Significance
Delta oscillations have been largely associated with slow-wave sleep and anesthesia, when no conscious functions take place during these states. However, our study demonstrates that coherent delta-band oscillations reflect the linkage between distant parietal and frontal cortical circuits during decision making. Thus, these findings open an avenue for investigating whether the activity between distant cortical circuits oscillates in the delta frequency range during other cognitive functions.
Keywords: synchrony, low-frequency rhythm, brain circuits
Abstract
Coherent oscillations in the theta-to-gamma frequency range have been proposed as a mechanism that coordinates neural activity in large-scale cortical networks in sensory, motor, and cognitive tasks. Whether this mechanism also involves coherent oscillations at delta frequencies (1–4 Hz) is not known. Rather, delta oscillations have been associated with slow-wave sleep. Here, we show coherent oscillations in the delta frequency band between parietal and frontal cortices during the decision-making component of a somatosensory discrimination task. Importantly, the magnitude of this delta-band coherence is modulated by the different decision alternatives. Furthermore, during control conditions not requiring decision making, delta-band coherences are typically much reduced. Our work indicates an important role for synchronous activity in the delta frequency band when large-scale, distant cortical networks coordinate their neural activity during decision making.
Studies of the neural correlates of decision making in behaving monkeys have mainly been based on the analysis of firing rate patterns of neurons in individual cortical circuits, recorded one by one in succession, while trained monkeys perform sensory, motor, and cognitive tasks (1–3). These studies showed that the neuronal activities distributed across parietal and frontal lobe cortices correlate with processes that lead to decision making (4–7). However, how these spatially distant, cortical circuits coordinate their activities into a unified functional network during decision making remains poorly understood.
It has been proposed that coherent oscillations of neuronal activities constitute a putative dynamical mechanism for mediating the interaction between different subsets of brain areas (8–11). Simultaneous recording from multiple intracortical areas in monkeys showed that the coherent higher frequency (beta and gamma bands) oscillations are linked to a broad variety of cognitive functions (12–18). Cortical oscillations at lower frequencies (theta and alpha bands) have also been discussed in terms of long-range integrative processes (19, 20). In fact, recent evidence showed theta-band coupling between visual area V4 and prefrontal cortex during short-term memory (21) and among the rat prefrontal cortex, ventral tegmental area, and hippocampus during working memory (22). It remains, however, probing whether coherent delta-band oscillations play a functional role in the interaction between cortical circuits. Delta-band oscillations are typically associated with slow-wave sleep (SWS; ref. 23), but an important question is whether delta-band oscillations during SWS and waking states represent the same underlying phenomenon (24). Recent findings associated delta-band oscillations in individual cortical areas with attention (25). In monkey primary visual cortex (26) and human motor cortex (27), delta-band oscillations entrain to the rhythm of external sensory events in an attention-dependent manner.
Here, we examined whether coherent oscillations coordinate the activity of five simultaneously recorded cortical areas in the monkey performing a somatosensory discrimination task (7). We specifically focused on whether coherent delta-band oscillations play a significant functional role in linking cortical circuits during decision making.
Results
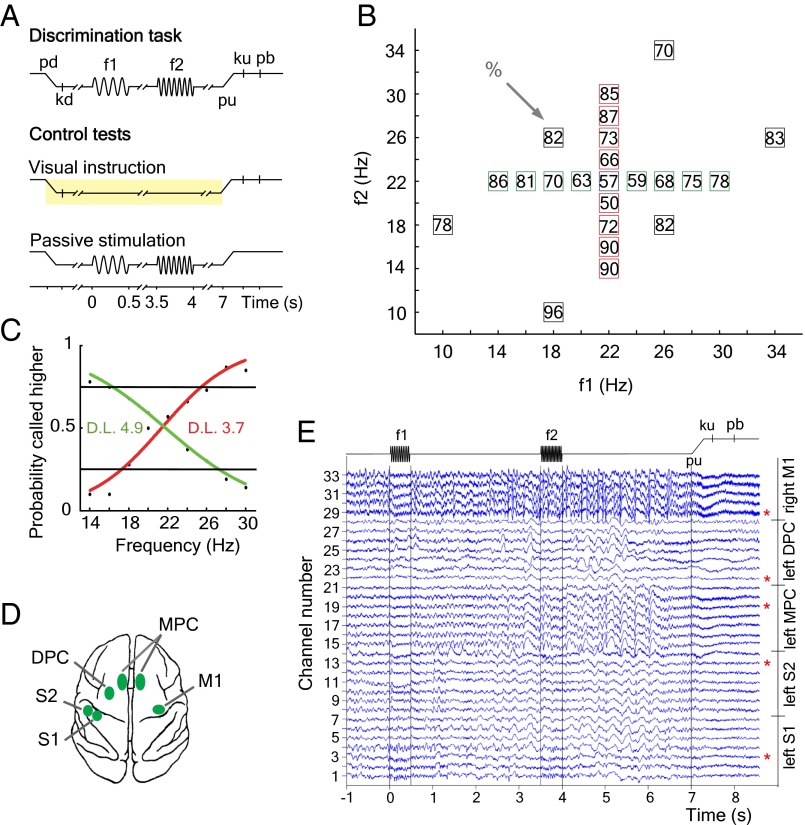
We analyzed coherence for frequencies between 1 and 45 Hz from local field potentials (LFPs) simultaneously recorded from five cortical areas in a trained monkey performing a somatosensory discrimination task (7) (Materials and Methods). In this task (Fig. 1A), the monkey discriminates the difference in frequency between two mechanical vibrations delivered sequentially to one fingertip. Crucially, the monkey must hold the first stimulus frequency (f1) in working memory, must compare the second stimulus frequency (f2) to the memory trace of f1 to form a decision of whether f2 > f1 or f2 < f1, and must postpone the decision report until a sensory cue triggers the motor report. The monkey was trained to perform the task up to its psychophysical threshold (Fig. 1 B and C).
Fig. 1.
Somatosensory discrimination task and cortical recording sites. (A) Sequence of events during the discrimination task and control tests (f1, first stimulus; f2, second stimulus; kd, key down; ku, key up; pb, push button; pd, probe down; pu, probe up; Materials and Methods). (B) Stimulus set during recordings. Each box indicates a (f1, f2) frequency stimulus pair. The numbers inside the box indicates overall percentage of correct trials for each (f1, f2) stimulus pair. (C) Psychophysical performance when f1 was maintained fixed at 22 Hz and f2 was variable (red curve), and when f2 was fixed at 22 Hz and f1 was variable (green curve). D.L., animal’s discrimination threshold in hertz. (D) Top view of the monkey brain and the cortical areas recorded (green spots). (E) Raw local field potentials simultaneously recorded during the discrimination task. We illustrate one single trial in one example recording session. LFPs are aligned to the f1 stimulus onset. For analysis purposes, we selected only one channel in each cortical area (red asterisks). Channel selection varied across sessions.
During each recording session, up to seven microelectrodes were individually inserted in each of the five cortical areas for simultaneous recordings of the LFPs and the activity of single neurons during the task. The selected cortical areas were the primary somatosensory cortex (S1), secondary somatosensory cortex (S2), medial premotor cortex [MPC; the MPC areas of both hemispheres were not simultaneously recorded (Materials and Methods)], dorsal premotor cortex (DPC), and primary motor cortex (M1) (Fig. 1D). We based this selection on the previously described responses of single neurons from these cortical areas associated with different components of the somatosensory discrimination task (7). Examples of the raw simultaneously recorded LFPs during the discrimination task are shown in Fig. 1E and Fig. S1.
Cortical Coherence Dynamics During the Discrimination Task.
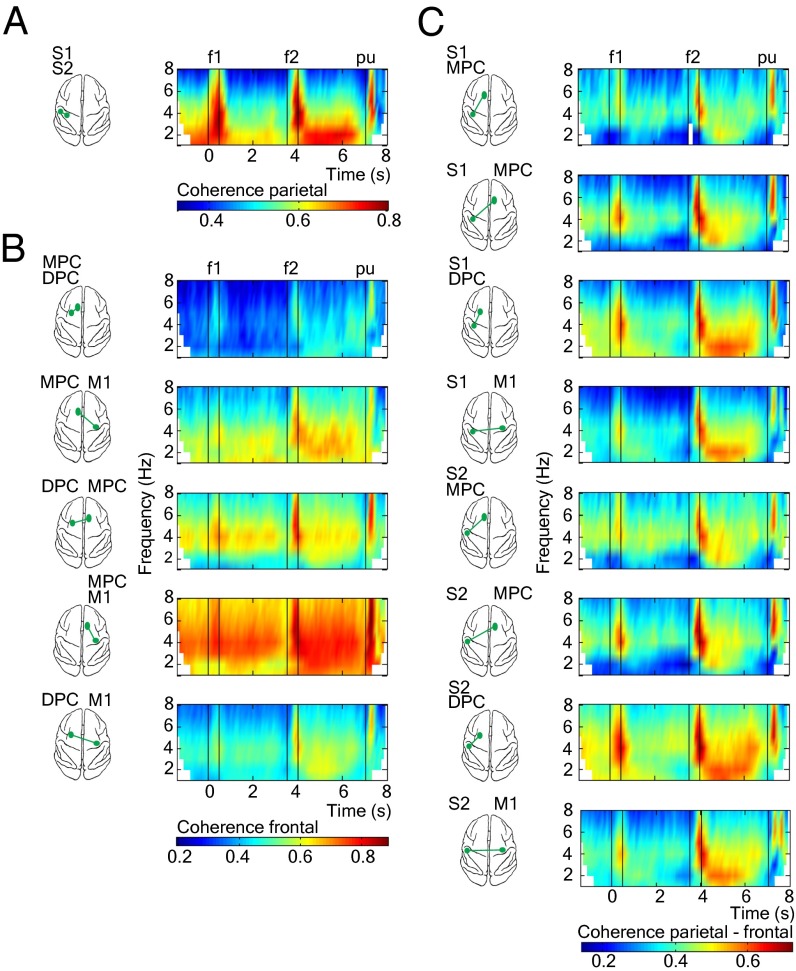
Next, to investigate low oscillatory (1–8 Hz) synchrony between LFPs from pairs of simultaneously recorded cortical areas during the task, we estimated the coherence between LFPs in short sliding windows (Materials and Method). Coherence is a measure of phase and amplitude consistency between two signals as a function of frequency, and coherence values ranges between 0 (no consistent relationship between the two signals) and 1 (a constant relationship). We note that a significant coherence between two cortical LFPs can result if the two areas receive similar external inputs or if the two areas are interconnected and communicate with each other (or a combination of these possibilities). We obtained a total of 14 parietal, frontal, and parietal-frontal interareal comparisons (Materials and Method). Time frequency maps of coherence for all pairs of areas revealed significant modulations in the low frequency range (1–8 Hz, Fig. 2; 1–45 Hz, Fig. S2) during the task. The low frequency range oscillations (delta, 1–4 Hz; theta, 4–8 Hz) between sensory areas in the parietal lobe (S1 and S2) showed stronger coherence modulations during the stimulation periods and the delays immediately afterward (Fig. 2A). Motor areas in the frontal lobe showed low frequency range coherence modulations throughout the task, but delta-band coherence was strongest during the delay period between the end of f2 and the cue that triggers the motor response (pu; i.e., postponed decision period, Fig. 2B). This elevated delta-band coherence during the postponed decision period was also observed between the cortical areas located in the parietal and frontal lobes (Fig. 2C). Thus, a pattern of delta-band synchronization varying in magnitude and time course for all pairs of cortical areas was observed during the postponed decision period. The synchronous delta oscillations in these cortical recorded areas are clearly present in the raw data (Fig. 1E and Fig. S1).
Fig. 2.
Cortical coherence dynamics during the discrimination task (1–8 Hz). (A) Parietal pair areas. (B) Frontal pair areas. (C) Parieto-frontal pair areas. Time-frequency plots show averaged LFP-LFP coherence over all sessions (n = 18) for simultaneously recorded cortical pairs. Coherence was calculated for hit trials by using an adaptive window that decreases in length with increased frequency in 100-ms consecutive window steps (note the boundary effects for lower frequencies). We show significant coherence results up to 8 Hz (P < 0.05, nonparametric randomization test; Materials and Methods). White time frequency points outside boundary effects indicate that coherence is not significant. Black vertical lines depict time task events: presentation of the first stimulus (0-0.5 s), presentation of the second stimulus (3.5–4 s), and probe up event (7 s).
Delta Coherence Is Modulated by Decision Making.
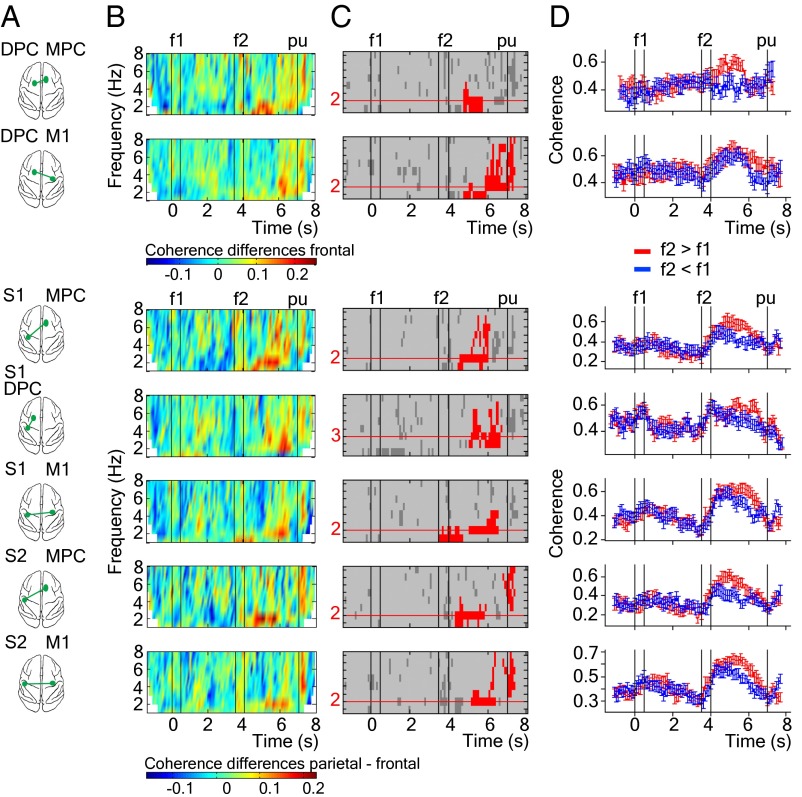
A fundamental question is whether the observed coherent oscillations between spatially segregated cortical areas are directly associated with the decision-making process of this task. To answer this question, we selected hit trials in which f2 is equal to 22 Hz. In this case, f2 (22 Hz) can be judged higher or lower depending on the variable f1 frequency. This selection allows us to discard effects due to the different f2 stimulus frequencies. Next, we computed coherences separately for trials corresponding to the two possible responses (f2 > f1 and f2 < f1) and subtracted the resulting coherence spectrograms (Fig. S3). To assess the statistical significance of these coherence differences, we performed a cluster-based nonparametric randomization test (Materials and Methods). This analysis identified the pairs of cortical areas showing significant coherence differences (Fig. 3A). For each time-frequency plot of coherence differences (Fig. 3B), we detected all connected time-frequency points above a threshold value and used the size of these clusters as the test statistic. The significant clusters are shown in red in Fig. 3C. Clusters spanned from f2 to reaction time period (∼500 ms after the pu) in delta and theta frequency bands. For each cortical area pair, we obtained the frequency values that best corresponded to the location of the significant clusters (the maximum length of the horizontal cluster dimension shown as red lines in Fig. 3C). Coherence for the two groups of trials (f2 > f1 and f2 < f1) as a function of time (Fig. 3D) revealed significant coherence differences during the postponed decision period, when the frequency values are in delta frequency range (2–3 Hz, red values in Fig. 3C). The consistency of these coherence differences across recording sessions are shown in Fig. S4. We next investigated whether these coherence differences are restricted to the delta band or whether they were also present in the theta frequency range (5–8 Hz). Fig. S5 shows that there were no significant coherence differences at theta frequency during the postponed decision period. However, significant coherence differences at theta frequency were found during the 500 ms immediately after pu (i.e., reaction time period). However, the time at which the monkey releases its hand from the key after the pu cue is variable, and we cannot discard a possible movement effect in the coherence differences found during this task period.
Fig. 3.
Significant coherence differences between f2 > f1 and f2 < f1 for groups of hit trials in which the f2 stimulus frequency is equal to 22 Hz. (A) Schematic view of the cortical pairs from which coherence is calculated (green spots). (B) Time-frequency plots of the coherence differences between both groups of trials (Fig. S3B). (C) Time-frequency plots showing the clusters where there are coherence differences between f2 > f1 minus f2 < f1 groups of trials. Only significant clusters are plotted in red color (P < 0.05, two-sided test; Materials and Methods). Horizontal red lines indicate the frequency values where the significant cluster has the maximum length. (D) Time course of coherence for each group of trials at frequency values (horizontal red lines). Error bars correspond to SEM (±2) over recording sessions (n = 18). Black vertical lines depict time task events: presentation of the first stimulus (0–0.5 s), presentation of the second stimulus (3.5–4 s), and probe up event (7 s).
To test whether delta-band coherence differences are explained by differences in power in the same frequency band, we applied the same cluster-based analysis for power in each recorded area. The parietal areas (S1 and S2) displayed differences in this frequency range at 2 Hz, but none of the frontal lobe areas recorded from the right hemisphere (MPC and M1) showed significant power differences for delta band as a function of the animal’s decision (Fig. S6). However, significant beta-band (13–30 Hz) power differences were found for right MPC and right M1 recorded areas during the postponed decision period, results that have been reported (28). Significant beta-band clusters observed during the f1 period for S1 and S2 areas are likely due to stimulus entrainment.
Delta Coherence Is Context Dependent.
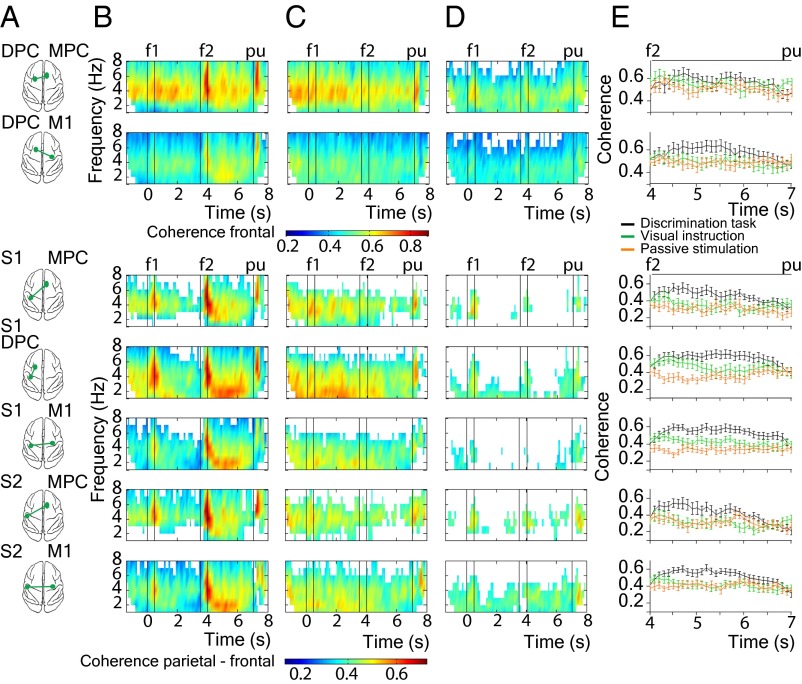
To further test whether delta-band coherences are directly associated with decision making, we also analyzed coherence in two control tasks: passive stimulation and visual instruction (Fig. 1A, Middle and Bottom; Materials and Methods). During passive stimulation, the same stimuli used during the discrimination task were delivered to the fingertip, but the monkey was not requested to perform the task. During visual instruction, the trials proceeded exactly as in the discrimination task, but the stimuli were not delivered to the skin and the movements were guided by visual cues present from the start of the trial. In this last condition, it is not possible for the monkey to elaborate a decision based on the evaluation of somatosensory information, but the same movements as in discrimination task were executed for reward. Compared with the discrimination task, both control conditions had reduced delta-band coherence, in particular during the postponed decision period (Fig. 4). To test whether this reduced coherence was caused by the fewer number of trials during control tasks (n = 30), we repeated the coherence analysis by selecting 30 random hit trials during the discrimination task (Fig. 4B). Similar coherence results were found compared with those results obtained during the discrimination task in which all hit trials were selected (Fig. 2). Fig. 4E shows the coherence differences among the three tasks during the postponed decision period. Quantification of these differences during the postponed decision period revealed that the magnitude of delta-band coherence in the discrimination task is significantly higher compared with both control sets (Table S1). Moreover, we explored coherence differences between f2 > f1 and f2 < f1 groups of trials by using the same cluster-based randomization test for the pairs of cortical areas as we did in the discrimination task (Fig. 3). There were no significant coherence differences in the frequency range studied (1–8 Hz) during the whole time task, neither for the visual instruction (Fig. S7 B and C), nor for the passive stimulation (Fig. S7 D and E). Thus, under control conditions, delta-band coherences differences did not depend on the animal’s decision report.
Fig. 4.
Coherence comparison between discrimination task and control tests. (A) Schematic view of the cortical pairs from which coherence is calculated (green spots). (B–D) Time-frequency plots of averaged LFP-LFP coherence over all sessions (n = 18) for the discrimination task in which 30 random hit trials were selected, visual instruction, and passive stimulation, respectively. We show significant coherence results up to 8 Hz (P < 0.05, nonparametric randomization test; Materials and Methods). White time frequency points outside boundary effects indicate that coherence is not significant. Black vertical lines depict time task events: presentation of the first stimulus (0–0.5 s), presentation of the second stimulus (3.5–4 s) and probe up event (7 s). (E) Time course of coherence at 2 Hz for each task type during the delay period between f2 and pu. Error bars correspond to SEM (± 2) over recording sessions (n = 18).
Spike-Field Synchrony.
To investigate whether the synchronous delta-band oscillations of the LFPs influence computations in the local neuronal circuits, we next made spike-triggered averages (STAs) of the LFPs (Materials and Methods). In many cases, STAs exhibited a strong oscillatory patterning in the frequency range of 1–3 Hz, indicating that temporal patterning of single neuron discharges were correlated with the phase of delta-band LFPs (Fig. S8). This result suggests that synchronized delta-band activity could coordinate the spiking activity of neurons in widespread cortical areas. Moreover, STAs were also modulated by the different decision alternatives (Fig. S8D), showing that the synchronization between spike times and LFPs carry decision related information.
Discussion
Our findings demonstrate that the simultaneously recorded activity from parietal and frontal cortical areas is synchronized in the delta-band frequency range during decision making. This synchronization is widespread, modulated by the different decision alternatives and context specific. Interestingly, these patterns of synchronization are prevalent for interactions between the sensory areas (S1 and S2) located in the left parietal lobe (contralateral to the stimulated finger) and the motor areas (MPC and M1) located in the right frontal lobe (contralateral to the responding hand/arm). Thus, this synchrony could facilitate the communication between the left and right hemisphere during decision making. However, further analysis is required to show whether this interaction is so.
It is worth mentioning that previous studies in which simultaneous recordings from multiple cortical areas were made, the functional role of delta-band was not explored. One possibility is that delta oscillations are hard to detect if the relevant task periods are of shorter duration (<1 s). The long postponed delay period (3 s) of our task allowed for the studying of delta-band oscillations. Another possibility is that delta oscillations have been considered of little functional relevance in cognition because they are consistently observed in SWS and during general anesthesia (23, 29). There is no definitive evidence regarding the origin of delta activity in the brain, but several studies place the site of waking delta-band generation in the anterior medial frontal cortex (30, 31). Moreover, recordings in waking animals show the existence of delta-band oscillations in the nucleus accumbens (32) and dopamine neurons of the ventral tegmental area (33), structures that are part of the brain reward system. These observations, together with those reported in attentional tasks (25–27), show that delta-band oscillations occurring in individual brain areas play a role in cognitive functions. Our results show that distant cortical circuits are linked in the delta-frequency band during decision making. However, whether the activity between distant cortical circuits oscillates in the delta frequency range during other cognitive functions is an open question.
Materials and Methods
This study was performed on one adult male monkey (Macaca mulatta), weighing 12 kg. All procedures followed the guidelines of the National Institutes of Health and Society for Neuroscience. Protocols were approved by the Institutional Animal Care and Use Committee of the Instituto de Fisiología Celular.
Discrimination Task.
The paradigm used here has been described (34, 35). The monkey sat on a primate chair with its head fixed in an isolated, sound-proof room. The right hand was restricted through a half-cast and kept in a palm-up position. The left hand operated an immovable key (elbow at ∼90°), and two push buttons were in front of the animal, 25 cm away from the shoulder and at eye level. The centers of the switches were located 7 and 10.5 cm to the left of the midsagittal plane. In all trials, the monkey first placed the left hand and later projected to one of the two switches. Stimuli were delivered to the skin of the distal segment of one digit of the right, restrained hand, via a computer-controlled stimulator (2 mm round tip; BME Systems). The initial probe indentation was 500 μm. Vibrotactile stimuli were trains of short mechanical pulses. Each of these pulses consisted of a single-cycle sinusoid lasting 20 ms. Stimulus amplitudes were adjusted to equal subjective intensities; for example, 71 μm at 12 Hz and 51 μm at 34 Hz (a decrease of ∼1.4% per Hz). During discrimination trials (Fig. 1A), the mechanical probe was lowered (probe down; pd), indenting the glabrous skin of one digit of the hand; the monkey placed its free hand on an immovable key (key down; kd); after a variable prestimulus delay (0.5–3 s) the probe oscillated vertically at the frequency of the first stimulus (f1); after a fixed delay (3 s), a second mechanical vibration was delivered at the second stimulus (f2) frequency; after another fixed delay (3 s) the probe is lifted off from the skin (probe up; pu); the monkey released the key (ku) and pressed either a lateral or a medial push button (pb) to indicate whether f2 was of higher or lower frequency than f1, respectively. The monkey was rewarded with a drop of liquid for correct discriminations. Performance was quantified through psychometric techniques (Fig. 1 B and C).
Control Tests.
Visual instruction.
Trials during the visual instruction begin exactly as described in the discrimination task, but when the probe touched the skin, one of the push buttons was illuminated. The monkey had to respond by holding the immovable key until the light was turned off and the probe was lifted off from the skin, which triggers the hand/arm movement. The monkey was rewarded for pressing the previously illuminated push button (100% correct responses). Note that in this condition, the vibratory stimuli are absent. The yellow box in Fig. 1A represents the time in which one of the push buttons remained illuminated.
Passive stimulation.
During this condition, the monkey was trained to maintain its free arm motionless during the trial (Fig. 1A). Stimuli were delivered to the fingertip and the animal remained alert by being rewarded with drops of liquid at different times, but no motor response with the free hand was required.
Recording.
Data acquisition, amplification, and filtering were described in detail (36). In brief, LFPs and the activity of single neurons were simultaneously recorded with an array of seven independent, movable microelectrodes (1–1.5 MΩ) inserted in each of five cortical areas. Electrodes within an area were spaced 305 or 500 μm apart (37). Spike sorting was performed manually on-line, and single neurons were selected if they responded to any of the different components of the discrimination task (7, 36). The cortical areas were the S1, S2, MPC, DPC, and M1 (Fig. 1D). Recordings in S1, S2, and DPC were made in the hemisphere contralateral to the stimulated hand (left hemisphere), and in M1 contralateral to the responding hand/arm (right hemisphere). Recordings in MPC were made either in the hemisphere contralateral or ipsilateral to the responding arm in different sessions (n = 11 and n = 7, respectively). MPC areas were not recorded simultaneously, leading a total of 14 interareal pairs. LFPs were simultaneously recorded by using a 250-Hz Butterworth digital low-pass filter of fourth order and were stored at 2 kHz for offline analysis. The neuronal signal of each microelectrode was sampled at 30 KHz. During the discrimination task, we collected data by using the stimulus set of Fig. 1B, 10 trials for each stimulus frequency pair. During control sets, we collected five trials for the six stimulus frequency pairs with black boxes. All LFPs recorded from S1 and S2 had cutaneous receptive fields confined to the distal segments of the glabrous skin of fingertips 2, 3, or 4. Recording sites changed from session to session.
Data Analysis.
Data were analyzed offline by using custom-build Matlab code (MathWorks). For the work presented here, we selected 18 experimental sessions in which the monkey had similar psychophysical thresholds (34). Before the LFP data were submitted to statistical analysis, the whole trials contaminated with artifacts (e.g., because of movement or electronic interference) were removed based on visual inspection of the data for all channels recorded simultaneously in each session. Similar raw LFPs signals from the same areas were observed (Fig. 1E). The most probable explanation could be the short distance between microelectrodes within the microelectrode array (38). The simplest strategy for across area comparisons was to select only one representative channel in each cortical area. For this purpose, two criteria used were as follows: (i) LFPs from S1 area should clearly show modulation as a function of stimulus frequency during the stimulus presentation, indicating that the microelectrode was placed in the center of the cutaneous receptive field and (ii) power spectra showing task-related modulations. Only trials with correct responses during the discrimination task were used for data analysis.
Data were filtered to have power between 1 and 45 Hz and down sampled to 200 Hz. We used a digital finite impulse response (FIR) filter as implemented in the Matlab functions fir1 and filtfilt to achieve this aim. Examples of filtered LFPs are shown in Fig. S1. Spectral quantities were estimated for each trial separately in moving windows by using bin sizes that depended on the frequency, i.e., shorter windows for higher frequencies. At each point in time (middle of a window), we estimated the best fitting complex exponential for frequencies between 1 and 45 Hz in steps of 1 Hz by using ordinary least squares. Given that we used short bin windows, which correspond to an oversampling of the spectra, similar results would be obtained by interpolating standard fast Fourier Transform-based estimates. The amplitude at each frequency and time point was taken as the trial average of the modulus (absolute value) of the exponentials. To investigate the linear dependence between each pair of areas, separately for each frequency, we computed the correlation (over trials) between the complex exponentials. Note that this correlation can be viewed as an estimator of coherence, and we refer to this quantity as “coherence.” To make this procedure more robust, we used Spearman’s rank correlation and the coherence estimate can hence be viewed as a semiparametric estimate of coherence. The results were similar when we used Pearson’s product-moment correlation coefficient (39). Furthermore, the results remained similar when we used the Matlab function mscohere for coherence estimates. Significance tests were based on a permutation procedure where the original (i.e., nonpermuted) results were compared with the tail of a distribution obtained by permuting the trials differently for the two areas under investigation (40). Time-frequency maps of coherence values were thresholded by using the N × P highest value obtained from in the permutation distribution (where N is the total number of permutations and P is the desired “P value”). Thus, we tested for coherence against a null hypothesis of complete independence and corrected for the multiple comparisons (one per frequency and time-bin) by using the maximum coherence value as the test statistic (40, 41).
To detect significant differences in coherence between groups of trials (f2 > f1 and f2 < f1 decision responses), we applied a cluster-based nonparametric randomization test (41), but this time across groups randomly taking the same number of trials for each condition. This step eliminated any possible bias from different sample sizes. By clustering neighboring samples, that is time-frequency points that presented significant coherence differences as a function of the animal’s decision (Fig. S3B), this test deals with the multiple comparisons problem (41). For each sample, a dependent-sample t value was computed. All samples were selected for which this t value exceeded a priori threshold (P < 0.05, two-sided test), and these samples were subsequently clustered on the basis of temporal-frequency adjacency (red clusters in Fig. 3B). The size of the largest cluster was used as the test statistic. This procedure was done within sessions (n = 18) and separately for each pair of areas. By randomizing the data across the two conditions and recalculating the test statistic a maximum of 10,000 times, we obtained a reference distribution of maximum cluster size values to evaluate the statistic of the actual data. This procedure is standard in the human brain imaging literature (e.g., ref. 42) and has been used before in electrophysiological studies (41).
For calculating the STA at each recording site, the LFP signals were band-pass filtered between 1 and 4 Hz. Neurons with stationary firing rates across trials that modulated their firing rates as a function of the decision report (except for S1 neurons) (7), and recorded from the same electrode selected for the LFP-LFP coherence analysis, were chosen. The STA analysis was done on data in an interval between 4.5–6.5 s after the onset of the first stimulus (the postponed decision period). This interval was divided into three intervals of 1 s each. The first 500 ms after the end of the second stimulus and the last 500 ms before the probe up event were not included in the analysis to prevent phase locking between spikes and LFPs due to stimulus and movement-related activity. STAs were calculated by averaging LFP segments ± 1 s around every spike recorded in correct hit trials. To characterize the variability of the STA results, the same analysis was applied to “surrogate data” where the order between LFP trials and spike -trials were randomly permuted. For each recorded cell, we made 100 such permutations (shown as gray curves in the background of Fig. S8 B and C). To explore STA modulations as a function of the animal’s decision, the STA analysis was done separately for f2 > f1 and f2 < f1 groups of hit trials in which the f2 stimulus frequency was equal to 22 Hz (Fig. S8D).
Supplementary Material
Acknowledgments
We thank R. Desimone for helpful comments on an earlier version of the manuscript. R.R.’s research was partially supported by International Research Scholars Award 55005959 from the Howard Hughes Medical Institute, Dirección de Personal Académico de la Universidad Nacional Autónoma de México Grant IN203210 and Consejo Nacional de Ciencia y Tecnología Grant CB-2009-01-130863. V.N. was supported by a Ministerio de Educación y Ciencia (MEC)-Fullbright Postdoctoral Fellowship from the Spanish Ministry of Science and Technology and Dirección de Personal Académico de la Universidad Nacional Autónoma de México. A.L. was supported by the Ramón y Cajal program from the Spanish government. G.D. was supported by the European Research Council Advanced Grant DYSTRUCTURE 295129 and European Union Seventh Framework Programme FP7/2007-2013 under Grant 269921 (BrainScaleS).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1314681110/-/DCSupplemental.
References
- 1.Schall JD. Neural basis of deciding, choosing and acting. Nat Rev Neurosci. 2001;2(1):33–42. doi: 10.1038/35049054. [DOI] [PubMed] [Google Scholar]
- 2.Romo R, de Lafuente V. Conversion of sensory signals into perceptual decisions. Prog Neurobiol. 2013;103:41–75. doi: 10.1016/j.pneurobio.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- 4.Kim JN, Shadlen MN. Neural correlates of a decision in the dorsolateral prefrontal cortex of the macaque. Nat Neurosci. 1999;2(2):176–185. doi: 10.1038/5739. [DOI] [PubMed] [Google Scholar]
- 5.Platt ML, Glimcher PW. Neural correlates of decision variables in parietal cortex. Nature. 1999;400(6741):233–238. doi: 10.1038/22268. [DOI] [PubMed] [Google Scholar]
- 6.Hoshi E, Tanji J. Differential roles of neuronal activity in the supplementary and presupplementary motor areas: From information retrieval to motor planning and execution. J Neurophysiol. 2004;92(6):3482–3499. doi: 10.1152/jn.00547.2004. [DOI] [PubMed] [Google Scholar]
- 7.Hernández A, et al. Decoding a perceptual decision process across cortex. Neuron. 2010;66(2):300–314. doi: 10.1016/j.neuron.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Fries P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9(10):474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Engel AK, Fries P, Singer W. Dynamic predictions: Oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2(10):704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 10.Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 11.Hipp JF, Engel AK, Siegel M. Oscillatory synchronization in large-scale cortical networks predicts perception. Neuron. 2011;69(2):387–396. doi: 10.1016/j.neuron.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 12.Murthy VN, Fetz EE. Coherent 25- to 35-Hz oscillations in the sensorimotor cortex of awake behaving monkeys. Proc Natl Acad Sci USA. 1992;89(12):5670–5674. doi: 10.1073/pnas.89.12.5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bressler SL, Coppola R, Nakamura R. Episodic multiregional cortical coherence at multiple frequencies during visual task performance. Nature. 1993;366(6451):153–156. doi: 10.1038/366153a0. [DOI] [PubMed] [Google Scholar]
- 14.Brovelli A, et al. Beta oscillations in a large-scale sensorimotor cortical network: Directional influences revealed by Granger causality. Proc Natl Acad Sci USA. 2004;101(26):9849–9854. doi: 10.1073/pnas.0308538101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315(5820):1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 16.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science. 2009;324(5931):1207–1210. doi: 10.1126/science.1171402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pesaran B, Nelson MJ, Andersen RA. Free choice activates a decision circuit between frontal and parietal cortex. Nature. 2008;453(7193):406–409. doi: 10.1038/nature06849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar RF, Dotson NM, Bressler SL, Gray CM. Content-specific fronto-parietal synchronization during visual working memory. Science. 2012;338(6110):1097–1100. doi: 10.1126/science.1224000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Stein A, Sarnthein J. Different frequencies for different scales of cortical integration: From local gamma to long range alpha/theta synchronization. Int J Psychophysiol. 2000;38(3):301–313. doi: 10.1016/s0167-8760(00)00172-0. [DOI] [PubMed] [Google Scholar]
- 20.Palva S, Palva JM. New vistas for alpha-frequency band oscillations. Trends Neurosci. 2007;30(4):150–158. doi: 10.1016/j.tins.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 21.Liebe S, Hoerzer GM, Logothetis NK, Rainer G. Theta coupling between V4 and prefrontal cortex predicts visual short-term memory performance. Nat Neurosci. 2012;15(3):456–462, S1–S2. doi: 10.1038/nn.3038. [DOI] [PubMed] [Google Scholar]
- 22.Fujisawa S, Buzsáki G. A 4 Hz oscillation adaptively synchronizes prefrontal, VTA, and hippocampal activities. Neuron. 2011;72(1):153–165. doi: 10.1016/j.neuron.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hobson JA, Pace-Schott EF. The cognitive neuroscience of sleep: Neuronal systems, consciousness and learning. Nat Rev Neurosci. 2002;3(9):679–693. doi: 10.1038/nrn915. [DOI] [PubMed] [Google Scholar]
- 24.Knyazev GG. EEG delta oscillations as a correlate of basic homeostatic and motivational processes. Neurosci Biobehav Rev. 2012;36(1):677–695. doi: 10.1016/j.neubiorev.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28(18):4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakatos P, Karmos G, Mehta AD, Ulbert I, Schroeder CE. Entrainment of neuronal oscillations as a mechanism of attentional selection. Science. 2008;320(5872):110–113. doi: 10.1126/science.1154735. [DOI] [PubMed] [Google Scholar]
- 27.Saleh M, Reimer J, Penn R, Ojakangas CL, Hatsopoulos NG. Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron. 2010;65(4):461–471. doi: 10.1016/j.neuron.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haegens S, et al. Beta oscillations in the monkey sensorimotor network reflect somatosensory decision making. Proc Natl Acad Sci USA. 2011;108(26):10708–10713. doi: 10.1073/pnas.1107297108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schomer DL, Lopes da Silva FH. Niedermeyer’s Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Philadelphia: Lippincot Williams & Wilkins; 2011. [Google Scholar]
- 30.Michel CM, Lehmann D, Henggeler B, Brandeis D. Localization of the sources of EEG delta, theta, alpha and beta frequency bands using the FFT dipole approximation. Electroencephalogr Clin Neurophysiol. 1992;82(1):38–44. doi: 10.1016/0013-4694(92)90180-p. [DOI] [PubMed] [Google Scholar]
- 31.Alper KR, et al. Correlation of PET and qEEG in normal subjects. Psychiatry Res. 2006;146(3):271–282. doi: 10.1016/j.pscychresns.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 32.Leung LS, Yim CY. Rhythmic delta-frequency activities in the nucleus accumbens of anesthetized and freely moving rats. Can J Physiol Pharmacol. 1993;71(5-6):311–320. doi: 10.1139/y93-049. [DOI] [PubMed] [Google Scholar]
- 33.Shi WX. Slow oscillatory firing: A major firing pattern of dopamine neurons in the ventral tegmental area. J Neurophysiol. 2005;94(5):3516–3522. doi: 10.1152/jn.00317.2005. [DOI] [PubMed] [Google Scholar]
- 34.Hernández A, Salinas E, García R, Romo R. Discrimination in the sense of flutter: New psychophysical measurements in monkeys. J Neurosci. 1997;17(16):6391–6400. doi: 10.1523/JNEUROSCI.17-16-06391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lemus L, et al. Neural correlates of a postponed decision report. Proc Natl Acad Sci USA. 2007;104(43):17174–17179. doi: 10.1073/pnas.0707961104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernández A, et al. Procedure for recording the simultaneous activity of single neurons distributed across cortical areas during sensory discrimination. Proc Natl Acad Sci USA. 2008;105(43):16785–16790. doi: 10.1073/pnas.0808702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eckhorn R, Thomas U. A new method for the insertion of multiple microprobes into neural and muscular tissue, including fiber electrodes, fine wires, needles and microsensors. J Neurosci Methods. 1993;49(3):175–179. doi: 10.1016/0165-0270(93)90121-7. [DOI] [PubMed] [Google Scholar]
- 38.Kajikawa Y, Schroeder CE. How local is the local field potential? Neuron. 2011;72(5):847–858. doi: 10.1016/j.neuron.2011.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derrick TR, Bates BT, Dufek JS. Evaluation of time-series data sets using the Pearson product-moment correlation coefficient. Med Sci Sports Exerc. 1994;26(7):919–928. [PubMed] [Google Scholar]
- 40.Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Maris E, Schoffelen JM, Fries P. Nonparametric statistical testing of coherence differences. J Neurosci Methods. 2007;163(1):161–175. doi: 10.1016/j.jneumeth.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 42.Ledberg A. Robust estimation of the probabilities of 3-D clusters in functional brain images: Application to PET data. Hum Brain Mapp. 2000;9(3):143–155. doi: 10.1002/(SICI)1097-0193(200003)9:3<143::AID-HBM3>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.