Abstract
Cognitive emotion regulation has been widely shown in the laboratory to be an effective way to alter the nature of emotional responses. Despite its success in experimental contexts, however, we often fail to use these strategies in everyday life where stress is pervasive. The successful execution of cognitive regulation relies on intact executive functioning and engagement of the prefrontal cortex, both of which are rapidly impaired by the deleterious effects of stress. Because it is specifically under stressful conditions that we may benefit most from such deliberate forms of emotion regulation, we tested the efficacy of cognitive regulation after stress exposure. Participants first underwent fear-conditioning, where they learned that one stimulus (CS+) predicted an aversive outcome but another predicted a neutral outcome (CS−). Cognitive regulation training directly followed where participants were taught to regulate fear responses to the aversive stimulus. The next day, participants underwent an acute stress induction or a control task before repeating the fear-conditioning task using these newly acquired regulation skills. Skin conductance served as an index of fear arousal, and salivary α-amylase and cortisol concentrations were assayed as neuroendocrine markers of stress response. Although groups showed no differences in fear arousal during initial fear learning, nonstressed participants demonstrated robust fear reduction following regulation training, whereas stressed participants showed no such reduction. Our results suggest that stress markedly impairs the cognitive regulation of emotion and highlights critical limitations of this technique to control affective responses under stress.
Whether we are running late to an appointment, arguing intensely with a loved one, or having a rough day at work, controlling our emotions when circumstances become stressful can be challenging. Although extensive work in the laboratory has demonstrated that we can cognitively alter emotional responses to foster more adaptive behavior (1–3), in real-world emotional contexts we often fail to do so. One potential reason for this regulatory failure might be that the pervasive presence of stress in daily life compromises our ability to effectively regulate emotions. Indeed, negative affect has long been proposed to play a key role in the failure to exert self-regulatory control over our thoughts and behavior (4, 5). However, as of yet, a direct relationship between the physiological stress response and the cognitive control of emotion has not been examined. Here, we sought to explore how the cognitive regulation of emotion is affected by an acute stress induction.
A large body of work has shown that responses to emotionally salient stimuli can be flexibly changed and controlled through cognitive emotion regulation (for review, see refs. 1–3 and 6). By targeting what has been described to be the initial appraisal of a salient cue or event (7, 8), cognitive regulation allows an individual to alter the relevance and meaning of a stimulus, subsequently shaping its emotional response (2, 3). Recruiting cognitive strategies to deliberately change the way a stimulus is evaluated, either by reinterpreting (i.e., reappraising) its meaning or focusing on its more positive aspects, has proven effective at reducing the subjective (2, 9, 10), physiological (2, 10, 11), and neural components (3, 9, 11, 12) of emotional arousal.
The cognitive regulation of emotion, however, is generally a complex and goal-directed process that depends on a number of higher cognitive functions, such as attention, cognitive flexibility, motivation, and working memory, which all facilitate the online maintenance of information needed to override initial affective reactions (2, 3, 13, 14). This regulatory capacity is critical to mental (15) and physical (16) health and its impairment strongly predicts vulnerability to an array of affective disorders (17, 18). Importantly, the principles underlying cognitive regulation also form the basis of cognitive-behavioral therapy (CBT), a tool widely used in the clinic to treat affective psychopathology. Like cognitive regulation, CBT relies on the tightly coupled relationship between thoughts and emotions and promotes the correction of irrational or distorted cognitive appraisals to engender more adaptive emotional responses (19). Although cognitive regulation has emerged as a highly effective technique for controlling emotional responses, its success relies on the availability of cognitive resources and intact executive function (3–5, 7, 13, 14). Critically, a growing body of work has revealed that exposure to acute stress impairs many of these higher cognitive processes (20–22), including cognitive flexibility (23, 24), goal-directed behavior (25), working memory (26–30), and self-control (5). These rapid cognitive effects of stress are thought to be mediated by neuroendocrine responses to acute stress exposure that impacts the functional integrity of the prefrontal cortex (PFC) (20, 21), which supports these processes (31, 32). Importantly, acute stress effects appear to preferentially target the dorsolateral PFC (20, 21, 33, 34), which has consistently been implicated as playing a key role in the successful execution of cognitive emotion regulation (3, 6, 12).
These findings suggest an important, yet unexplored, paradox: in the stressful situations in which we might benefit most from deliberate, active forms of emotional control, the mechanisms required to support such cognitive regulation may be impaired. Thus, cognitive regulation may be ineffective at controlling emotional responses precisely when such control is needed most. Here, we test whether acute stress influences the ability to use cognitive regulation to diminish conditioned fear responses. Given the pervasive nature of stress in daily life, characterizing how stress influences our ability to modify emotional responding is critical for understanding the boundaries within which existing regulation techniques are effective, and offer insight into treatment options for those who suffer from stress-related psychological disorders.
We investigated the effect of stress on cognitive emotion regulation using a 2-d protocol. On day 1 (learning session), participants underwent a fear-conditioning paradigm using visual cues as conditioned stimuli (CS) and a mild electric wrist-shock as the unconditioned stimulus (US). Skin conductance response (SCR) served as an index of physiological fear arousal. One image (CS+) was paired with a shock on a subset of trials, but the other (CS−) was never paired with shock and served as a baseline measure of arousal. Directly after fear-conditioning, participants reported three emotions that they associated with each CS and rated the intensity of these emotions on a scale from 1 (least intense) to 10 (most intense). Participants were then trained to use a cognitive regulation strategy that incorporated elements of reappraisal and was based on the principles of CBT. This regulation strategy has been shown in previous work to result in the persistent attenuation of conditioned fear compared with sustained fear seen in those participants who underwent a control task (10) (Methods). After cognitive regulation training (CRT), participants rerated the intensity of their self-reported emotions and were asked to return the next day to undergo the same fear-conditioning task using their newly acquired regulation techniques.
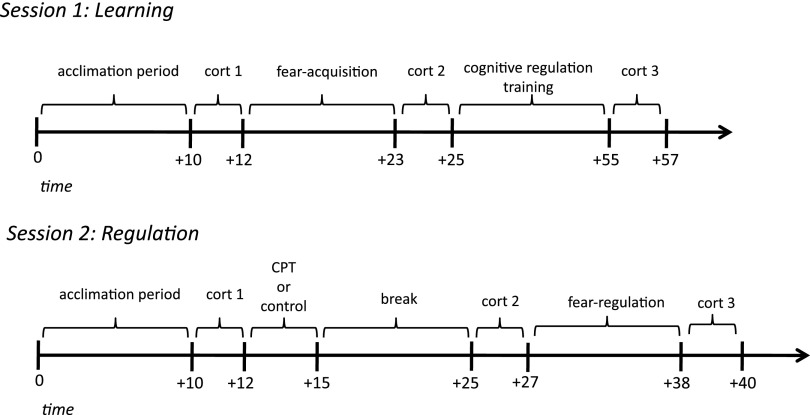
On day 2 (regulation session), participants who showed adequate fear-learning the previous day (Methods) were randomly assigned to the stress or control group. Critically, we manipulated stress levels by having participants undergo either the cold-pressor (CP) task (35), an acute stress induction in which participants submerged their arms in ice-cold water for 3 min, or a control task using room-temperature water, before repeating the fear-conditioning session. To ensure that our stress manipulation elicited hypothalamic-pituitary-adrenal (HPA) axis activity, the hallmark of an acute stress response, we measured cortisol concentrations in participants’ saliva throughout the experiment. HPA-axis activation triggers the release of stress hormones that peak ∼10–20 min after a stressor (20, 36–39). Therefore, we collected salivary samples at baseline (immediately before the CP/control task), again 10 min later (after the CP/control task but before the fear-conditioning task), and, finally, 20 min after the CP/control task (immediately after the fear-conditioning task) (see Fig. 1 for a schematic of experimental protocol). Cortisol release is preceded and triggered by an earlier wave of catecholamines (i.e., noradrenaline) that reflects autonomic nervous system arousal and is released rapidly after a stressor to facilitate preparatory responses to stress (20, 21, 39). To measure this response, we also assayed α-amylase, a salivary enzyme that serves as an index of noradrenergic activity (40–42) in each of these samples. Participants reported subjective levels of stress after the CP/control task on a scale from 1 (least stressful) to 10 (most stressful). Self-reported emotions and intensity ratings were collected on day 2 after the CP/control manipulation, but before participants repeated the fear-conditioning task. We hypothesized that after undergoing CRT nonstressed participants would successfully regulate fear to the CS+ on day 2 (10), whereas fear responses in stressed participants would persist.
Fig. 1.
Experimental timeline. Schematic of experimental procedure and timeline of neuroendocrine assessments for both day 1 (learning) and day 2 (regulation).
Results
Physiological Fear Responses.
To ensure that we could measure the influence of stress on fear regulation the following day, only participants who showed evidence of acquiring a conditioned fear response (CS+ > CS− by 0.1 μS) were included (see Methods for detailed exclusion criteria). To confirm that fear acquisition on day 1 did not differ between conditions, we conducted a repeated-measures ANOVA using a within-subject factor of CS (CS+, CS−), and a between-subjects factor of condition, using mean SCRs from the fear-conditioning session. As expected, given our exclusion criteria, we found a significant main effect of CS [F(1, 78) = 190.20, P < 0.000001]. Importantly, there was no effect of condition and no interaction. Independent t tests confirmed that participants in both groups showed greater mean SCRs to the CS+ than CS− [stress: t(35) = 9.24, P < 0.000001; control: t(43) = 10.24, P < 0.0000001] and that fear responses did not differ between groups for either CS [CS+: t(78) = −0.28, P = 0.78; CS−: t(78) =0.62 P = 0.54].
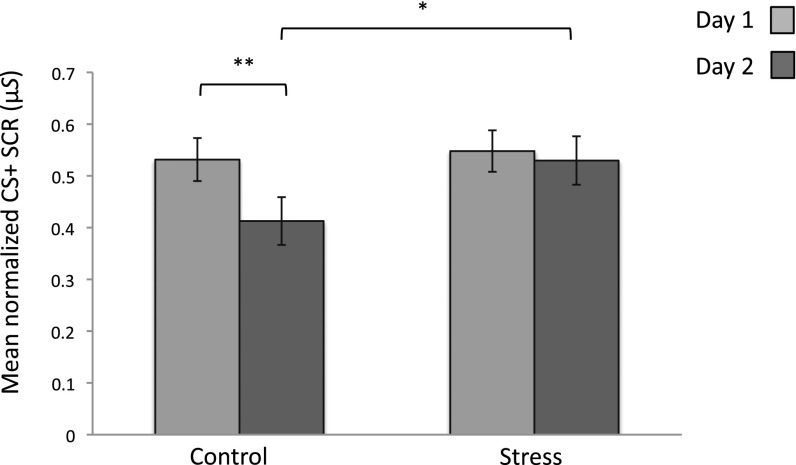
Our primary analysis of interest was how fear responses to the CS+ decreased across sessions for each condition. We conducted a repeated-measures ANOVA using condition (stress, control) as a between-subject factor and session (day 1, day 2) as a within-subject factor. We observed a main effect of session [F(1, 78) = 8.45, P = 0.005], no effect of condition [F(1, 78) = 1.32, P = 0.25], and a significant session X condition interaction [F(1, 78) = 4.55, P = 0.03]. Paired samples t-tests confirmed that the control group showed a significant decrease in SCR to the CS+ across sessions [t(43) = 3.65, P = 0.001], whereas the stress group showed no such reduction [t(35) = 0.546, P = 0.59]. Mean SCR to the CS+ did not differ between groups on day 1 [t(78) = −0.28, P = 0.78, one-tailed]; however, on day 2 stressed participants showed significantly stronger SCR to the CS+ than did the control group, despite both groups undergoing the regulation training [t(78) = −1.76, P = 0.04, one-tailed] (Fig. 2). An additional analysis of our baseline stimulus (CS−) yielded no interaction or group differences across sessions (Fig. S1 and SI Results).
Fig. 2.
Conditioned fear response for each group. Mean SCR for the CS+ for each group across sessions. On day 1, groups demonstrated equivalent levels of SCR. On day 2, fear arousal to the CS+ was successfully diminished for the control group only; no such reduction was shown in the stress group. *P < 0.05; **P < 0.01; error bars denote SEM.
Self-Reported Fear Responses.
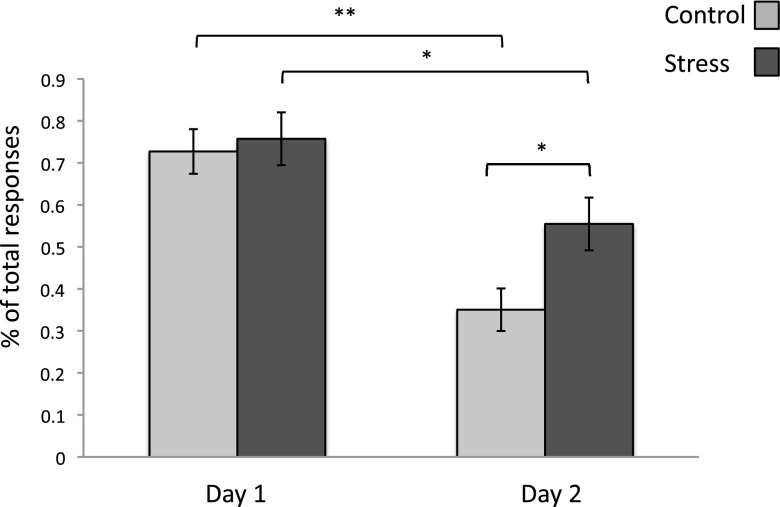
Subjective reports of emotional experience were also consistent with our physiological findings. We examined the influence of stress on self-reported fear by assessing the proportion of fear-related emotions that participants assigned to the CS+ across sessions (Methods). A session X condition repeated-measures ANOVA revealed a main effect of session [day 1, day 2; F(1, 78)= 28.53, P = 0.0000002], a marginally significant main effect of condition [stress, control; F(1, 78) = 3.94, P = 0.050], and a trend toward an interaction [F(1, 78) = 2.57, P = 0.11]. Planned comparisons confirmed that the proportion of fear-related words assigned to the CS+ were equivalent after fear conditioning on day 1 [t(78) = −0.372, P = 0.71], verifying that both groups initially considered the CS+ equally fearful. On day 2, although both groups assigned fewer fear-related words overall to the CS+ [stress: t(35) = 2.38, P = 0.02; control: t(43) = 5.44, P = 0.00001], stressed participants reported a higher number of fear-related emotions for the CS+ than did controls [t(78) = −2.57, P = 0.01], indicating that stress influenced self-reported fear in addition to physiological fear arousal (Fig. 3). The stress group not only assigned significantly more fear-related emotions to the CS+ on day 2, but the average intensity rating for those self-reported fear emotions was also marginally higher (Fig. S2 and SI Results).
Fig. 3.
Average proportion of fear-related words assigned to the CS+ across sessions. Groups did not differ on day 1 before regulation training; however, after the CP/control manipulation on day 2, stressed participants reported a higher proportion of fear-related words for the CS+ than did controls. Both groups significantly reduced the proportion of fear-related words reported across sessions. *P < 0.05, **P < 0.00001; error bars denote SEM.
Stress Analyses.
Self-reported stress.
As expected, stressed participants rated the CP task with significantly higher levels of discomfort than those participants who underwent the control manipulation [t(76) = 10.42, P < 0.00001, two-tailed] (Fig. S3). To assess whether these subjective ratings translated to larger increases in cortisol after the CP/control task, we conducted a correlation analysis between increases in cortisol relative to baseline and self-reported stress ratings. We found a positive correlation between the increase in cortisol and stress ratings both 10 min (r = 0.43, P = 0.0002) and 20 min (r = 0.48, P = 0.00001) after the CP/control task. (Fig. S4). This relationship did not emerge between increases in α-amylase and stress ratings at either time-point (+10 min: r = 0.13, P = 0.28; +20 min: r = 0.04, P = 0.68). No relation was found between these ratings and subsequent fear arousal responses (i.e., SCR) to either the CS+ (r = 0.14; P = 0.20), or the CS− (r = −0.04, P = 0.72).
Neuroendocrine responses.
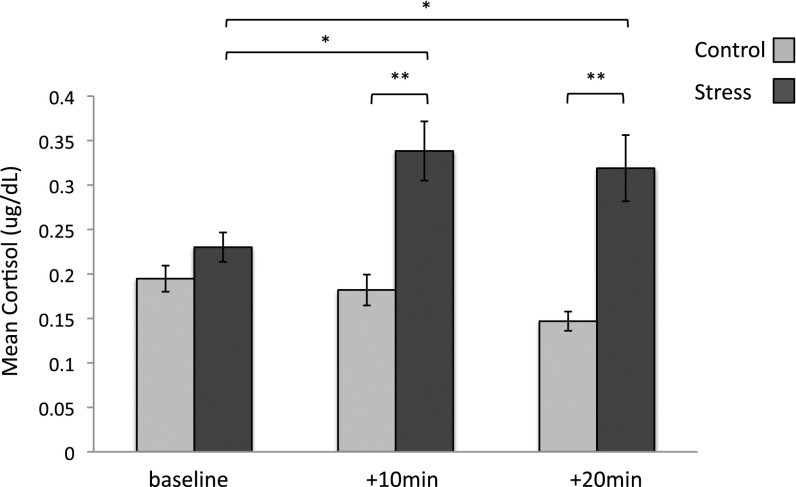
Our two neuroendocrine measures of stress response included cortisol, a reliable measure of HPA-axis activity, and α-amylase, which reflects noradrenergic response. Groups did not differ in α-amylase or cortisol concentrations on day 1 (SI Results). On day 2 we examined how cortisol concentrations differed between groups to confirm the efficacy of our stress manipulation. (Any samples that contained insufficient saliva could not be analyzed and were excluded from this analysis.) A repeated-measures ANOVA with a between-subject factor of condition (stress, control) and within-subject factor of time (baseline, +10 min, +20 min) revealed a main effect of time [F(2, 134) = 5.16, P = 0.007] and condition [F(1, 67) = 16.36, P = 0.0001], as well as a time X condition interaction [F(2, 134) = 13.23, P = 0.000005]. Independent samples t tests confirmed that cortisol levels differed between groups at each time-point measured after the CP/control task [+10 min: t(69) = −4.19, P = 0.00008; +20 min: t(72) = −4.74, P = 0.00001], but not at baseline. Only the stress group showed significantly higher cortisol compared with baseline 10 min [t(34) = −3.69, P = 0.001] and 20 min [t(34) = −3.04, P = 0.005] after the stressor. Participants in the control condition showed no cortisol change 10 min after the control task [t(34) = 1.62, P = 0.11], and demonstrated a significant decrease in cortisol relative to baseline 20 min after the task [t(37) = 5.93, P = 0.0000007] (Fig. 4). A similar analysis using alpha-amylase concentrations yielded no group differences or interaction (SI Results).
Fig. 4.
Mean cortisol levels at baseline, as well as 10 min and 20 min after the CP/control task. *P < 0.01; **P < 0.001; error bars denote SEM.
Neuroendocrine responses and fear regulation.
To explore whether increases in glucocorticoids influenced fear regulation on day 2, we conducted a linear regression using participants’ mean cortisol level 10 and 20 min after the CP/control task as independent variables and physiological fear arousal to the CS+ (i.e., SCR) as our dependent variable. We found no relation between cortisol increase and fear arousal responses across participants either 10 min (β = −0.03, P = 0.77), or 20 min (β = 0.09, P = 0.43), after the CP/control manipulation.
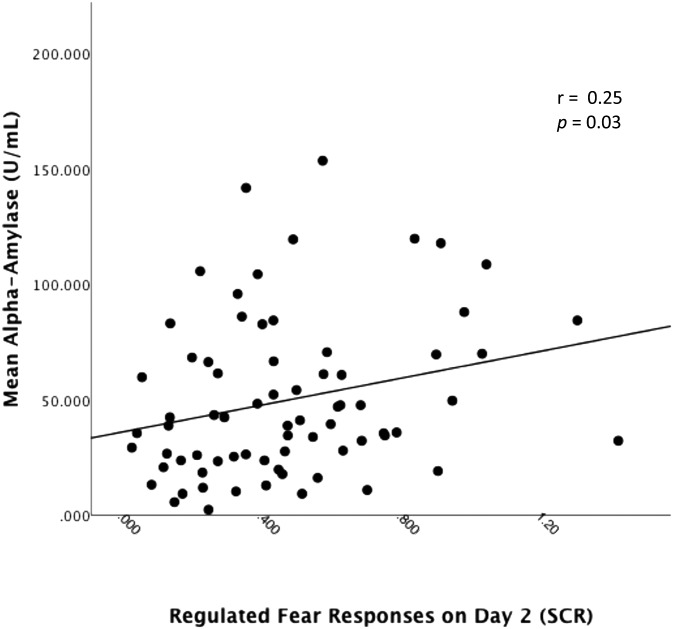
We then examined whether α-amylase levels, indicative of noradrenergic activity, were related to fear regulation responses across participants by using participants’ mean α-amylase level after the CP/control manipulation. Because α-amylase is characterized by a rapid onset and exerts effects more quickly than does cortisol (36–38), we hypothesized that only α-amylase samples taken 10 min after the CP/control task (but not 20 min after) would potentially influence fear arousal. We conducted a linear regression using participants’ mean α-amylase level both 10 and 20 min after the CP/control task on mean SCR during the regulation session. α-Amylase levels assessed 10 min after the CP/control task predicted fear arousal responses across participants (β = 0.25, P = 0.03) (Fig. 5). However, for α-amylase levels 20 min after the CP/control task, this correlation was only trending (β = 0.17, P = 0.13).
Fig. 5.
α-Amylase predicts regulated fear arousal. Mean fear arousal responses to the CS+ during the regulation session as a function of α-amylase levels 10 min after the CP/control manipulation.
Discussion
These results suggest that acute stress impairs the ability to recruit cognitive regulation to control fear responses to aversive cues. Although both groups underwent CRT following initial fear acquisition, only the nonstressed group was able to successfully use these regulation techniques to diminish fear arousal on a subsequent test. In contrast, fear responses in the stress group were comparable to those during the initial fear acquisition, before any regulation training.
Although our data cannot speak to the precise neural mechanisms underlying these fear regulation impairments, we can take advantage of our understanding of the neurobiology of the stress response to hypothesize as to why these effects might emerge. Regulated fear responses on day 2 were correlated with salivary α-amylase, a marker of noradrenergic activity. This finding suggests that early catecholamine responses driven by sympathetic nervous system arousal may be one mechanism by which cognitive control over fear responses is impaired. This finding is consistent with work both in animals (20, 21, 29) and humans (23, 27, 29, 30, 33, 43) showing that elevated noradrenaline levels during stress lead to rapid alterations in executive functioning and impair the PFC. Indeed, a recent pharmacological study conducted in humans showed that exogenously administering a noradrenergic antagonist reduced stress-related neural responsiveness and interconnectivity, whereas a cortisol antagonist had no such effect (35). The finding is also consistent with clinical investigations showing successful treatment of posttraumatic stress disorder symptoms after administration of prazosin, which blocks α-1-adrenoreceptor activity, enhancing prefrontal functioning and dampening amygdala activity (44, 45).
We did not observe a similar correlation between cortisol and fear responses during regulation. The elevations we show in cortisol are indicative of HPA-axis activity and are widely known to facilitate an organism’s recovery from a stressor, but cortisol can also influence prefrontal function by potentiating earlier catecholamine release (20, 29, 46). It has been suggested that cortisol may preserve or exaggerate the effects of catecholamines by inhibiting their clearance from the PFC, thus altering their brain concentration (20, 29, 33, 46). Although only α-amylase levels correlated with fear arousal 10 min after the stressor, it is possible that the lasting elevated cortisol responses in the stress group exacerbated the effects of these noradrenergic inputs in the brain, allowing their effects on PFC function to persist even after detectable differential salivary levels of alpha amylase between the groups dissipated. Therefore, it is possible that both noradrenergic and glucocorticoid responses to stress, and the interacting influence they exert on one another in the brain, serve as a potential mechanism for the impact of stress on the cognitive control of fear. Nonetheless, a clearer understanding of the neural mechanisms that underlie this stress-induced cognitive regulation impairment requires further study.
Although preliminary evidence has shown that individuals with high trait anxiety are impaired at regulating emotions (47), this investigation is unique in explicitly manipulating stress levels in healthy humans during a cognitive emotion regulation task, precisely when this capacity may be compromised. The regulatory deficits described here are consistent with a growing body of evidence suggesting that stress impairs cognitive control and flexibility, presumably by disrupting PFC functioning (20, 21). It is also consistent with theories of self-regulation failure, most notably, those derived from Baumeister and Heatherton et al. (4, 5) that describe self-regulatory capacities as a limited resource that may become weak and depleted either over time (48) or when exposed to negative emotions (4, 5). This influential model of self-regulation asserts that regulatory capacities rely on top-down prefrontal control and may be weakest when the PFC is impaired, or when subcortical regions involved in the automatic emotional response behavior are enhanced (5). Our findings are consistent with this model in a broader context of self-regulation failure and provide a unique demonstration of one underlying reason such self-regulation failure may be common in everyday life.
Considering that situations that require such deliberate effort to control emotions are typically those characterized by stress, the present results highlight important limitations on the use of cognitive strategies to change emotional responses, suggesting that such strategies may be largely ineffective in the face of stress.
Asserting control over our emotional responses to subsequently alter behavior has been of considerable interest to a number of research domains. The stress-induced impairments seen here highlight significant limitations in the real-world application of cognitive regulation in the domains of reward processing (4, 5, 49, 50), decision-making (51–53), and intergroup attitude and bias change (54, 55), all of which have effectively used cognitive regulation to change or influence behavior in the laboratory.
That cognitive emotion regulation techniques are rendered ineffective under stress has broad implications for the efficacy of cognitive regulation to change behavior in everyday life. Importantly, these results offer insight into why strategies taught in the clinic may not always generalize to the real world, where stress exposure is ubiquitous. Alternative forms of emotion regulation that are less reliant on the PFC may be more suitable for changing emotion responses under stress (6). Additionally, under longer durations of training or practice, the recruitment of cognitive regulation strategies might become easier and more habitual, thus relying less on the top-down cognitive control and executive functioning that are compromised by stress (56). Furthering our understanding of how emotion regulation malfunctions under stress may lead to better interventions that foster resistance to stress-induced regulatory impairments and offer better treatment options for clinical populations.
Methods
Participants.
Participants were ineligible for the study if they were pregnant, had experienced heart or blood pressure problems in the past, or were taking any antidepressant or antianxiety medication. All participants signed a consent form approved by New York University’s Committee on Activities Involving Human Subjects and were compensated $30 for their participation. Participants were assigned either two images of spiders or two images of snakes to serve as the CS+ and CS−. To ensure participants were not phobic of these stimuli, they completed the Snake Phobic Questionnaire (SNAQ) and Spider Phobic Questionnaire (SPQ) (57) on-line before participating in the study. Those who scored above 15 on either of these questionnaires (scores range from 0 to 31; higher score indicates increased fear) were considered “phobic” and were thus ineligible to participate. This cut-off is less than that which has been designated for phobic individuals (SNAQ: M = 24.44, SD = 2.95; SPQ: M = 23.76; SD = 3.8) (58). The stimulus category with the higher score was used to ensure the stimuli were emotionally arousing. Participants included in our final analysis had a mean SNAQ and SPQ score of 8.4 (SD = 6.68) and 6.95 (SD = 5.44), respectively, both of which are comparable to that of healthy populations (57).
Because SCRs served as our index of fear arousal, participants who failed to show measurable electrodermal signal to the shock (US) or on >75% of nonreinforced CS+ trials were classified as nonresponders and excluded before the second session (n = 25). Participants who failed to demonstrate adequate differential conditioning (mean CS+ > mean CS− by at least 0.1 μS) across the acquisition session were categorized as nonacquirers and excluded before the second session (n = 44). This criterion was necessary because we could not assess fear regulation without first confirming that participants acquired differential fear to the CS+ vs. CS−; similar criteria have been used in previous fear-conditioning studies (10, 59). Four participants were excluded because of technical errors with data acquisition software and two were excluded for failing to follow experimental instructions. Additionally, four participants from the stress group were unable to complete the CP task and were thus excluded. Two final participants were removed from the stress group before analysis because their cortisol concentrations at baseline were greater than 3 SDs from the mean. Our final analysis included a total of 78 healthy participants (39 females) with a mean age of 23.2 (SD = 8.18; range = 18–54).
Fear Conditioning.
A delay, discrimination fear-conditioning procedure with partial reinforcement was used. CSs were either two distinct images of snakes or two distinct images of spiders (Fig. S5). CS category assignment (i.e., snakes vs. spiders) was counterbalanced across participants, as was the assignment of images within each category as CS+ or CS−. These “prepared stimuli” were used because they are emotionally engaging without eliciting fear, as verified by the SNAQ and SPQ. Two distinct, pseudorandomized trial orders were used such that no trial of the same kind occurred more than two times consecutively; these trial orders were also counterbalanced across participants.
A mild electric wrist-shock (200 ms) served as the US. One image (CS+) coterminated with the US on a subset of trials, whereas the other image (CS−) was never paired with shock (42 trials total; 17 CS+, 17 CS−, 8 CS-US pairings). Partial reinforcement (∼33%) was used to allow unreinforced trials to be analyzed for anticipatory fear arousal without contamination from US responses. On each trial, the CS was presented for 4 s, followed by an 8- to 12-s intertrial interval, during which a fixation cross was presented at the center of the screen. Participants were instructed to pay attention to the relationship between the image and shock, and that this relationship would be discussed with the experimenter afterward. This fear-conditioning protocol was repeated on day 2 (regulation phase) with instructions to use the CRT from the previous day.
Psychophysiological Stimulation and Assessment.
Mild electric shocks (50 pulses per second) were delivered through a bar electrode (Biopac Systems) attached with a Velcro strap to participants’ wrist. The electrode wells were manually filled with NaCl electrolyte gel to enhance conductance and attached to a SD9 Square Pulse Stimulator (Grass Technologies). To identify each individual’s shock level, mild shocks were manually triggered and increased in increments of 5 V until the participant reported the shock to be uncomfortable, but not painful, or 60 V were reached. Our index of fear arousal was SCR, an assay of sympathetic nervous system arousal that reflects changes over discrete intervals consistent with the autonomic arousal characteristic of fear (12, 60). To record SCR, shielded Ag-AgCl electrodes filled with standard NaCl electrolyte gel were first applied between the first and second phalanges of the participant’s left index and middle fingers. SCR was sampled at a rate of 200 Hz and recorded using an MP100 Data Acquisition module (Biopac Systems) connected to an Apple computer. Raw SCR amplitudes were square root transformed to reduce skew and were subsequently divided by individual mean US responses to account for individual differences in shock reactivity (61). We assessed conditioned responding off-line using AcqKnowledge software (Biopac Systems) by analyzing unreinforced trials only. The level of skin conductance was assessed for each trial by taking largest base-to-peak waveform amplitude response (in microsiemens, µS) within the 0.5- to 4.5-s interval after stimulus onset. Responses lower than a predetermined criterion of 0.02 μS were recorded as zero. Each individual’s SCR data were preprocessed using AcqKnowledge software (Biopac Systems) before analysis by low-pass filtering (cut-off frequency 25 Hz) and mean-value smoothing using a three-sample window.
CRT Session.
The CRT session incorporated elements of reappraisal (1–3) and CBT and has been shown previously to persistently attenuate conditioned fear responses (10) (see SI Methods and Figs. S6–S8 for details regarding this protocol). The primary objective of the CRT session was to illustrate how thoughts directly influence emotional responses. Participants were trained to recognize this relationship as well as cognitive errors people typically make when dealing with emotionally charged stimuli or events (i.e., catastrophizing). The participants were then instructed to reappraise the CS+ as less threatening by generating alternative, more positive ways in which they could think about the image, and the fear-conditioning session overall. After this training, participants rerated the intensity of the emotions previously reported to be associated with the CS+. All participants were informed that they would return in 24 h to undergo the same fear-conditioning protocol, during which they should consider and try to incorporate the strategies reviewed during the CRT session.
Stress Manipulation.
On day 2 (regulation phase), participants were randomly assigned to the stress group, or the control group. Participants in the stress group underwent the CP task, during which they submerged their right hand to elbow in a 0–4 °C ice-water bath for 3 min. The CP task is used widely in the laboratory to model the effects of mild to moderate acute stress that participants might encounter in everyday life (35–37) and has been shown to reliably activate sympathetic nervous system and HPA axis arousal as measured by increased physiological, endocrine (i.e., cortisol), and subjective levels of stress (35–38). If a participant was unable to keep their arm in the water, the experiment was terminated (n = 4; see Participants). The control participants submerged their right arms in room-temperature water for 3 min. To assess subjective levels of stress after the task, all participants rated how stressful they found the stress or control task on a scale from 1 (not stressful) to 10 (most stressful) (SI Results). Participants waited 10 min after the CP/control task before repeating the fear-conditioning task on day 2 to allow time for cortisol levels to rise and to ensure participants’ arms in the CP condition were not cold before the fear-conditioning session. Shock levels were recalibrated to ensure that participants still found the US to be uncomfortable but not painful.
Neuroendocrine Analysis.
Cortisol levels were measured using an oral swab that participants placed under their tongue for 2 min. A baseline sample was collected 10 min after participants’ arrival each day to allow for contextual acclimation of the laboratory. On day 1, samples were also collected directly after the fear-conditioning and CRT session. On day 2, samples were collected 10 min after the CP/control manipulation (+10 min) and directly after the fear-conditioning session (+20 min). All samples were stored immediately in a sterile tube in a freezer set to −20 °C for preservation. Samples were analyzed using Salimetrics Testing Services (State College, PA). Samples were brought to room temperature and centrifuged at ∼1500 × g for 15 min before testing.
Self-Reported Fear.
Self-reported emotions and intensity ratings were collected on day 1 after fear-conditioning. To confirm that regulation training reduced the intensity of these reported emotions, participants rerated each emotion after the CRT session. On day 2, after the CP/control manipulation, all participants filled out a worksheet that reviewed the CRT session from the previous day. Here, participants again reported and rated the intensity of three emotions related to each CS. The participants then adjusted any residually fear-related emotions or thoughts to those more positive and adaptive ones that were generated during the CRT session the previous day. Self-reported emotions were categorized into one of six basic affective categories (i.e., fear, disgust, sadness, anger, surprise, happiness), or as neutral (10). Because our objective was to examine how stress affects the regulation of fear, only those emotions categorized under “fear” were analyzed. The proportion of reported emotions categorized as fearful was used to confirm that participants found the CS+ subjectively fearful. Intensity ratings for these fear-related words were averaged across participants and served as an estimate of subjective fear intensity.
Questionnaires.
Participants completed self-report questionnaires at the start of day 1 that included the State and Trait Anxiety Inventory (STAI-S, STAI-T) (62), the Intolerance of Uncertainty Scale (IUS) (63), the Need for Closure Scale (NFCS) (64), the Emotional Regulation Questionnaire (ERQ) (65), and the Pain Catastrophizing Scale (PCS) (66). On day 2, participants repeated the STAI-S, IUS, and PCS. None of these questionnaires yielded any group differences on either day (SI Results).
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health Grants R01 AG039283, MH080756, and MH097085 (to E.A.P.), and a Henry M. MacCracken Graduate Fellowship (to C.M.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305706110/-/DCSupplemental.
References
- 1.Gross JJ. The emerging field of emotion regulation: An integrative review. Rev Gen Psychol. 1998;2(3):271–299. [Google Scholar]
- 2.Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. pp. 3–24. [Google Scholar]
- 3.Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: A synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumeister RF, Heatherton TF. Self-regulation failure: An overview. Psychol Inq. 1996;7(4):1–15. [Google Scholar]
- 5.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartley CA, Phelps EA. Changing fear: The neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35(1):136–146. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frijda NH. The laws of emotion. Am Psychol. 1988;43(5):349–358. doi: 10.1037//0003-066x.43.5.349. [DOI] [PubMed] [Google Scholar]
- 8.Smith CA, Lazarus RS. Appraisal components, core relational themes, and the emotions. Cogn Emotion. 1988;7(3):233–269. [Google Scholar]
- 9.Ochsner KN, Bunge SA, Gross JJ, Gabrieli JDE. Rethinking feelings: An FMRI study of the cognitive regulation of emotion. J Cogn Neurosci. 2002;14(8):1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- 10.Shurick AA, et al. Durable effects of cognitive restructuring on conditioned fear. Emotion. 2012;12(6):1393–1397. doi: 10.1037/a0029143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schiller D, Delgado MR. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16(3):174–180. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 14.Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. J Pers Soc Psychol. 2008;95(6):1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- 15.Gross JJ, Muñoz RF. Emotion regulation and mental health. Clin Psychol Sci Pract. 1995;2:151–164. [Google Scholar]
- 16.Sapolsky RM. Stress, stress-related disease, and emotional regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: Guilford; 2007. [Google Scholar]
- 17.Cisler JM, Olatunji BO, Feldner MT, Forsyth JP. Emotion regulation and the anxiety disorders: An integrative review. J Psychopathol Behav Assess. 2010;32(1):68–82. doi: 10.1007/s10862-009-9161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27(33):8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck AT, Emery G. Anxiety Disorders and Phobias: A Cognitive Perspective. New York, NY: Basic Books; 1985. [Google Scholar]
- 20.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10(6):410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arnsten AFT, Wang MJ, Paspalas CD. Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10(6):423–433. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JK, Hillier A, Smith RM, Tivarus ME, Beversdorf DQ. Beta-adrenergic modulation of cognitive flexibility during stress. J Cogn Neurosci. 2007;19(3):468–478. doi: 10.1162/jocn.2007.19.3.468. [DOI] [PubMed] [Google Scholar]
- 24.Plessow F, Fischer R, Kirschbaum C, Goschke T. Inflexibly focused under stress: Acute psychosocial stress increases shielding of action goals at the expense of reduced cognitive flexibility with increasing time lag to the stressor. J Cogn Neurosci. 2011;23(11):3218–3227. doi: 10.1162/jocn_a_00024. [DOI] [PubMed] [Google Scholar]
- 25.Plessow F, Kiesel A, Kirschbaum C. The stressed prefrontal cortex and goal-directed behaviour: Acute psychosocial stress impairs the flexible implementation of task goals. Exp Brain Res. 2012;216(3):397–408. doi: 10.1007/s00221-011-2943-1. [DOI] [PubMed] [Google Scholar]
- 26.Duncko R, Johnson L, Merikangas K, Grillon C. Working memory performance after acute exposure to the cold pressor stress in healthy volunteers. Neurobiol Learn Mem. 2009;91(4):377–381. doi: 10.1016/j.nlm.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behav Neurosci. 2005;119(1):98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- 28.Luethi M, Meier B, Sandi C. Stress effects on working memory, explicit memory, and implicit memory for neutral and emotional stimuli in healthy men. Front Behav Neurosci. 2009;15:1–9. doi: 10.3389/neuro.08.005.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24(6):1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schoofs D, Wolf OT, Smeets T. Cold pressor stress impairs performance on working memory tasks requiring executive functions in healthy young men. Behav Neurosci. 2009;123(5):1066–1075. doi: 10.1037/a0016980. [DOI] [PubMed] [Google Scholar]
- 31.Goldman-Rakic PS. The prefrontal landscape: Implications of functional architecture for understanding human mentation and the central executive. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1445–1453. doi: 10.1098/rstb.1996.0129. [DOI] [PubMed] [Google Scholar]
- 32.Fuster JM. The Prefrontal Cortex. London: Academic; 2008. [Google Scholar]
- 33.Qin S, Hermans EJ, van Marle HJ, Luo J, Fernández G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66(1):25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 34.Kern S, et al. Glucose metabolic changes in the prefrontal cortex are associated with HPA axis response to a psychosocial stressor. Psychoneuroendocrinology. 2008;33(4):517–529. doi: 10.1016/j.psyneuen.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovallo W. The cold pressor test and autonomic function: A review and integration. Psychophysiology. 1975;12(3):268–282. doi: 10.1111/j.1469-8986.1975.tb01289.x. [DOI] [PubMed] [Google Scholar]
- 36.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: Implications for the field of brain and cognition. Brain Cogn. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Velasco M, Gómez J, Blanco M, Rodriguez I. The cold pressor test: Pharmacological and therapeutic aspects. Am J Ther. 1997;4(1):34–38. doi: 10.1097/00045391-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 38.McRae AL, et al. Stress reactivity: Biological and subjective responses to the cold pressor and trier social stressors. Hum Psychopharmacol. 2006;21(6):377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- 39.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 40.Thoma MV, Kirschbaum C, Wolf JM, Rohleder N. Acute stress responses in salivary alpha-amylase predict increases of plasma norepinephrine. Biol Psychol. 2012;91(3):342–348. doi: 10.1016/j.biopsycho.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 41.Nater UM, Rohleder N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology. 2009;34(4):486–496. doi: 10.1016/j.psyneuen.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 42.van Stegeren A, Rohleder N, Everaerd W, Wolf OT. Salivary alpha amylase as marker for adrenergic activity during stress: Effect of betablockade. Psychoneuroendocrinology. 2006;31(1):137–141. doi: 10.1016/j.psyneuen.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 43.Hermans EJ, et al. Stress-related noradrenergic activity prompts large-scale neural network reconfiguration. Science. 2011;334(6059):1151–1153. doi: 10.1126/science.1209603. [DOI] [PubMed] [Google Scholar]
- 44.Taylor F, Raskind MA. The alpha1-adrenergic antagonist prazosin improves sleep and nightmares in civilian trauma posttraumatic stress disorder. J Clinical Psychopharmacol. 2002;22(1):82–85. doi: 10.1097/00004714-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Raskind MA, et al. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: A placebo-controlled study. Am J Psychiatry. 2003;160(2):371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- 46.Gründemann D, Schechinger B, Rappold GA, Schömig E. Molecular identification of the corticosterone-sensitive extraneuronal catecholamine transporter. Nat Neurosci. 1998;1(5):349–351. doi: 10.1038/1557. [DOI] [PubMed] [Google Scholar]
- 47.Indovina I, Robbins TW, Núñez-Elizalde AO, Dunn BD, Bishop SJ. Fear-conditioning mechanisms associated with trait vulnerability to anxiety in humans. Neuron. 2011;69(3):563–571. doi: 10.1016/j.neuron.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vohs KD, Heatherton TF. Self-regulatory failure: A resource-depletion approach. Psychol Sci. 2000;11(3):249–254. doi: 10.1111/1467-9280.00250. [DOI] [PubMed] [Google Scholar]
- 49.Delgado MR, Gillis MM, Phelps EA. Regulating the expectation of reward via cognitive strategies. Nat Neurosci. 2008;11(8):880–881. doi: 10.1038/nn.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li CS, Sinha R. Inhibitory control and emotional stress regulation: Neuroimaging evidence for frontal-limbic dysfunction in psycho-stimulant addiction. Neurosci Biobehav Rev. 2008;32(3):581–597. doi: 10.1016/j.neubiorev.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hutcherson CA, Plassmann H, Gross JJ, Rangel A. Cognitive regulation during decision making shifts behavioral control between ventromedial and dorsolateral prefrontal value systems. J Neurosci. 2012;32(39):13543–13554. doi: 10.1523/JNEUROSCI.6387-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mitchell DGV. The nexus between decision making and emotion regulation: A review of convergent neurocognitive substrates. Behav Brain Res. 2011;217(1):215–231. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- 53.Sokol-Hessner P, et al. Thinking like a trader selectively reduces individuals’ loss aversion. Proc Natl Acad Sci USA. 2009;106(13):5035–5040. doi: 10.1073/pnas.0806761106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amodio DM, Devine PG. Control in the regulation of intergroup bias. In: Hassin RR, Ochsner KN, Trope Y, editors. Self Control in Society, Mind and Brain. NY: Oxford Univ Press; 2010. [Google Scholar]
- 55.Krendl AC, Kensinger EA, Ambady N. How does the brain regulate negative bias to stigma? Soc Cogn Affect Neurosci. 2012;7(6):715–726. doi: 10.1093/scan/nsr046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hauner KK, Mineka S, Voss JL, Paller KA. Exposure therapy triggers lasting reorganization of neural fear processing. Proc Natl Acad Sci USA. 2012;109(23):9203–9208. doi: 10.1073/pnas.1205242109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Klorman R, Weerts TC, Hastings JE, Melamed BG, Lang PJ. Psychometric description of some specific-fear questionnaires. Behav Ther. 1974;5(3):401–409. [Google Scholar]
- 58.Fredrikson M. Reliability and validity of some specific fear questionnaires. Scand J Psychol. 1983;24(4):331–334. doi: 10.1111/j.1467-9450.1983.tb00507.x. [DOI] [PubMed] [Google Scholar]
- 59.Schiller D, et al. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2010;463(7277):49–53. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Critchley HD. Electrodermal responses: What happens in the brain. Neuroscientist. 2002;8(2):132–142. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- 61.Lykken DT, Venables PH. Direct measurement of skin conductance: A proposal for standardization. Psychophysiology. 1971;8(5):656–672. doi: 10.1111/j.1469-8986.1971.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 62.Spielberger CD, Gorsuch RL, Lusthene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 63.Buhr K, Dugas MJ. The Intolerance of Uncertainty Scale: Psychometric properties of the English version. Behav Res Ther. 2002;40(8):931–945. doi: 10.1016/s0005-7967(01)00092-4. [DOI] [PubMed] [Google Scholar]
- 64.Kruglanski AW, Webster DM, Klem A. Motivated resistance and openness to persuasion in the presence or absence of prior information. J Pers Soc Psychol. 1993;65(5):861–876. doi: 10.1037//0022-3514.65.5.861. [DOI] [PubMed] [Google Scholar]
- 65.Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- 66.Sullivan MJL, Bishop S, Pivik J. The Pain Catastrophizing Scale: Development and validation. Psychol Assess. 1995;7(4):524–532. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.