Abstract
The rationale of α1-antitrypsin (AAT) augmentation therapy to treat progressive emphysema in AAT-deficient patients is based on inhibition of neutrophil elastase; however, the benefit of this treatment remains unclear. Here we show that clinical grade AAT (with elastase inhibitory activity) and a recombinant form of AAT (rAAT) without anti-elastase activity reduces lung inflammatory responses to LPS in elastase-deficient mice. WT and elastase-deficient mice treated with either native AAT or rAAT exhibited significant reductions in infiltrating neutrophils (23% and 68%), lavage fluid levels of TNF-α (70% and 80%), and the neutrophil chemokine KC (CXCL1) (64% and 90%), respectively. Lung parenchyma TNF-α, DNA damage-inducible transcript 3 and X-box binding protein-1 mRNA levels were reduced in both mouse strains treated with AAT; significantly lower levels of these genes, as well as IL-1β gene expression, were observed in lungs of AAT-deficient patients treated with AAT therapy compared with untreated patients. In vitro, LPS-induced cytokines from WT and elastase-deficient mouse neutrophils, as well as neutrophils of healthy humans, were similarly reduced by AAT or rAAT; human neutrophils adhering to endothelial cells were decreased by 60–80% (P < 0.001) with either AAT or rAAT. In mouse pancreatic islet macrophages, LPS-induced surface expression of MHC II, Toll-like receptor-2 and -4 were markedly lower (80%, P < 0.001) when exposed to either AAT or rAAT. Consistently, in vivo and in vitro, rAAT reduced inflammatory responses at concentrations 40- to 100-fold lower than native plasma-derived AAT. These data provide evidence that the anti-inflammatory and immunomodulatory properties of AAT can be independent of elastase inhibition.
Keywords: alpha 1-antitrypsin, inflammation, immunomodulation
Alpha1-antitrypsin (AAT) is the prototypic member of the serpin superfamily and one of the most abundant serine protease inhibitors in the circulation (1). As an acute-phase protein, AAT is thought to play an important role in limiting host-tissue injury triggered by proteases, particularly neutrophil elastase (NE). The clinical relevance of AAT is highlighted in individuals with inherited deficiency in circulating AAT, who have increased susceptibility to early-onset pulmonary emphysema, liver and pancreatic diseases, and in rare cases to panniculitis and vasculitis (2). It has been assumed that in AAT-deficiency the protease/antiprotease balance is shifted toward NE, which leads to extensive tissue damage, particularly in causing emphysema. Therefore, augmentation of circulating AAT was introduced 25 y ago to treat emphysema patients with severe PiZZ (Glu342Lys) AAT deficiency (3). Because clinical trials of AAT augmentation therapy use historical data as controls, the benefit of AAT therapy for PiZZ patients remains under debate (4–6), although in most cases therapy offers disease stabilization. The uncertainty surrounding the efficacy of augmentation therapy also reflects the incomplete understanding of the properties of the AAT protein, which can be affected by the isolation methods from plasma.
Although the administration of exogenous human plasma-derived AAT is used in experimental models to validate the putative benefit of AAT augmentation therapy, plasma-derived AAT also suppresses inflammatory and immunomodulating pathways that appear to be independent of elastase inhibition (reviewed in refs. 7 and 8). For example, the addition of exogenous AAT in vitro inhibits the release of IL-8 by monocytes (9) and the expression of HIV-1 (10). In animal models, the administration of AAT prevents murine islet cell allografts from rejection (11), blocks β-cell apoptosis (12), and suppresses alloreactivity in allogeneic marrow transplantation models (13, 14). In other models, AAT therapy reduces TNF-α– or endotoxin-induced lethality, cigarette smoke-induced emphysema and inflammation, and suppresses bacterial proliferation during infections (15–17).
Whereas studies suggest that the anti-inflammatory properties and immunomodulating effects of AAT are unrelated to inhibition of elastase, there is no direct proof of this. Therefore, we examined the effects of clinical grade AAT with anti-elastase activity and a recombinant form of AAT (rAAT), which lacks the ability to inhibit elastase. We used the model of LPS-induced acute lung injury in WT and NE-deficient mice. Additional studies were carried out in blood-derived neutrophils isolated from healthy humans or bone marrow neutrophils from either WT or NE-deficient mice. Changes in surface expression of immune markers Toll-like receptor (TLR)4, TLR2, and MHC II on macrophages from mouse pancreatic islet cells were also studied.
Results
AAT Reduces LPS-Induced Acute Lung Inflammation in WT and NE-Deficient Mice.
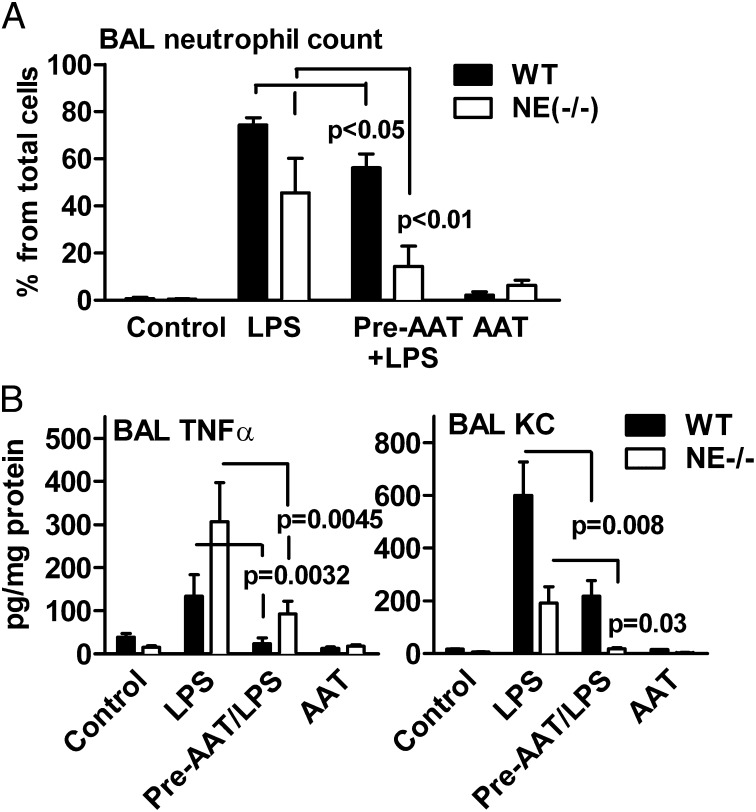
As expected, the inflammatory response in WT mice challenged with LPS resulted in a significant increase in bronchoalveolar lavage (BAL) elastase activity, whereas mice pretreated with AAT (Prolastin) exhibited no elastase activity (Fig. S1A). As shown in Table 1 and Fig. 1A, intranasal LPS increased total BAL cells by sixfold (P < 0.001) and the percent of neutrophils (by 80%) relative to controls or Prolastin-treated mice. Treatment with LPS also increased numbers of eosinophils (by 2.5%, P < 0.05) and lymphocytes (by 2%), whereas there was a decrease of 88% (P < 0.001) in macrophages (Table S1). However, 24 h after LPS, WT mice pretreated with 2 mg of Prolastin exhibited reduced BAL neutrophils of 23% (P < 0.05) and markedly lower levels of BAL TNF-α (70%) and cytokine KC (CXCL1) (64%) compared with LPS plus vehicle (Fig. 1). There was a similar decrease in total BAL cells (by about 45%) as well as BAL neutrophils (65%), TNF-α (77%), and KC (86%) in NE-deficient mice pretreated with Prolastin (Fig. 1 and Table 1). Noticeably, baseline levels of lymphocytes were found to be higher in NE-deficient mice relative to WT mice. However, when NE-deficient mice were challenged with LPS or with LPS after pretreatment with Prolastin, lymphocyte numbers did not change significantly (Table S1).
Table 1.
BAL cells in WT and NE-deficient mice
| Condition | WT | NE-deficient |
| Vehicle | 2.66 ± 1.3* | 7.87 ± 0.63 |
| LPS | 16.27 ± 6.8** | 35.40 ± 14.4** |
| AAT-LPS | 7.66 ± 3.4*** | 19.98 ± 8.0*** |
| AAT | 4.96 ± 1.3 | 9.70 ± 6.7 |
n = 8 per group. *BAL cells × 105; **P < 0.001 difference between vehicle and LPS; ***P = 0.008 and P = 0.037, difference between LPS and AAT-LPS in WT and NE deficient, respectively.
Fig. 1.
Neutrophil infiltration and cytokine levels in BAL fluid in WT and NE-deficient mice. Twenty-four hours before LPS challenge, WT mice were treated with 2 mg of AAT (Prolastin). (A) Mean ± SD percent of neutrophils of total cells in WT (n = 8) and NE-deficient mice (n = 7). (B) Mean ± SD levels of TNF-α and KC in WT and NE-deficient mice (n = 8 per group). The statistical significance values are between LPS in the presence and absence of AAT treatment. The data are from the same samples shown in Table 1.
Recombinant AAT Lacking Elastase Inhibition Suppresses Acute Lung Injury.
We next examined the effects of rAAT, which is fused to the Fc of IgG1 and lacks the ability to inhibit elastase (SI Materials and Methods and Fig. S1B). WT mice were pretreated with 50 µg of rAAT intranasally for 24 h followed by intranasal challenge with LPS. There was a decrease of 30% (P < 0.05) in BAL neutrophils after 24 h. Similarly, there were lower levels of TNF-α and KC in the BAL fluid; mean KC decreased from 153 ± 30.5 to 21.8 ± 8.7 pg/mg protein (86% decrease, P < 0.01) and TNF-α decreased from 149 ± 35 to 27 ± 10 pg/mg protein (89% decrease, P < 0.01).
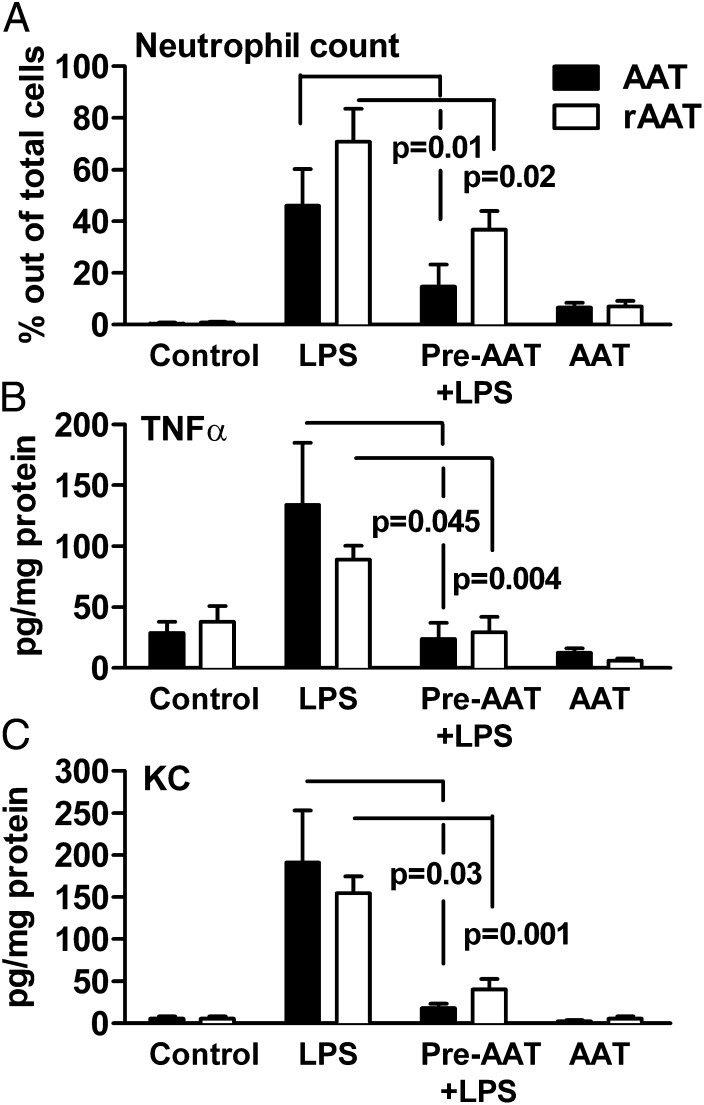
The experiment was repeated in mice deficient in NE. As shown in Fig. 2A, pretreatment of NE-deficient mice with 50 µg rAAT suppressed LPS-induced BAL fluid neutrophils by 48% (Fig. 2A, open bars). In comparison, in mice pretreated with 2 mg of Prolastin (40-fold more than rAAT), neutrophil numbers decreased by 68%. We also observed a decrease in BAL fluid TNF-α of 64% (Fig. 2B) and KC of 56% (Fig. 2C) in the NE-deficient mice receiving 50 µg rAAT. For comparison, the decreases in BAL fluid KC and TNF-α in NE-deficient mice pretreated with 2 mg of Prolastin from Fig. 1 are shown in Fig. 2. Thus, rAAT without the ability to inhibit elastase reduced cellular infiltration and cytokine levels at a dose 40-fold lower than that of Prolastin.
Fig. 2.
Comparison of AAT to rAAT in LPS-induced acute lung injury in NE-deficient mice. NE-deficient mice were intranasally pretreated with rAAT (50 µg per mouse, 13 per group, open bars) or AAT (2 mg Prolastin, 7 per group, filled bars) 24 h before LPS challenge and BAL fluid obtained after an additional 24 h. (A) Mean ± SD percent of neutrophils. (B) Mean ± SD TNF-α levels per milligram of BAL protein. (C) Mean ± SD KC levels. Data for AAT (Prolastin)-treated NE-deficient mice are taken from Fig. 1 and shown for comparison.
Lower Proinflammatory Gene Expression in Lung Tissue of Mice Pretreated with AAT.
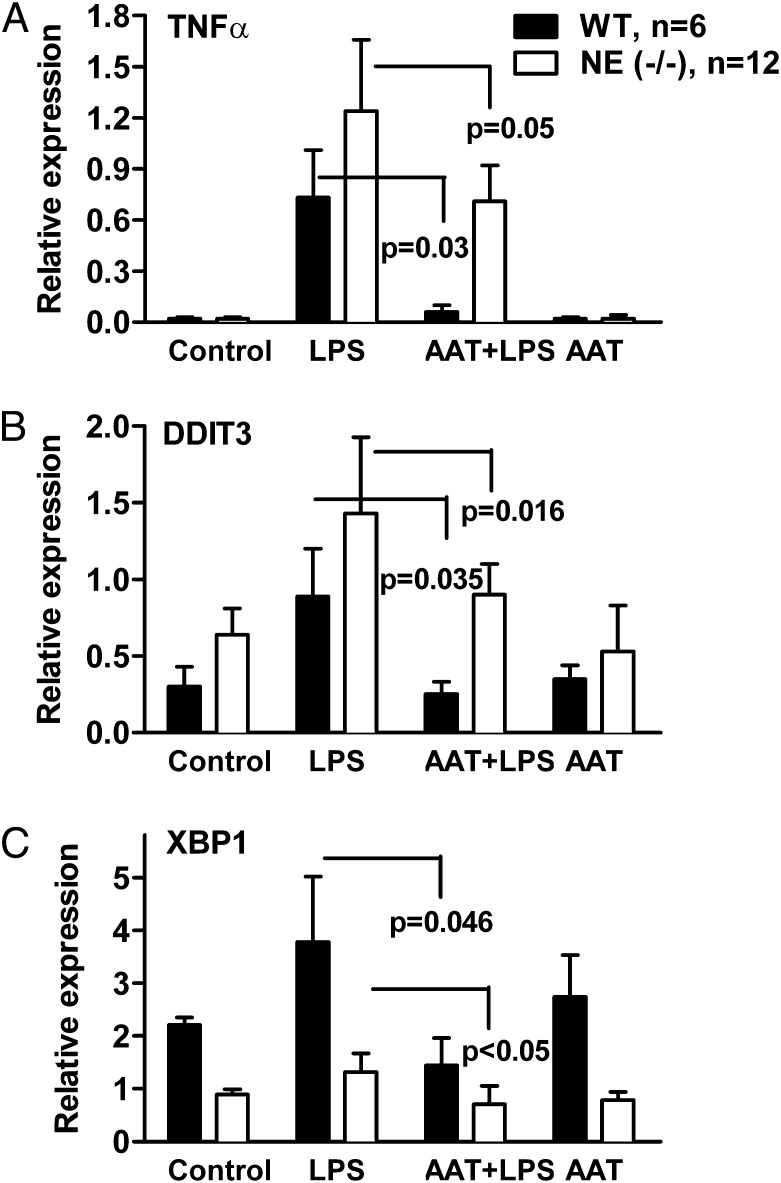
mRNA was prepared from whole lung tissue and expression of inflammation-associated genes was measured using quantitative real-time RT-PCR. Compared with controls (Fig. 3A), WT mice challenged with LPS increased the relative expression of TNF-α (24.5-fold, P = 0.002), DNA damage-inducible transcript 3 (DDIT3; 6.6-fold, P = 0.01) and X-box binding protein-1 (XBP1; 1.6-fold, not significant). Similarly, treatment of NE-deficient mice with LPS resulted in enhanced relative expression of TNF-α (42.8-fold, P < 0.001), DDIT3 (2.25-fold, P < 0.001), and XBP1 (1.36-fold). WT mice pretreated with Prolastin exhibited a decrease in LPS-induced mRNA levels of 92% for TNF-α, 90% for DDIT3, and 59% for XBP1. In NE-deficient mice, pretreatment with Prolastin led to a reduction in LPS-induced expression of 26% for TNF-α, 33% for DDIT3, and 26% for XBP1. Thus, Prolastin reduces the expression of selected genes following inflammatory response to LPS in either WT or NE-deficient mice.
Fig. 3.
(A–C) AAT reduces proinflammatory gene expression in lung tissue following LPS challenge in WT and NE-deficient mice. Before LPS challenge, WT mice (n = 6) and NE-deficient mice (n = 12) were pretreated for 24 h with 2 mg of intranasal AAT (Prolastin) per mouse. Twenty-four hours after LPS challenge, mRNA was prepared from whole lung tissue. The relative gene expression in each group is shown as the mean ± SD.
Lower Proinflammatory Gene Expression in Lungs from AAT-Deficient Patients Treated with Augmentation Therapy.
The changes in gene expression observed in the lungs of mice treated with Prolastin following LPS were mirrored in the laser-microdissected areas of lungs from ZZ AAT deficiency-related emphysema patients treated with augmentation therapy. There was lower expression of DDIT3 (2.4-fold, P = 0.025), XBP1 (52.1-fold, P = 0.052), activating transcription factor 4 (ATF4) (10.7-fold, P = 0.05), and IL-1β (18%, P = 0.054) compared with nontreated patients (Figs. S2 and S3).
Comparison of AAT and rAAT on LPS-Induced Release of TNF-α and KC from WT and NE-Deficient Mouse Bone Marrow Neutrophils.
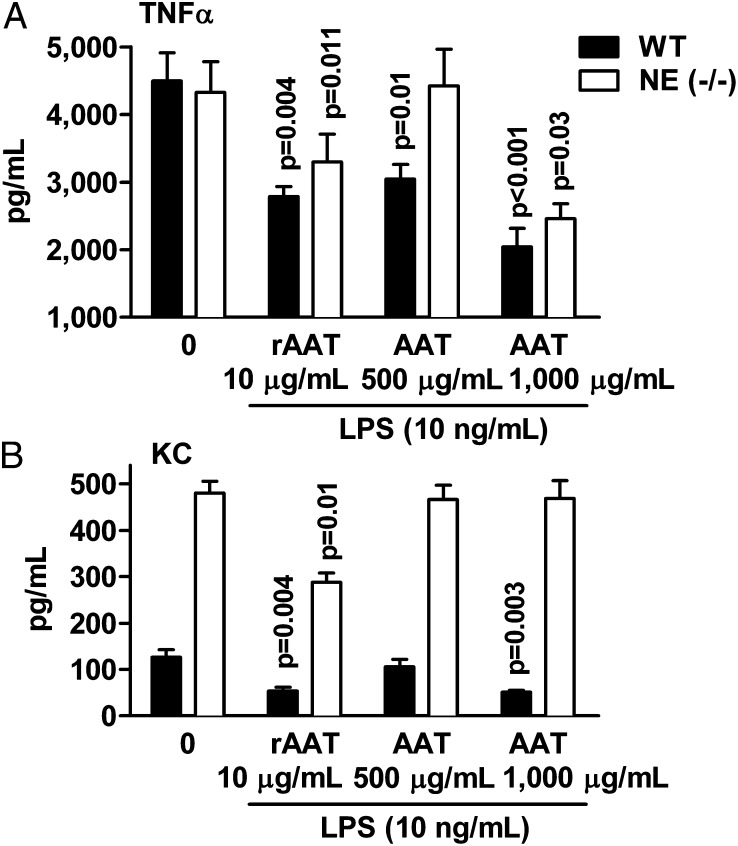
As illustrated in Fig. 4, when WT or NE-deficient neutrophils were pretreated with rAAT before the addition of LPS, the release of TNF-α was decreased (42% and 24%, respectively) compared with LPS. The release of KC also decreased (57% and 40%, respectively). We observed a similar reduction in TNF-α from either WT or NE-deficient neutrophils exposed to Prolastin but at a higher concentration compared with rAAT (Fig. 4A). Increasing the concentration of Prolastin to 1 mg/mL lowered LPS-induced TNF-α (by 55% and 43%, respectively) and KC (by 60%, P = 0.03) in WT mice. At 2 mg/mL of Prolastin, LPS-induced KC release in NE-deficient mice decreased (47%, P = 0.04).
Fig. 4.
AAT and rAAT reduce LPS-induced release of cytokines from mouse neutrophils in vitro. Bone marrow-derived mouse neutrophils (2 × 106 per mL) were preincubated for 1 h with and without AAT (Prolastin) or rAAT and then stimulated with LPS (10 ng/mL) for 8 h at 37 °C. Mean ± SE levels of (A) TNF-α and (B) KC released from neutrophils of WT (n = 6) and NE-deficient (n = 8) mice. Concentrations of Prolastin and rAAT are indicated. Zero indicates LPS without either AAT or rAAT.
Comparison of AAT and rAAT on LPS-Induced Release of TNF-α and IL-8 from Human Neutrophils.
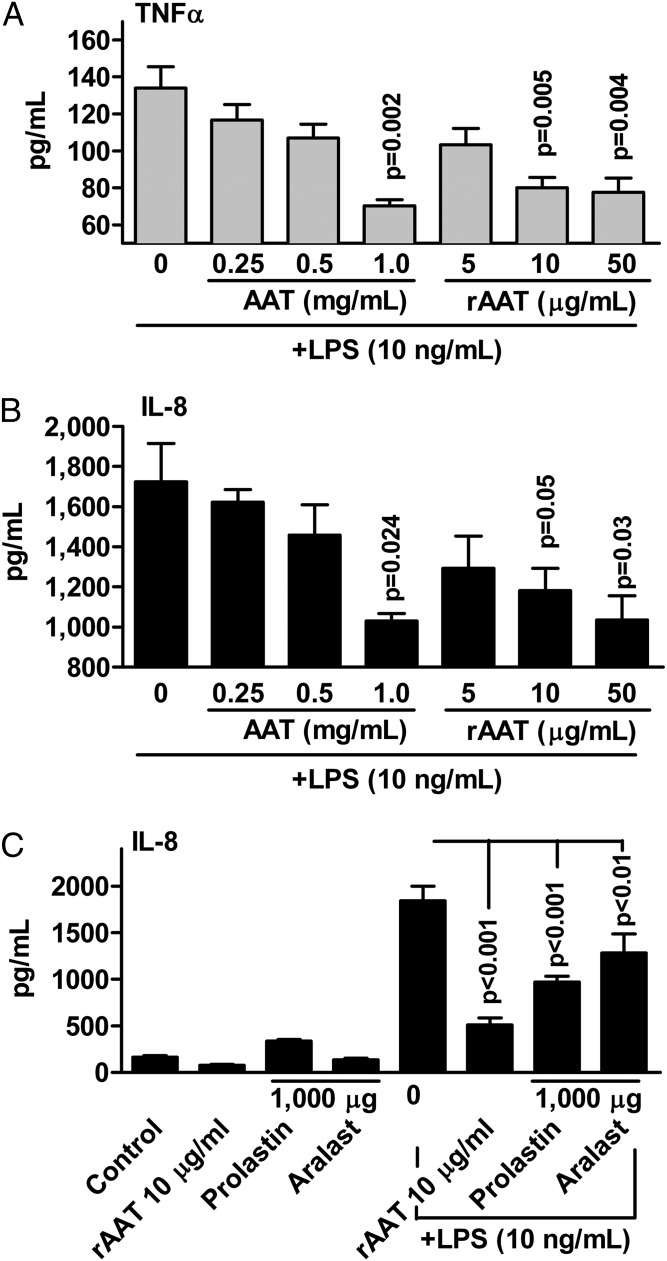
Freshly isolated human blood neutrophils from healthy subjects were incubated for 8 h with LPS with and without increasing concentrations of AAT (Prolastin) or rAAT. As shown in Fig. 5A, at 1 mg/mL Prolastin reduced the release of LPS-induced TNF-α by 46% (P = 0.002) and of IL-8 by 29% (P = 0.024). However, rAAT at markedly lower concentrations of 10 μg/mL reduced TNF-α by 41% (P = 0.005) and IL-8 release by 40% (P = 0.05). For IL-8, a similar reduction was observed with either Prolastin or Aralast concentrations of 1 mg/mL, compared with 10 μg/mL of rAAT (a 100-fold difference) (Fig. 5C). For TNF-α, comparable reductions were achieved at 200-fold lower concentrations of rAAT relative to Prolastin and Aralast.
Fig. 5.
AAT and rAAT reduce LPS-induced release of cytokines from human neutrophils in vitro. Human blood neutrophils (2 × 106 per mL) isolated from six donors were preincubated with AAT (Prolastin in A and B) or rAAT for 1 h and then stimulated with LPS (10 ng/mL) for 8 h. (A) Mean ± SE TNF-α release at 8 h. (B) Mean ± SE IL-8 release. (C) Mean ± SE IL-8 release in an additional three donors comparing Prolastin, Aralast, and rAAT. Concentrations are micrograms per milliliter (µg/mL).
Because engagement of Fc receptors on neutrophils can result in release of IL-8 (18), we blocked the Fc domain on rAAT. Recombinant AAT retained its ability to reduce LPS-induced IL-8 in the presence of an Fc receptor blocker (Figs. S4 and S5) or 1% serum (Fig. S6), supporting the concept that the Fc domain of rAAT does not contribute to the anti-inflammatory potency of rAAT. In addition, we used IgG Fc fragments as a control for rAAT and found that these have no effect on LPS-induced IL-8 release (Fig. S7).
Neutrophils are a source of the IL-1 receptor antagonist (IL-1Ra). We measured IL-1Ra release in cells incubated with Prolastin or rAAT only or with LPS. As shown in Fig. S8, Prolastin and rAAT each induced the release of IL-1Ra alone but also augmented LPS-induced IL-1Ra secretion by 2.5- and 4.6-fold, respectively. Under these experimental conditions, cell viability remained unaffected by Prolastin (88.2%) or rAAT (86.5%), compared with nontreated controls (82.1%) (Fig. S9).
AAT and rAAT Prevent Adhesion of Activated Human Neutrophils to Human Lung Microvascular Endothelial Cells.
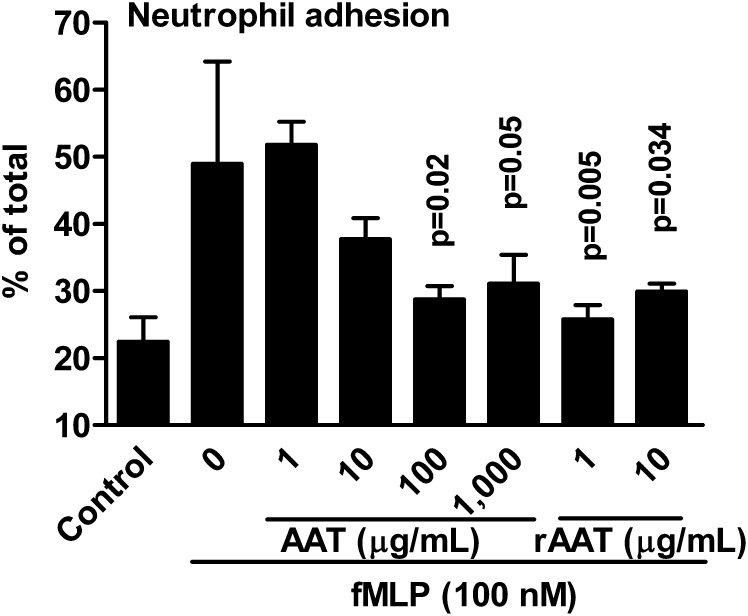
The oxidative stress-induced activation of neutrophils increases adhesion to endothelial cells (19). Because AAT inhibits IL-8 production and possesses antioxidant activities (20), we investigated whether AAT affects neutrophil adhesion to lung endothelial cells. Human neutrophils were labeled with calcein and stimulated with N-formyl-methionyl-leucyl-phenylalanine (fMLP). Calcein-labeled human neutrophils were first preincubated with increasing concentrations of either AAT (Prolastin) or rAAT for 1 h, exposed to fMLP, and then added to human lung microvascular endothelial cells (HMVEC-L). As shown in Fig. 6, a significant reduction (40%) was achieved at 100μg/mL Prolastin, compared with 1 μg/mL rAAT.
Fig. 6.
AAT and rAAT inhibit human neutrophil adhesion to primary human lung microvascular endothelial cells. Mean ± SE percent-change of fMLP activated human blood neutrophils adhering to the human lung HMVEC. The neutrophils were pretreated with or without AAT (Prolastin) or rAAT. Concentrations of Prolastin and rAAT are depicted under the horizontal axis. The data are derived from the neutrophils of three donors, each performed in quadruplicate. The P values are in comparison with fMLP-stimulated neutrophils without exposure to either AAT or rAAT.
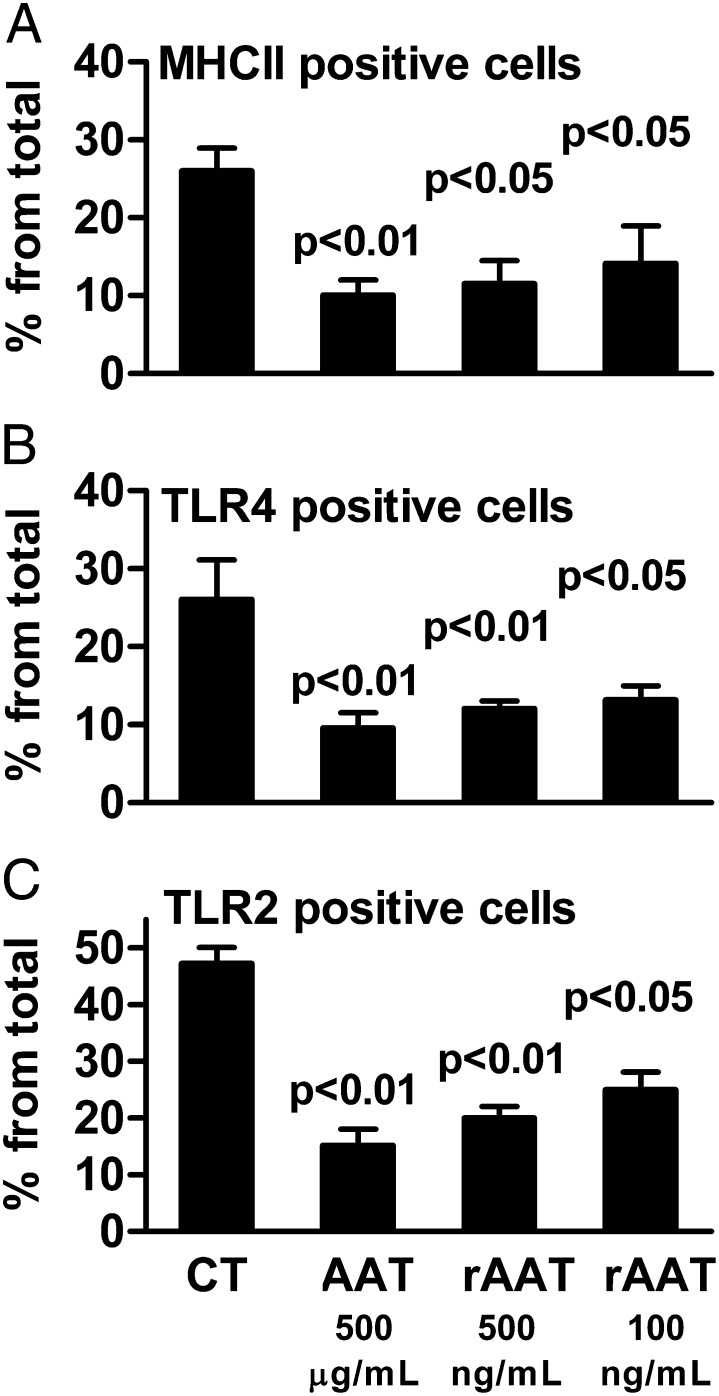
AAT and rAAT Reduce MHC II, TLR4, and TLR2 Expression on Mouse Pancreatic Islet Macrophages.
Plasma-derived AAT (Aralast) monotherapy prolongs survival of islet allograft transplants (11), induces immune tolerance to allografts (14), and prevents the development of diabetes in the nonobese diabetic mouse (12). We incubated LPS-stimulated mouse pancreatic islets with AAT (Aralast) as well as rAAT and determined the surface expression of MHC II, TLR4, and TLR2 on islet macrophages. As shown in Fig. 7, there are significant reductions in these markers at concentrations of rAAT significantly lower than that of AAT.
Fig. 7.
Expression of macrophage surface receptors on islet cell macrophages exposed to AAT or rAAT. Mean ± SE change in the percent of cells from CD45+CD11b+ cells expressing surface markers for MHC II (A), TLR4 (B), and TLR2 (C) 72 h after exposure to LPS (10 ng/mL) in the presence of AAT (Aralast) or rAAT at the indicated concentrations. The data are one of three similar experiments, each performed in triplicate.
Discussion
The preferred target of AAT is NE (21); therefore, the dominant theory for the pathogenesis of AAT deficiency-related emphysema is reduced pulmonary protection against NE. This concept finds support by the high neutrophil burden and increased levels of free elastase in patients with AAT deficiency-related emphysema (22). Thus, inhibition of NE has consistently been the objective in the development of therapeutic strategies for these patients. However, the broad anti-inflammatory and immunomodulating properties of AAT (7, 8, 23) raise the question whether elastase inhibition accounts for the spectrum of biological activities of AAT. Here, we compared in vitro and in vivo anti-inflammatory effects of clinical grade AAT with elastase inhibitory activity to a form of recombinant AAT that does not inhibit elastase. We also compared LPS-induced acute lung injury and neutrophils in WT mice and elastase-deficient mice.
As expected, 24 h following LPS there were markedly increased numbers of BAL neutrophils, enhanced TNF-α and KC levels in lung tissue, and significantly elevated levels of BAL elastase in WT mice. Mice deficient in NE also exhibited the same changes but an even greater degree of inflammation was observed. However, administration of AAT (Prolastin) ameliorated the LPS-induced inflammatory effects in the lungs of WT and NE-deficient mice to the same extent. Furthermore, pretreatment of neutrophils from WT or NE-deficient mice with either Prolastin or rAAT reduced or abolished LPS-induced TNF-α and IL-8. In addition to the reduction in LPS-induced IL-8, human neutrophils released increased levels of the anti-inflammatory cytokine IL-1Ra when exposed to either Prolastin or rAAT (Fig. S8).
Both Prolastin and rAAT reduced fMLP-activated human neutrophil adhesion to lung-derived endothelial cells. It is known that AAT suppresses neutrophil function, including IL-8 release and adherence to endothelial cells (24, 25). Indeed, the absence of elastase had no effect on neutrophil recruitment (26), which supports our findings. Circulating AAT enters cells (27) and can act as an inhibitor for matriptase (28), caspases-1 and -3 (29, 30), TNF-α–converting enzyme (31), and intracellular calpain I (32).
It is now generally accepted that LPS induces the endoplasmic reticulum (ER) stress-DDIT pathway in the mouse lung (33). During this inflammatory response, activation of ER stress-related gene expression, such as DDIT3 (also termed CHOP) and XBP-1, occurs in a time course similar to that for the production of TNF-α and KC. Originally, DDIT3 was thought to induce apoptosis; however, evidence reveals that LPS-induced DDIT3 activates the IL-1β pathway (34), which plays a central role in the early stages of the inflammatory response (35). Remarkably, pretreatment of WT or NE-deficient mice with Prolastin reduced LPS-induced DDIT3 and XBP-1 expression in lung tissue. Based on these findings, we examined whether ZZ AAT deficiency-related emphysema patients treated with Prolastin had lower DDIT3 expression in their lungs. Indeed, we found that augmentation therapy significantly lowered DDIT3, as well as XBP-1 and IL-1β expression, compared with matched but untreated patients. Increased inflammation can be a manifestation of ER stress resulting from misfolded proteins, and may contribute to the pathophysiology of chronic obstructive pulmonary disease (36).
The decrease in cytokine/chemokine release from mouse or human neutrophils, the reduced adhesion of neutrophils to endothelial cells, and the lower surface expression in LPS-induced MHC II, TLR4, and TLR2 in islet macrophages were observed at concentrations of rAAT markedly lower than those of plasma-derived AAT (Prolastin or Aralast). One explanation is that AAT purified from human plasma has lost much of its anti-inflammatory and immunomodulating properties because of methods such as low pH, ionic strength, and oxidation (37); the cold ethanol precipitation of plasma during the purification may impair the nonprotease, anti-inflammatory, and immunomodulating domains of AAT. High heat and detergents associated with reducing viral contamination of human plasma products may also affect these domains. In fact, Prolastin contains latent forms that affect activity (38). In contrast, the rAAT used in the present study required a single, gentle purification step, which may allow preservation of the anti-inflammatory and immunomodulating domains of the molecule (39).
We conclude that AAT protein possesses at least two independent functions: inhibition of elastase and an anti-inflammatory/immunomodulatory function, the latter of which is independent of elastase inhibition. Therefore, clinical preparations of AAT should be validated for both anti-inflammatory/immunomodulatory as well as anti-elastase activities.
Materials and Methods
See SI Materials and Methods for a more detailed discussion.
Mice.
Animal experiments were approved by the German authority for animal protection (33.9–42502-04–09/1766). The intranasal administrations were performed as previously described (40–42).
Laser-Assisted Microdissection of Surgical Lung Explants.
Surgical lung explants from end-stage ZZ AAT emphysema cases with AAT augmentation therapy (n = 11, males, age 53.5 ± 2.4 y) and without AAT therapy (n = 8, males, age 44.7 ± 5.3 y) were retrieved from the Institute of Pathology of Hannover Medical School with the approval of the Hannover Medical School Ethics Committee (No. 990–2011). Fig. S9 illustrates laser-assisted microdissection of lung tissue (IX 71 microscope Olympus Europa) with CellCut Plus system (MMI Molecular Machines and Industries) (43).
Gene Expression in Lung Tissue.
Five-micrometer whole-lung tissue sections from mice or microdissected tissue from human lungs were suspended overnight in a proteinase K digestion buffer and RNA was isolated by phenol-chloroform extraction. cDNA was synthesized and levels of mRNA determined by real-time PCR (43).
Preparation of Mouse Bone Marrow Neutrophils.
WT and NE-deficient mice were killed, femurs, tibiae, and humeri were removed, and bone marrow was harvested by flushing with HBSS supplemented with 0.5% FCS. The cells (>90% neutrophils) were resuspended in RPMI medium 1640 at a concentration of 2 × 106 cells per mL.
Preparation of Human Blood Neutrophils.
Human neutrophils were isolated from the peripheral blood of healthy volunteers using PolymorphprepTM (Axis-Shield PoC AS). The cells were resuspended in RPMI medium 1640 at a concentration of 2 × 106 cells per mL and distributed on cell culture plates precoated with 10% (wt/vol) FCS. In some experiments, purified neutrophils (5 × 106 cells per mL) were labeled in suspension with 5 μg/mL calcein-AM.
Human Neutrophil Adhesion to Endothelial Cells.
HMVEC-L were derived from lung tissues (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) and were grown in medium with 5% (wt/vol) FCS.
Recombinant Human AAT Fc-1 Fusion Protein.
Recombinant human AAT-Fc-1 (rAAT) was produced in a stable clone of Chinese Hamster Ovary cells expressing the cDNA sequence of human AAT in frame with the sequence of human Fc of IgG1 at the C terminus, as previously described (39) (SI Materials and Methods).
Statistics.
Statistical analysis was performed using SPSS software (v19 for Windows, SPSS).
Supplementary Material
Acknowledgments
The authors thank Tania Azam for her contribution to these studies on recombinant α1-antitrypsin. This work was supported in part by Fundacion Federico; Deutsche Forschungsgemeinschaft [SFB 587, A18 (to S.J.); Grant JO743/2-1 (to D.J. and F.L.)]; Deutsches Zentrum für Lungenforschung; Hannover Biomedical Research School Graduate School (S.J. and M.A.-O.); Integriertes Forschungs- und Behandlungszentrum Transplantation (01EO0802); the Cambridge National Institute of Health Research Biomedical Research Institute (R.M.); National Institutes of Health Grants AI-15614, AR-45584, and CA-04 6934 (to C.A.D.); National Research Foundation Grant WCU: R33-2008-000-10022-0 (to K.H., J.H., and S.-H.K.); Korea Healthcare Technology Research and Development Project A100460 (to K.H., J.H., and S.-H.K.); and the Israel Science Foundation (E.C.L.).
Footnotes
Conflict of interest statement: S.-H.K., E.C.L., and C.A.D. own stocks in Omni Bio Pharmaceutical.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1309648110/-/DCSupplemental.
References
- 1.Carrell RW, Lomas DA. Alpha1-antitrypsin deficiency—A model for conformational diseases. N Engl J Med. 2002;346(1):45–53. doi: 10.1056/NEJMra010772. [DOI] [PubMed] [Google Scholar]
- 2.Luisetti M, Seersholm N. Alpha1-antitrypsin deficiency. 1: Epidemiology of alpha1-antitrypsin deficiency. Thorax. 2004;59(2):164–169. doi: 10.1136/thorax.2003.006494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gadek JE, Klein HG, Holland PV, Crystal RG. Replacement therapy of alpha 1-antitrypsin deficiency. Reversal of protease-antiprotease imbalance within the alveolar structures of PiZ subjects. J Clin Invest. 1981;68(5):1158–1165. doi: 10.1172/JCI110360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miravitlles M. Alpha-1-antitrypsin and other proteinase inhibitors. Curr Opin Pharmacol. 2012;12(3):309–314. doi: 10.1016/j.coph.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 5.Wewers MD, Crystal RG. Alpha-1 antitrypsin augmentation therapy. COPD. 2013;10(Suppl 1):64–67. doi: 10.3109/15412555.2013.764402. [DOI] [PubMed] [Google Scholar]
- 6.Stockley RA, et al. Therapeutic efficacy of α-1 antitrypsin augmentation therapy on the loss of lung tissue: an integrated analysis of 2 randomised clinical trials using computed tomography densitometry. Respir Res. 2010;11:136–144. doi: 10.1186/1465-9921-11-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bergin DA, Hurley K, McElvaney NG, Reeves EP. Alpha-1 antitrypsin: A potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz) 2012;60(2):81–97. doi: 10.1007/s00005-012-0162-5. [DOI] [PubMed] [Google Scholar]
- 8.Lewis EC. Expanding the clinical indications for α(1)-antitrypsin therapy. Mol Med. 2012;18:957–970. doi: 10.2119/molmed.2011.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ralston DR, Marsh CB, Lowe MP, Wewers MD. Antineutrophil cytoplasmic antibodies induce monocyte IL-8 release. Role of surface proteinase-3, alpha1-antitrypsin, and Fcgamma receptors. J Clin Invest. 1997;100(6):1416–1424. doi: 10.1172/JCI119662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapiro L, Pott GB, Ralston AH. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J. 2001;15(1):115–122. doi: 10.1096/fj.00-0311com. [DOI] [PubMed] [Google Scholar]
- 11.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102(34):12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes. 2007;56(5):1316–1323. doi: 10.2337/db06-1273. [DOI] [PubMed] [Google Scholar]
- 13.Marcondes AM, et al. Inhibition of IL-32 activation by α-1 antitrypsin suppresses alloreactivity and increases survival in an allogeneic murine marrow transplantation model. Blood. 2011;118(18):5031–5039. doi: 10.1182/blood-2011-07-365247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tawara I, et al. Alpha-1-antitrypsin monotherapy reduces graft-versus-host disease after experimental allogeneic bone marrow transplantation. Proc Natl Acad Sci USA. 2012;109(2):564–569. doi: 10.1073/pnas.1117665109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Libert C, Van Molle W, Brouckaert P, Fiers W. Alpha1-antitrypsin inhibits the lethal response to TNF in mice. J Immunol. 1996;157(11):5126–5129. [PubMed] [Google Scholar]
- 16.Churg A, Wang RD, Xie C, Wright JL. Alpha-1-antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med. 2003;168(2):199–207. doi: 10.1164/rccm.200302-203OC. [DOI] [PubMed] [Google Scholar]
- 17.Cantin AM, Woods DE. Aerosolized prolastin suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med. 1999;160(4):1130–1135. doi: 10.1164/ajrccm.160.4.9807166. [DOI] [PubMed] [Google Scholar]
- 18.Marsh CB, Gadek JE, Kindt GC, Moore SA, Wewers MD. Monocyte Fc gamma receptor cross-linking induces IL-8 production. J Immunol. 1995;155(6):3161–3167. [PubMed] [Google Scholar]
- 19.Baggiolini M, Walz A, Kunkel SL. Neutrophil-activating peptide-1/interleukin 8, a novel cytokine that activates neutrophils. J Clin Invest. 1989;84(4):1045–1049. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brantly M. Alpha1-antitrypsin: Not just an antiprotease: Extending the half-life of a natural anti-inflammatory molecule by conjugation with polyethylene glycol. Am J Respir Cell Mol Biol. 2002;27(6):652–654. doi: 10.1165/rcmb.F250. [DOI] [PubMed] [Google Scholar]
- 21.Korkmaz B, et al. Competition between elastase and related proteases from human neutrophil for binding to alpha1-protease inhibitor. Am J Respir Cell Mol Biol. 2005;32(6):553–559. doi: 10.1165/rcmb.2004-0374OC. [DOI] [PubMed] [Google Scholar]
- 22.Stoller JK, Aboussouan LS. Alpha1-antitrypsin deficiency. Lancet. 2005;365(9478):2225–2236. doi: 10.1016/S0140-6736(05)66781-5. [DOI] [PubMed] [Google Scholar]
- 23.Janciauskiene SM, et al. The discovery of α1-antitrypsin and its role in health and disease. Respir Med. 2011;105(8):1129–1139. doi: 10.1016/j.rmed.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Rainger GE, Rowley AF, Nash GB. Adhesion-dependent release of elastase from human neutrophils in a novel, flow-based model: Specificity of different chemotactic agents. Blood. 1998;92(12):4819–4827. [PubMed] [Google Scholar]
- 25.Houston DS, Carson CW, Esmon CT. Endothelial cells and extracellular calmodulin inhibit monocyte tumor necrosis factor release and augment neutrophil elastase release. J Biol Chem. 1997;272(18):11778–11785. doi: 10.1074/jbc.272.18.11778. [DOI] [PubMed] [Google Scholar]
- 26.DiStasi MR, Ley K. Opening the flood-gates: How neutrophil-endothelial interactions regulate permeability. Trends Immunol. 2009;30(11):547–556. doi: 10.1016/j.it.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramaniyam D, et al. Cholesterol rich lipid raft microdomains are gateway for acute phase protein, SERPINA1. Int J Biochem Cell Biol. 2010;42(9):1562–1570. doi: 10.1016/j.biocel.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Petrache I, et al. alpha-1 antitrypsin inhibits caspase-3 activity, preventing lung endothelial cell apoptosis. Am J Pathol. 2006;169(4):1155–1166. doi: 10.2353/ajpath.2006.060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janciauskiene S, et al. Alpha1-antitrypsin inhibits the activity of the matriptase catalytic domain in vitro. Am J Respir Cell Mol Biol. 2008;39(6):631–637. doi: 10.1165/rcmb.2008-0015RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toldo S, et al. Alpha-1 antitrypsin inhibits caspase-1 and protects from acute myocardial ischemia-reperfusion injury. J Mol Cell Cardiol. 2011;51(2):244–251. doi: 10.1016/j.yjmcc.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Bergin DA, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest. 2010;120(12):4236–4250. doi: 10.1172/JCI41196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Omari M, et al. Acute-phase protein α1-antitrypsin inhibits neutrophil calpain I and induces random migration. Mol Med. 2011;17(9–10):865–874. doi: 10.2119/molmed.2011.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Endo M, Oyadomari S, Suga M, Mori M, Gotoh T. The ER stress pathway involving CHOP is activated in the lungs of LPS-treated mice. J Biochem. 2005;138(4):501–507. doi: 10.1093/jb/mvi143. [DOI] [PubMed] [Google Scholar]
- 34.Nakayama Y, et al. Molecular mechanisms of the LPS-induced non-apoptotic ER stress-CHOP pathway. J Biochem. 2010;147(4):471–483. doi: 10.1093/jb/mvp189. [DOI] [PubMed] [Google Scholar]
- 35.Dinarello CA, Simon A, van der Meer JW. Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov. 2012;11(8):633–652. doi: 10.1038/nrd3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greene CM, McElvaney NG. Protein misfolding and obstructive lung disease. Proc Am Thorac Soc. 2010;7(6):346–355. doi: 10.1513/pats.201002-019AW. [DOI] [PubMed] [Google Scholar]
- 37.De Simone A, et al. Intrinsic disorder modulates protein self-assembly and aggregation. Proc Natl Acad Sci USA. 2012;109(18):6951–6956. doi: 10.1073/pnas.1118048109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lomas DA, Elliott PR, Carrell RW. Commercial plasma alpha1-antitrypsin (Prolastin) contains a conformationally inactive, latent component. Eur Respir J. 1997;10(3):672–675. [PubMed] [Google Scholar]
- 39.Lee S, et al. Effect of recombinant α1-antitrypsin Fc-fused (AAT-Fc)protein on the inhibition of inflammatory cytokine production and streptozotocin-induced diabetes. Mol Med. 2013;19:65–71. doi: 10.2119/molmed.2012.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chignard M, Balloy V. Neutrophil recruitment and increased permeability during acute lung injury induced by lipopolysaccharide. Am J Physiol Lung Cell Mol Physiol. 2000;279(6):L1083–L1090. doi: 10.1152/ajplung.2000.279.6.L1083. [DOI] [PubMed] [Google Scholar]
- 41.Southam DS, Dolovich M, O’Byrne PM, Inman MD. Distribution of intranasal instillations in mice: Effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol. 2002;282(4):L833–L839. doi: 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 42.Ozeri E, Mizrahi M, Shahaf G, Lewis EC. α-1 Antitrypsin promotes semimature, IL-10-producing and readily migrating tolerogenic dendritic cells. J Immunol. 2012;189(1):146–153. doi: 10.4049/jimmunol.1101340. [DOI] [PubMed] [Google Scholar]
- 43.Theophile K, Jonigk D, Kreipe H, Bock O. Amplification of mRNA from laser-microdissected single or clustered cells in formalin-fixed and paraffin-embedded tissues for application in quantitative real-time PCR. Diagn Mol Pathol. 2008;17(2):101–106. doi: 10.1097/PDM.0b013e318163f26e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.