Significance
Species display a range of plastic phenotypes that presumably have evolved as a result of adaptation to heterogeneous environments. We asked whether the genetic mechanisms that underlie adaptation across populations also determine the response of an individual plant to environmental cues in Arabidopsis. Using an integrative root phenotyping approach, genes that underlie natural variation in root architecture across populations were shown to control plasticity responses within an individual. Together, our results uncover a genetic mechanism underlying the phenotypic plasticity of an individual and phenotypic diversity across natural variants.
Keywords: GWAS, morphometrics, GxE interaction, QTL, RootScape
Abstract
Phenotypic plasticity is presumed to be involved in adaptive change toward species diversification. We thus examined how candidate genes underlying natural variation across populations might also mediate plasticity within an individual. Our implementation of an integrative “plasticity space” approach revealed that the root plasticity of a single Arabidopsis accession exposed to distinct environments broadly recapitulates the natural variation “space.” Genome-wide association mapping identified the known gene PHOSPHATE 1 (PHO1) and other genes such as Root System Architecture 1 (RSA1) associated with differences in root allometry, a highly plastic trait capturing the distribution of lateral roots along the primary axis. The response of mutants in the Columbia-0 background suggests their involvement in signaling key modulators of root development including auxin, abscisic acid, and nitrate. Moreover, genotype-by-environment interactions for the PHO1 and RSA1 genes in Columbia-0 phenocopy the root allometry of other natural variants. This finding supports a role for plasticity responses in phenotypic evolution in natural environments.
A long-standing debate in evolutionary biology is the relevance of phenotypic plasticity as a mechanism leading to species diversity (1). It has been argued that selection on plasticity responses to environment pressures could underlie fixed phenotypic changes between natural variants (2), providing a potentially rapid mechanism of evolutionary change (3). Thus, we tested the hypothesis that genes that enable a functional response to the environment within a population also underlie adaptive changes across natural variants. Arabidopsis thaliana offers ample opportunity to study genes involved in phenotypic plasticity in response to experimental laboratory perturbations, whereas its natural variants offer the opportunity to study the genetic basis for developmental variation observed in nature. We took a unique approach to integrating results from these two perspectives to uncover the molecular basis underlying individual plasticity and variation among natural variants. We focused on the root architectural system because it shows a high degree of plasticity under diverse environmental conditions (4–9). We used a quantitative phenotyping model to capture and integrate plastic changes in root system architecture in response to a range of experimental treatments within the laboratory reference accession Columbia-0 (Col-0). We next cross-referenced this plasticity space derived for an individual accession (Col-0) to the range of phenotypic differences in root architecture observed across Arabidopsis accessions that represent the extent of natural variation under one condition. This allowed us to identify candidate genes underlying root systems architecture using genome-wide association mapping and to test their role in individual plasticity using mutants.
Results
Integrative Characterization of Phenotypic Plasticity and Natural Variation in Root Architecture.
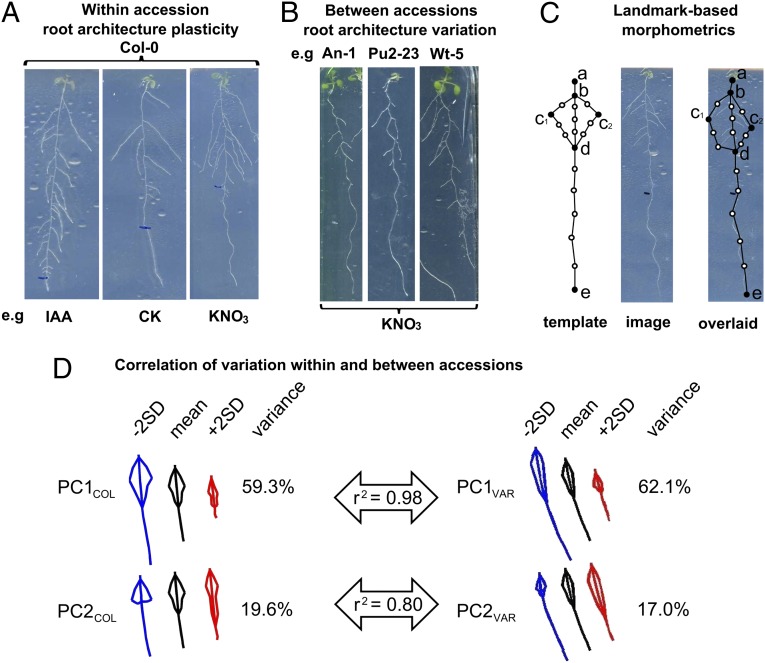
To characterize the root plasticity “space” within an accession, we grew the A. thaliana laboratory strain Col-0 under a set of treatments using nutrients and hormones known to mediate different aspects of root development. This uncovered a range of plasticity within a single genotype across multiple treatments: control (KCl), auxin [Indol acetic acid (IAA)], cytokinin (CK), abscisic acid (ABA), nitrate (KNO3), and ammonium chloride (NH4Cl) (10) (Fig. 1A). Next, we characterized natural variation of root system architecture in 69 genotyped Arabidopsis accessions (12) grown in a single environment (KNO3) under which they exhibit a large breadth of natural phenotypic root variation (Fig. 1B). Root systems architecture was quantified holistically with a method (RootScape) that uses landmark-based morphometrics and has the advantage of being blind to the relevance of conventional morphological characters (11, 13). This method uses a 20-point landmark template that consists of a set of reference points fixed to developmental landmarks such as the base of the primary root and to points that capture plasticity to a greater degree, such as the end of the primary root, the widest lateral roots, and the end of the lateral roots. Intermediate points are added evenly between the fixed landmarks. In this way the template captures the main features of root architecture as an integrated system (11) (Fig. 1C).
Fig. 1.
Root architecture plasticity within an accession (Col-0) broadly recapitulates natural variation quantified across 69 accessions. (A) Root phenotypes of Col-0 plants were grown under five conditions: IAA, CK, ABA, KNO3, and NH4Cl plus a control (KCl); roots of three treatment conditions are shown. (B) Root variation between 69 accessions grown under one condition (KNO3). (C) Landmark template to capture the root system architecture (11). Primary landmarks (black circles) are defined according to corresponding features in all roots; secondary landmarks (white circles) are evenly spaced between primary landmarks (11). (D) Two PCs capture more than 75% of the variation both within Col-0 (PCCOL) and between accessions (PCVAR). PC1COL and PC2COL have high correlation to PC1VAR and PC2VAR, respectively. PC1COL is mainly a size effect, whereas PC2COL captures mainly root allometry, the length and distribution of lateral roots long the primary root. SD, standard deviation.
To create a framework to compare natural variation and plasticity responses, we used data from the landmark-based morphological root models and ran Principal Component Analyses (PCA). The resulting PCs uncovered the main trends of phenotypic plasticity in roots of the laboratory reference accession Col-0 (PCACOL) and among the A. thaliana natural VARiants (PCAVAR). In both root models (PCCOL and PCVAR), PC1 and PC2 covered more than 75% of the variation in root systems architecture (Fig. 1D). PC1COL captures a size effect; PC2COL is an orthogonal axis to PC1COL and therefore captures a type of variation unrelated to size. Thus, PC2COL seems to capture the proportion of the primary root that contains visible lateral roots, in other words, the distribution of lateral roots in the proximal–distal axis regardless or root size (Fig. 1D). Similarly, a PCA of the VAR space across 69 natural Arabidopsis accessions captures a size-effect trend of the variation (PC1VAR) and the lateral root allometry effect (PC2VAR). Overall, the PC analyses suggest that the variation captured within an individual accession (PCCOL) is similar to the range of variation observed in natural accessions (PCVAR) under our growth conditions (Fig. 1D). This suggests a shared genetic mechanism controlling both phenotypic plasticity and natural variation, which would imply an important role of plasticity in adaptive change.
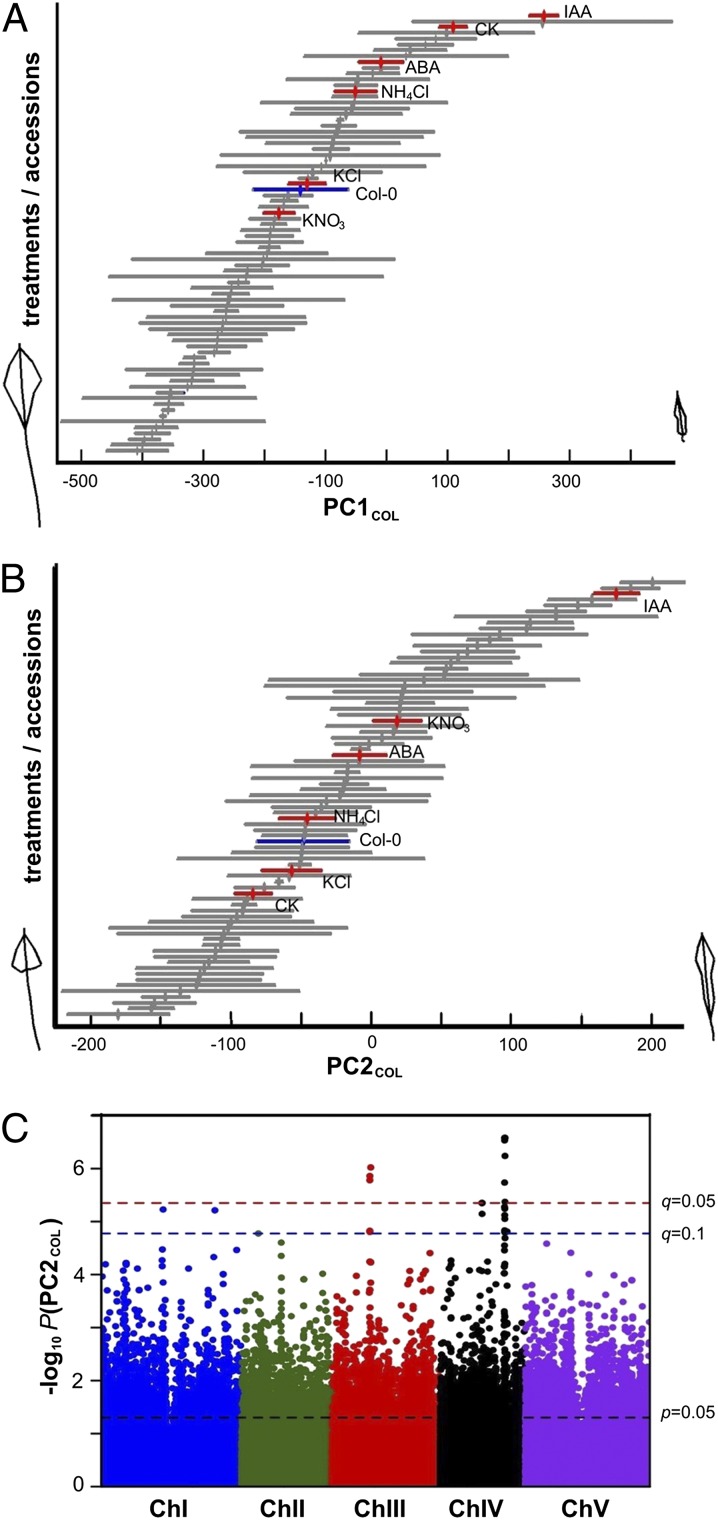
The similarity of variation in root systems architecture observed between the plasticity space of Col-0 and across the natural accessions—(PC1COL ∼ PC1VAR) and (PC2COL ∼ PC2VAR)—allowed us to use these PC axes as phenotypic metrics to perform quantitative comparisons. We projected the PC values for both datasets COL and VAR into the PC1COL and PC2COL axes (reference plasticity space) (Fig. 2). Surprisingly, the laboratory reference accession Col-0 (Fig. 2 A and B, blue bar), which has been historically selected for other purposes, localized roughly to the middle of the phenotypic axes of both PCs, corresponding in root phenotype to a “mean accession” relative to the other natural variants (Fig. 2 A and B, gray and blue bars). However, the treatments (Fig. 2 A and B, red bars) expanded the phenotypic breadth of Col-0, pushing its plasticity mainly toward the higher ends of the PCCOL axes, relative to the mean. This distribution suggests underlying developmental constraints for the plasticity to explore the lower end of the PCCOL space. We tested this using other Arabidopsis accessions and found that their plasticity is mostly directed toward the higher end of the PC axis (i.e., PC2COL) (Fig. S1). PC2COL captures the distribution of the lateral roots on the primary roots. The lower end shows a narrower distribution on a range of accessions (i.e., Tamm-27) and Col-0 plasticity phenotypes (i.e., CK). The absence of phenotypes with lower PC2COL might reflect a bias of our selected treatments.
Fig. 2.
Root plasticity variation within an accession (Col-0) spans a range of plasticity exhibited by Arabidopsis natural variants and maps to regions in chromosome III and IV. (A and B) Bars indicate SE. Red bars, Col-0 in the five treatments IAA, CK, ABA, KNO3, and NH4Cl and a KCl control (n = 20); gray bars, phenotypes of 69 Arabidopsis accessions grown under a single (KNO3) condition (n = 3–4); blue bars, reference Col-0 accession. Morphometrics modeled root systems architecture phenotypes; those corresponding to extreme root PC1COL and PC2COL phenotypes are shown. (C) Manhattan plot illustrating the GWAS mapping of the PC2COL phenotype.
The projection onto the PC1COL and PC2COL phenotypic spaces (Fig. 2 A and B) also allowed us to establish that the distribution of Col-0 phenotypes resulting from experimental treatments (Fig. 2 A and B, red bars) covers ∼50% of the distribution of phenotypes exhibited by the natural accessions along PC1COL and ∼65% of the distribution in PC2COL (Fig. 2 A and B, gray and blue bars). We performed the reciprocal analysis and projected the same datasets into the PC1VAR and PC2VAR axes (“natural variation space”) obtaining similar results (Fig. S2). A pair-wise comparison of the phenotype projections showed that PC1COL and PC2COL are highly correlated to PC1VAR and PC2VAR, respectively (Fig. 1D). Thus, the plasticity space of individual Arabidopsis accessions recapitulates natural variation in root architecture when exposed to distinct laboratory-induced physiological environments.
Identification of Candidate Genes Underlying Natural Variation in Root Architecture.
The projections of the PC values from the natural variants into the PC1COL and PC2COL phenotypic space were next used to map genes underlying both natural variation and phenotypic plasticity. To do this, we looked for associations between the PC1COL or PC2COL phenotypes exhibited in the natural accessions and the 214,000 SNP dataset of polymorphisms in Arabidopsis (14). No significant genome-wide association study (GWAS) associations were identified for the PC1COL trait (overall root size), suggesting that size might be controlled by many loci of small effect. However, the PC2COL trait (allometry of lateral roots) revealed 12 significant GWAS associations at the false-discovery rate (FDR) level of q < 0.05 or 19 associations at q < 0.1. Most of these SNPs mapped to two genomic regions in chromosomes III and IV (Fig. 2C), partially overlapping regions with previously identified root morphology quantitative trait loci (QTL) (4–6, 15–18). We assumed a 5-kb window before the first and after the last significant SNPs, given that linkage disequilibrium decays more than 50% in Arabidopsis in that range (19). Thus, the region in chromosome (Chr) III had five SNP associations in a 212-kb interval (Chr III: 8181817–8393525 bp) containing 49 genes (Table S1, Chromosome III region). The region in chromosome IV had 18 significant SNPs in a 112-kb interval (Chr IV: 13988547–14100946 bp) containing 39 genes (Table S1, Chromosome IV region). We focused on a subset of 11 genes that each contain highly significant SNP associations (FDR: q < 0.05) (Table S2). Among these candidate genes is PHOSPHATE 1 or PHO1 (At3g23430) shown previously to play a role in inorganic phosphate loading into the xylem and aspects of root development (20, 21). Several other uncharacterized genes were also identified.
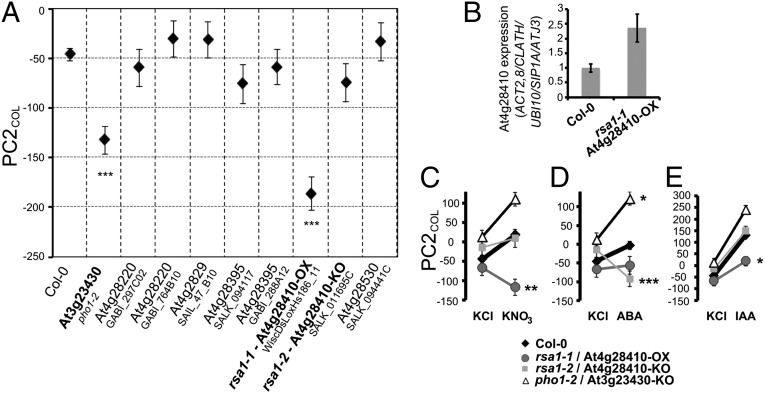
To test whether the corresponding mutant alleles show phenotypic effects for PC2COL, we characterized the root phenotypes in mutants of six candidate genes in the chromosome III and IV regions (Fig. 3A). pho1-2 (22) showed a significantly lower PC2COL phenotype than wild-type Col-0 (Fig. 3A), supporting the hypothesis that PHO1 is one of the underlying QTL in chromosome III. A second gene that showed a significant phenotype in PC2COL is At4g28410 or Root System Architecture 1 (RSA1). RSA1, a gene identified in this screen, encodes a protein that belongs to the tyrosine transaminase family, with similarity to SUPERROOT 1 (SUR1; At2g20610) (23), a gene involved in glucosinolate biosynthesis from tryptophan derivatives (24).
Fig. 3.
Candidate genes have PC2COL mutant phenotypes; PHO1/At3g23430 and RSA1/At4g28410 show G × E interaction for KNO3, ABA, and IAA conditions. (A) PC2COL values of mutant alleles for six candidate genes (n = 22–31) grown in KNO3 media plates, compared with wild-type Col-0 (n = 36). (B) Quantitative PCR on the rsa1-1 gain-of-function mutant allele (n = 3). (C–E) Reaction norms testing G × E interactions for IAA, ABA, CK, KNO3, and NH4Cl in pho1-2, rsa1-1 gain-, and rsa1-2 loss- of function mutants, respectively (n ≥ 11). Only significant interactions (KNO3, ABA, and IAA) are shown: *P < 0.05, **P < 0.005, ***P < 0.0005. Error bars: SE.
Among other defects, the sur1 mutant overproduces auxin because the conversion to glucosinolates is blocked, which canalizes tryptophan derivates toward auxin production, thus exhibiting extensive proliferation of lateral roots (25). We thus postulate that the SUR1-like gene, RSA1, has a redundant function to SUR1, and loss-of-function mutations (rsa1-2 = At4g28410-KO) are unlikely to show strong phenotypic effects; however, overexpression alleles (rsa1-1 = Atg428410-OX) would be expected to have an auxin deficiency-like phenotype, consistent with the phenotype of the rsa1-1 gain-of-function mutant (Fig. 3 A and B). rsa1-1 is a tDNA insertion mutant mapping to the 5′ UTR of A4g28410, whereas rsa1-2 is a tDNA insertion mapping to 969 bp from the start codon, producing a predicted truncated protein of 327 amino acids (full length is 447 amino acids). To test the possible role of RSA1 in auxin homeostasis, we obtained the genome-wide expression profile of the rsa1-1 overexpression mutant. We found that 104 (P < 0.001) genes or 492 (P < 0.01) genes are more than 1.5-fold differentially expressed compared with the sibling wild type (Table S3). We compared the list of misregulated genes in the rsa1-1 mutant to a list of 3,186 genes regulated by the synthetic auxin naphthalene-1-acetic acid in the root (26). We found significant over-representations of the intersections of auxin-regulated genes with genes misregulated in the rsa1-1 mutant for both of our datasets (P < 0.001), compared with random generated gene lists of the same size (27, 28), further suggesting a rsa1-1 role in auxin homeostasis. To further test whether expression of RSA1 mRNA is causative to changes in root allometry, we tested whether natural variation in RSA1 activity is correlated with changes in PC2COL. To do this, we obtained normalized RSA1 expression in 10 Arabidopsis accessions (Fig. S3) and compared them to their respective PC2COL phenotypes. Consistent with the result on the rsa1-1 overexpression allele, we found a negative correlation between RSA1 mRNA levels and the PC2COL phenotype (Pearson correlation: −0.55). This correlation of gene activity and phenotypic changes in PC2COL in the rsa1-1 mutant, and the correlation between RSA1 expression and PC2COL phenotypes in the natural accessions also supports the notion that RSA1 (At4g28410) underlies the QTL for PC2COL that maps to chromosome IV.
Using the Arabidopsis 1,001 Genomes browser (1001genomes.org), we identified SNP positions in the RSA1 and PHO1 loci. For RSA1, the SNP at position 14051900 in chromosome IV is 348 bp upstream of the 5′ UTR. This opens the possibility that cis-regulatory variation at RSA1 is driving variation in root allometry that is supported by our studies of RSA1 expression in the rsa1-1 mutant (Fig. 3 A and B) and in natural variants (Fig. S3). For PHO1, we found that the SNP at position 8388425 in chromosome III (TAIR 8 annotation) is within an exon of the coding region. Furthermore, the SNP is nonsynonymous; a cytosine at that position codes for histidine, whereas a thymine at that position codes for tyrosine. Histidine is a basic amino acid and tyrosine is an aromatic amino acid, however, the physiological ramifications of this nonsynonymous change are not clear.
Two Candidate Genes Underlying Natural Variation in Root Architecture Have Phenotypic Plasticity Responses.
Genes involved in phenotypic plasticity exert environmental control over other genes that affect the phenotypic response (29). Thus, impaired activities of these genes are predicted to exhibit atypical phenotypic responses to their respective environment. To test our initial hypothesis that genes underlying natural variation can also be responsible for phenotypic plasticity, we investigated the root phenotypic response of rsa1 and pho1 mutant alleles to stimuli affecting the plant physiological “environment.” Genotype-by-Environment interactions (G × E) in the rsa1 and pho1 mutants would suggest a role of RSA1/At4g28410 and PHO1 in plasticity responses, as well as its associated role in natural variation. To test this, we exposed the rsa1 mutants and the pho1-2 mutant allele to the five experimental treatments (IAA, CK, ABA, KNO3, and NH4Cl) and the control (KCl) and evaluated the resulting phenotypes along the PC2COL phenotypic axis. A two-way analysis of variance (ANOVA) for PC2COL gave a significant G × E interaction term (P < 0.0001) for the several treatments. For RSA1, significant G × E interaction effects were determined for KNO3 (P = 0.0031, standardized-β = −0.203) (Fig. 3C) and IAA (P = 0.0093, standardized-β = −0.177) (Fig. 3E) in the rsa1-1 overexpression mutant. ABA gave significant interactions in the rsa1-2 (P < 0.0001, standardized-β = −0.310) (Fig. 3D). This is illustrated by the reaction norms where rsa1-1 has a lower PC2COL phenotype, compared with Col-0, and the KNO3 treatment increases the difference in the PC2COL phenotype of Col-0 vs. rsa1-1, relative to controls (e.g., KCl; Fig. 3D). The rsa1-2 mutant had a lower PC2COL phenotype in the ABA condition compared with Col-0 (Fig. 3D). The rsa1-2 mutant was not as responsive to the IAA treatment as Col-0 (Fig. 3E), consistent with its predicted SUR1-like function.
The pho1-2 mutant showed a significant interaction in the ABA treatment (P = 0.0228, standardized-β = 0.144) (Fig. 3D), illustrated by the higher PC2COL phenotype of the pho1-2 mutant compared with Col-0, specifically in our control environment (e.g., KCl). The pho1-2 phenotype was enhanced in the presence of ABA, supporting the activity dependency between PHO1 and ABA recently shown in stomatal responses (30). To further study the interaction of ABA and phosphate availability in the pho1-2 mutant, we performed a combinatorial experiment in the presence or absence of ABA and phosphate. However, no significant interactions or additional phenotypes were revealed under the conditions that we tested (Fig. S4). Together, these results suggest that the role of RSA1 and PHO1 in mediating lateral root plasticity acts through its interplay with KNO3, ABA, and IAA signaling. Moreover, these results show that RSA1 and PHO1, candidate genes identified to be associated with natural variation in root architecture, also play roles in mediating plasticity responses within an accession Arabidopsis.
Finally, we tested the possibility that RSA1 and PHO1 natural alleles might be associated with changes in the environment using a “Landscape Genetics” approach (31), described in more detail in Methods and Table S4. Using environmental variables that are related to temperature and humidity (32–34), we found that none of the evaluated environmental factors (Table S4) were significantly associated with the SNP near RSA1 in the tested accessions (Table S5). For PHO1, we discovered that “wet day frequency” was significantly associated with the SNP in PHO1 (P = 0.0027). Specifically, the SNP allele in PHO1 marked by a cytosine was predicted to be much less frequent in environments with fewer than 10 wet days per month compared with environments with higher wet day frequencies, whereas the SNP allele marked by a thymine was predicted to be the most frequent in environments with fewer than 10 wet days per month compared with environments with higher wet day frequencies (Fig. S5). The findings associating specific SNPs within PHO1 with response to the environment in the field suggest future experiments to better understand this phenomenon.
Discussion
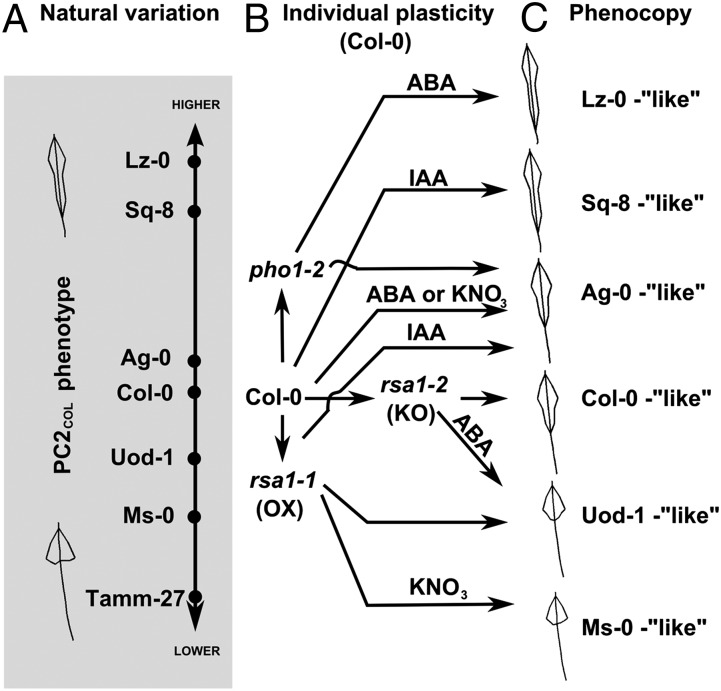
In this paper, we provide a comprehensive framework to dissect how the extent of the phenotypic response of an individual Arabidopsis laboratory accession relates to phenotypic plasticity intrinsic to its natural variants. Our results show that a phenotypic plasticity trait quantified in the laboratory strain Col-0 (PC2COL) broadly recapitulates that found in natural accessions and can be used to quantify and map variation in natural Arabidopsis accessions (Fig. 4A). This intersection of individual plasticity and natural variation spaces enabled us to identify candidate genes (i.e., RSA1 and PHO1) controlling variation of root allometry. Previous genome-wide studies have identified QTL for root architecture (4, 6, 15–18), some of them overlapping with our identified regions in chromosomes III and IV; but only one locus on chromosome I, BREVIS RADIX, has been characterized as controlling natural variation (5, 7). We now provide evidence of two more candidate genes controlling natural variation in root system architecture in Arabidopsis (RSA1 and PHO1) and provide evidence suggesting that the environment (wet day frequency) has driven allelic variation in PHO1 (Fig. S5).
Fig. 4.
The PHO1-RSA1-dependent G × E interaction expands the root plasticity space of Col-0 to phenocopy a range of natural accessions. (A) Axis representing natural variation on PC2COL phenotype with examples of accession’s positions and root allometry phenotypes. (B) Treatments with KNO3 ABA, or IAA in rsa1-1 gain-of-function (OX) and rsa1-2 loss-of-function (KO) mutants have contrasting effects to Col-0. (C) Col-0 and rsa1 mutant alleles treated with KNO3, ABA, or IAA phenocopy the root architecture of natural variants.
In addition, we found that the phenotypic effects of RSA1 and PHO1 activities on root allometry as captured by PC2COL are conditional to specific environments, e.g., KNO3, ABA, or IAA (Fig. 4B). Thus, a genotype-by-environment interaction mediated through the RSA1 and PHO1 genes determines whether the root response to ABA, IAA, and KNO3 increases, reduces, or maintains the distribution of lateral root allometry. Moreover, these genetic and environmentally induced changes in root allometry in the Col-0 accession in turn phenocopies the root allometry trait of a range of natural accessions (Fig. 4C). This suggests that the mechanisms controlling phenotypic plasticity of root allometry Col-0 are similar to the ones controlling natural variation in Arabidopsis accessions. Moreover, the accessibility of the phenotypic plasticity space of an individual accession depends on the activity of genes controlling natural variation, as represented by RSA1 and PHO1. These findings reopen the discussion of the relevance of phenotypic plasticity genes in natural variation and adaptive evolution (1–3, 29).
Our study also highlights a strategy for intersecting experimentally perturbed phenotypes within a laboratory strain with those observed across a panel of natural variants to uncover genetic mechanisms underlying plasticity and natural variation for any evolved trait of interest. Contextualizing root plasticity within laboratory variants and natural variation in accessions under laboratory conditions is a powerful strategy to dissect the influence of factors on plasticity typically composed of complex multigene traits, such as the impact of hormones on root system architecture. In developing a method to cross-reference the genes involved in phenotypic plasticity and natural variation, we open a window to understanding the possible mechanisms that characterize adaptive change in Arabidopsis.
Methods
Growth Conditions and Phenotyping.
Seeds were disinfected with a solution of ethanol, bleach, and water 4:1:3 and three rinses of sterilized water. Seeds were sown on square plates of custom-made MS media (−sucrose/−nitrogen) supplemented with sucrose 0.1%, nitrogen (as indicated below), MES sodium salts 0.05% (Gibco BRL), and agar 1% (Bacto Agar BD). Plates were kept at 4 °C for 4 d in the dark and then placed vertically in a growth chamber under 22 °C, long-day 16h/8h condition at 125 μmol⋅m−2s−1 light intensity (Percival Scientific). For the phenotypic characterization of accessions and mutants, the custom MS media was supplemented with 5 mM of KNO3 and 1% of sucrose as carbon source, grown at 50 mol⋅m−2s−1 light intensity; roots were imaged 16 d after sowing. For the IAA, CK, ABA, KNO3, NH4Cl, and control (KCl) treatments in Col-0 and mutants, the seeds were sown on media containing 1 mM of KCl, and seedlings were transferred to the fresh plates containing 500 nM of IAA, 500 nM of kinetin (CK), 1 μM of ABA, 1 mM of KNO3, 1 mM of NH4Cl, or 1 mM KCl (control), and roots were imaged 14 d after germination (4 d after transfer to treatment plates).
Plate images were obtained at 300-dpi resolution using a scanner. Landmark data and morphometric analysis were obtained using the software “Shape Model Toolbox” (12) in Matlab as described (13). Before analyzing the root variation, procrustes for translation and rotation were applied to the datasets (11, 13). This was done by aligning the datasets according to their centroid and then rotating them about the centroid to minimize the distance between corresponding landmarks; no scaling was applied. PCA was carried out on the covariance matrix to obtain the main trends of the variation (PCs), which were used as traits. Projections of other datasets were used to quantify phenotypes according to the corresponding PCCOL. For details on the PCA methods and projections, see refs. 13 and 35. Landmark datasets to generate the models are provided in Dataset S1.
Sampling and Statistical Tests.
The phenotypic plasticity space PCCOL was created by growing Col-0 in the treatments described above with 20 plants per treatment. The accession data were obtained by growing three to four plants of each of the 96 Nordborg lines. Only 69 were phenotyped with the RootScape method (11), as the rest did not outgrow visible lateral roots under our conditions. To characterize the mutant phenotypes of candidate genes, homozygote tDNA insertion mutants and wild-type siblings were identified using primers obtained using default settings on the iSect tool (http://signal.salk.edu/tdnaprimers.2.html). Plates were sown side by side with the mutant and wild-type siblings to account for background and plate effects. The dataset consisted of n = 239 plants of Col-0; 36 of pho1-2; 23 of GABI_297C02; 25 of GABI_764B10; 24 of SAIL_47_B10; 27 of SALK_094117; 22 of GABI_288A12; 31 of WiscDsLoxHs186_11; 22 of SALK_011695C; and 22 of SALK_094441C. The projected PC2COL data were analyzed according to the following REML mixed model: PC2COL = βgenotype + βplate + ε (Fig. 3A). To characterize the plasticity response of the RSA1/At4g28410 mutants, a minimum of 11 plants per treatment per genotype were analyzed, for a total of 291 plants. This dataset was analyzed together with the data to generate the PCCOL space (above). G × E interactions were tested according to the following two-way ANOVA model: PC2COL = βgenotype + βtreatment + βgenotype*treatment + ε (Fig. 3 C–E). To further test pho1-2 plasticity in a phosphate-depleted environment, we custom-made MS media following the manufacturer’s recipe, but also substituted the moles of –PO4 in KH2PO4 for KOH and imaged the roots 4 d after transfer to phosphate-depleted media.
Genome-Wide Association Study.
After standardizing each PCCOL root trait to a mean of zero and a SD of 1, we performed genome-wide association mapping using the SNP database from Atwell et al. (14), which documents over 214,000 SNPs (an average of 1 SNP/500 bp) in 69 different inbred lines from the wild. We filtered the database to include only SNPs with a minor allele frequency greater than 0.10, which left 177,623 SNPs for mapping.
To account for genome-wide patterns of relatedness that can confound the results of GWAS studies (36), we used all 214,000 SNPs to construct a similarity matrix representing the proportion of loci that is identical in state between any pair of lines (K; 36). For each trait, we then separately fit the model y = Xα + Zu + ε, where y is a vector of phenotypes, X is a matrix of single-locus genotypes, α is a vector of allele effects to be estimated, Z is an identity matrix, u is a matrix of random deviates due to genome-wide relatedness (as inferred from K), and ε is a vector of residual errors. The analysis was conducted using the EMMA approach (37) in R version 3.0.1 (R Development Core Team 2013). To account for multiple simultaneous tests (because α is modeled separately for each SNP), we calculated P values that were adjusted for the genome-wide FDR (q-values) using the q-value package (38) in R (R Core Team 2013) (39).
Gene Expression Analysis.
For expression analysis using quantitative PCR, the RNA extraction were carried out on roots collected from a rsa1-2 mutant and sibling wild type at day 12, grown side by side on vertical agar plates, 5 mM of KNO3, and 1% of sucrose at 50 mol⋅m−2s−1 light intensity. For each of the three replicates, we pooled tissue from three roots. For assays in accessions, plants were grown in liquid media in phytatrays, and total RNA was extracted using RNeasy minikit (Qiagen). Double-stranded cDNA was synthesized by the SuperScript RT-PCR system (Invitrogen). PCR were performed using the LightCyclerFastStart DNA masterPLUS SYBR Green I (Roche) in a LightCycler 480 (Roche). Expression level of At4g28410 was quantified using the oligos 5′-GTGGTGATAATGAATCCTCACAAC-3′ and 5′-CCATCGGGACAAATTTATTCTCT-3′. Five standard reference genes were used to quantify relative expression: Clathrin/At4g24550 (5′-AGCATACACTGCGTGCAAAG-3′ and 5′-TCGCCTGTGTCACATATCTC-3′), ACT2,8/At3g18780,At1g49240 (5′-GGTAACATTGTGCTCAGRGGTGG-3′ and 5′-AACGACCTTAATCTTCATGCTGC-3′), SIP1A/At3g04090 (5′-TCCTTGTCATTGTTTAGATCCACAC-3′ and 5′-TAAATGTTTCTAAACCGGAAGAGAGTC-3′), ATJ3/A3g44110 (5′-TCCAACCAATTTGTCTCTTGCT-3′ and 5′-AACAAGTTTCGATGTTCCACC-3′), and UBI10/At4g0532 (5′-GGCCTTGTATAATCCCTGATGAATAAG-3′ and 5′-AAAGAGATAACAGGAACGGAAACATAGT-3′). All PCRs were performed with annealing of 60 °C. PCR efficiency of At4g28410 was tested with a standard curve in each plate, using four serial dilutions of a wild-type sample: 1/1, 1/10, 1/100, and 1/1000. For genome-wide expression in the rsa1-1 mutant allele (n = 3) and the sibling wild type (n = 3), we used standard protocols from Affymetrics to amplify, label, and hybridize RNA samples to the ATH1 Affymetrics chip. Raw data were processed in MASv5.0 and two-tailed t test was performed in R (R Core Team 2013) (40).
Landscape Genetic Analysis.
We took the significant SNP in PHO1 (At3g23430) and the significant SNP near RSA1 (At4g28410) and examined how they were structured on the landscape as a function of several environmental factors. We used a list of accessions and geographic coordinates of their origins (41). We intersected this list with lists of genes for which SNP genotype information is available at the two SNP loci of interest (14). We limited this list to accessions falling within the native range of A. thaliana in Europe and Asia (−11° to 86° E and 35° to 71° N), as estimated based on ref. 42. We then filtered this list by the “red list” and the “yellow list” of putative misidentified accessions previously flagged (41). A total of 726 accessions remained after intersecting and filtering (Fig. S5A and Table S5).
Separately for each SNP, we examined how the SNP was structured on the landscape as a function of temperature and humidity variables used in previous studies (Table S4). All environmental factors were at a 10 arc-minute (∼13 km) scale worldwide. We extracted the values of the environmental factors at the locations origin of the accessions using ArcMap version 9.3 (Esri Inc.). We then thinned the list of environmental factors to those with correlation coefficients less than |0.6| among the locations of the accessions (Table S4).
The logistic regression model was based on the logistic function y = 1/(1 + e−z), where y is a binary dependent variable (one SNP allele or the other); and z = β0 + β1S1 +…+ βxSx+ βx + 1E1 +…+ βx + yEz + ε, where β0 is the intercept, S1 − Sx are the covariates to account for spatial autocorrelation (Table S4), E1 − Ez are the environmental variables, ε is the residual error, and the β terms are the corresponding regression coefficients. The analysis was performed using the lrm function of the rms package (39) in R (R Development Core Team 2013) (40).
Supplementary Material
Acknowledgments
The pho1-2 was line was kindly provided by Ives Poirier (Faculté de Biologie et de Médicine, Université de Lausanne). Rongchen Wang (Division of Biological Sciences, University of California San Diego) provided mutant lines for candidate genes. We thank Tara Moran and Nancy Francoeur for their help on Affy chips of rsa1-1. We thank Joan Doidy for his advice on custom media preparation. We acknowledge the feedback of Dennis Shasha and Daniel Tranchina on statistical analysis. This work was supported by National Science Foundation (NSF) Grant MCB-0929338 (to G.M.C. and K.D.B.); NSF Grant DEB-0917489 (to M.D.P.); National Institutes of Health (NIH) Grant R01 GM032877 (to G.M.C.); NIH Grant R01 GM078279 (to K.D.B.); a Human Frontier Postdoctoral Fellowship (to U.R.); a Fulbright Science and Technology award (to D.R.); a Marie Curie postdoctoral fellowship, Agence Nationale de Recherches (ANR) (NitroNet: ANR 11 PDOC 020 01); Centre National de la Recherche Scientifique (Projets Exploratoires Pluridisciplinaires Bio math Info 2012–2013: SuperRegNet) grants (to G.K.); European Molecular Biology Organization postdoctoral “A Long Term Fellowship” 107-2005; and Biotechnology and Biological Sciences Research Council Grant BB/H109502/1 (to M.L.G.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305883110/-/DCSupplemental.
References
- 1.Pigliucci M, Murren CJ, Schlichting CD. Phenotypic plasticity and evolution by genetic assimilation. J Exp Biol. 2006;209(Pt 12):2362–2367. doi: 10.1242/jeb.02070. [DOI] [PubMed] [Google Scholar]
- 2.Waddington CH. Genetic assimilation for an acquired character. Evolution. 1953;7(2):118–126. [Google Scholar]
- 3.Pigliucci M, Murren CJ. Perspective: Genetic assimilation and a possible evolutionary paradox: Can macroevolution sometimes be so fast as to pass us by? Evolution. 2003;57(7):1455–1464. doi: 10.1111/j.0014-3820.2003.tb00354.x. [DOI] [PubMed] [Google Scholar]
- 4.Rauh L, Basten CB, Buckler S., IV Quantitative trait loci analysis of growth response to varying nitrogen sources in Arabidopsis thaliana. Theor Appl Genet. 2002;104(5):743–750. doi: 10.1007/s00122-001-0815-y. [DOI] [PubMed] [Google Scholar]
- 5.Mouchel CF, Briggs GC, Hardtke CS. Natural genetic variation in Arabidopsis identifies BREVIS RADIX, a novel regulator of cell proliferation and elongation in the root. Genes Dev. 2004;18(6):700–714. doi: 10.1101/gad.1187704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant Cell Environ. 2006;29(1):115–125. doi: 10.1111/j.1365-3040.2005.01405.x. [DOI] [PubMed] [Google Scholar]
- 7.Beuchat J, et al. A hyperactive quantitative trait locus allele of Arabidopsis BRX contributes to natural variation in root growth vigor. Proc Natl Acad Sci USA. 2010;107(18):8475–8480. doi: 10.1073/pnas.0913207107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikram S, Bedu M, Daniel-Vedele F, Chaillou S, Chardon F. Natural variation of Arabidopsis response to nitrogen availability. J Exp Bot. 2012;63(1):91–105. doi: 10.1093/jxb/err244. [DOI] [PubMed] [Google Scholar]
- 9. Gruber BD, Giehl RFH, Friedel S, von Wirén N (2013) Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiol, 10.1104/pp.113.218453. [DOI] [PMC free article] [PubMed]
- 10.Krouk G, et al. A framework integrating plant growth with hormones and nutrients. Trends Plant Sci. 2011;16(4):178–182. doi: 10.1016/j.tplants.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Ristova D, et al. RootScape: A landmark-based system for high-throughput screening of root architecture in Arabidopsis thaliana. Plant Physiol. 2013;161(3):1086–1096. doi: 10.1104/pp.112.210872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nordborg M, et al. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 2005;3(7):e196. doi: 10.1371/journal.pbio.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langlade NB, et al. Evolution through genetically controlled allometry space. Proc Natl Acad Sci USA. 2005;102(29):10221–10226. doi: 10.1073/pnas.0504210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atwell S, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465(7298):627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi Y, Koyama H. QTL analysis of Al tolerance in recombinant inbred lines of Arabidopsis thaliana. Plant Cell Physiol. 2002;43(12):1526–1533. doi: 10.1093/pcp/pcf174. [DOI] [PubMed] [Google Scholar]
- 16.Loudet O, Gaudon V, Trubuil A, Daniel-Vedele F. Quantitative trait loci controlling root growth and architecture in Arabidopsis thaliana confirmed by heterogeneous inbred family. Theor Appl Genet. 2005;110(4):742–753. doi: 10.1007/s00122-004-1900-9. [DOI] [PubMed] [Google Scholar]
- 17.Fitz Gerald JN, et al. Identification of quantitative trait loci that regulate Arabidopsis root system size and plasticity. Genetics. 2006;172(1):485–498. doi: 10.1534/genetics.105.047555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El-Lithy ME, Reymond M, Stich B, Koornneef M, Vreugdenhil D. Relation among plant growth, carbohydrates and flowering time in the Arabidopsis Landsberg erecta × Kondara recombinant inbred line population. Plant Cell Environ. 2010;33(8):1369–1382. doi: 10.1111/j.1365-3040.2010.02155.x. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, et al. Recombination and linkage disequilibrium in Arabidopsis thaliana. Nat Genet. 2007;39(9):1151–1155. doi: 10.1038/ng2115. [DOI] [PubMed] [Google Scholar]
- 20.Poirier Y, Thoma S, Somerville C, Schiefelbein J. Mutant of Arabidopsis deficient in xylem loading of phosphate. Plant Physiol. 1991;97(3):1087–1093. doi: 10.1104/pp.97.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamburger D, Rezzonico E, MacDonald-Comber Petétot J, Somerville C, Poirier Y. Identification and characterization of the Arabidopsis PHO1 gene involved in phosphate loading to the xylem. Plant Cell. 2002;14(4):889–902. doi: 10.1105/tpc.000745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delhaize E, Randall PJ. Characterization of a phosphate accumulator mutant of Arabidopsis thaliana. Plant Physiol. 1995;107(1):207–213. doi: 10.1104/pp.107.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boerjan W, et al. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell. 1995;7(9):1405–1419. doi: 10.1105/tpc.7.9.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mikkelsen MD, Naur P, Halkier BA. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. Plant J. 2004;37(5):770–777. doi: 10.1111/j.1365-313x.2004.02002.x. [DOI] [PubMed] [Google Scholar]
- 25.Seo M, et al. Higher activity of an aldehyde oxidase in the auxin-overproducing superroot1 mutant of Arabidopsis thaliana. Plant Physiol. 1998;116(2):687–693. doi: 10.1104/pp.116.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanneste S, et al. Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell. 2005;17(11):3035–3050. doi: 10.1105/tpc.105.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katari MS, et al. VirtualPlant: A software platform to support systems biology research. Plant Physiol. 2010;152(2):500–515. doi: 10.1104/pp.109.147025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krouk G, Mirowski P, LeCun Y, Shasha DE, Coruzzi GM. Predictive network modeling of the high-resolution dynamic plant transcriptome in response to nitrate. Genome Biol. 2010;11(12):R123. doi: 10.1186/gb-2010-11-12-r123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlichting CD, Pigliucci M. Control of phenotypic plasticity via regulatory genes. Am Nat. 1993;142(2):366–370. doi: 10.1086/285543. [DOI] [PubMed] [Google Scholar]
- 30.Zimmerli C, et al. PHO1 expression in guard cells mediates the stomatal response to abscisic acid in Arabidopsis. Plant J. 2012;72(2):199–211. doi: 10.1111/j.1365-313X.2012.05058.x. [DOI] [PubMed] [Google Scholar]
- 31.Banta JA, et al. Climate envelope modelling reveals intraspecific relationships among flowering phenology, niche breadth and potential range size in Arabidopsis thaliana. Ecol Lett. 2012;15(8):769–777. doi: 10.1111/j.1461-0248.2012.01796.x. [DOI] [PubMed] [Google Scholar]
- 32.New M, Lister D, Hulme M, Makin I. A high-resolution data set of surface climate over global land areas. Clim Res. 2002;21:1–25. [Google Scholar]
- 33.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965–1978. [Google Scholar]
- 34.Fournier-Level A, et al. A map of local adaptation in Arabidopsis thaliana. Science. 2011;334(6052):86–89. doi: 10.1126/science.1209271. [DOI] [PubMed] [Google Scholar]
- 35.Bensmihen S, et al. Mutational spaces for leaf shape and size. HFSP J. 2008;2(2):110–120. doi: 10.2976/1.2836738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu J, et al. A unified mixed-model method for association mapping that accounts for multiple levels of relatedness. Nat Genet. 2006;38(2):203–208. doi: 10.1038/ng1702. [DOI] [PubMed] [Google Scholar]
- 37.Kang HM, et al. Efficient control of population structure in model organism association mapping. Genetics. 2008;178(3):1709–1723. doi: 10.1534/genetics.107.080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dabney A, Storey JD (2010) qvalue: Q-Value Estimation for False Discovery Rate Control. (Princeton University, Princeton), R package version 1.22.0. Available at www.bioconductor.org/packages/release/bioc/html/qvalue.html. Accessed August 16, 2013.
- 39. Harrell FE, Jr. (2013) rms: Regression Modelling Strategies. (Vanderbilt University, Nashville), R package version 3.6-3. Available at http://CRAN.R-project.org/package=rms. Accessed August 15, 2013.
- 40.Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation; 2013. [Google Scholar]
- 41.Anastasio AE, et al. Source verification of mis-identified Arabidopsis thaliana accessions. Plant J. 2011;67(3):554–566. doi: 10.1111/j.1365-313X.2011.04606.x. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman MH. Biogeography of Arabidopsis thaliana (L.) Heynh. (Brassicaceae) 2002 Journal of Biogeography 29(1):125–134. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.