Significance
Cognitive decline during aging impairs life quality and may lead to dementia. It is associated with a dysfunction of the brain acetylcholinergic system. Here we demonstrate that pharmacological stimulation of neurokinin3 receptors improves learning and memory in aged rats by enhancing acetylcholinergic function in the brain. In a human association study we show that a single-nucleotide polymorphism in the neurokinin3-receptor–coding gene TACR3 can predict learning and memory in elderly patients with cognitive impairments and their hippocampus volume. These findings suggest the neurokinin3 receptor as a potential biomarker and treatment target for cognitive enhancement in the elderly.
Keywords: senktide, in vivo microdialysis
Abstract
Impaired learning and memory performance is often found in aging as an early sign of dementia. It is associated with neuronal loss and reduced functioning of cholinergic networks. Here we present evidence that the neurokinin3 receptors (NK3-R) and their influence on acetylcholine (ACh) release may represent a crucial mechanism that underlies age-related deficits in learning and memory. Repeated pharmacological stimulation of NK3-R in aged rats was found to improve learning in the water maze and in object-place recognition. This treatment also enhanced in vivo acetylcholinergic activity in the frontal cortex, hippocampus, and amygdala but reduced NK3-R mRNA expression in the hippocampus. Furthermore, NK3-R agonism incurred a significantly higher increase in ACh levels in aged animals that showed superior learning than in those that were most deficient in learning. Our findings suggest that the induced activation of ACh, rather than basal ACh activity, is associated with superior learning in the aged. To test whether natural variation in NK3-R function also determines learning and memory performance in aged humans, we investigated 209 elderly patients with cognitive impairments. We found that of the 15 analyzed single single-nucleotide ploymorphism (SNPs) of the NK3-R–coding gene, TACR3, the rs2765 SNP predicted the degree of impairment of learning and memory in these patients. This relationship could be partially explained by a reduced right hippocampus volume in a subsample of 111 tested dementia patients. These data indicate the NK3-R as an important target to predict and improve learning and memory performance in the aged organism.
Humans exhibit a decline in cognitive capacity with age, which may progressively lead to dementia (1). Age-dependent morphological and physiological changes within the cholinergic system of the brain have been described as a major mechanism underlying impairments of learning and memory (2, 3). Numerous studies in aged rats have revealed that a degeneration of cholinergic neurons in the basal forebrain is associated with learning and >memory deficits (4). Likewise, the consistent correlations found between cholinergic degeneration and cognitive impairments indicate a high correspondence between acetylcholinergic activity and the magnitude of cognitive decline (4–6). This relationship has also been shown in patients with Alzheimer’s disease (7, 8).
The neuropeptide neurokinins (NKs) substance P, neurokinin A, neurokinin B (NK-B), neuropeptide K, neuropeptide γ, and hemokinin 1 bind to the three known NK-receptors (NK3-R) (NK1-, NK2-, and NK3-R) with different degrees of affinity, whereby NK-B is the preferred ligand to NK3-R (9). NK3-Rs are widespread in the brain, including areas that are implicated in processes governing learning and memory such as the hippocampus, frontal cortex (FC), and medial septum (10, 11). There is a close interaction between NK3-R and the cholinergic system. NK3-R are localized on cholinacetyltransferase-containing neurons in the basal forebrain (12), and the activity of the septo-hippocampal cholinergic system was found to be stimulated by NK3-R agonists in adult animals (13, 14). An involvement of NK3-Rs in learning and memory processes has also been indicated in rats and mice (14–17).
We therefore hypothesized that NK3-R stimulation would counteract cholinergic deficits and, by this means, improve learning and memory in the aged organism and carried out a functional analysis of NK3-R agonist effects on behavior and neurotransmitter activity in aged rats. To assess a potential relevance of this mechanism for humans also, we analyzed whether single-nucleotide polymorphisms (SNPs) of the NK3-R–coding gene, TACR3, are related to learning and memory performance as well as hippocampal morphology in aged humans with cognitive deficits.
Results
NK3-Receptor Agonism Improves Learning in Aged Rats.
Aged rats show deficits in water-maze and object recognition learning compared with adult rats (see SI Materials and Methods, SI Results, and Fig. S1 for more details). The NK3-R agonist senktide administered posttrial significantly improved learning in an object-place recognition task (Fig. 1A) in aged rats (17–22 mo old). This effect was blocked by the NK3-R antagonist SR142801 (Fig. 1B). Paired sample t tests showed that aged animals treated with vehicle-senktide (P = 0.001) and 3 mg/kg SR142801-senktide (P = 0.004) explored the spatial displaced object longer than the spatial stationary object with no difference in total exploratory activity (P > 0.05; Table S1). This indicates that both groups displayed an intact object-place recognition memory. In contrast, the animals treated with vehicle-vehicle, 3 mg/kg SR142801-vehicle, 6 mg/kg SR142801-vehicle, and 6 mg/kg SR142801-senktide could not distinguish between a spatial stationary and spatial displaced object within the test trial (P > 0.05).
Fig. 1.
The neurokinin3-receptor agonist, senktide, improves object-place and water-maze learning in aged rats. Object-place recognition and water-maze learning of aged rats treated with vehicle + vehicle (VE +VE), 3 mg/kg SR142801+vehicle (3 mg SR + VE), 6 mg/kg SR142801 + vehicle (6 mg SR + VE), vehicle + 0.2 mg/kg senktide (VE + Senk), 3 mg/kg SR142801 + 0.2 mg/kg senktide (3 mg SR + Senk), or 6 mg/kg SR142801+0.2 mg/kg senktide (6 mg SR + Senk). (A) Object-place recognition design. The schematic picture shows possible object locations in the object-place recognition task. The animals received two trials with a 5-min duration and an intertrial interval (ITI) of 25 min. During the test trial, two objects known from the sample trial were presented, one in a novel position (A1, spatial displaced) and one in the same position (A2, spatial stationary) relative to the sample trials. (B) Object-place recognition. The data are represented as means plus SE of object-exploration time (seconds) of spatial stationary and spatial displaced objects in the test trial (#P < 0.01). (C) Water maze: mean escape latencies onto a hidden platform over 12 trials of water-maze acquisition of aged rats (#P < 0.01; $P < 0.001 vs. VE + VE).
In the water-maze task repeated posttrial administration of the NK3-R agonist senktide also significantly improved learning in the aged rats. This effect was blocked by the NK3-R antagonist SR142801 (Fig. 1C). The two-way repeated measure analysis of variance (ANOVA) revealed a main effect of trials (F11,484 = 18.519, P < 0.001), group (F5,44 = 60.598, P < 0.001), and a trial × group interaction (F55,484 = 2.128, P < 0.001). Post hoc Dunnett tests revealed significant improvements in learning in the animals treated with vehicle-senktide (P < 0.001) and 3 mg/kg SR142801-senktide (P < 0.001) compared with those given vehicle-vehicle (see SI Materials and Methods and SI Results for more statistical details). The treatment had no effect on swim velocity (Fig. S2). There was also no significant difference between groups in the probe trial, indicating no differences between groups to remember the location of the platform and also no significant differences between groups in the cued trial, suggesting no effects of the treatment on perception and locomotor activity.
NK3-Receptor Agonism Reduces NK3-Receptor mRNA Expression in the Hippocampus of Aged Rats.
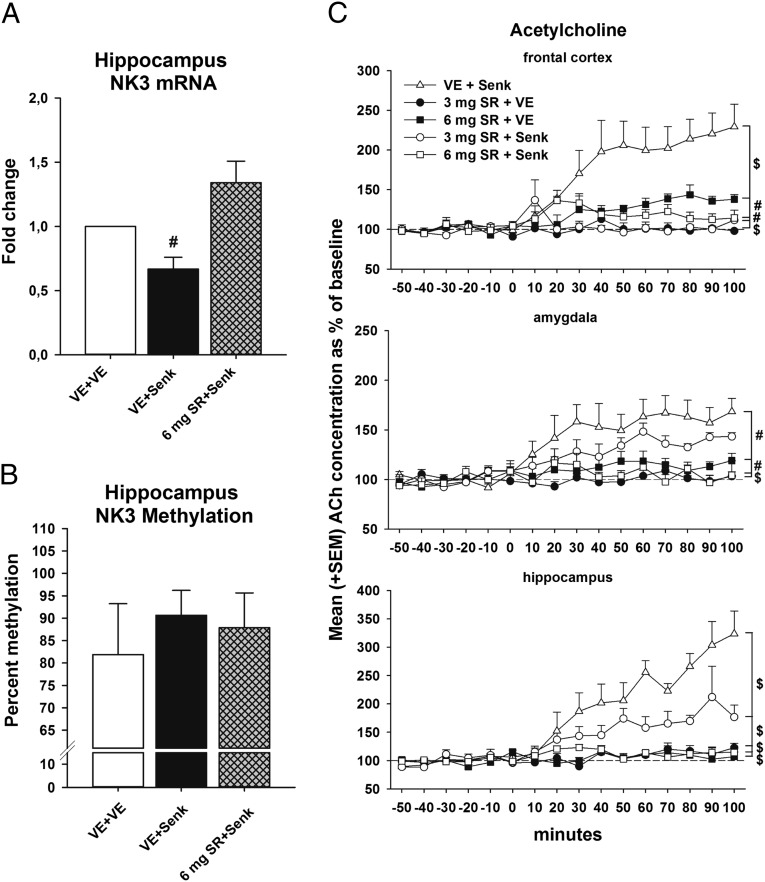
Repeated treatment with the NK3-R agonist senktide reduced NK3-R mRNA expression in the hippocampus of the aged rats (Student t test, t = 3.63, P = 0.0054). This effect was blocked by pretreatment with the NK3-R antagonist SR142801 (Fig. 2A). The agonist alone did not influence NK3-R DNA promoter methylation in the hippocampus (Fig. 2B; P > 0.05).
Fig. 2.
The neurokinin3-receptor agonist senktide increases extracellular acetylcholine levels in the brain. (A) Neurokinin3-receptor mRNA expression in the hippocampus. (B) TACR3 promoter methylation in the hippocampus. (C) Extracellular acetylcholine levels in the frontal cortex, amygdala, and hippocampus as measured by in vivo microdialysis in anesthetized animals (n = 5–6/group). The values are presented as the percentage of baseline with the six baseline samples taken as 100% (mean + SEM). Vehicle + 0.2 mg/kg senktide (VE + Senk), 3 mg/kg SR142801 + vehicle (3 mg SR + VE), 6 mg/kg SR142801 + vehicle (6 mg SR + VE), 3 mg/kg SR142801 + 0.2 mg/kg senktide (3 mg SR + Senk), or 6 mg/kg SR142801 + 0.2 mg/kg senktide (6 mg SR + Senk). #P < 0.01; $P < 0.001. [In a previous microdialysis study with aged animals, we showed that systemic vehicle treatment alone had no effect on ACh neurotransmission in the frontal cortex, amygdala, and hippocampus (14).]
NK3-Receptor Agonism Enhances Extracellular Acetylcholine Levels in Aged Rats.
A systemic injection of the NK3-R agonist senktide led to a lasting increase in levels of extracellular acetylcholine (ACh) in the hippocampus, the FC, and the amygdala of aged rats as assessed by in vivo microdialysis. Whereas in the FC and hippocampus both doses of SR142801 blocked the effects of senktide, in the amygdala only the highest dose of SR142801 was effective (Fig. 2C; for more statistical details see SI Materials and Methods and SI Results).
NK3-Receptor Agonist Effects on ACh Are Most Pronounced in Superior Aged Learners.
We next asked whether the neurochemical effects of NK3-R agonism on ACh depend on the preserved learning capacity in the aged rat and compared the effectiveness of NK3-R agonist treatment in aged superior learners vs. inferior learners. We thus screened animals for object-place recognition memory (Fig. 3A) and water-maze learning (Fig. 3B). This procedure identified n = 12 superior and n = 8 inferior learners with identical assignment of the animals to the inferior vs. superior learner groups in both tasks. In vivo microdialysis showed that there was no difference in basal extracellular ACh levels in the FC, hippocampus, or amygdala between aged superior and inferior learners (P > 0.05, Table S2). The NK3-R agonist senktide increased extracellular ACh levels predominantly in the superior learners in all three brain areas (Fig. 3C; for more statistical details see SI Materials and Methods and SI Results). mRNA analysis also showed no differences in NK3-R mRNA expression in the three brain areas analyzed (P > 0.05; Fig. S3).
Fig. 3.
Aged superior learners show a more pronounced acetylcholine response to neurokinin3-receptor agonist treatment than aged inferior learners. Aged rats were divided into groups of superior and inferior learners according to their performance in (A) object-place recognition and (B) water-maze escape learning. (C) Extracellular acetylcholine levels in the frontal cortex, amygdala, and the hippocampus as measured by in vivo microdialysis in anesthetized animals after treatment with the neurokinin3-receptor agonist senktide (0.2 mg/kg, s.c.). The values are presented as the percentage of baseline with the six baseline samples taken as 100% (mean + SEM). *P < 0.05; #P < 0.01; $P < 0.001.
TACR3 Polymorphism Predicts Learning in Elderly Patients.
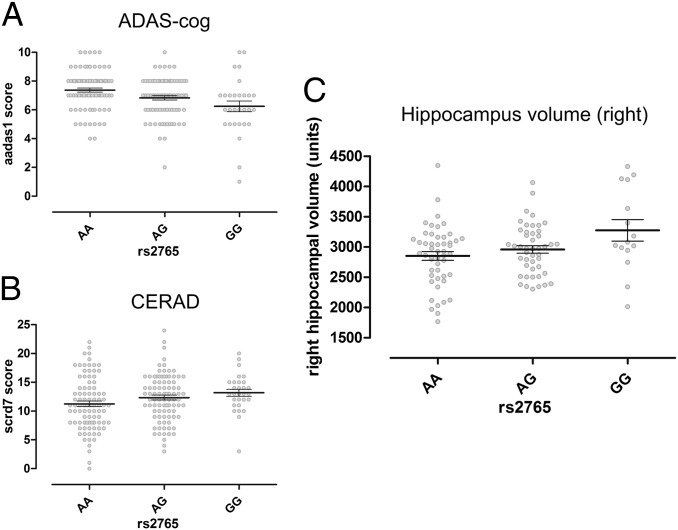
Of the 15 SNPs analyzed from the TACR3 gene (Fig. S4), rs2765 and rs11722288 (linkage disequilibrium, R2 = 0.7) were significantly associated with the Alzheimer’s Disease Assessment Scale–cognitive subscale (ADAS-cog) “word-list learning” task (n = 198; rs2765: Spearman’s Rho = −0.251, P = 0.0004, pcorr < 0.05; rs11722288: Spearman’s Rho = −0.223, P = 0.0016, pcorr < 0.05; Table S3). The subsequent Kruskal–Wallis tests showed gene dose–response effects between the genotype carriers [rs2765: n(AA) = 86, n(AG) = 83, n(GG) = 29, χ2 = 12.6, P = 0.0018 (Fig. 4A); rs11722288: n(GG) = 105, n(GA) = 75, n(AA) = 18, χ2 = 10.1, P = 0.0063]. Age, sex, and mild cognitive impairment vs. dementia status did not differ significantly among the rs2765 and the rs11722288 genotypes (P > 0.05) and thus were considered not to be major confounders. To confirm these findings, we further investigated the relationship of the two SNPs with word-list learning of the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) neuropsychology assessment. The Pearson correlation confirmed a significant association only between rs2765 and word-list learning (n = 209; r = 0.165, P = 0.0169; Fig. 4B). Both tests showed an impaired performance when age-matched reference values were considered. As expected, the scores of both immediate recall tests correlated inversely with each other (n = 198, Rho = −0.527, P < 0.0001). The allele-specific analyses further corroborated the association between rs2765 and neuropsychological test results. Compared with the G-allele, the A-allele was related to a significantly higher ADAS-cog score (Mann–Whitney U test, U = 14,028, P = 0.0002) and a significantly lower CERAD score (Student t-test, t = −2.6, P = 0.0099).
Fig. 4.
Significant associations of the TACR3 polymorphism, rs2765, with verbal memory and right hippocampal volume in aged humans (mean ± SEM and entire distribution). Word-list learning in (A) the ADAS-cog and (B) CERAD tests. (C) Right hippocampus volume. The AA homozygotes showed the worst performance in verbal memory as indicated by high scores in the ADAS-cog and low scores in the CERAD. They also had the smallest right hippocampal volumes.
TACR3 Polymorphism Predicts Right Hippocampal Volume in Elderly Patients.
Working memory performance is correlated with hippocampus volume in healthy volunteers (18) and psychiatric patients (19, 20). We used structural magnetic resonance imaging in a subsample of 111 individuals and found an association of rs2765 with right hippocampal volume [genotype analysis (n(AA) = 50, n(AG) = 46, n(GG) = 15: Pearson correlation, r = 0.252, P = 0.0075, ANOVA, F2,108 = 4.2, P = 0.0170 (Fig. 4C); allelic analysis (A/G): Student t test, t = −2.8, P = 0.0056]. There was no significant association with left hippocampal volume. The hierarchical linear regression analyses revealed that the rs2675 SNP explained 1.6% of the behavioral variance in the CERAD word-list learning task, 1% of which was explained by right hippocampal volume. This suggests that about 62.5% (1%/1.6%) of the association between rs2765 and word-list learning (CERAD) can be explained by variation in the volume of the right hippocampus. Furthermore, about 22% (2.2%/10.2%) of the association between rs2765 and word-list learning (ADAS-cog) can be accounted for by right hippocampal volume. Altogether these data indicate that variance in the NK3-R–coding gene TACR3 is associated with reduced right hippocampal volume and learning performance in elderly patients with a broad range of cognitive impairments.
Discussion
We show that pharmacological stimulation of NK3-R can significantly enhance learning and memory in aged animals. These effects are most likely mediated by an increase of ACh levels in the major cholinergic projection target areas of the basal forebrain, together with a down-regulation of NK3-R in the hippocampus. We also show that cholinergic effects of NK3-R stimulation are most pronounced in those aged animals with an above-average learning capacity. In aged humans with cognitive impairments, we found that the rs2765 SNP in the NK3-R–coding gene TACR3 correlated with performance in learning and memory tests along with reduced right hippocampal volume.
In animal models, diverse age-dependent impairments have been reported in learning and memory tasks, such as the Morris water maze (21, 22) and object recognition test (23, 24). Aged rats exhibit deficits in water-maze acquisition as well as in discrimination between spatial stationary and spatial displaced objects compared with adult animals (SI Materials and Methods and SI Results). Our data show that the poor learning and memory performance of aged animals can be significantly enhanced by posttrial treatment with the NK3-R agonist senktide. The injection of senktide promoted memory for object location in the object-place recognition task and for the location of a hidden platform in the Morris water-maze test when given immediately after the sample trial and after each acquisition trial, respectively. This suggests that senktide was effective during the period after the learning trial when labile memory traces are liable to be facilitated or disrupted (25). This effect was completely blocked by pretreatment with an NK3-R antagonist. The NK3-R antagonist itself did not affect learning and memory in either paradigm. These results suggest a use of NK3-R agonists as a chronic treatment to improve learning and memory performance in aged organisms that have already developed cognitive impairments.
We found that NK3-R agonism also enhances ACh activity in brain regions relevant to learning and memory. This effect was NK3-R specific because it was completely blocked by an NK3-R antagonist. NK3 receptors are located on cholinergic neurons (11, 12), and the activity of the cholinergic system can be stimulated by NK3-R agonism in adult animals (14). Although one cannot rule out that the increase in cholinergic activity is due to an indirect activation of cholinergic cells of the basal forebrain, it is possible that such an effect is the consequence of an excitatory activation of these cells by a direct action on NK3-Rs located on cell bodies of cholinergic cells of the basal forebrain. We found that a subchronic treatment with the NK3-R agonist, as it was done during the learning tests, also decreased NK3-R mRNA expression in the hippocampus. This adaptive effect seems to be directly linked to the activation of NK3-Rs because such activation was prevented by NK3-R antagonist pretreatment. Transcriptional activity is controlled by the methylation of the promoter DNA of the NK3-R–coding gene. However, we did not find evidence for a change in TACR3 promoter methylation, which suggests an alternative regulatory mechanism.
Previous findings reported the facilitation of age-associated learning deficits by the NK1-R ligand substance P (SP) (26). Substance P was also found to increase ACh neurotransmission (27, 28). However, it is possible that the memory-facilitating effect of SP is not solely modulated by NK1-Rs on cholinergic neurons, but also by NK3-Rs, because SP, although with highest affinity to NK1-Rs, can also bind to NK3-Rs (9, 29). Our current finding of memory facilitation via NK3-R activation by senktide and the consequential increase of ACh release might also account for previously described memory-promoting effects of SP in aged animals (26).
The pharmacological inhibition of the metabolizing enzyme acetylcholine esterase (AChE) was shown to enhance ACh levels and to improve learning and memory in the aged organism. However, AChE inhibitors can have profound side effects due to abundant AChE activity in the periphery and in the autonomous nervous system (30). NK3-R agonists have a low impact on ACh-mediated peripheral mechanisms (31) and therefore may offer a way to improve brain ACh activity with a reduced risk for side effects. A recent study in nonhuman primates did not find adverse behavioral effects after acute or subchronic i.p. senktide treatment, but a sensitization for the arousal-enhancing effects of cocaine (32). Subchronic treatment in adult rats had neither aversive nor appetitive effects on its own and did not impair the action of other reinforcers (33). In the present study we observed neither hyperlocomotion nor other behavioral effects that had been reported after, e.g., i.c.v. application in gerbils (34).
Aged humans and animals show considerable individual differences in performance in cognitive tasks (21, 22, 26, 27). Age-associated differences in sensitivity to NK3-R agonism most likely reflect differences in cholinergic degeneration (35). The cholinergic projection neurons in the basal forebrain region are reported to undergo significant atrophy in the aged rodent. The degree of atrophy is highly correlated with the cognitive impairment exhibited in the Morris water-maze task (36). However, we did not see differences in basal ACh activity or NK3-R mRNA expression. We found that the NK3-R agonist was most effective in enhancing cholinergic activity in the basal forebrain projection areas in the superior learners. The aging-associated loss of cholinergic neurons can be prevented by the endogenous NK3-R agonist NK-B (37). NK-B stimulates AChE activity in the aged brain (38). Therefore, an attenuated increase in ACh level upon senktide administration in the inferior learners may have at least partially resulted from additional AChE activation. Overall, the present data suggest that superior learning in aged animals is not due to higher basal ACh activity but rather due to its enhanced sensitivity for activation. As such, NK3-R agonists may also serve to prevent aging-associated decline in cholinergic function. However, although adult rats and nonhuman primates appear to tolerate NK3-R agonists well after subchronic treatment (32, 33), little is known about tolerance in the aged organism. Recent animal studies suggest that the use of NK3-R agonists may be limited in Parkinsons’s disease (39).
Although natural genetic variance can predict learning and memory performance in adults (18), little is known about performance in the aged organism. In the present study, we investigated the genetic association of 15 SNPs in the human NK3-R–coding gene TACR3 with performance in tests of verbal learning. In a human sample of 209 patients who all exhibited cognitive impairments, we found that the r2765 SNP in the TACR3 gene predicted the degree of learning and memory impairment in two neuropsychological tests of word-list learning. Mutations in the human TACR3 gene have been shown to play an important role in puberty development and are associated with hypogonadotrophic hypogonadism (40). An association with cognitive performance has also been suggested by animal studies using NK3-R knockout mice (15). The TACR3 polymorphism was related to encoding of new information rather than to retrieval of previously consolidated information. However, one limitation of this study is that no retrieval from long-term memory could be tested. In that, the latter possibility cannot be ruled out.
We also discovered a significant association of rs2765 with right hippocampal volume. Parts of the association of rs2765 with learning and memory performance were thereby explained by the volume of the right hippocampus. An association of word-list learning with hippocampus volume was reported previously in multiple sclerosis patients (19). Also, patients with Alzheimer’s disease show a pronounced volume loss of the hippocampus and associated areas compared with patients with mild cognitive impairments (20). However, from our data we cannot rule out an association of TACR3 polymorphisms with learning and memory in healthy aged subjects. Altogether, current data may suggest that the rs2765 polymorphism of the TACR3 gene may provide some protection from a decline in hippocampal volume and associated loss of verbal learning capacity when dementia develops at old age.
In summary, our combined approach provides evidence for the NK3-R as a target to predict and improve learning and memory performance in aged humans and rodents. Our study identifies cholinergic mechanisms as the most likely mechanisms of action, with aged superior learners benefiting in their ACh stimulation the most from NK3-R agonism. Approaches to the use of natural genetic variance as a predictor for aging-related changes in brain morphology and for the decline of learning and memory performance may support the diagnosis of dementia, and NK3-R agonism with suitable substances may provide a principle to treat learning and memory impairments in the aged.
Materials and Methods
For a detailed methodological description, see SI Materials and Methods.
Subjects and Drugs.
Eighty-two aged male Wistar rats (weight 350–750 g), 17–21 mo of age, were used for the behavioral tests and 30 animals for the in vivo microdialysis experiment. Experiments were carried out during the animals’ active period. The studies were performed in accordance with the German Animal Protection Law which was approved by the Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen.
The NK3-R agonist senktide ([succinyl-Asp6-Me-Phe8]SP6–11; Bachem) and the NK3-R antagonist SR142801 ((R)-(N)-[1-[3-[1-benzoyl-3-(3,4-dichlorophenyl) piperidin-3-yl]propyl]-4-phenylpiperidin-4-yl]-N-methylacetamide; Axon Medchem BV) were used.
Effects of NK3-R Agonism on Learning and Memory, NK3-R mRNA Expression, and Promoter Methylation.
For behavioral testing, 50 aged Wistar rats were randomly assigned to one of the following groups: vehicle + vehicle (n = 8), vehicle + senktide (n = 9), 3 mg/kg SR142801 + vehicle (n = 8), 6 mg/kg SR142801 + vehicle (n = 8), 3 mg/kg SR142801 + senktide (n = 9), or 6 mg/kg SR142801 + senktide (n = 8). The animals were tested in the object-place recognition and the Morris water-maze tasks (Fig. 1A). The treatment was given after the sample trial in the case of the object-place recognition test and shortly after each acquisition trial for the Morris water-maze task. Treatment consisted of systemic administration of SR142801 (3 mg or 6 mg/kg) or its vehicle (i.p.) followed 1 min later by an injection of senktide (0.2 mg/kg) or its vehicle (s.c.). Intact object-place recognition memory was defined as increased exploration time of the spatial displaced compared with the spatial stationary object (41, 42). For the water-maze test, the acquisition phase was followed by a probe trial without a platform. Twenty-four hours later, the rats were tested in one trial in the cued platform version (43). Two weeks after the last maze test, the animals were killed and the hippocampus was dissected out. For NK3-R mRNA expression, the total RNA was extracted using a modified Qiagen protocol as described previously (44). Analysis of NK3-R promoter DNA methylation was performed via a methylation-sensitive digestion assay followed by real-time PCR as previously described (45) (for details, see SI Materials and Methods).
Effects of NK3-R Agonism on Basal Forebrain Cholinergic Activity.
For the in vivo microdialysis, experimentally naive aged Wistar rats were randomly assigned to one of the following groups: vehicle + senktide (n = 5), 3 mg/kg SR142801 + vehicle (n = 5), 6 mg/kg SR142801 + vehicle (n = 6), 3 mg/kg SR142801 + senktide (n = 5) or 6 mg/kg SR142801 + senktide (n = 6). Samples were collected from the FC, amygdala, and hippocampus and analyzed for their content of ACh using high-performance liquid chromatography with electrochemical detection (HPLC-EC) as described in previous studies (14, 28).
Effects of NK3-R Agonism on Cholinergic Activity in Superior and Inferior Aged Learners.
We tested 20 naive rats for their learning and memory performance in the object-place recognition and Morris water maze, but without any treatment. The rats were then divided into two subgroups, superior (n = 12) and inferior (n = 8) learners, on the basis of their performance in the object-place recognition test and water-maze escape task. The criterion for superior and inferior performance in the water-maze task was specified a priori and was based on the animal’s performance in our previous water-maze studies (14, 16). Next, the in vivo microdialysis was conducted with superior vs. inferior learners as independent groups. Each animal received a 0.2-mg/kg senktide (s.c.) treatment.
NK3-R mRNA Expression in Superior and Inferior Aged Learners.
We measured NK3-R mRNA expression in the brain of superior and inferior learners. A new group of 12 aged animals was tested for object recognition learning (46). According to their performance, we identified six superior and six inferior learners. The day after behavioral testing, these animals were killed, and NK3-R mRNA expression was investigated in the FC, the hippocampus, and the adjacent perirhinal cortex.
Genetic Association Analysis in Patients with Cognitive Impairments.
Performance in verbal learning was measured by the item word-list learning of two neuropsychological tests (aadas1, scrd7) in a sample of 209 elderly patients (50–90 y old, n = 91 males, n = 118 females), diagnosed with mild cognitive impairment or dementia (n = 59 mild cognitive impairment, n = 150 dementia) (47, 48). SNPs of the NK3-R coding gene TACR3 were genotyped using the Illumina Omni-1M-Quad array. In 111 of those participants, hippocampus volume was determined from high-resolution structural magnetic resonance images using the FMRIB’s Integrated Registration and Segmentation Tool (FIRST) (49) from the FMRIB Software Library (FSL) package of tools (50).
Statistical Analysis.
Data were analyzed by one- or two-way ANOVA with appropriate post hoc tests. For not normally distributed data, nonparametric methods (Spearman correlation, Mann–Whitney U-test, Kruskal–Wallis test) were used. The P values were considered significant if α ≤ 0.05. P values were Bonferroni-corrected for multiple testing. IBM SPSS Statistic 20.0 was used for all analyses (for more details, see SI Materials and Methods).
Supplementary Material
Acknowledgments
This work was supported by grants from the German National Science Foundation (Deutsche Forschungsgemeinschaft: DFG DE 792/2-4; Research Training Group GK1033-2) and funds from the Friedrich-Alexander-University of Erlangen-Nuremberg. This study used data of the German Dementia Competence Network, which was funded by Grant O1GI 0102 from the German Federal Ministry for Education and Research (Bundesministerium für Bildung und Forschung).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1306884110/-/DCSupplemental.
References
- 1.Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38(1–2):61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- 2.Araujo DM, Lapchak PA, Meaney MJ, Collier B, Quirion R. Effects of aging on nicotinic and muscarinic autoreceptor function in the rat brain: Relationship to presynaptic cholinergic markers and binding sites. J Neurosci. 1990;10(9):3069–3078. doi: 10.1523/JNEUROSCI.10-09-03069.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sarter M, Bruno JP. Developmental origins of the age-related decline in cortical cholinergic function and associated cognitive abilities. Neurobiol Aging. 2004;25(9):1127–1139. doi: 10.1016/j.neurobiolaging.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 4.Fischer W, Gage FH, Björklund A. Degenerative changes in forebrain cholinergic nuclei correlate with cognitive impairments in aged rats. Eur J Neurosci. 1989;1(1):34–45. doi: 10.1111/j.1460-9568.1989.tb00772.x. [DOI] [PubMed] [Google Scholar]
- 5.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp Neurol. 2000;163(2):495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 6.De Jaeger X, et al. Decreased acetylcholine release delays the consolidation of object recognition memory. Behav Brain Res. 2013;238:62–68. doi: 10.1016/j.bbr.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Patel S, Tariot PN. Pharmacologic models of Alzheimer’s disease. Psychiatr Clin North Am. 1991;14(2):287–308. [PubMed] [Google Scholar]
- 8.VanDenBerg CM, Kazmi Y, Jann MW. Cholinesterase inhibitors for the treatment of Alzheimer’s disease in the elderly. Drugs Aging. 2000;16(2):123–138. doi: 10.2165/00002512-200016020-00004. [DOI] [PubMed] [Google Scholar]
- 9.Maggi CA. The mammalian tachykinin receptors. Gen Pharmacol. 1995;26(5):911–944. doi: 10.1016/0306-3623(94)00292-u. [DOI] [PubMed] [Google Scholar]
- 10.Ding YQ, et al. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. J Comp Neurol. 1996;364(2):290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 11.Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. J Comp Neurol. 1996;372(3):395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 12.Chen LW, et al. Cholinergic neurons expressing neuromedin K receptor (NK3) in the basal forebrain of the rat: A double immunofluorescence study. Neuroscience. 2001;103(2):413–422. doi: 10.1016/s0306-4522(00)00568-6. [DOI] [PubMed] [Google Scholar]
- 13.Morozova E, Wu M, Dumalska I, Alreja M. Neurokinins robustly activate the majority of septohippocampal cholinergic neurons. Eur J Neurosci. 2008;27(1):114–122. doi: 10.1111/j.1460-9568.2007.05993.x. [DOI] [PubMed] [Google Scholar]
- 14.Schäble S, et al. Neurokinin3-R agonism in aged rats has anxiolytic-, antidepressant-, and promnestic-like effects and stimulates ACh release in frontal cortex, amygdala and hippocampus. Eur Neuropsychopharmacol. 2011;21(6):484–494. doi: 10.1016/j.euroneuro.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 15.Siuciak JA, et al. Disruption of the neurokinin-3 receptor (NK3) in mice leads to cognitive deficits. Psychopharmacology (Berl) 2007;194(2):185–195. doi: 10.1007/s00213-007-0828-6. [DOI] [PubMed] [Google Scholar]
- 16.Schäble S, Huston JP, Barros M, Tomaz C, de Souza Silva MA. The NK3 receptor agonist senktide ameliorates scopolamine-induced deficits in memory for object, place and temporal order. Neurobiol Learn Mem. 2012;97(2):235–240. doi: 10.1016/j.nlm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Zlomuzica A, Dere E, Huston JP, de Souza Silva MA. NK(3) receptor agonism promotes episodic-like memory in mice. Neurobiol Learn Mem. 2008;90(2):420–425. doi: 10.1016/j.nlm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 18.Papassotiropoulos A, et al. A genome-wide survey and functional brain imaging study identify CTNNBL1 as a memory-related gene. Mol Psychiatry. 2013;18(2):255–263. doi: 10.1038/mp.2011.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fuentes A, et al. Memory performance and normalized regional brain volumes in patients with pediatric-onset multiple sclerosis. J Int Neuropsychol Soc. 2012;18(3):471–480. doi: 10.1017/S1355617711001913. [DOI] [PubMed] [Google Scholar]
- 20.Miettinen PS, et al. Structure and function of medial temporal and posteromedial cortices in early Alzheimer’s disease. Eur J Neurosci. 2011;34(2):320–330. doi: 10.1111/j.1460-9568.2011.07745.x. [DOI] [PubMed] [Google Scholar]
- 21.Schulz D, et al. Water maze performance, exploratory activity, inhibitory avoidance and hippocampal plasticity in aged superior and inferior learners. Eur J Neurosci. 2002;16(11):2175–2185. doi: 10.1046/j.1460-9568.2002.02282.x. [DOI] [PubMed] [Google Scholar]
- 22.Topic B, et al. Aged and adult rats compared in acquisition and extinction of escape from the water maze: Focus on individual differences. Behav Neurosci. 2005;119(1):127–144. doi: 10.1037/0735-7044.119.1.127. [DOI] [PubMed] [Google Scholar]
- 23.de Lima MN, et al. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp Gerontol. 2005;40(6):506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Kameyama T, Ukai M, Shinkai N. Ameliorative effects of tachykinins on scopolamine-induced impairment of spontaneous alternation performance in mice. Methods Find Exp Clin Pharmacol. 1998;20(7):555–560. doi: 10.1358/mf.1998.20.7.485718. [DOI] [PubMed] [Google Scholar]
- 25.McGaugh JL. Dissociating learning and performance: Drug and hormone enhancement of memory storage. Brain Res Bull. 1989;23(4–5):339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 26.Hasenöhrl RU, Huston JP, Schuurman T. Neuropeptide substance P improves water maze performance in aged rats. Psychopharmacology (Berl) 1990;101(1):23–26. doi: 10.1007/BF02253712. [DOI] [PubMed] [Google Scholar]
- 27.Steinberg R, et al. Expression and presence of septal neurokinin-2 receptors controlling hippocampal acetylcholine release during sensory stimulation in rat. Eur J Neurosci. 1998;10(7):2337–2345. doi: 10.1046/j.1460-9568.1998.00244.x. [DOI] [PubMed] [Google Scholar]
- 28.de Souza Silva MA, Hasenöhrl RU, Tomaz C, Schwarting RK, Huston JP. Differential modulation of frontal cortex acetylcholine by injection of substance P into the nucleus basalis magnocellularis region in the freely-moving vs. the anesthetized preparation. Synapse. 2000;38(3):243–253. doi: 10.1002/1098-2396(20001201)38:3<243::AID-SYN3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 29.Regoli D, Boudon A, Fauchére JL. Receptors and antagonists for substance P and related peptides. Pharmacol Rev. 1994;46(4):551–599. [PubMed] [Google Scholar]
- 30.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng FH, Chan SW, Rudd JA. Contractile effect of tachykinins on Suncus murinus (house musk shrew) isolated ileum. Neuropeptides. 2008;42(5–6):671–679. doi: 10.1016/j.npep.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Melamed JL, et al. Sensitization of hypervigilance effects of cocaine can be induced by NK3 receptor activation in marmoset monkeys. Drug Alcohol Depend. 2013;128(1–2):155–160. doi: 10.1016/j.drugalcdep.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 33.Jocham G, Lauber AC, Müller CP, Huston JP, de Souza Silva MA. Neurokinin 3 receptor activation potentiates the psychomotor and nucleus accumbens dopamine response to cocaine, but not its place conditioning effects. Eur J Neurosci. 2007;25(8):2457–2472. doi: 10.1111/j.1460-9568.2007.05491.x. [DOI] [PubMed] [Google Scholar]
- 34.Nordquist RE, Durkin S, Jacquet A, Spooren W. The tachykinin NK3 receptor agonist senktide induces locomotor activity in male Mongolian gerbils. Eur J Pharmacol. 2008;600(1–3):87–92. doi: 10.1016/j.ejphar.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 35.Gage FH, Chen KS, Buzsaki G, Armstrong D. Experimental approaches to age-related cognitive impairments. Neurobiol Aging. 1988;9(5–6):645–655. doi: 10.1016/s0197-4580(88)80129-5. [DOI] [PubMed] [Google Scholar]
- 36.Gallagher M, Nicolle MM. Animal models of normal aging: Relationship between cognitive decline and markers in hippocampal circuitry. Behav Brain Res. 1993;57(2):155–162. doi: 10.1016/0166-4328(93)90131-9. [DOI] [PubMed] [Google Scholar]
- 37.Wenk GL, Zajaczkowski W, Danysz W. Neuroprotection of acetylcholinergic basal forebrain neurons by memantine and neurokinin B. Behav Brain Res. 1997;83(1–2):129–133. doi: 10.1016/s0166-4328(97)86056-1. [DOI] [PubMed] [Google Scholar]
- 38.Mantha AK, Moorthy K, Cowsik SM, Baquer NZ. Membrane associated functions of neurokinin B (NKB) on Abeta (25-35) induced toxicity in aging rat brain synaptosomes. Biogerontology. 2006;7(1):19–33. doi: 10.1007/s10522-005-6044-z. [DOI] [PubMed] [Google Scholar]
- 39.Chu JM, Chan YS, Chen LW, Yung KK. Neurokinin receptor 3 peptide exacerbates 6-hydroxydopamine-induced dopaminergic degeneration in rats through JNK pathway. J Neurochem. 2012;123(3):417–427. doi: 10.1111/j.1471-4159.2012.07858.x. [DOI] [PubMed] [Google Scholar]
- 40.Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: Closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. doi: 10.1210/en.2011-1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ennaceur A, Neave N, Aggleton JP. Spontaneous object recognition and object location memory in rats: The effects of lesions in the cingulate cortices, the medial prefrontal cortex, the cingulum bundle and the fornix. Exp Brain Res. 1997;113(3):509–519. doi: 10.1007/pl00005603. [DOI] [PubMed] [Google Scholar]
- 42.Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neurosci Biobehav Rev. 2007;31(5):673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 44.Rotter A, et al. Glucocorticoid receptor antagonism blocks ethanol-induced place preference learning in mice and attenuates dopamine D2 receptor adaptation in the frontal cortex. Brain Res Bull. 2012;88(5):519–524. doi: 10.1016/j.brainresbull.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Bleich S, et al. Epigenetic DNA hypermethylation of the HERP gene promoter induces down-regulation of its mRNA expression in patients with alcohol dependence. Alcohol Clin Exp Res. 2006;30(4):587–591. doi: 10.1111/j.1530-0277.2006.00068.x. [DOI] [PubMed] [Google Scholar]
- 46.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 47.Kornhuber J, et al. Early and differential diagnosis of dementia and mild cognitive impairment: Design and cohort baseline characteristics of the German Dementia Competence Network. Dement Geriatr Cogn Disord. 2009;27(5):404–417. doi: 10.1159/000210388. [DOI] [PubMed] [Google Scholar]
- 48.Jessen F, et al. A multicenter (1)H-MRS study of the medial temporal lobe in AD and MCI. Neurology. 2009;72(20):1735–1740. doi: 10.1212/WNL.0b013e3181a60a20. [DOI] [PubMed] [Google Scholar]
- 49.Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–922. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SM, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.