell bodies that coincide in 1 and 2. Yellow arrow point on neuronal cell body that have F-H fluorescence but have no ASP+ fluorescence. Blue arrows point to blood vessels. Scale:100 µm. (See also web content for animated picture).
D. External application of 20 µM of dopamine produced visible inhibition of fluorescent neuron activity.
MultiClamp 700A patch-clap amplifier with DigiData 1322A interface (Axon Instruments, now part of Molecular devices, CA), was used for recording and stimulation. On-line data acquisition and analysis was done with pClamp 10 software package (Axon Instrument, CA).
Ih current was elicited in voltage-clamp mode using standard step protocol applying 10 mV (1 sec duration) steps from −40 mV to + 140 mV, and in current clamp 200 pA (1 sec duration) steps from +200 pA to – 1,2 nA [12] (Fig.1 A, upper trace).
Borosilicate glass patch pipettes (O.D. 1.5 mm, I.D. 1,0 mm; WPI, Sarasota, FL) were pulled to the final resistance of 4–5 MΩ for neuronal recordings in four steps, applying a Sutter P-97 puller (Novato, CA). Electrodes were filed with the following solution (in mM): 130 K-gluc, 10 Na- gluc, 4 NaCl, 4 phosphocreatine, 0.3 GTP-Na, 4 Mg–ATP, 10 HEPES, and the pH was adjusted to 7.2 adjusted with KOH.
Experiments with dopamine (DA) perfusion were performed with no light in order to reduce auto oxidation.
Chemicals
All chemicals were purchased from Sigma (St Louis, MO), except for ASP+ or 4-(4-(dimethylamino) styryl)-N-methylpyridinium iodide (Invitrogen, CA).
Results
After midbrain slices being held for 30 min in 100 nM ASP+ solution, fluorescent neurons in VTA became clearly visible and could be patched in an usual way switching between infra-red and fluorescent image. It allowed to find out what particular cell has fluorescence (Fig.1 B, red arrow). Staining with ASP+ in VTA revealed that about 38% of all neuronal cell bodies visible in infra-red microscope in VTA area got fluorescence (n=6 slices, 183 neurons counted). Recordings from 28 fluorescent neurons in VTA area slices from 5 different animals showed that all fluorescent cells possessed strong Ih-current (Fig1. A). Application of 10 mV voltage steps (Fig.1A, upper trace, see.Methods) revealed a steep development of Ih current alongside with the development of hyperpolarization from −40 to −140 mV (Fig 1A, middle trace). A steady-state level was reached at about 0.8–1 sec, and a significant tail current was recorded after abrupt end of hyperpolarization pulse. In current clamp mode, application of 200 pA current steps to the membrane of fluorescent cells elicited well-defined sag response upon hyperpolarization (Fig.1 A, lower trace), one of the important characteristics of Ih-current [12]. All 28 (100%) of fluorescent cells recorded from in VTA had prominent Ih current (> 200 pA at −140 mV). These cells exhibited pacemaker firing within 1–3 Hz rate (Fig1 A, low trace, also Fig.1 D). Application of 20 µM DA by extracellular perfusion significantly reduced firing rate 86±8% (n=6) and produced hyperpolarization 4±1.8 mV (n=6).
There weren’t any changes in neuronal activity during 30–60 min recordings, confirming that a low concentration (100 nM) of ASP+ is not harming a cell during 1–2 hours after the application. A higher concentration (10µM) of ASP+ acutely produced inhibition of spiking activity in VTA DA neurons, and later (3–4 hour) reduction of spike amplitudes and membrane potential. After 30 min staining with 100 nM ASP+, confocal microscope exhibited that fluorescent cell bodies can be found in VTA zone and in SNc at low magnification (10× objective)(Fig.2 C). High magnification in VTA (40× objective, Fig2 D) revealed that fluorescence level of neuron bodies was different: some were fluorescent, and the others had no accumulation of ASP+. Control staining of hippocampus desplayed low fluorescence in majority of neurons (Fig.2 A, B, also see Web content).
Fig.2.
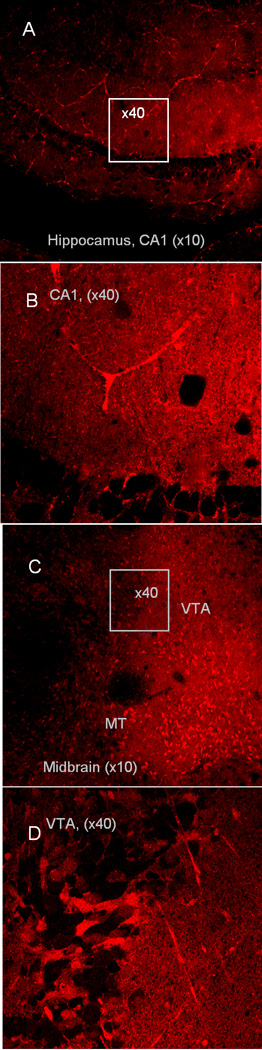
Confocal images of live brain slices stained with 100 nM ASP+. A - Control staining of hippocampus CA3 zone observed with 10× objective. B. The same CA3 zone with 40× oil objective. Stratum radiatum (with fluorescent astrocytes) and pyramidal cells layer with low fluorescent neurons can be seen (see also Web content Fig 2 for 3D image of this zone).
C- Low magnification (10 × objective) of VT midbrain slice, showing overall layout of fluorescent cells in that zone. D - Fluorescent neurons and blood vessels in VTA at higher magnification (40× oil objective), while some of neurons have fluorescence lower, than background (see also Web content Fig 2 for 3D image of this zone).
Double staining of DA-containing neurons with F-H method and ASP+ accumulation in neurons with DA-transporter, indicated that 84% of neurons (66 neurons out of 79, counted in 6 slices) with F-H fluorescence along with ASP+ fluorescence (Fig.1 C1) while neurons with no F-H fluorescence didn’t have ASP+ staining, either (Fig.1 C2, see also Fig. 1 C1+2, also Web content).
In additional, ASP+ fluorescence accumulation in VTA slices was analyzed in the presence of 5 µM cocaine, a well known blocker of neuronal type sodium-dependent catecholamine transporters. In those experiments, a general luminance in VTA area was assayed, since it can be attributed mainly to fluorescence in neurons because neurons were the main source of ASP+ fluorescence in that area (Fig.2 C). 30 min staining with 100 nM ASP+ in the presence of 5 µM of cocaine produced significantly reduced fluorescence (42%, n=4, p<0.01) than in the control staining without transporter blocker.
Discussion
In this study, we used a known neuronal dopamine transporter (DAT) fluorescent substrate (ASP+) to visualize cell bodies of DA neurons possessing this transporter in VTA region in rat brain slices. Uptake of 100 nM of ASP+ in brain slices of rat VTA region marked 38% of visible neurons, while other neurons from this region have low fluorescence (Fig.2 D). All neurons from hippocampus slices were not fluorescent (Fig.2 B). Interestingly, there was clear ASP+ staining of neuronal cells bodies while the majority of DA release and uptake is situated in neuronal processes [15]. ASP+ is a substrate for DAT transporter and also for both neuronal norepineprine transporter and serotonine transporter [9]. However, in VTA and SNc there are no other monaminergic cells except of dopaminergic ones [3]. It is known that astrocytes have sodium dependent neuronal type monoamine transporters, as well as sodium independent extraneuronal monoamine transporters, such as organic cation transporter type3, that can uptake ASP+ [16]. Astrocyte endfeet on blood vessels, thus, clearly mark blood vessels boundaries (Fig. 1 B2)
Fluorescent neurons in VTA showed Ih-current and other electrophysiological characteristics of DA neurons (Fig.1 A).
Electrophysiological recordings during ASP+ application have also confirmed that low concentrations (100 nM) of ASP+ have no visible effect on neuronal activity during 1–2 hours after staining while higher concentrations have acute effect on spike frequency. During 3–4 hours 10 µM (probably due to transporter pumping substantial concentration ASP+ inside the cell) create a drop of membrane potential.
Accumulation of ASP+ fluorescence could be prevented by cocaine, a well known blocker of DAT transport.
It is important to stress, that ASP+ revealed neurons that have functional DAT transporter. Some DA neurons (calbindin-positive) in VTA have very low density of DAT transporter [17]. Similarly, calbindin-positive DA neurons showed negligible Ih-current [18]. These neurons probably have no uptake of ASP+ or at least they cannot be seen using standard fluorescent microscope with limited sensitivity. Interestingly, calbindin-positive DA neurons survive better during Parkison’s disease [19]. The lack of the DAT could be one of the major reasons for this phenomenon what makes a further research of DAT distribution among dopaminergic neurons of special interest. The proposed method can serve as an important tool.
Confocal microscope, unlike the microscope used in electrophysiological experiments, reveals a variety in ASP+ staining in VTA, assumingly due to different expression of DAT in DA neurons (Fig.2D, see also Web content).
It was shown, that dopaminergic neurons from striatum olfactory bulb with tyrosine hydroxylase (TH) immunoreactivity produced γ-butyric acid (GABA) inhibitory mediator and form inhibitory synapses [20;21]. They had well expressed DAT transporter. In VTA some DA neurons produced excitatory mediator glutamate and respective synapses [22;23;24]. Presumably, there are also a pure DA neurons producing only dopamine. There is no information on how these different DA neurons populations express DAT and on how it is distributed on the cell.
Recently, it was shown that patch-clamp recording electrodes with an internal solution with high Cl2 concentration reduced the intensity of TH co-labeling, in some cases to background [25;26]. This had raised some doubts even for TH immunostaining.
Taken together, our data suggest that uptake of fluorescent monoamine analog ASP+ by neurons with DAT can be an additional criterion for DA neuron identification in slices, although it is limited to cells with functional dopamine transporter. Our method may be also applied to study monoamine transporters expression on the neurons.
Acknowledgments
This work was supported by NIH- G12RR03035 and 8G12MD007583-27 grants.
Literature
- 1.Grace AA, Floresco SB, Goto Y, Lodge DJ. Regulation of firing of dopaminergic neurons and control of goal-directed behaviors. Trends Neurosci. 2007;30:220–227. doi: 10.1016/j.tins.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Aghajanian G, Bunney BC. Central dopaminergic neurons: Neurophysiologic identification and responses to drugs. In: Usdin E, Snyder SH, editors. Frontiers in catecholamine research; proceedings. Elmsford, N.Y.: Pergamon Press; 1973. pp. 643–648. [Google Scholar]
- 3.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons--1. Identification and characterization. Neuroscience. 1983;10:301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 4.Lacey MG, Mercuri NB, North RA. Two cell types in rat substantia nigra zona compacta distinguished by membrane properties and the actions of dopamine and opioids. J Neurosci. 1989;9:1233–1241. doi: 10.1523/JNEUROSCI.09-04-01233.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- 6.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegemental area revisited: is there an electrophysiological marker for dopaminergic neurons. J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz JW, Blakely RD, DeFelice LJ. Binding and transport in norepinephrine transporters. Real-time, spatially resolved analysis in single cells using a fluorescent substrate. J Biol Chem. 2003;278(11):9768–9777. doi: 10.1074/jbc.M209824200. [DOI] [PubMed] [Google Scholar]
- 10.Mallajosyula JK, Kaur D, Chinta SJ, Rajagopalan S, Rane A, Nicholls DG, Di Monte DA, Macarthur H, Andersen JK. MAO-B elevation in mouse brain astrocytes results in Parkinson's pathology. PLoS ONE. 2008;3(2):e1616. doi: 10.1371/journal.pone.0001616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- 12.Inyushin MU, Arencibia-Albite F, Vázquez-Torres R, Vélez-Hernández ME, Jiménez-Rivera CA. Alpha-2 noradrenergic receptor activation inhibits the hyperpolarization-activated cation current (Ih) in neurons of the ventral tegmental area. Neuroscience. 2010;167(2):287–297. doi: 10.1016/j.neuroscience.2010.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahlström A, Fuxe K. Evidence for the existence of monoamine containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of the brain stem neurons. Acta Physiol Scand. 1964;62(Suppl. 232):1–55. [PubMed] [Google Scholar]
- 14.Torre JC, De la An improved approach to histofluorescence using the SPG method for tissue monoamines. J Neurosci Methods. 1980;1:1–5. doi: 10.1016/0165-0270(80)90029-1. [DOI] [PubMed] [Google Scholar]
- 15.Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- 16.Inazu M, Kubota N, Takeda H, Zhang J, Kiuchi Y, Oguchi K, Matsumiya T. Pharmacological characterization of dopamine transport in cultured rat astrocytes. Life Sci. 1999;64:2239–2245. doi: 10.1016/s0024-3205(99)00175-7. [DOI] [PubMed] [Google Scholar]
- 17.Haber SN, Ryoo H, Cox C, Lu W. Subsets of midbrain dopaminergic neurons in monkeys are distinguished by different levels of mRNA for the dopamine transporter: Comparison with the mRNA for the D2 receptor, tyrosine hydroxylase and calbindin immunoreactivity. J. Comp Neurology. 1995;3:400–410. doi: 10.1002/cne.903620308. 362. [DOI] [PubMed] [Google Scholar]
- 18.Neuhoff H, Neu A, Liss B, Roeper J. I(h) channels contribute to the different functional properties of identified dopaminergic subpopulations in the midbrain. J Neurosci. 2002 Feb 15;22(4):1290–1302. doi: 10.1523/JNEUROSCI.22-04-01290.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada T, McGeer PL, Baimbridge KG, McGeer EG. Relative sparing in Parkinson's disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res. 1990 Sep 3;526(2):303–307. doi: 10.1016/0006-8993(90)91236-a. [DOI] [PubMed] [Google Scholar]
- 20.Betarbet R, Turner R, Chockkan V, DeLong MR, Allers KA, Walters J, Levey AI, Greenamyre JT. Dopaminergic neurons intrinsic to the primate striatum. J Neurosci. 1997 Sep 1;17(17):6761–6768. doi: 10.1523/JNEUROSCI.17-17-06761.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maher BJ, Westbrook GL. Co-transmission of dopamine and GABA in periglomerular cells. J Neurophysiol. 2008 Mar;99(3):1559–1564. doi: 10.1152/jn.00636.2007. Epub 2008 Jan 23. [DOI] [PubMed] [Google Scholar]
- 22.Sulzer D, Joyce MP, Lin L, Geldwert D, Haber SN, Hattori T, Rayport S. Dopamine neurons make glutamatergic synapses in vitro. J Neurosci. 1998 Jun 15;18(12):4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyce MP, Rayport S. Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic. Neuroscience. 2000;99(3):445–456. doi: 10.1016/s0306-4522(00)00219-0. [DOI] [PubMed] [Google Scholar]
- 24.Chuhma N, Zhang H, Masson J, Zhuang X, Sulzer D, Hen R, Rayport S. Dopamine neurons mediate a fast excitatory signal via their glutamatergic synapses. J Neurosci. 2004 Jan 28;24(4):972–981. doi: 10.1523/JNEUROSCI.4317-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang TA, Placzek AN, Dani JA. In vitro identification and electrophysiological characterization of dopamine neurons in the ventral tegmental area. Neuropharmacology. 2010 Nov;59(6):431–436. doi: 10.1016/j.neuropharm.2010.06.004. Epub 2010 Jun 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis EB, Coker AR, Driscoll JR, Lemaître AI, Fields HL. Reliability in the identification of midbrain dopamine neurons. PLoS One. 2010 Dec 9;5(12):e15222. doi: 10.1371/journal.pone.0015222. [DOI] [PMC free article] [PubMed] [Google Scholar]