Abstract
The cellular control of cholesterol metabolism mediated by lipoproteins was first appreciated in pioneering work published in a 1974 PNAS Classic by Michael Brown and Joseph Goldstein. We know from this paper that the LDL binds to a cell surface receptor and dampens the activity of a key enzyme in cholesterol biosynthesis and that a receptor deficiency is responsible for a major genetic cause of hypercholesterolemia and premature atherosclerosis.
Keywords: endocytosis, HMG-CoA, reductase, lysosome
In the early 1970s, the young physician-scientist team of Michael Brown and Joseph Goldstein embarked on an adventure that led to their discovery of the role of lipoproteins and a novel cell surface receptor—the LDL receptor—in cholesterol biosynthesis through feedback inhibition of a key enzyme, hydroxymethylglutaryl CoA reductase (HMG-CoA reductase). Many of their crucial discoveries were, and continue to be, published in PNAS. In the 1974 PNAS Classic paper discussed here, they reported a saturable LDL receptor binding site and a defect in LDL binding in fibroblasts cultured from patients with familial hypercholesterolemia (FH) (1). The work serves as an example of their many landmark publications.
Control by Cell Surface Receptors Through 1972
Although their achievement represented a singular milestone in the history of receptor biology and medicine, several interweaving precedents framed the Brown and Goldstein discovery in the context of cellular control mechanisms and the cellular uptake of extracellular macromolecules. In the mid-1950s, Earl Sutherland and colleagues pioneered the notion of ligand control of cell metabolism. His discovery of a connection between hormone (epinephrine and glucagon) contact at the cell surface with the intracellular machinery of glycogen breakdown represents a crucial starting point in what has become a dominant theme in our understanding of the mechanism of cellular metabolic control (2). In a parallel development, Cohen et al. highlighted the role of growth factors and control of cell surface receptors (3). In yet another landmark paper published in PNAS, Pedro Cuatrecasas developed the powerful approach of affinity purification to isolate the insulin cell surface receptor from liver membranes (4). Although this was not appreciated at the outset, we now know the action of these hormones is modulated by ligand–receptor internalization into intracellular membrane compartments.
An independent thread of discovery may be traced to the careful electron microscopic observations of Roth and Porter, who examined developing mosquito oocytes as they acquire yolk storage proteins secreted by the mother. Without the advantage of modern immunocytochemical or fluorescence microscopy techniques, Roth and Porter were able to visualize yolk protein clustered at the oocyte cell surface in invaginations that are marked on their cytoplasmic side by a distinct electron dense coat material (5). They speculated that this cytoplasmic coat could be responsible for the selective internalization of yolk proteins for subsequent intracellular breakdown and use in the developing embryo. Later and quite independently, Barbara Pearse discovered that the coat consists of a structural protein, clathrin, which has the unusual property of forming a regular lattice cage polyhedron (6). Pearse proposed that clathrin might assemble on the cytoplasmic face of the plasma membrane and, by subunit rearrangement, deform a cell surface patch into a coated bud and vesicle and thus capture cell surface–bound ligands into a coated transport vesicle.
A third theme of discovery comes from the work of Gilbert Ashwell and Elizabeth Neufeld, who studied the tissue and cellular uptake of glycoproteins. Ashwell’s group found that removal of the terminal sialic acid residue on N-glycans greatly reduced the lifetime in circulation of asialoglycoproteins injected into rats, which they speculated was due to a saturable internalization receptor on the surface of hepatocytes that recognized exposed galactosyl residues (7). Following shortly on this discovery, Neufeld and colleagues reported the unusual fate of lysosomal hydrolases produced by fibroblasts cultured from patients with a rare carbohydrate storage disorder: I-cell disease. Hickman and Neufeld observed that the fraction of lysosomal enzymes secreted by normal cultured fibroblasts could be recaptured into cells and then to the lysosome dependent on a selective process of uptake at the cell surface (8). Fibroblasts from patients with I-cell disease fail to localize lysosomal proteins that are instead secreted into the growth medium. Crucially, they showed that lysosomal enzymes secreted by I-cell fibroblasts fail to be recaptured by normal or I-cell fibroblasts, whereas the corresponding enzymes secreted by normal cells are taken up by I-cells. Neufeld and colleagues speculated on the existence of a cell surface receptor responsible for the recognition and internalization of lysosomal precursor proteins and that I-cell disease must affect some covalent feature of the lysosomal enzyme required for interaction with the receptor (9). Subsequent work by Sly and Kornfeld and colleagues revealed the nature of the mannose-6-phosphate (M6P) tag on a lysosomal precursor protein glycan, the M6P receptor responsible for precursor protein internalization in intracellular traffic and the I-cell gene, which encodes a Golgi membrane enzyme that tags lysosomal glycoprotein precursors en route to the lysosome (10, 11).
Cellular Control of Cholesterol Biosynthesis
Whatever influence the precedents of receptor control and ligand internalization may have had on Brown and Goldstein’s path to discovery, their immediate objectives were framed by knowledge of the pathway of cholesterol biosynthesis and of a key regulatory step catalyzed by HMG CoA reductase, all revealed by pioneering enzymologic studies by Konrad Bloch. Further, they knew that FH patients have much higher than normal levels of cholesterol and that elevated levels of LDL, one of the major carriers of cholesterol in the blood, potentiates heart attacks, a pathology that is drastically accelerated in FH homozygotes and markedly elevated even in FH heterozygotes.
In 1972, Brown and Goldstein (Fig. 1) joined forces to tackle the cholesterol regulatory problem in relation to FH with two bold predictions: that cellular control of and by cholesterol could be reduced to the biochemical assay of HMG CoA reductase activity in cell lysates and that the lipoprotein regulation of HMG CoA reductase could be recapitulated in cultured fibroblasts. This choice of cells was crucial because of the potential to examine fibroblasts from skin biopsies of homozygous and heterozygous FH patients. Within a year, the team established the HMG CoA reductase assay, showed LDL regulation of activity in fibroblasts, and most importantly, demonstrated a defect in enzyme regulation in homozygous FH fibroblasts cultured in the presence of LDL particles (12, 13).
Fig. 1.
Michael Brown and Joseph Goldstein in their shared laboratory, circa mid-1970s.
The evidence suggested an FH defect not associated with HMG CoA reductase per se, but rather with some other aspect of regulation. In their classic 1974 PNAS paper, the team reported that LDL binds saturably to normal fibroblasts but much less well to cells cultured from five different homozygous FH patients. An excess of very-low-density lipoprotein (a precursor to LDL) competed with LDL in saturation binding tests, but HDL did not, consistent with a lipoprotein selective receptor. These results correlated exactly with a report they published a year earlier on a defect in HMG CoA reductase regulation in FH fibroblasts. In a hint of things to come, the team found LDL bound in a saturable manner to normal fibroblasts incubated at 4 °C and 37 °C, but at 37 °C, they found a 30-fold greater binding capacity followed by the formation of acid-soluble ApoB (the protein subunit of LDL particles) peptides indicative of a temperature-dependent proteolytic event. Although nonradioactive LDL competed with radioactive LDL for binding to intact cells, cells exposed to radioactive LDL at 37 °C and then transferred to medium with nonradioactive LDL continued to release radioactive fragments even in the presence of excess LDL. In a remarkable exercise of restraint, they avoided speculating, but to any student of receptor biology at the time, the obvious explanation of their data was a time- and temperature-dependent internalization of LDL to a compartment housing lipases (to expose the ApoB protein) and proteases, i.e., the lysosome.
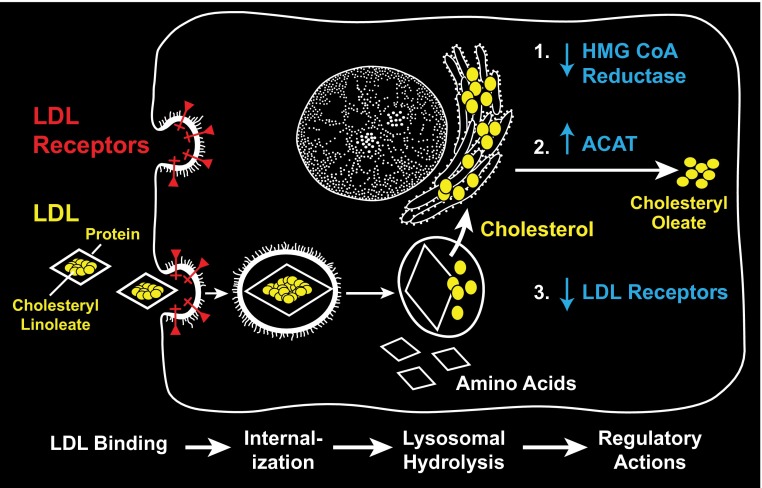
At this point, just 3 years into their careers as independent investigators, the team attracted a great deal of attention for their discoveries. However, this was only a glimmer of things to come. Over the ensuing decade, they documented the pathway of internalization of LDL to the lysosome and the corresponding cycle of receptor uptake, acid pH–dependent dissociation of LDL from the receptor, and apo-receptor retrieval to the cell surface (Fig. 2). They purified the receptor protein, cloned the gene, and sequenced hundreds of mutant alleles from FH patients around the world. Although most mutant alleles define aspects of the N-terminal ligand binding domain, two rare patients contain mutations in the C-terminal cytoplasmic domain that had no effect on ligand binding but that display mutant protein that is uniformly spread on the cell surface and is not restricted to the coated pits normally associated with receptor internalization (14, 15). These mutants, one a chain-terminating mutation that results in a truncated receptor and the other a missense mutation that changes a crucial tyrosine residue, revealed a cytoplasmic sorting signal responsible for clustering the receptor in coated pits. For all these pioneering studies, Brown and Goldstein received the Lasker Award and the Nobel Prize in the same year, 1985, just 13 years from their first efforts as beginning Assistant Professors in Dallas!
Fig. 2.
Molecular control of cholesterol biosynthesis and the LDL receptor pathway in animal cells.
Molecular Control of Cholesterol Biosynthesis
For some, the early attention and fame proves a distraction. However, Brown and Goldstein recognized that they had yet to understand how cholesterol released by the breakdown of LDL in the lysosome controls HMG CoA reductase activity. Over the next 20 years, culminating in two more landmark PNAS papers (15, 16), they solved this problem. In mapping a cholesterol-responsive DNA sequence upstream of the HMG-CoA reductase and LDL receptor genes, they discovered a transferable sterol regulatory element (SRE), which they used to isolate an SRE binding protein (SREBP). On cloning the SREBP gene, they discovered the protein they isolated corresponds to an N-terminal proteolytic fragment of a larger integral membrane protein precursor. Surprisingly, in cells grown under conditions where HMG-CoA reductase activity and gene expression are repressed (high exogenous cholesterol), the SREBP precursor remains lodged in the endoplasmic reticulum (ER) membrane. When sterols are removed from the growth medium, the SREBP precursor becomes packaged into transport vesicles for traffic to the Golgi complex. In that location, SREBP experiences two proteolytic cleavages, releasing a soluble N-terminal fragment corresponding to the protein they isolated that binds the SRE control element. Once freed from its membrane anchor, the fragment is transported into the nucleus and on to the many genes whose transcription it elevates.
However, exactly how does cholesterol work to promote the vesicular packaging of SREBP precursor? In the course of genetic studies to identify novel regulators of SREBP function, the team found genes encoding the two Golgi membrane proteases, but also another integral membrane protein, SCAP, whose presence in the ER is crucial for SREBP traffic to the Golgi. SCAP contains a cholesterol-binding pocket that, when occupied, retains the SCAP–SREBP complex in the ER. This retention is maintained by a resident ER membrane protein, INSIG, which itself is a sterol binding protein with specificity for 25-OH cholesterol (15). When sterols are removed from the growth medium, INSIG dissociates from SCAP, and a cytoplasmically exposed protein loop of apo-SCAP changes its conformation to expose a sorting signal that is recognized by the sorting subunit of a coat protein complex, COPII, responsible for traffic of all secretory cargo proteins from the ER to the Golgi complex (16). Thus, the puzzle of cholesterol regulation may be explained by a conformational change in SCAP that liberates a SCAP–SREBP complex in a form that is packaged for transport to the Golgi complex where the proteases responsible for SREBP maturation are housed.
Closing the Cholesterol Regulatory Loop
A remaining mystery, and one that Brown and Goldstein claim they must solve before they retire, is how cholesterol liberated from LDL cholesteryl-esters is transported out of the lysosome and then to the ER for interaction with SCAP.
The first breakthrough came with the discovery that another cholesterol metabolic disease, Nieman-Pick type C, results from a defect in one of two genes that encode cholesterol carrier and membrane transporter proteins that convey cholesterol to the inner surface and then through the lysosomal membrane to the cytoplasmic face of the organelle. The process whereby cholesterol then is passed through the cytoplasm to the ER membrane remains somewhat uncertain. Studies in yeast suggest that the traffic of ergosterol from the ER to other organelles is regulated by one of seven different OSH genes. The OSH proteins are believed to bridge different membranes, permitting direct sterol transfer without a freely soluble cytoplasmic carrier intermediate (17, 18). Other studies in mammalian cells suggest another protein that could serve as a soluble carrier (19).
This remains an area of active investigation to which Brown and Goldstein will no doubt make crucial contributions.
Conclusion
Over a 40-year span, the team of Brown and Goldstein has solved a significant but elusive problem in cardiovascular disease, the role of a lipoprotein, LDL, and cholesterol in the regulation of cholesterol biosynthesis. Few examples in basic biomedical science have led to such deep cellular and biochemical mechanistic insights that impact health and disease. The 1974 PNAS Classic paper featured here is just one step in a long path that led them to our current understanding of how cholesterol regulates the activity of HMG-CoA reductase. The beauty of their approach, the focus on one enzyme and pathway, and the precision of their analysis stand as a model of clarity in all of the life sciences. Few scholars can model their success, but all can benefit by their wisdom.
Footnotes
The author declares no conflict of interest.
This article is a PNAS Direct Submission.
This Perspective is published as part of a series highlighting landmark papers published in PNAS. Read more about this classic PNAS article online at www.pnas.org/site/classics/pnas_classics.xhtml.
See Classic Article “Familial hypercholesterolemia: Defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity” on page 788 in issue 3 of volume 71.
See Classic Profile on page 14829.
References
- 1.Brown MS, Goldstein JL. Familial hypercholesterolemia: Defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. Proc Natl Acad Sci USA. 1974;71(3):788–792. doi: 10.1073/pnas.71.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland EW, Wosilait WD. The relationship of epinephrine and glucagon to liver phosphorylase. I. Liver phosphorylase; preparation and properties. J Biol Chem. 1956;218(1):459–468. [PubMed] [Google Scholar]
- 3.Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc Natl Acad Sci USA. 1954;40(10):1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cuatrecasas P. Affinity chromatography and purification of the insulin receptor of liver cell membranes. Proc Natl Acad Sci USA. 1972;69(5):1277–1281. doi: 10.1073/pnas.69.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth TF, Porter KR. Yolk protein uptake in the oocyte of the mosquito Aedes aegypti. L. J Cell Biol. 1964;20:313–332. doi: 10.1083/jcb.20.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearse BMF. Coated vesicles from pig brain: Purification and biochemical characterization. J Mol Biol. 1975;97(1):93–98. doi: 10.1016/s0022-2836(75)80024-6. [DOI] [PubMed] [Google Scholar]
- 7.Morell AG, Gregoriadis G, Scheinberg IH, Hickman J, Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971;246(5):1461–1467. [PubMed] [Google Scholar]
- 8.Hickman S, Neufeld EF. A hypothesis for I-cell disease: Defective hydrolases that do not enter lysosomes. Biochem Biophys Res Commun. 1972;49(4):992–999. doi: 10.1016/0006-291x(72)90310-5. [DOI] [PubMed] [Google Scholar]
- 9.Hickman S, Shapiro LJ, Neufeld EF. A recognition marker required for uptake of a lysosomal enzyme by cultured fibroblasts. Biochem Biophys Res Commun. 1974;57(1):55–61. doi: 10.1016/s0006-291x(74)80356-6. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan A, Achord DT, Sly WS. Phosphohexosyl components of a lysosomal enzyme are recognized by pinocytosis receptors on human fibroblasts. Proc Natl Acad Sci USA. 1977;74(5):2026–2030. doi: 10.1073/pnas.74.5.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kornfeld S, Reitman ML, Varki A, Goldberg D, Gabel CA. Steps in the phosphorylation of the high mannose oligosaccharides of lysosomal enzymes. Ciba Found Symp. 1982;92(92):138–156. doi: 10.1002/9780470720745.ch8. [DOI] [PubMed] [Google Scholar]
- 12.Brown MS, Dana SE, Goldstein JL. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in human fibroblasts by lipoproteins. Proc Natl Acad Sci USA. 1973;70(7):2162–2166. doi: 10.1073/pnas.70.7.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldstein JL, Brown MS. Familial hypercholesterolemia: Identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc Natl Acad Sci USA. 1973;70(10):2804–2808. doi: 10.1073/pnas.70.10.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson RG, Goldstein JL, Brown MS. A mutation that impairs the ability of lipoprotein receptors to localise in coated pits on the cell surface of human fibroblasts. Nature. 1977;270(5639):695–699. doi: 10.1038/270695a0. [DOI] [PubMed] [Google Scholar]
- 15. Radhakrishnan A, Ikeda Y, Kwon HJ, Brown MS, Goldstein JL (2007) Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc Natl Acad Sci USA 104(16):6511–6518. [DOI] [PMC free article] [PubMed]
- 16.Sun L-P, Seemann J, Goldstein JL, Brown MS. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Insig renders sorting signal in Scap inaccessible to COPII proteins. Proc Natl Acad Sci USA. 2007;104(16):6519–6526. doi: 10.1073/pnas.0700907104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sullivan DP, Ohvo-Rekilä H, Baumann NA, Beh CT, Menon AK. Sterol trafficking between the endoplasmic reticulum and plasma membrane in yeast. Biochem Soc Trans. 2006;34(Pt 3):356–358. doi: 10.1042/BST0340356. [DOI] [PubMed] [Google Scholar]
- 18.Raychaudhuri S, Im YJ, Hurley JH, Prinz WA. Nonvesicular sterol movement from plasma membrane to ER requires oxysterol-binding protein-related proteins and phosphoinositides. J Cell Biol. 2006;173(1):107–119. doi: 10.1083/jcb.200510084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mesmin B, et al. STARD4 abundance regulates sterol transport and sensing. Mol Biol Cell. 2011;22(21):4004–4015. doi: 10.1091/mbc.E11-04-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]