Significance
It is recognized that the risk of malaria in dry areas is increased in the proximity of irrigation infrastructure. Although historical evidence shows that eventually malaria risks subside on the road to greater prosperity and food security, how this comes about remains poorly understood. We studied changes in land use and malaria risk in a large irrigation project in a semidesert region in northwest India. The transition phase we describe, characterized by elevated malaria despite raised control efforts, has already lasted for over a decade. The protracted nature of this transition highlights the need for a long-term commitment by health authorities and international agencies supporting irrigation schemes, to monitor health impacts and sustain control measures.
Keywords: vector-borne diseases, agricultural development, epidemic malaria, irrigation gradient, environmental health
Abstract
In arid areas, people living in the proximity of irrigation infrastructure are potentially exposed to a higher risk of malaria due to changes in ecohydrological conditions that lead to increased vector abundance. However, irrigation provides a pathway to economic prosperity that over longer time scales is expected to counteract these negative effects. A better understanding of this transition between increased malaria risk and regional elimination, in particular whether it is slow or abrupt, is relevant to sustainable development and disease management. By relying on space as a surrogate for stages of time, we investigate this transition in a semidesert region of India where a megairrigation project is underway and expected to cover more than 1,900 million hectares and benefit around 1 million farmers. Based on spatio-temporal epidemiological cases of Plasmodium vivax malaria and land-use irrigation from remote sensing sources, we show that this transition is characterized by an enhanced risk in areas adjacent to the trunk of the irrigation network, despite a forceful and costly insecticide-based control. Moreover, this transition between climate-driven epidemics and sustained low risk has already lasted a decade. Given the magnitude of these projects, these results suggest that increased health costs have to be planned for over a long time horizon. They further highlight the need to integrate assessments of both health and environmental impacts to guide adaptive mitigation strategies. Our results should help to define and track these transitions in other arid parts of the world subjected to similar tradeoffs.
In agricultural economies, food insecurity imposes a strong pressure to extend agriculture to marginal areas. In low-rainfall regions, irrigation offers considerable rewards, creating water resources for irrigation and other uses. On either side of the border of India and Pakistan, an extensive arid region is intersected by large rivers carrying water from the Himalayan glaciers and rainwater from a short rainy season. Hundreds of millions of people depend on the southwestern monsoon for their survival. Over the centuries, its periodic failure and severe ensuing drought and famine conditions have provided a strong incentive to develop and extend irrigation. The continuing expansion of the Indian population in the 21st century adds further pressure to optimize the country’s water resources for agriculture, fisheries, and industrial and general use.
The development of water resources, in the context of malaria and dengue, exemplifies a central challenge in sustainability science: How can we achieve socioeconomic development based on land-use transformations, with concomitant increases in human well-being, when the transformations can compromise ecosystem services and human health for present and future generations?
For arid regions, concerns have been raised about the consequences for malaria epidemiology resulting from ecological changes subsequent to the arrival of irrigation, with several studies reporting local increases in prevalence and parasitemia (1–4). By increasing surface water levels, irrigation modifies ecohydrological conditions of the landscape, creating more standing bodies of water for longer periods of time (5), thereby increasing the abundance of mosquito breeding sites and adult vector populations (6–13). In addition, agricultural development can increase the frequency of human–vector contact, when human labor and mosquito breeding seasons are synchronized (14), and promotes migration to newly irrigated areas (15), thus changing the spatial scale of malaria transmission. The global population at risk for contracting malaria due to proximity to irrigation infrastructure has been estimated at around 800 million, which represents ∼12% of the global malaria burden (16).
In these dry, fragile ecosystems, where increase in water availability from rainfall is the limiting factor for malaria transmission, irrigation infrastructure can drastically alter mosquito population abundance to levels above the threshold needed to maintain malaria transmission. In northwestern India, an increase in domestic and paradomestic water storage support Anopheles stephensi, India's urban malaria vector. In the desert areas of Rajasthan, a rise in malaria associated with Anopheles culicifacies has been reported following large-scale irrigation development by the Indira Gandhi canal (17, 18). Currently, seasonal epidemics of mainly Plasmodium vivax occur in these semideserts, at the edge of the geographic distribution of the disease. At these fringes, P. vivax, with its relapses (19), has a competitive advantage over Plasmodium falciparum, the more malignant form of malaria that also occurs in India. P. falciparum, less able to persist in unfavorable transmission conditions, is less consistently present in this region and displays more interannual fluctuations. Because we are mostly concerned with the seminal changes of malaria resulting from irrigation in the area, we have in this study focused on changes of P. vivax.
Despite these environmental changes favoring transmission, historical studies in the semiarid regions of Pakistan and the former Punjab Province have also documented irrigation-based development having the opposite effect. With the arrival of irrigation in the latter half of the 19th century, large-scale migration and colonization followed, and the regional malaria burden initially rose dramatically (20, 21). However, the Eastern and Western Punjab of former British India, part of India and Pakistan, respectively, since 1947, are now rich agricultural regions with low malaria prevalence.
Apparently, ecological and socioeconomic factors alter the dynamics and distribution of the parasite, the vector, and human susceptibility, from the arrival of irrigation to its long-term stabilization. Although previous studies taken together suggest that different effects operate over different time horizons (22), observations typically correspond to different stages of the developmental process, local in time or space. No study to date has followed remotely sensed irrigation characteristics and malaria levels simultaneously over a period that encompasses these different stages or over large regions whose irrigation gradient provides a surrogate for these temporal stages.
The long-term malaria surveillance program in arid northwestern India provides a unique spatio-temporal dataset for considering just such a gradient in irrigation intensification over the last 15 y. By following the changes in malaria incidence, vegetation, and socioeconomic data at the level of subdistricts, we identify a transition phase toward sustainable low risk (elimination) lasting for more than a decade, and characterized by an enhanced environmental malaria risk despite intensive mosquito control efforts. This protracted phase highlights the need for considering health impacts in the long-term planning, assessment, and mitigation of projects related to water resources.
Results
Study Area.
This research was conducted in a semiarid area of the northeast part of the state of Gujarat, in the districts of Kutch, Banas Kantha, and Patan (SI Appendix, Fig. S1). These districts are divided into 25 subdistricts, or talukas, for which the epidemiological surveillance data are aggregated (Fig. 1A). Due to the influence of the southwest monsoon, rainfall is extremely variable from year to year in the region. Annual rainfall ranges from 120 mm in the western part of Kutch to 600 mm in the eastern border of Banas Kantha and is concentrated between the months of June and September. This climatic pattern creates strong seasonal malaria and high variability between years (23, 24). The peak of the epidemics usually varies from August to November, depending on the parasite species and the timing and length of the rainfall season (25).
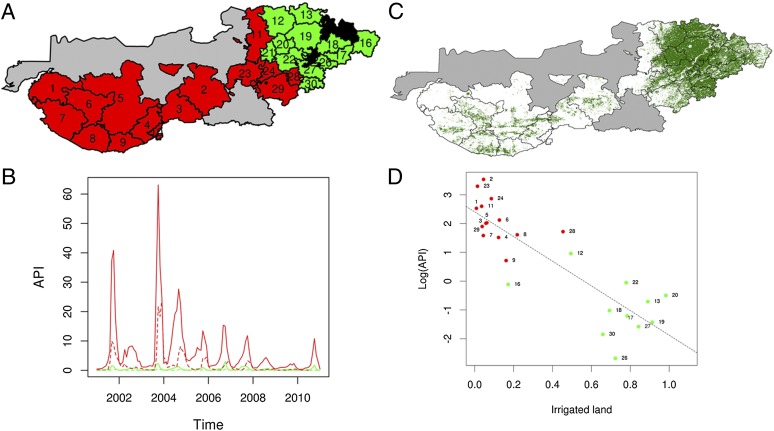
Fig. 1.
Spatio-temporal pattern in malaria population dynamics and its relationship to irrigation development. (A) Two groups in the configuration of malaria risk obtained by the Bayesian grouping algorithm. Areas of high risk are colored in red and those of low risk areas, in green. (The overall results on groupings are robust to the choice of parasite species, as well as to the number of levels for the different quintile divisions of the epidemiological data). (B) The time series of accumulated cases are shown for the two groups (red and green) and for the two malaria species (solid line, P. vivax and dashed line, P. falciparum). Most of the malaria burden in the region corresponds to P. vivax. For comparison with A, C shows the irrigation pattern for the year 2009, for which a detailed irrigation map was available. This comparison shows that the high and low malaria groups map respectively onto the non- (or low-) irrigated (in white) and irrigated areas (in green) respectively. Although the map is for 2009, a similar broad pattern of irrigation holds across years (SI Appendix, Fig. S8), and the eastern region has been irrigated for at least three decades (SI Appendix, Fig. S2). The detailed pattern of how irrigation has changed in the more recent decade is addressed in Fig. 2. (D) An example of the malaria–irrigation relationship for the large epidemic of the year 2003. In this plot, each point corresponds to Annual Parasite Incidence (API, cases per 1,000 people) in log scale in a particular taluka, during the epidemic season (September–December), as a function of the proportion of the land classified as irrigated, based on January’s vegetation from remote sensing (Methods). The numbers in A and D correspond to the name of a taluka as follows: 1, Lakhpat; 2, Rapar; 3, Bhachau; 4, Anjar and Gandihan; 5, Bhuj; 6, Nakhatrana; 7, Abdasa; 8, Mandavi; 9, Mundra; 11, Cac; 12, Tharad; 13, Dhanera; 16, Danta; 17, Vadgam; 18, Palanpur; 19, Deesa; 20, Deodar; 21, Bhabhar; 22, Kankrej; 23, Santalpur; 24, Radhanpur; 26, Sidhpur; 27, Patan; 28, Harij; 29, Sami; and 30, Chanasma.
Spatio-Temporal Patterns of Malaria and Irrigation.
Strong and long-lasting differences exist in malaria population dynamics between the talukas located in the eastern and the western parts of the study area. We identified these two main regions (depicted in red and green colors in Fig. 1A using a Bayesian statistical method that identifies groups of spatial locations (talukas) whose temporal disease dynamics are similar (26) (Methods). These differences, observed for P. vivax reflect distinct overall incidence levels, as illustrated by the time series of the monthly cases accumulated for each group (Fig. 1B). The identified grouping was robust to changes in modeling assumptions (Methods). A similar pattern was also observed for P. falciparum, the species with the lower and less consistent regional presence. Throughout the entire region a slow declining trend is also apparent, presumably as the result of the intense level of mosquito control intervention in the area (27). However, due to the dynamic interplay between rainfall and control intervention in the region, which can cause a tendency to cycle at decadal time scales (28), caution is needed in extrapolating this trend to the future.
The identified differences in malaria population dynamics are strongly coherent with long-term irrigation patterns. Fig. 1C shows a map of irrigated areas for the year 2009 obtained from remote sensing information and the spectral signature of important crops in the area (Methods). In Fig. 1C, two regions are also apparent and closely map onto the malaria clusters. Whereas the westernmost subdistricts in Banas Kantha and Patan are intensively irrigated (mainly from deep wells) and have been for over 30 years (SI Appendix, Fig. S2), the eastern ones, in Kutch and parts of Patan and Banas Kantha, have little irrigation. Thus, we refer to these two regions as “mature irrigated” and “low irrigated,” respectively. Fig. 1D shows the epidemic vulnerability of the low irrigated areas after above normal monsoon rains for the year 2003. The incidence of malaria recorded in the low-irrigated zone (red dots) was significantly higher than that observed in the mature-irrigated one (green dots). This particular large epidemic followed a very dry year (2002) with little malaria, and a reduced (reactive) insecticide coverage response in 2003 (see Methods for insecticide application policy). Thus, although this particular year exhibits an increase in incidence at every location, the ranking in epidemic size is consistent with the two spatial clusters defined over the whole period of study, despite differences in local climatic conditions or control intervention.
Are these differences in malaria risk between the mature-irrigated and low-irrigated areas associated with the overall level of development and wealth of these two main regions? Table 1 summarizes the results from a statistical comparison between high and low malaria risk zones in terms of socioeconomic indicators for the year 2001 (Methods). In general, high-risk talukas had a lower proportion of literate people and more limited access to sources of improved drinking water. In addition, no significant differences were observed between the percentage of people with access to state-supplied public health and medical facilities and education. The differences between the proportion of people with access to credit from agricultural societies, however, are pronounced, with 80% of the farming communities living in the low-risk malaria area having access to credit for improving agricultural practices, compared with only 60% of those living in the high-risk area.
Table 1.
Statistical analysis of differences between mature irrigated and nonirrigated areas
| Variable | High risk | Low risk | t value | df | P value | W | W P value |
| Literates/illiterates | 0.7577 | 0.8935 | −0.8827 | 16.1796 | 0.3903 | 71 | 0.7675 |
| Improved drinking water | 0.9227 | 0.9903 | −2.2142 | 13.9951 | 0.0439 | 36.5 | 0.0282 |
| Agricultural credit societies | 0.6106 | 0.8183 | −2.7805 | 18.2579 | 0.0122 | 18 | 7.00E-04 |
| Banks access | 0.2565 | 0.2464 | 0.1966 | 19.1836 | 0.8462 | 83 | 0.7675 |
| Education access | 0.9946 | 0.9975 | −1.0629 | 22.9806 | 0.2989 | 51 | 0.1492 |
| Medical access | 0.7705 | 0.6615 | 1.5383 | 22.9944 | 0.1376 | 102 | 0.1832 |
| % irrigated land | 0.1615 | 0.7172 | −9.1045 | 21.25 | <0.0001 | 2 | <0.0001 |
| IRS control | 0.4558 | 0.069 | 4.6783 | 16.176 | <0.0001 | 146 | 2.00E-04 |
Socioeconomic variables consist of the ratio between literate and illiterate populations, the proportion of the population in each taluka with access to potable water, agricultural credit societies, banks, and education, public health, and medical facilities. Education facilities encompass all primary and secondary schools. Medical access corresponds to the community health centers, primary health centers, subcenters, and hospitals available in each taluka. A two-sample location unpaired Welch’s t test, with the level of malaria risk obtained from the cluster algorithm as a categorical variable, was used to test if the socioeconomic indicators from the talukas were sampled from two different normal distributions based on the level of the malaria risk. We also performed a nonparametric Wilcoxon test that does not assume normality. We applied these tests for indoor residual spray (IRS) control and for the percentage of the talukas under irrigation in 2001. W, the Wilcoxon statistic.
Change in Irrigation and Malaria Risk in Last Decade.
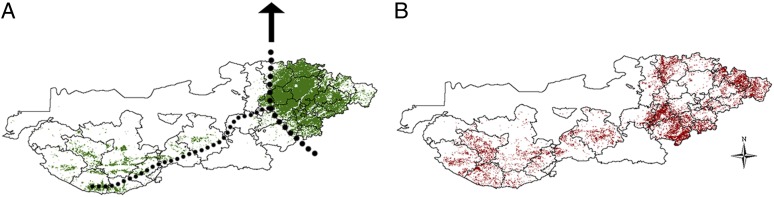
Currently, a large irrigation project is under expansion at the edge of the mature-irrigated and low-irrigated subdistricts (Fig. 2A). Most of the low-irrigated territory is expected to receive the complete intended water supply for agriculture and human consumption by the year 2014. Because we do not have direct information on yearly irrigation, and therefore, on the annual change that has occurred during the same period for which the epidemiological and control data were obtained, we estimated these changes by relying on the yearly variation in vegetation coverage during the peak of the irrigation (rabi) season (January). During this period without rain, most of the satellite-observed vegetation in this arid environment should be the result of irrigated crops, an assumption that is supported by the observation of the seasonality of the Normalized Difference Vegetation Index (NDVI) in the area (SI Appendix, Fig. S3) and the spatial clustering of the vegetation outside the rainy season. Taking advantage of this seasonality and the map of true irrigation from the year 2009, we develop a classification based on a threshold value of NDVI to separate mature-irrigated and low-irrigated locations (pixels) (Methods). Fig. 2A shows the area classified as irrigated and nonirrigated for the year 2001, and Fig. 2B shows in red the area classified as irrigated in 2009 but not in 2001. This latter map highlights that most of the change in irrigation (outside the monsoon season) during the last decade took place in the fringe zone between the mature-irrigated and low-irrigated regions. These ecological changes occurring on the border match the path of the main irrigation canal of this megairrigation project taking place in the area of study (Fig. 2A). This increment in vegetation was especially pronounced in the southernmost talukas where the principal canal first arrived more than a decade ago (Fig. 2B and SI Appendix, Fig. S4), but the canal is expanding to the north, to the unirrigated districts of Rajasthan, with a side branch to the talukas in Kutch (Fig. 2A).
Fig. 2.
Irrigation pattern and its change over time. (A) Area classified as irrigated agriculture (in green) for 2001, based on the NDVI classification of irrigated pixels outside the monsoon season (at a 250-m2 resolution), as described in Methods. The black dotted lines in A represent the position of the trunk of the canal and its main branch to the west. (B) Areas that have experienced the most pronounced variation in irrigation levels between 2001 and 2009. Specifically, areas in red correspond to those classified as irrigated in 2009 but nonirrigated in 2001 (see also SI Appendix, Figs. S8 and S9).
How do these changes in irrigation within the last decade correlate to the changes in malaria risk during the same period? The spatial distribution of malaria burden, especially within the most malarious and low-irrigated talukas, can be examined in more detail by dividing the time series into two periods (2000–2005 and 2006–2010), accumulating the incidence during the two periods and then normalizing them by the corresponding value for the subdistrict with the highest burden. In this way we can compare the spatial distribution of the cases independently from the yearly variation in malaria due to climate conditions or control application. These maps of spatial relative risk (Fig. 3A for 2006–2010 and SI Appendix, Fig. S6 for 2000–2005) also highlight this same boundary region in the middle of the mature-irrigated and low-irrigated clusters. In this fringe zone, the incidence of P. vivax was higher than in the low-irrigated more western talukas of Kutch, particularly in the last part of the decade. P. falciparum does not show this clear distinction especially for the more recent years (SI Appendix, Fig. S5), given its low incidence.
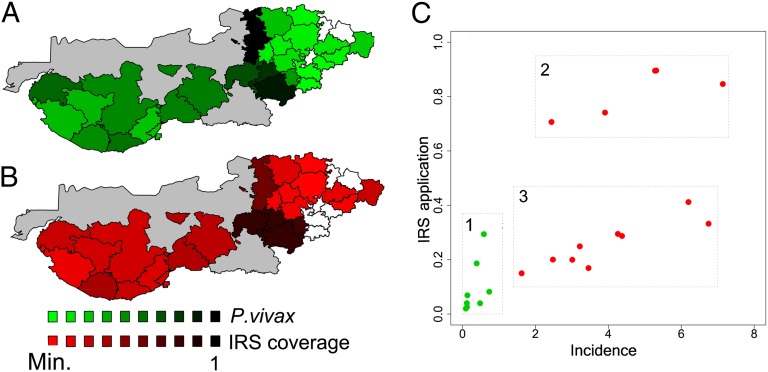
Fig. 3.
Spatial distribution of relative malaria risk and control application. (A)The spatial distribution of total incidence for Plasmodium vivax relative to its maximum value for the period of 2006 to 2010. The scale of green tones corresponds to values of malaria incidence relative to this maximum and spans values of “risk” from the lowest (0.006, in light green) to the highest (up to 1, in dark green). (B) Similar spatial distribution of the IRS effort based on the mean proportion of the population covered, again relative to the maximum value observed between the period of 2006 and 2010. Colors from light to dark red represent population covered with insecticide house spraying (IRS) according to the national policy, from a low (0.0004) to a high percentage, respectively. Talukas with no information are shown in white; gray areas represent the uninhabited Rann of Kutch. (Similar spatial patterns are seen for the period of 2000–2005 in SI Appendix, Figs. S6 and S7). (C) The three malaria epidemiological zones defined based on incidence, control, and irrigation. The mean proportion of population covered by IRS is shown as a function of median incidence from 2000 to 2010. Green dots correspond to the talukas that have been irrigated for a long time and present low malaria risk and low IRS coverage (zone 1). Differences in the level of IRS coverage for areas of high risk (red dots) can be observed between the talukas in Kutch with relative low control but high risk (zone 3) and those in Banas Kantha and Patan, the transition zone, that exhibit both high IRS application and high incidence (zone 2), with coverage reaching values of around 80%. Despite the downwards trend in cases, the existence of these three zones persist throughout the decade (SI Appendix, Fig. S10).
Strikingly, this transition region with highest levels of malaria is also observed in the efforts to control the disease. Fig. 3B shows the percentage of population covered by mosquito indoor residual spray (IRS) application in each subdistrict between 2006 and 2010 (see SI Appendix, Fig. S7 for 2000 and 2005). In the transition zone, up to 80% of the population qualifies for spraying; signifying the raised levels of public health efforts to address increased levels of malaria. This contrasts with the low-irrigated regions to the west, and particularly with the mature-irrigated areas that required the least intervention. This clearly highlights that this zone is epidemiologically different from the other two regions previously described.
Based on the incidence and control of malaria and the ecological changes observed, three main ecoepidemiological zones can be recognized (Fig. 3C): (i) an area of low disease burden and low requirement of control coverage, corresponding to subdistricts that have been irrigated over a long period (several decades; SI Appendix, Fig. S2); (ii) a transition region with high incidence despite high control coverage (IRS coverage of 80–90% of the targeted population) in the subdistricts adjacent to the advancing irrigation project; and (iii) and a low-irrigation area in Kutch with variable rainfall-dependent seasonal outbreaks and intermediate (variable) levels of required intervention.
The transition zone in malaria risk is characterized by an increased environmental impact that has now already lasted for at least a decade.
Discussion
We have shown that enhanced disease risk despite heightened intervention is concentrated in the subdistricts adjacent to the main canal that have experienced the most pronounced change in irrigation levels in the last decade. By contrast, a sustained low disease burden, not requiring high coverage with vector control, is found in neighboring subdistricts that have been irrigated for at least three decades. This long-lasting transition phase is consistent with the historical changes reported for the Punjab, once the center of some of the most devastating malaria epidemics on record (29, 30) and today one of the more prosperous food-producing parts of India, with low endemic levels of the disease. These historical changes took place in a period in excess of half a century and their dynamics remain only partially understood.
A better understanding of socioeconomic and ecological differences between recently irrigated and mature irrigation areas could provide the means to reduce the malaria burden and shorten the transition phase (31). On the environmental side, changes in vectors’ ecology following increases in surface water levels and soil salinity have been proposed for the decrease in malaria risk in the Punjab (32, 33). Historically an enhanced malaria risk has also been related to construction activities, such as the local production of bricks and road works that create vectors’ habitats by altering the landscape and fall under the “tropical aggregation of labor” (34). For the expanding population in the command area of the Sardar Sarovar Project in Gujarat, this has been recognized as a problem (4), along with the seepage of water from improperly constructed and maintained irrigation structures.
On the socioeconomic side, our observations based on the 2001 census data, show that in the cluster of subdistricts irrigated for at least three decades, farmers had easier access to agricultural credit, and populations benefited from higher literacy levels and better access to clean water. (Similar analyses for the 2011 census would be of interest when these data become available). By extending periods of water availability beyond the rainy season, irrigation creates the possibility of multicrop rotations and also facilitates the use of high yield varieties with superior economic return (35). Over the years, these changes should ensure food security and more stable income, leading to improved socioeconomic conditions and the ability of the population to seek health care and afford preventive measures, eventually spiraling out of the “malaria poverty trap” (36, 37).
Regardless of specific mechanisms, this long-lasting transition from high risk to low disease prevalence is usually accompanied by a resource-intensive vector control operation mainly based on the use of insecticides. Given the timescale of this transition, efficient and long-term policies and sustainable intervention capability is required, especially in usually poor semiarid areas undergoing intensive and rapid ecological change, where resources to support long-term control interventions can be limited. The exacerbation of malaria risk combined with a deceleration of economic development or even the temporary relaxation of control (38) may lengthen the transition phase and allow for epidemic surprises in years of anomalous high rainfall (28), in a regression back to climate-driven dynamics. This is a concern especially in areas where groundwater extraction surpasses the current capacity of water sources (39).
Based on the high cost of interventions and the large areas involved, environmental impact assessments (EIAs) in irrigation developments should include health impact assessments (HIAs) at all different phases of the projects (40). Ongoing monitoring, surveillance, and adaptive mitigation of the negative consequences on water-borne and vector-borne diseases are needed as the projects evolve over time, as well as provision for the cost of these activities over a significant time horizon (41). During the construction phase of the Sardar Sarovar Project (1979–1985), malaria incidence increased significantly (4). Later, during the developmental activities in the command area, considerations regarding water- and vector-borne diseases were incorporated and mitigation measures implemented. Even though HIA is generally included in EIA for large-scale developmental projects, the sustained implementation of recommended measures to decrease these impacts is often incomplete (42, 43). The situation is generally more critical for small- to medium-scale developmental projects in Southeast Asia, such as the construction of smaller irrigation canals and ponds for water collection and storage. In these settings, HIAs, and specifically monitoring and surveillance, are more limited, despite their potential to affect extensive areas and large populations.
The observations on the long transition phase in Gujarat reinforce the insight that development of water resources requires a strong binding commitment to finance and implement projects that maintain public health and safety (44). Environmental management methods for sustainable disease control are strongly needed. Several of these methods have proved to be cheaper, more effective, and feasible to implement at local scales, including those that manipulate water flow in irrigation systems, such as intermittent irrigation or canal flushing (45). The challenge ahead, then, will be to apply these methods over extensive regions and maintain them for long enough periods. Already in the early 1980s, the interdisciplinary and multisectorial nature of health problems related to irrigation were recognized. A joint World Health Organization/Food and Agriculture Organization/United Nations Environment Programme Panel of Experts on Environmental Management was established, which produced guidelines and raised main issues at stake with policy-setting institutions worldwide (46). The likely decline of global food security with predicted climate, and the rise of food prices in recent years, should be an incentive to reinvigorate initiatives to develop methods that mobilize global water resources without compromising human and environmental health.
Methods
Study Area and Epidemiological Data.
The western district of Kutch contains 9 subdistricts or talukas. In the eastern districts of Banas Kantha, and Patan, 16 talukas complete our data set. Taluka Bhabhar was part of taluka Deodhar until the year 2000. For this analysis, cases for both talukas were merged and included as a single unit.
The epidemiological data consists of time series of microscopically confirmed cases of P. falciparum and P. vivax from rural areas for the 25 talukas in these three districts. These monthly time series span a period of 12–15 y, from 1997 to 2011 for Kutch and Banas Kantha, and between 2000 and 2011 for Patan. The malaria data based on active surveillance (health workers’ home visits twice a month) and passive surveillance (self-reporting at public health facilities), were obtained from the office of the Joint Director (Malaria), Commissionerate of Health and Family Welfare, Gandhinagar (Gujarat). All of the analyses were conducted using incidence rates (cases per 1,000 people). Rural population data for each taluka were obtained by linear interpolation from three national district decadal population census reports for 1991, 2001, and 2011, by removing large urban agglomeration and cities (for the robustness of the results to other means of estimating population numbers, see SI Appendix, Figs. S6 and S7). Socioeconomic data were obtained from the District Census Handbook of the concerned district for the year 2001 from Directorate of Census Operations, Gujarat (Table 1).
Indoor Residual Spraying Data and Control in the Region.
IRS with an effective insecticide is applied to control malaria in high-risk villages and to contain malaria outbreaks. The national policy requires IRS application in high-risk villages only, for which villages are stratified by risk at the beginning of each calendar year (high risk reported in the previous year: villages reporting two or more malaria cases per 1,000 people; those reporting confirmed malaria death or cumulative increase in falciparum in the past 3 y). Usually beginning in late May/early June, two rounds of IRS 3 mo apart with a pyrethroid insecticide or three rounds 45 d apart with malathion are applied. For this study, we obtained IRS data of each taluka for 2001 through 2010 from the office of the Joint Director (Malaria) Gandhinagar.
In outbreak years, IRS intervention efforts are complemented through intensive active case detection and prompt treatment and better coverage by insecticides. Until recently, chloroquine has been the first line of treatment for P. falciparum and P. vivax cases. Recent changes in control efforts for the district of Kutch include the introduction of deltamethrin impregnated nets at a small scale since 2004, of long-lasting nets after our study period, and of artemisinin-based combination therapy for the treatment of P. falciparum cases in the year 2010.
Remote Sensing Data and NDVI-Based Irrigation Product.
Irrigation map for the year 2009.
A procedure to discriminate irrigated from nonirrigated crops using remote sensing information is to compare the empirical spectral signature of crops, obtained by ground control measurements, against the spectral signature of vegetation in the area of interest gathered by satellite sensors (47). The irrigation map presented in Fig. 1C was developed in this way by the Bhaskaracharya Institute for Space Application and Geo-Informatics (BISAG) Gandhinagar, Gujarat, and constructed using the Advanced Wide Field Sensor (AWiFS), at a resolution of 56 m and with ground control points from the BISAG data library.
Reconstructing irrigation maps and estimating change in irrigated areas based on remote sensing.
Agricultural seasons can be divided into kharif and rabi, with crops in the former usually planted in June/July and consisting of a mixture of irrigation and rain-fed crops, and those in the latter (mainly wheat) planted in October/November and harvested in March/April, relying mostly on irrigation.
To reconstruct areas of irrigated agriculture outside the year of 2009, we used the 250-m resolution NDVI images from the moderate-resolution imaging spectroradiometer instrument (MODIS) aboard the Terra satellite. The data were provided by the Land Processes Distributed Active Archive Center located at the US Geological Survey (USGS) Earth Resources Observation and Science (EROS) Center (https://lpdaac.usgs.gov). Our method is based on the observation of the seasonal temporal pattern of vegetation in the region: in highly-irrigated areas, NDVI time series show a bimodal seasonal pattern with one peak in September (end of kharif crop) and another in January (middle of rabi crop) reflecting irrigation (SI Appendix, Fig. S3). Thus, the NDVI image for the month of January 2009 shows a spatial pattern similar to that of the irrigated map for that same year (SI Appendix, Fig. S8). Based on the comparison of NDVI values in January 2009 with the irrigation map, we developed a classification that differentiates irrigated and nonirrigated pixels based on a cutoff value in NDVI. Thus, by collecting images from the MODIS instrument, we generated a set of Boolean images between 2001 and 2013 (Fig. 2A for 2001 and SI Appendix, Fig. S8 for other years, including 2009) that were then used to quantify the change in the proportion of irrigated land in each subdistrict (Fig. 2B for 2001–2009 and SI Appendix, Fig. S9 for other years). The best cutoff value was obtained when the classification performance was the most accurate in predicting true occurrence of irrigated and nonirrigated locations simultaneously.
Specifically, let Pi and Pn be univariate density functions of NDVI-MODIS images for irrigated and nonirrigated classes, respectively, and Fi and Fn, their corresponding cumulative distribution functions at different cutoff values of NDVI ∈ [0,1]. For our purposes, Fi is the empirical cumulative distribution function of the predicted probability for pixels of MODIS-NDVI images when irrigation actually occurred (BISAG map), and Fn is the empirical cumulative distribution function for areas where irrigation did not occur. We determined the value of NDVI that maximizes the Kolmogorov-Smirnov distance D(τ) = max|Fi − Fn|, where τ is the value of NDVI with the greatest distance D between the two curves. A threshold of NDVI = 0.34 (F statistic = 0.8) was found as the best value for this binary classification.
Group Inference via a Markov Transition Model.
To identify groups of locations with similar dynamics, we used a nonparametric Markov transition model (48) in a Bayesian framework (26). Under this model, the data are discretized into a set of finite levels by putting all zeros in the lowest level and then dividing the remaining data into observed quantiles of cases per capita. Transitions between levels over time are described by a Markov transition matrix. Locations are assigned to different groups, and within a group each location’s time series is assumed to follow the same transition matrix. Good groupings thus have locations with similar dynamics assigned to the same groups, and the goal is to identify the best grouping, defined as the one with the highest marginal likelihood. Transition-matrix rows were assigned noninformative Jeffreys priors (49), and the marginal likelihood was evaluated analytically for all 224 = 16,777,216 different arrangements into one or two groups. We checked results for robustness to changes in transition-matrix priors, to the number of disease levels, and to changes in the number of groups. We also compared the results to an equivalent maximum-likelihood analysis, which yielded identical results.
Supplementary Material
Acknowledgments
We thank the Director of the National Institute for Malaria Research (New Delhi) for support, the Office of the Joint Director (Malaria) Gandhinagar (Gujarat), the district malaria officers for supplying the malaria and the insecticide use data, Dr. Shrivastava for insightful discussions, and Dr. T. P. Singh (Director of the Bhaskaracharya Institute for Space Application and Geo-Informatics) for providing the irrigation map. E.B.B. is a Department of Energy Computational Science Graduate Fellow (Grant DE-FG02-97ER25308). M.P. is an Investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1305728110/-/DCSupplemental.
References
- 1.Jayaraman TK. Malarial impact of surface irrigation projects: A case study from Gujarat, India. Agric Environ. 1982;7(1):23–34. [Google Scholar]
- 2.Yohannes M, et al. Can source reduction of mosquito larval habitat reduce malaria transmission in Tigray, Ethiopia? Trop Med Int Health. 2005;10(12):1274–1285. doi: 10.1111/j.1365-3156.2005.01512.x. [DOI] [PubMed] [Google Scholar]
- 3.Yewhalaw D, et al. Malaria and water resource development: The case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar J. 2009;8:21. doi: 10.1186/1475-2875-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Srivastava HC, Bhatt RM, Kant R, Yadav RS. Malaria associated with the construction of the Sardar Sarovar Project for water-resources development, in Gujarat, India. Ann Trop Med Parasitol. 2009;103(7):653–657. doi: 10.1179/000349809X12502035776199. [DOI] [PubMed] [Google Scholar]
- 5. Sharma KD (2001) Indira Gandhi Nahar Pariyojana: Lessons learnt from past management practices in the Indian arid zone. Iahs-Aish P Iahs Publication, eds Schumann AH, Acreman MC, Davis R, Marino MA, Rosbjerg D, Jun X (Int Assoc Hydrological Sciences, Wallingford, UK), pp 49–55.
- 6.Yadav RS, Sharma RC, Bhatt RM, Sharma VP. Studies on the anopheline fauna of Kheda district and species specific breeding habitats. Indian J Malariol. 1989;26(2):65–74. [PubMed] [Google Scholar]
- 7.Amerasinghe PH, et al. Malaria transmission by Anopheles subpictus (Diptera: Culicidae) in a new irrigation project in Sri Lanka. J Med Entomol. 1992;29(4):577–581. doi: 10.1093/jmedent/29.4.577. [DOI] [PubMed] [Google Scholar]
- 8.Amerasinghe FP, Indrajith NG. Postirrigation breeding patterns of surface water mosquitoes in the Mahaweli Project, Sri Lanka, and comparisons with preceding developmental phases. J Med Entomol. 1994;31(4):516–523. doi: 10.1093/jmedent/31.4.516. [DOI] [PubMed] [Google Scholar]
- 9.Konradsen F, Stobberup KA, Sharma SK, Gulati OT, van der Hoek W. Irrigation water releases and Anopheles culicifacies abundance in Gujarat, India. Acta Trop. 1998;71(2):195–197. doi: 10.1016/s0001-706x(98)00059-x. [DOI] [PubMed] [Google Scholar]
- 10.Herrel N, et al. Breeding of Anopheles mosquitoes in irrigated areas of South Punjab, Pakistan. Med Vet Entomol. 2001;15(3):236–248. doi: 10.1046/j.0269-283x.2001.00312.x. [DOI] [PubMed] [Google Scholar]
- 11.Claborn DM, et al. Environmental factors associated with larval habitats of malaria vectors in northern Kyunggi Province, Republic of Korea. J Am Mosq Control Assoc. 2002;18(3):178–185. [PubMed] [Google Scholar]
- 12.Dia I, Samb B, Konate L, Fontenille D. Population structure of newly established Anopheles funestus populations in the Senegal River basin using paracentric chromosomal inversions. Acta Trop. 2010;115(1–2):90–94. doi: 10.1016/j.actatropica.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 13.Kibret S, et al. The impact of a small-scale irrigation scheme on malaria transmission in Ziway area, Central Ethiopia. Trop Med Int Health. 2010;15(1):41–50. doi: 10.1111/j.1365-3156.2009.02423.x. [DOI] [PubMed] [Google Scholar]
- 14.Doannio JMC, et al. Malaria transmission in the rice growing area of Kafine village, Cote d'Ivoire. Bull Soc Pathol Exot. 2002;95(1):11–16. [PubMed] [Google Scholar]
- 15.Shah T, Singh OP. Irrigation development and rural poverty in Gujarat, India: A disaggregated analysis. Water Int. 2004;29(2):167–177. [Google Scholar]
- 16.Keiser J, et al. Effect of irrigation and large dams on the burden of malaria on a global and regional scale. Am J Trop Med Hyg. 2005;72(4):392–406. [PubMed] [Google Scholar]
- 17.Tyagi BK, Yadav SP. Bionomics of malaria vectors in two physiographically different areas of the epidemic-prone Thar Desert, north-western Rajasthan (India) J Arid Environ. 2001;47(2):161–172. [Google Scholar]
- 18.Tyagi BK. A review of the emergence of Plasmodium falciparum-dominated malaria in irrigated areas of the Thar Desert, India. Acta Trop. 2004;89(2):227–239. doi: 10.1016/j.actatropica.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 19.White NJ. Determinants of relapse periodicity in Plasmodium vivax malaria. Malar J. 2011;10:297. doi: 10.1186/1475-2875-10-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darling ML. The Punjab Peasant in Prosperity and Debt. London: Oxford Univ Press; 1925. [Google Scholar]
- 21.de Zulueta J, Mujtaba SM, Shah IH. Malaria control and long-term periodicity of the disease in Pakistan. Trans R Soc Trop Med Hyg. 1980;74(5):624–632. doi: 10.1016/0035-9203(80)90153-4. [DOI] [PubMed] [Google Scholar]
- 22.Klinkenberg E, van der Hoek W, Amerasinghe FP. A malaria risk analysis in an irrigated area in Sri Lanka. Acta Trop. 2004;89(2):215–225. doi: 10.1016/j.actatropica.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 23.Laneri K, et al. Forcing versus feedback: Epidemic malaria and monsoon rains in northwest India. PLOS Comput Biol. 2010;6(9):e1000898. doi: 10.1371/journal.pcbi.1000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeza A, et al. Climate forcing and desert malaria: The effect of irrigation. Malar J. 2011;10:190. doi: 10.1186/1475-2875-10-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatt RM, Shrivastava HC, Rajnikant, Yadav RS. Dynamics of Anopheles culicifacies transmitted malaria in the absence of effective zooprophylaxis in a riverine settlement in Gujarat, India. Curr Sci. 2008;95:82–87. [Google Scholar]
- 26. Baskerville EB, Bedford T, Reiner RC, Pascual M (2013) Nonparametric Bayesian grouping methods for spatial time-series data. arXiv:1306.5202.
- 27.Dattani M, Prajapati P, Raval D. Impact of indoor residual spray with synthetic pyrethroid in gandhinagar district, gujarat. Indian J Community Med. 2009;34(4):288–292. doi: 10.4103/0970-0218.58384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Baeza A, Bouma MJ, Dhiman R, Pascual M (2013) Malaria control under unstable dynamics: Reactive vs. climate-based strategies. Acta Trop, S0001-706X(13)00087–9. [DOI] [PubMed]
- 29.Swaroop S. Forecasting of epidemic malaria in the Punjab, India. Am J Trop Med Hyg. 1949;29(1):1–17. doi: 10.4269/ajtmh.1949.s1-29.1. [DOI] [PubMed] [Google Scholar]
- 30.Bouma MJ, van der Kaay HJ. The El Niño Southern Oscillation and the historic malaria epidemics on the Indian subcontinent and Sri Lanka: An early warning system for future epidemics? Trop Med Int Health. 1996;1(1):86–96. doi: 10.1046/j.1365-3156.1996.d01-7.x. [DOI] [PubMed] [Google Scholar]
- 31.Russell PF, Ramachandra Rao T. A study of the density of Anopheles culicifacies in relation to malaria endemicity. Am J Trop Med Hyg. 1942;22:535–558. [Google Scholar]
- 32.Herrel N, et al. Adult anopheline ecology and malaria transmission in irrigated areas of South Punjab, Pakistan. Med Vet Entomol. 2004;18(2):141–152. doi: 10.1111/j.0269-283X.2004.00481.x. [DOI] [PubMed] [Google Scholar]
- 33.Klinkenberg E, et al. Malaria vectors in the changing environment of the southern Punjab, Pakistan. Trans R Soc Trop Med Hyg. 2004;98(7):442–449. doi: 10.1016/j.trstmh.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 34. Molineaux L (1998) The epidemiology of human malaria as an explanation of its distribution, including some implications for its control. Malaria: Principles and Practice of Malariology, eds Wernsdorfer WH, McGregor I (Churchill Livingstone, Edinburgh)
- 35. Food and Agriculture Organization (2003) Rethinking the approach to Ground Water and Food Security in Water Reports (FAO, Rome). Available at www.fao.org/docrep/005/y4495e/y4495e00.htm. Accessed March 15, 2013.
- 36. Bonds MH, Keenan DC, Rohani P, Sachs JD (2010) Poverty trap formed by the ecology of infectious diseases. P Roy Soc B Biol Sci 277(1685):1185–1192. [DOI] [PMC free article] [PubMed]
- 37.Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. doi: 10.1038/415680a. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JM, et al. Malaria resurgence: A systematic review and assessment of its causes. Malar J. 2012;11:122. doi: 10.1186/1475-2875-11-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rodell M, Velicogna I, Famiglietti JS. Satellite-based estimates of groundwater depletion in India. Nature. 2009;460(7258):999–1002. doi: 10.1038/nature08238. [DOI] [PubMed] [Google Scholar]
- 40. World Health Organization (2012) Health 2020: A European policy framework supporting action across government and society for health and well-being (WHO, Geneva). Available at http://222.euro.who.int/_data/assets/pdf_file/0009/169803/RC62wd09_Eng.pdf. Accessed July 3, 2013.
- 41. Nam Theun 2 Power Company (2005) Project Implementation Plan. Chapter 2: Health Programs. (NTPC, Khammouane Province, Laos). Available at www.namtheun2.com/images/stories/pip/PIP%20Final%20-%20Part%20B%20Chapter%202_Health_050527.pdf. Accessed July 3, 2013.
- 42.Caussy D, Kumar P, Than Sein U. Health impact assessment needs in south-east Asian countries. Bull World Health Organ. 2003;81(6):439–443. [PMC free article] [PubMed] [Google Scholar]
- 43.Garg A, Dhiman RC, Bhattacharya S, Shukla PR. Development, malaria and adaptation to climate change: A case study from India. Environ Manage. 2009;43(5):779–789. doi: 10.1007/s00267-008-9242-z. [DOI] [PubMed] [Google Scholar]
- 44. Birley MH (1991) Methods of forecasting the vector-borne disease implications in development of a water-resources project. Techniques for Environmentally Sound Water Resources Development (Pentech Press, London), pp 50–63.
- 45.Keiser J, Singer BH, Utzinger J. Reducing the burden of malaria in different eco-epidemiological settings with environmental management: A systematic review. Lancet Infect Dis. 2005;5(11):695–708. doi: 10.1016/S1473-3099(05)70268-1. [DOI] [PubMed] [Google Scholar]
- 46.Slooff R. Towards healthier water resources management. Water Lines. 1990;9(2):2–2. [Google Scholar]
- 47.Dheeravath V, et al. Irrigated areas of India derived using MODIS 500 m time series for the years 2001-2003. Isprs J Photogramm. 2010;65(1):42–59. [Google Scholar]
- 48.Reiner RC, Jr, et al. Highly localized sensitivity to climate forcing drives endemic cholera in a megacity. Proc Natl Acad Sci USA. 2012;109(6):2033–2036. doi: 10.1073/pnas.1108438109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jeffreys H (1946) An invariant form for the prior probability in estimation problems. Proc R Soc Lon Ser A 186(1007):453–461. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.