Significance
A unique facet of arthropod-borne virus infection is that the pathogens are orally acquired by insects during the taking of a blood meal. Hence, there is a direct link between nutrient acquisition and pathogen challenge. We found that the classic nutrient-responsive ERK pathway is a molecular determinant of this “midgut barrier”; ERK signaling is essential for antiviral defense in the insect intestinal epithelial cells. Surprisingly, we also found vertebrate insulin, which activates ERK signaling during a blood meal, both restricts viral infection in insect cells and protects against viral invasion of the gut epithelium. ERK signaling in the insect intestines restricts viral infection, suggesting that insects may take advantage of cross-species signals in the meal to preemptively activate antiviral immunity.
Keywords: innate immunity, enterocytes
Abstract
A unique facet of arthropod-borne virus (arbovirus) infection is that the pathogens are orally acquired by an insect vector during the taking of a blood meal, which directly links nutrient acquisition and pathogen challenge. We show that the nutrient responsive ERK pathway is both induced by and restricts disparate arboviruses in Drosophila intestines, providing insight into the molecular determinants of the antiviral “midgut barrier.” Wild-type flies are refractory to oral infection by arboviruses, including Sindbis virus and vesicular stomatitis virus, but this innate restriction can be overcome chemically by oral administration of an ERK pathway inhibitor or genetically via the specific loss of ERK in Drosophila intestinal epithelial cells. In addition, we found that vertebrate insulin, which activates ERK in the mosquito gut during a blood meal, restricts viral infection in Drosophila cells and against viral invasion of the insect gut epithelium. We find that ERK’s antiviral signaling activity is likely conserved in Aedes mosquitoes, because genetic or pharmacologic manipulation of the ERK pathway affects viral infection of mosquito cells. These studies demonstrate that ERK signaling has a broadly antiviral role in insects and suggest that insects take advantage of cross-species signals in the meal to trigger antiviral immunity.
Many (re)emerging viral pathogens are arthropod borne, transmitted via an insect vector, and cause significant global health and agricultural problems (1). When the insect takes an infectious blood meal, the initial host encounters with pathogens occur locally, usually at an epithelial surface (1–3). Although studies have successfully infected a range of vectors by directly injecting arboviruses into the thoracic cavity, oral challenge often does not result in productive infection (3–5). This distinguishing characteristic has led to the description of a midgut barrier, whereby it is thought that the arbovirus is unable to establish a productive infection in the midgut cells due to restriction by local defenses. This midgut barrier has long been recognized as a major determinant of vector competence (3, 4, 6), but can be overcome in some cases, by increasing the dose of the pathogen (4). Below a particular threshold, few vectors ingesting the blood meal become infected; above this threshold, significant numbers become infected (4). At the molecular level, transcriptional profiling of disparate insects challenged orally by viral pathogens suggest that there is an active immune response that includes the induction or down-regulation of many known insect immune pathways such as the JAK/STAT, Toll, and JNK signaling (7–9). However, less is known about whether the induction of these pathways have a direct role in viral restriction in the gut epithelium and whether there are additional pathways that play important roles in barrier immunity.
Indeed, there are few clear molecular determinants known to be active within the gut epithelial cells that specifically protect against viral invasion (6, 8, 10, 11). This lack of knowledge is due in part to the difficult nature of performing molecular and genetic mechanistic studies in insect vectors, including hematophagous mosquitoes. Drosophila offers several advantages as a model insect for identifying and studying antiviral mechanisms that play important roles in insect vectors (12, 13), because it shares a high degree of conservation with these organisms, exhibiting similar metamorphic life cycles and genetic pathways (12, 13). These features have allowed researchers to take advantage of powerful Drosophila genetic tools to extend our understanding of insect antiviral immunity (14). Indeed, many viral restriction pathways, including RNA interference (RNAi) and JAK/STAT signaling, were first identified in Drosophila and subsequently shown to be antiviral in mosquito vectors (15, 16). Using this system, we identified the ERK pathway as providing a mechanistic link between nutrient acquisition and antiviral innate immunity in insects. Not only do we find that the nutrient responsive ERK pathway is both induced by and restricts disparate viral infections, including human arboviruses, in Drosophila cells, but also that ERK signaling is essential for antiviral defense in the insect intestinal epithelium. We found that this antiviral ERK signaling is conserved in Aedes mosquito cells. Furthermore, vertebrate insulin, which triggers ERK signaling in the mosquito gut during a blood meal, can both restrict viral infection in insect cells and protect against viral invasion of the gut epithelium. These studies collectively demonstrate that this nutrient-responsive pathway may have evolved a secondary role to protect against viral invasion of the insect gut.
Results
Using cell-based RNAi screening for novel antiviral factors in Drosophila cells, we previously discovered (17) that NELF-dependent transcriptional pausing controls early responses to infection by disparate medically relevant arboviruses. This transcriptional response includes genes from all known Drosophila antiviral pathways, suggesting that additional genes within this gene set may confer antiviral activity. MAPK signaling components were both transcriptionally induced and overrepresented (SI Appendix, Fig. S1) (17). There are three related and conserved MAPK signaling pathways (ERK, JNK, and p38) (18), two of which have clear positive roles in immunity spanning flies to humans (JNK and p38) (19–22). Because genome-wide RNAi screens against viruses in Drosophila have not been optimized to identify antiviral factors but rather required factors (23–25), we performed a directed RNAi screen of 11 core components of these pathways optimized to determine their cell-intrinsic activity against vesicular stomatitis virus (VSV), Sindbis virus (SINV), and Drosophila C virus (DCV) (SI Appendix, Fig. S1 and Table S1) (26, 27). VSV and SINV are arboviruses belonging to two disparate families (Rhabodoviridae and Alphaviridae, respectively) whose natural cycle involves transmission between insects and vertebrates. DCV is a natural Drosophila pathogen that does not infect vertebrates (28).
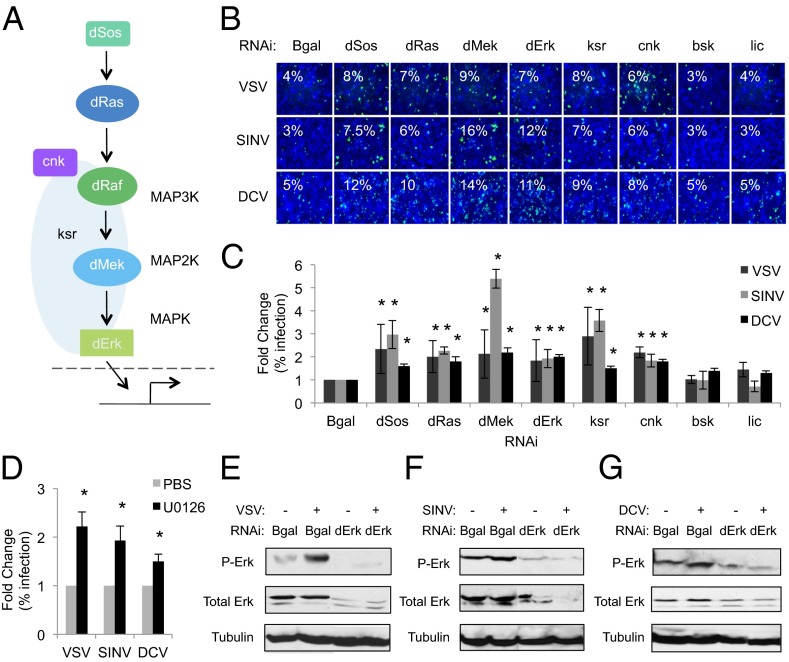
We found that six canonical ERK signaling components [dSos, dRas (Ras85D), dMek (Dsor1), dErk (rl), ksr, and cnk] restrict VSV, SINV, and DCV infection of Drosophila cells, because their depletion causes a significant increase in the percentage of infected cells compared with controls (Fig. 1 A–C and SI Appendix, Figs. S1 and S2 and Tables S1 and S2). We also observed increased viral protein and viral RNA levels in cells depleted of ERK pathway components, as measured by immunoblot and quantitative RT-PCR (qRT-PCR), respectively (SI Appendix, Figs. S3 and S4). In contrast, depletion of core components of the other two MAPK pathways (JNK, p38) did not alter the percentage of VSV, SINV, or DCV infected cells (Fig. 1B and C and SI Appendix, Table S1). We verified knock-down in dSos and dMek-depleted cells by qRT-PCR (SI Appendix, Fig. S5A). And immunoblot analysis of dErk levels also demonstrate robust knockdown (SI Appendix, Fig. S5B). Lastly, we used independent dsRNAs targeting dSos, dMek, dErk, and bsk and observed similar results where depletion of dSos, dMek, or dErk led to increased viral infection, but not bsk (SI Appendix, Fig. S5C).
Fig. 1.
The ERK pathway is broadly activated by and restricts viral infections in Drosophila cells. (A) ERK pathway schematic. (B) Drosophila cells treated with the indicated dsRNAs challenged with VSV [multiplicity of infection (MOI) = 0.2], SINV (MOI = 5), or DCV (MOI = 1.5) and monitored by fluorescence microscopy (virus in green, nuclei in blue). (C) Quantification of images in A with mean ± SD of three independent experiments; *P < 0.05. (D) Drosophila cells treated with PBS or U0126 (10 µM) challenged with VSV, SINV, or DCV as in A. Quantification of images with mean ± SD of three independent experiments; *P < 0.05. (E–G) Immunoblot analysis of Drosophila cells infected with VSV, SINV, or DCV, as indicated.
Using an orthogonal approach, we took advantage of the ERK pathway inhibitor U0126 that blocks MEK activation across diverse species (29, 30), including Drosophila (31, 32). Using an antibody that recognizes activated Drosophila Erk, we found that vertebrate insulin induces Erk activation within 15 min (SI Appendix, Fig. S6A). Furthermore, Drosophila cells treated with U0126 were more permissive to infection because there were higher levels of infections of VSV, SINV, and DCV compared with controls (Fig. 1D and SI Appendix, Fig. S6B). This treatment had no impact on cell viability (SI Appendix, Fig. S6C), and was dose dependent (SI Appendix, Fig. S7).
Next, we tested whether ERK signaling is activated by viral infection in Drosophila cells. We found VSV, SINV, and DCV activated Erk within 60 min as measured by increased phospho-Erk levels (Fig. 1 E–G and SI Appendix, Figs. S8 and S9), a time point before viral replication (17), whereas total Erk levels did not change. These findings suggest that viral infection is sensed by Drosophila cells leading to the rapid induction of an antiviral ERK signaling cascade.
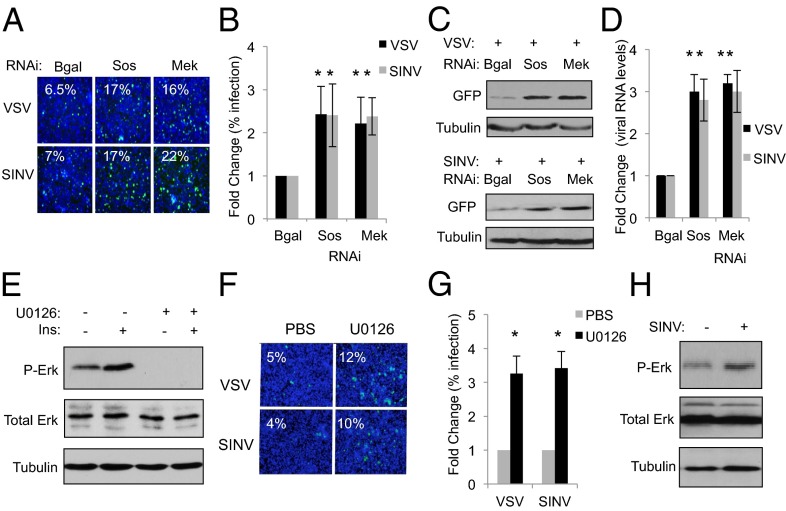
Because insect vectors, including Aedes aegypti mosquitoes, can transmit arboviruses such as SINV (33), we set out to determine whether the antiviral activity of the ERK pathway is conserved in mosquito cells. We depleted A. aegypti Aag2 cells of Sos (AAEL001165) or Mek (AAEL012723) by RNAi and compared the percentage of infected cells to nontargeting controls using microscopy. We found that depletion of Sos or Mek led to increased viral infection by VSV or SINV in mosquito cells (Fig. 2 A and B and SI Appendix, Fig. S10). Moreover, we observed higher viral protein expression and viral RNA levels upon VSV and SINV infection in Sos- and Mek-depleted Aag2 cells, as measured by immunoblot and qRT-PCR, respectively (Fig. 2 C and D). We could not infect mosquito cells with DCV, which is consistent with the fact that DCV is a natural pathogen of Drosophila with a narrow host range (28). As we observed in Drosophila, vertebrate insulin induces Erk activation within 15 min in Aag2 cells (Fig. 2E and SI Appendix, Fig. S11) and treatment with the ERK pathway inhibitor U0126 also prevents both basal and insulin-induced phospho-Erk, similar to published findings in other mosquito species (Fig. 2E and SI Appendix, Fig. S11) (30). We found that cell number was not affected by U0126 treatment of Aag2 cells (SI Appendix, Fig. S12). Treatment of mosquito cells with U0126 led to increases in VSV and SINV infection compared with control cells (Fig. 2 F and G and SI Appendix, Fig. S13). Furthermore, we found that viral infection also rapidly activates the ERK pathway in mosquito cells as measured by phospho-Erk (Fig. 2H and SI Appendix, Fig. S14). Altogether, our findings suggest that ERK signaling plays a conserved antiviral role in diverse insects.
Fig. 2.
The ERK pathway is antiviral in mosquito cells. (A) A. aegypti Aag2 cells treated with the indicated dsRNAs were challenged with VSV (MOI = 0.01) or SINV (MOI = 0.5) and monitored by microscopy (virus, green; nuclei, blue). (B) Quantification of A with mean ± SD of three independent experiments; *P < 0.05. (C) Immunoblot analysis of Aag2 cells treated as in A. (D) qRT-PCR analysis of viral RNA normalized to Rp49 and shown relative to control cells treated as in A. Mean ± SD of three independent experiments; *P < 0.05. (E) Immunoblot analysis of Aag2 cells treated with insulin (3.4 µM) or U0126 (10 µM) for 15 min. (F) Aag2 cells treated with PBS or U0126 (10 µM), infected with the indicated virus, and monitored by microscopy. (G) Quantification of images in F with mean ± SD of three independent experiments; *P < 0.05. (H) Immunoblot analysis of Aag2 cells infected with SINV for 60 min.
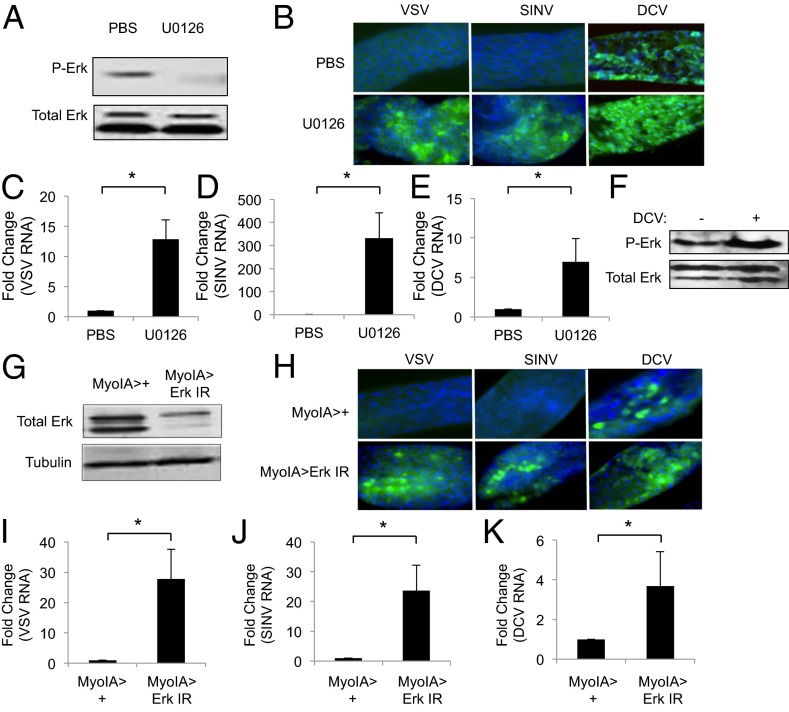
Arboviruses are naturally transmitted to insect vectors during the ingestion of a nutrient-rich blood meal (1). Because ERK signaling can be activated in the mosquito digestive tract by nutrients and insulin in the blood meal (30, 34), we reasoned that the ERK pathway may couple signals in the meal with antiviral defense to protect insects from orally acquired viral infections, including arboviruses. To determine whether ERK activity in the gut impacts local viral susceptibility, we took advantage of U0126 to inhibit ERK signaling in the gut. We fed wild-type flies with either vehicle or U0126 and found that drug treatment attenuated intestinal phospho-Erk levels while having no impact on total Erk levels (Fig. 3A and SI Appendix, Fig. S15). Moreover, we found that oral administration of adult flies with U0126 did not alter feeding as measured by the ingestion of dye or survival compared with vehicle-fed flies during the time course of our infection assays (SI Appendix, Fig. S16). Next, we fed flies with vehicle or U0216 along with the arboviruses VSV or SINV. At 7 d after infection, we found that vehicle-fed flies had undetectable levels of viral infection in their midguts as measured by microscopy that monitors viral antigen expression in infected intestinal cells (Fig. 3B) or qRT-PCR that monitors viral RNA levels in whole intestines (Fig. 3 C and D). In contrast, flies fed with the ERK pathway inhibitor had increased viral infection of midgut cells as measured by microscopy without evident mortality (Fig. 3B). Furthermore, we observed increased viral RNA in ERK pathway inhibited intestines as measured by qRT-PCR (Fig. 3 C and D).
Fig. 3.
ERK signaling protects against viral infection of the insect gut. (A) Immunoblot analysis of wild-type flies fed PBS or U0126 (10 µM) for 1 h. (B) Representative images of midguts from flies fed PBS or U0126 (500 µM) and orally challenged with the indicated virus for 7 d. (virus, green; nuclei, blue). (C–E) qRT-PCR analysis of viral RNA normalized to Rp49 and shown as relative to the controls from midguts 7 d after infection in flies treated as in B. Mean ± SD of three independent experiments; *P < 0.05. (F) Immunoblot analysis of wild-type flies fed PBS or DCV for 1 h. (G) Immunoblot analysis of MyoIA>+ and MyoIA > Erk IR midguts. (H) Representative images of midguts from MyoIA>+ or MyoIA > Erk IR flies 7 d after infection (virus, green; nuclei, blue). (I–K) qRT-PCR analysis of viral RNA normalized to Rp49 and shown relative to the control from midguts isolated at 7 d after infection as in G. Mean ± SD of three independent experiments; *P < 0.05.
Although VSV and SINV do not naturally infect Drosophila, DCV is a natural pathogen of Drosophila melanogaster that can be orally transmitted (35). In contrast to the infections with VSV and SINV, vehicle-fed flies orally challenged with DCV had detectable infection of the midgut by 7 d after infection, as measured by immunofluorescence (Fig. 3B), and viral RNA by qRT-PCR but not at earlier time points (Fig. 3E). Furthermore, we observed increased phospho-Erk in the gut at 2 h after DCV ingestion compared with controls (Fig. 3F and SI Appendix, Fig. S17). As we observed with VSV and SINV, ERK inhibition led to significantly increased DCV infection, as measured both by microscopy and qRT-PCR (Fig. 3 B and E). Hence, ERK signaling is both activated by and restricts viral infection in the gut epithelium.
DCV infection of flies by injection in the body cavity leads to systemic viremia, and the visceral muscles surrounding the gut are robustly infected (36). Thus, we were able to monitor infection of this tissue as a readout for viral spread outside of the gut epithelium. These visceral muscles are morphologically quite distinct and localized outside of, and surrounding, the monolayer of epithelia. Because they have a clearly distinct location and morphology, we used these criteria to quantify the number of guts that had staining in this compartment. We found that whereas 4% of the wild-type animals fed DCV had detectable infection in the intestinal visceral muscles at 7 d after infection, infection was increased to 24% upon cofeeding with the ERK inhibitor U0126 without evident mortality at this time point (SI Appendix, Fig. S18). Therefore, increased infection of the midgut epithelium also allows increased viral spread.
The Drosophila midgut is a simple epithelium, where multipotent stem cells give rise to enteroblasts that differentiate into either enterocytes that are characterized by large nuclei or secretory enteroendorcine cells (37). Midgut enterocytes are responsible for nutrient uptake and are the initial cell type infected by arboviruses in vector mosquitoes (8, 38, 39). We observed that the midgut enterocytes were infected by all three viruses in U0126-fed flies (SI Appendix, Fig. S19) and hypothesized that the Erk-dependent antiviral function is active within enterocytes. To test this hypothesis, we took advantage of the MyoIA-Gal4 driver that selectively expresses Gal4 in midgut enterocytes (40, 41). We used this system to drive expression of an inverted repeat transgene to perform in vivo RNAi against Erk and found that total Erk levels in MyoIA > Erk IR guts were reduced compared with controls (Fig. 3G). Flies with enterocyte-specific Erk depletion had no changes in feeding behavior or increased mortality compared with controls within the time course of our assays (SI Appendix, Fig. S20). Next, we fed MyoIA > Erk IR flies with VSV, SINV, or DCV and observed significantly increased infection in midgut enterocytes compared with controls, as measured both by microscopy and qRT-PCR (Fig. 3 H–K). These studies demonstrate that ERK signaling specifically within enterocytes restricts orally acquired viral infections in the insect digestive tract.
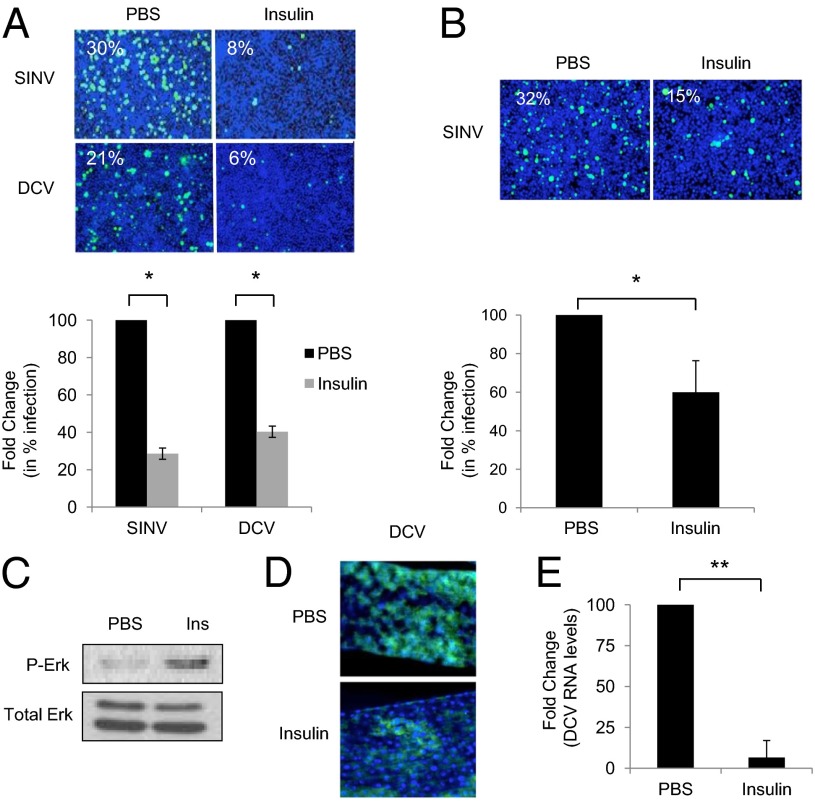
Previous studies have shown that growth factors found within the blood meal, including vertebrate insulin, can activate the ERK pathway in the mosquito intestines (30). Hence, we hypothesized that the ERK pathway may couple nutrient-associated vertebrate signals to trigger antiviral defense in gut enterocytes. Importantly, the dose dependence of insulin-mediated activation of phospho-ERK was similar in Drosophila and mosquito cells (SI Appendix, Fig. S21). Thus, we tested whether insulin-induced ERK activation in cell culture can protect against viral infection. Indeed, we found that insulin protects Drosophila cells from SINV and DCV infection (Fig. 4A and SI Appendix, Fig. S22A) while having no impact on cell number (SI Appendix, Fig. S22B). Likewise, mosquito cells are insulin responsive and protected from SINV infection by insulin treatment (Fig. 4B and SI Appendix, Fig. S23A) while having no impact on cell number (SI Appendix, Fig. S23B). We next took a genetic approach to determine whether the antiviral activity of insulin was ERK dependent. We found that insulin-mediated protection was lost upon knockdown of ERK signaling components (SI Appendix, Fig. S24). These findings collectively suggest that insulin-dependent protection against viral infection is mediated through Erk signaling and is conserved across insect species.
Fig. 4.
Insulin protects against viral infection of the gut. (A) Drosophila cells were either mock insulin-treated (3.4 µM), infected with SINV (MOI = 20) or DCV (MOI = 6), and monitored by microscopy (virus, green; nuclei, blue). Quantification with mean ± SD of three independent experiments; *P < 0.05. (B) Mosquito Aag2 cells were either mock or insulin-treated (3.4 µM), infected with SINV (MOI = 2), and monitored by microscopy (virus, green; nuclei, blue). Quantification with mean ± SD of three independent experiments; *P < 0.05. (C) Immunoblot analysis of intestines from wild-type flies fed PBS or insulin (83.5 µM) for 30 min. (D and E) Flies were fed either PBS or insulin (83.5 µM) and orally challenged with DCV (2 µL of 1 × 1013 IU/mL) for 7 d. DCV infection of intestines were analyzed by microscopy (virus, green; nuclei, blue) (D) or qRT-PCR normalized to Rp49 (E) and shown relative to the control. Mean ± SD of five independent experiments; *P < 0.05.
Next, we tested whether feeding insulin to flies can trigger antiviral ERK signaling in the gut. Perhaps surprisingly, we observed robust Erk activation within 30 min after insulin feeding as measured by phospho-Erk (Fig. 4C and SI Appendix, Fig. S25), similar to previous reports in the vector mosquito Anopheles stephensi (34). Furthermore, we found that oral administration of insulin did not alter feeding behavior or increase mortality compared with vehicle-fed flies within the time course of our assays (SI Appendix, Fig. S26). Next, we tested whether insulin-induced ERK activation altered viral infection of midgut enterocytes. To this end, we fed flies with either vehicle or insulin along with DCV for 7 d. We found that insulin-fed flies had decreased infection of their intestines compared with controls, both by microscopy and qRT-PCR (Fig. 4 D and E). Because the levels of insulin in a blood meal have been measured at 0.07–0.7 µM, we tested whether doses within this range would also impact infection. We found that doses as low as 0.7 µM suppressed infection (SI Appendix, Fig. S27). Furthermore, insulin-mediated antiviral activity in the fly gut depended on ERK signaling in enterocytes (SI Appendix, Fig. S28). We were unable to test insulin-dependent attenuation of VSV or SINV infection of the gut, because we could not detect infection by these viruses in untreated wild-type intestines (Fig. 3 B–D).
Lastly, because ERK is a negative regulator of antibacterial gene expression through the immune deficiency (IMD) pathway in Drosophila, although not in enterocytes per se (42), we set out to determine whether the IMD pathway plays a role in enterocyte-mediated ERK antiviral immunity. First, we tested whether the canonical antimicrobial peptide Diptericin B (DiptB) that is downstream of IMD signaling is induced by ERK depletion in enterocytes. We found that the there is no basal induction of DiptB upon Erk loss in enterocytes, consistent with a previous study (SI Appendix, Fig. S29A) (41). Furthermore, we found that viral infection of the gut did not induce IMD-dependent DiptB expression (SI Appendix, Fig. S29 B–D). Therefore, it is unlikely that the IMD pathway plays a role in ERK-dependent antiviral immunity. Future studies should define the downstream mechanism by which ERK exerts its antiviral effects.
Discussion
The intestinal epithelium has been long recognized as a major determinant of vector competence for a variety of pathogens, not only arboviruses. This midgut barrier acts as a blockade to prevent pathogenic invasion and systemic dissemination of pathogens in insects (3, 43). This barrier sets the threshold for infectivity. Although studies have found genetic associations with parasitic and bacterial infections, including Plasmodium species and bacterial gut pathogens, fewer molecular antiviral determinants have been identified that are responsible for enterocyte-mediated defense (3, 8, 11, 39, 44, 45). Although enterocytes secrete antimicrobial peptides and reactive oxygen species upon pathogenic bacterial infection, intestinal stem cell self-renewal also confers protection upon epithelial damage from such infections (40, 46, 47). However, the spectrum of intestinal epithelial cell-intrinsic antiviral responses are largely unexplored (3), albeit transcriptional profiling of disparate insect species orally challenged by viral pathogens indicate that a potentially wide array of host responses are actively induced.
Through RNAi screening in Drosophila and subsequent studies in vivo, we identified the ERK pathway as a cell-intrinsic regulator of intestinal immunity against a panel of viral pathogens. Through pharmacologic and genetic manipulation of ERK signaling, we find that ERK pathway components restrict VSV, SINV, and DCV infection in Drosophila cells and in the intestinal epithelia of adult flies. In addition, our studies suggest that ERK is one pathway active in the gut epithelium. This finding provides molecular insight into immune barrier function that prevents both the establishment of infection and systemic viral spread. Moreover, the broad antiviral activity of the ERK pathway is likely conserved in other insects, because Aag2 cells derived from Aedes mosquitoes become increasingly susceptible to VSV and SINV infections when ERK signaling is disrupted. Further studies directly in mosquitoes and other organisms can reveal the extent of this conservation both in cells and in the intestinal epithelium. Interestingly, all three viruses restricted by ERK signaling in our studies are RNA viruses. A study in Bombyx mori found that ERK signaling facilitates replication of the dsDNA virus baculovirus (48), suggesting differences in how viruses intersect with this pathway. Hence, determining the full spectrum of viruses restricted by ERK may elucidate the upstream viral recognition and signaling molecules that trigger antiviral ERK activation in insects.
We find that vertebrate insulin, which activates ERK signaling, confers protection against disparate RNA viruses in Drosophila cells and DCV in the fly intestinal tract. Both mosquitoes and Drosophila are similarly responsive to insulin-mediated ERK activation and antiviral protection in vitro. And lastly, insulin-mediated protection in Drosophila is via the ERK pathway both in cell culture and in the enterocytes of the digestive tract. Because arboviral challenge of the insect gut, as well as other enteric viruses, occurs during feeding, the nutrient responsive ERK pathway may allow the organism to couple cross-species nutrient signals with immune defense in the epithelium. Given the energetic costs in mounting an immune response (49) and the potentially toxic production of immune effectors (50), the coupling of signals in the meal with antiviral defenses restricts the response to times when the organism is most vulnerable. Thus, the set point of ERK signaling in the gut may alter the permissivity to viral infection. In contrast, studies of Plasmodium falciparum in mosquitoes demonstrate that ingested human insulin increases the susceptibility of Anopheles stephensi by promoting replication of Plasmodium via the down-regulation of NF-κB signaling (51, 52). Furthermore, ERK pathway activation by another mammalian blood product, TGF-β, in Anopheles mosquitoes has been found to down-regulate ROS production and also promote parasite growth (30). Hence, the insulin-mediated activation of ERK signaling may have different effects depending on the pathogen type, because it promotes Plasmodium replication while inhibiting replication of disparate viruses. Further identification of both the upstream and downstream players involved in antiviral ERK activation will expand our understanding of these differences. Nevertheless, insects have developed a sophisticated strategy involving recognition of particular vertebrate specific molecules to facilitate antiviral defenses. Insulin is likely one of many factors that are directly sensed by insects, and further studies should elucidate the full spectrum of cross-species immune regulators.
Experimental Procedures
Cells, Viruses, and Reagents.
Insect cells were grown and maintained as described (27). VSV-GFP, SINV-GFP, and DCV were grown as described (17). U0126 and Erk antibodies (Phospho-Erk and total Erk) were obtained from Cell Signaling. Anti-DCV capsid antibody was used as described (36). Additional chemicals were from Sigma.
RNAi and Viral Infections in Cell Culture.
dsRNA are described at http://flyrnai.org, and RNAi was performed as described (17). The experimental methods and image analysis have been extensively described (26, 27, 53). In brief, cells were passaged into serum-free media (SFM) and plated into wells containing dsRNA at 250 ng/16,500 cells in 384-well plates. After 1 h in SFM, complete media was added and cells were incubated at 25 °C for 3 d to allow for gene knockdown. Three days after dsRNA bathing, cells were infected with the indicated viral inoculum. VSV, SINV, or DCV inocula were added to the existing media in 384-well plates in 10 μL of serum-free Schneider’s media. VSV and DCV-infected cells were processed at 24 h after infection. SINV was spinoculated as follows: Existing media was removed, virus inoculum was added to wells in 10 μL of serum-free Schneider’s media, cells were spun at 290 relative centrifugal force for 2 h, then 20 μL of 10% (vol/vol) serum Schneider’s media was added to cells. SINV-infected cells were processed at 36 h after infection. Cells were fixed for 10 min in 4% (vol/vol) formaldehyde, washed in PBS/0.1% Triton X-100 (PBST) twice for 10 min each, and blocked in 2% (vol/vol) BSA/PBST for 30 min. Primary antibody was diluted in block and incubated with cells overnight at 4°C. Cells were washed three times in PBST and incubated in secondary antibody for 1 h at room temperature. Cells were counterstained with Hoescht 33342 (Sigma). Following secondary antibody staining and counterstaining, cells were washed an additional three times in PBST and imaged using an automated microscope at 20× (ImageXpress Micro). Images of three sites per wavelength for each well were collected and with a minimum of three wells per treatment. Automated image analysis was performed by using MetaXpress image analysis software (Cell Scoring) to identify nuclei (Hoescht+) and virus-infected (GFP+ or antibody+) cells and thresholding on uninfected wells on each plate. The percent infection was calculated for each site and averaged to obtain a single aggregate value for each well. A Student’s t test was performed for significance per condition for each plate. Lastly, there were three independent biological replicates performed in this manner for each gene. The data are represented as the average fold change and SD from the three independent biological replicates, *P < 0.05 for three independent experiments. Cells were treated with 3.4 µM bovine insulin and 10 µM U0126 as described (31, 54). For phospho-Erk studies in cells, infections were synchronized by prebinding virus at 16 °C for 1 h, then brought to 25 °C and processed at the indicated time points.
RNA and RT-PCR.
Total RNA was extracted from cells or 15 fly guts using TRIzol (Invitrogen). qRT-PCR was performed as described (17). The data are represented as relative RNA expression compared with the untreated samples and displayed as the mean ± SD for three independent experiments.
Immunoblotting.
Cells or 15 guts were prepared by using RIPA buffer with protease inhibitors, and blotted as described (26).
Fly Infections.
Seven- to ten-day-old adult female flies were used. Wild-type (w1118) flies were used for drug studies. Myo1A-Gal4 was obtained from E. Baehrecke (University of Massachusetts Medical School, Worcester, MA) and UAS-Erk IR (109108) was obtained from the VDRC. Before the day of infection, flies were restricted to a water-only diet for 12 h and starved for 30 min before feeding to synchronize ingestion. The food contained 5% (vol/vol) sucrose plus dye in addition to the indicated treatment. Bovine insulin (83.5 µM) was used as described (34), and mixing experiments demonstrated no change in infectivity of VSV when mixed with insulin (SI Appendix, Fig. S30). U0126 (500 μM) was used as described (31, 32). Flies were fed 10 µL of the following concentrations of virus: VSV (1 × 108 pfu/mL), SINV (1 × 109 pfu/mL), and DCV (1 × 1012 IU/mL), unless otherwise indicated. For the U0126 experiments, food and virus was changed every 3 d. For the insulin experiments, insulin was only provided for the first 3 d, subsequently food and virus was changed every 3 d. For immunoblots, 15 fly guts were dissected in PBS and processed in RIPA buffer, protease inhibitor mixture (PP2, PP3) and PMSF as described (17). For RNA, 15 fly guts were dissected in PBS and then processed by using TRIzol as per the manufacturer’s protocol and as described (53). For microscopy, fly guts were dissected as described (55). In brief, 5–10 guts per experiment for at least three independent experiments were dissected in PBS, fixed in 4% (vol/vol) formaldehyde solution for 30 min, rinsed three times in PBS, and blocked with 5% (vol/vol) normal donkey serum for 45 min at room temperature. Primary antibodies were incubated overnight at 4 °C (DCV capsid 1:5,000), secondary antibodies with Hoescht 33342 were used at 1:1,000 and incubated for 1 h at room temperature. Samples were subsequently washed three times. Coverslips were mounted by using Vectashield and imaged by using 20× and 40× objectives with a Leica DMI 4000 B fluorescent microscope.
Statistics.
For qRT-PCR studies, P values were obtained by comparing delta CT values for three independent experiments. For other experiments, the Student’s two-tailed t test was used to measure the statistical significance in each experiment and then considered significant if P < 0.05 in three independent experiments.
Supplementary Material
Acknowledgments
We thank M. Tudor and M. Markstein for critical reading of the manuscript, members of the S.C. laboratory for discussions, and the Bloomington Drosophila Stock Center and the Vienna Drosophila RNAi Center for fly stocks. This work was supported by National Institutes of Health Grants R01AI074951, R01AI095500, and U54AI057168 (to S.C.). S.C. is a recipient of the Burroughs Wellcome Investigators in the Pathogenesis of Infectious Disease Award. J.X. is a Howard Hughes Medical Institute International Student Research Fellow.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1303193110/-/DCSupplemental.
References
- 1.Weaver SC, Barrett AD. Transmission cycles, host range, evolution and emergence of arboviral disease. Nat Rev Microbiol. 2004;2(10):789–801. doi: 10.1038/nrmicro1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinert S, Levashina EA. Intracellular immune responses of dipteran insects. Immunol Rev. 2011;240(1):129–140. doi: 10.1111/j.1600-065X.2010.00985.x. [DOI] [PubMed] [Google Scholar]
- 3.Davis MM, Engström Y. Immune response in the barrier epithelia: Lessons from the fruit fly Drosophila melanogaster. J Innate Immun. 2012;4(3):273–283. doi: 10.1159/000332947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaty BaMW . The Biology of Disease Vectors. Niwot, CO: Univ Press of Colorado; 1996. [Google Scholar]
- 5.Charroux B, Royet J. Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly (Austin) 2010;4(1):40–47. doi: 10.4161/fly.4.1.10810. [DOI] [PubMed] [Google Scholar]
- 6.Hardy JL, Houk EJ, Kramer LD, Reeves WC. Intrinsic factors affecting vector competence of mosquitoes for arboviruses. Annu Rev Entomol. 1983;28:229–262. doi: 10.1146/annurev.en.28.010183.001305. [DOI] [PubMed] [Google Scholar]
- 7.Ramirez JL, Dimopoulos G. The Toll immune signaling pathway control conserved anti-dengue defenses across diverse Ae. aegypti strains and against multiple dengue virus serotypes. Dev Comp Immunol. 2010;34(6):625–629. doi: 10.1016/j.dci.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souza-Neto JA, Sim S, Dimopoulos G. An evolutionary conserved function of the JAK-STAT pathway in anti-dengue defense. Proc Natl Acad Sci USA. 2009;106(42):17841–17846. doi: 10.1073/pnas.0905006106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luan JB, et al. Global analysis of the transcriptional response of whitefly to tomato yellow leaf curl China virus reveals the relationship of coevolved adaptations. J Virol. 2011;85(7):3330–3340. doi: 10.1128/JVI.02507-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girard YA, Klingler KA, Higgs S. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector Borne Zoonotic Dis. 2004;4(2):109–122. doi: 10.1089/1530366041210729. [DOI] [PubMed] [Google Scholar]
- 11.Xi Z, Ramirez JL, Dimopoulos G. The Aedes aegypti toll pathway controls dengue virus infection. PLoS Pathog. 2008;4(7):e1000098. doi: 10.1371/journal.ppat.1000098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cherry S, Silverman N. Host-pathogen interactions in drosophila: New tricks from an old friend. Nat Immunol. 2006;7(9):911–917. doi: 10.1038/ni1388. [DOI] [PubMed] [Google Scholar]
- 13.Eleftherianos I, Schneider D. Drosophila immunity research on the move. Fly (Austin) 2011;5(3):247–254. doi: 10.4161/fly.5.3.17028. [DOI] [PubMed] [Google Scholar]
- 14.Lemaitre B, Hoffmann J. The host defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- 15.Sabin LR, Hanna SL, Cherry S. Innate antiviral immunity in Drosophila. Curr Opin Immunol. 2010;22(1):4–9. doi: 10.1016/j.coi.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Christophides GK, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298(5591):159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- 17.Xu J, et al. Transcriptional pausing controls a rapid antiviral innate immune response in Drosophila. Cell Host Microbe. 2012;12(4):531–543. doi: 10.1016/j.chom.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, et al. Participation of the p38 pathway in Drosophila host defense against pathogenic bacteria and fungi. Proc Natl Acad Sci USA. 2010;107(48):20774–20779. doi: 10.1073/pnas.1009223107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sluss HK, et al. A JNK signal transduction pathway that mediates morphogenesis and an immune response in Drosophila. Genes Dev. 1996;10(21):2745–2758. doi: 10.1101/gad.10.21.2745. [DOI] [PubMed] [Google Scholar]
- 21.Park JM, et al. Targeting of TAK1 by the NF-kappa B protein Relish regulates the JNK-mediated immune response in Drosophila. Genes Dev. 2004;18(5):584–594. doi: 10.1101/gad.1168104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang G, Shi LZ, Chi H. Regulation of JNK and p38 MAPK in the immune system: Signal integration, propagation and termination. Cytokine. 2009;48(3):161–169. doi: 10.1016/j.cyto.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cherry S. RNAi screening for host factors involved in viral infection using Drosophila cells. Methods Mol Biol. 2011;721:375–382. doi: 10.1007/978-1-61779-037-9_23. [DOI] [PubMed] [Google Scholar]
- 24.Sessions OM, et al. Discovery of insect and human dengue virus host factors. Nature. 2009;458(7241):1047–1050. doi: 10.1038/nature07967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karlas A, et al. Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature. 2010;463(7282):818–822. doi: 10.1038/nature08760. [DOI] [PubMed] [Google Scholar]
- 26.Sabin LR, et al. Ars2 regulates both miRNA- and siRNA- dependent silencing and suppresses RNA virus infection in Drosophila. Cell. 2009;138(2):340–351. doi: 10.1016/j.cell.2009.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose PP, et al. Natural resistance-associated macrophage protein is a cellular receptor for sindbis virus in both insect and mammalian hosts. Cell Host Microbe. 2011;10(2):97–104. doi: 10.1016/j.chom.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jousset FX. [Host range of drosophila melanogaster C virus among diptera and lepidoptera (author’s transl)] Ann Microbiol (Paris) 1976;127(4):529–544. [PubMed] [Google Scholar]
- 29.Favata MF, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273(29):18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- 30.Surachetpong W, Singh N, Cheung KW, Luckhart S. MAPK ERK signaling regulates the TGF-beta1-dependent mosquito response to Plasmodium falciparum. PLoS Pathog. 2009;5(4):e1000366. doi: 10.1371/journal.ppat.1000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Thompson BJ, Hietakangas V, Cohen SM. MAPK/ERK signaling regulates insulin sensitivity to control glucose metabolism in Drosophila. PLoS Genet. 2011;7(12):e1002429. doi: 10.1371/journal.pgen.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bangi E, Garza D, Hild M. In vivo analysis of compound activity and mechanism of action using epistasis in Drosophila. J Chem Biol. 2011;4(2):55–68. doi: 10.1007/s12154-010-0051-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson AC, Bowen JC, Downe AE. Experimental infection of Aedes aegypti (Diptera: Culicidae) by the oral route with Sindbis virus. J Med Entomol. 1993;30(2):332–337. doi: 10.1093/jmedent/30.2.332. [DOI] [PubMed] [Google Scholar]
- 34.Kang MA, Mott TM, Tapley EC, Lewis EE, Luckhart S. Insulin regulates aging and oxidative stress in Anopheles stephensi. J Exp Biol. 2008;211(Pt 5):741–748. doi: 10.1242/jeb.012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomariz-Zilber E, Poras M, Thomas-Orillard M. Drosophila C virus: Experimental study of infectious yields and underlying pathology in Drosophila melanogaster laboratory populations. J Invertebr Pathol. 1995;65(3):243–247. doi: 10.1006/jipa.1995.1037. [DOI] [PubMed] [Google Scholar]
- 36.Cherry S, Perrimon N. Entry is a rate-limiting step for viral infection in a Drosophila melanogaster model of pathogenesis. Nat Immunol. 2004;5(1):81–87. doi: 10.1038/ni1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pitsouli C, Perrimon N. Developmental biology: Our fly cousins’ gut. Nature. 2008;454(7204):592–593. doi: 10.1038/454592a. [DOI] [PubMed] [Google Scholar]
- 38.Pierro DJ, Myles KM, Foy BD, Beaty BJ, Olson KE. Development of an orally infectious Sindbis virus transducing system that efficiently disseminates and expresses green fluorescent protein in Aedes aegypti. Insect Mol Biol. 2003;12(2):107–116. doi: 10.1046/j.1365-2583.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- 39.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. Dengue virus type 2: Replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC Microbiol. 2007;7:9. doi: 10.1186/1471-2180-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137(7):1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morgan NS, Skovronsky DM, Artavanis-Tsakonas S, Mooseker MS. The molecular cloning and characterization of Drosophila melanogaster myosin-IA and myosin-IB. J Mol Biol. 1994;239(3):347–356. doi: 10.1006/jmbi.1994.1376. [DOI] [PubMed] [Google Scholar]
- 42.Ragab A, et al. Drosophila Ras/MAPK signalling regulates innate immune responses in immune and intestinal stem cells. EMBO J. 2011;30(6):1123–1136. doi: 10.1038/emboj.2011.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Forrester NL, Guerbois M, Seymour RL, Spratt H, Weaver SC. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS Pathog. 2012;8(9):e1002897. doi: 10.1371/journal.ppat.1002897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Black WC, 4th, et al. Flavivirus susceptibility in Aedes aegypti. Arch Med Res. 2002;33(4):379–388. doi: 10.1016/s0188-4409(02)00373-9. [DOI] [PubMed] [Google Scholar]
- 45.Vazeille-Falcoz M, Rosen L, Mousson L, Rodhain F. Replication of dengue type 2 virus in Culex quinquefasciatus (Diptera: Culicidae) Am J Trop Med Hyg. 1999;60(2):319–321. doi: 10.4269/ajtmh.1999.60.319. [DOI] [PubMed] [Google Scholar]
- 46.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Katsuma S, Mita K, Shimada T. ERK- and JNK-dependent signaling pathways contribute to Bombyx mori nucleopolyhedrovirus infection. J Virol. 2007;81(24):13700–13709. doi: 10.1128/JVI.01683-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 50.Nappi AJ, Ottaviani E. Cytotoxicity and cytotoxic molecules in invertebrates. Bioessays. 2000;22(5):469–480. doi: 10.1002/(SICI)1521-1878(200005)22:5<469::AID-BIES9>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 51.Pakpour N, et al. Ingested human insulin inhibits the mosquito NF-κB-dependent immune response to Plasmodium falciparum. Infect Immun. 2012;80(6):2141–2149. doi: 10.1128/IAI.00024-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Surachetpong W, Pakpour N, Cheung KW, Luckhart S. Reactive oxygen species-dependent cell signaling regulates the mosquito immune response to Plasmodium falciparum. Antioxid Redox Signal. 2011;14(6):943–955. doi: 10.1089/ars.2010.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cherry S, et al. Genome-wide RNAi screen reveals a specific sensitivity of IRES-containing RNA viruses to host translation inhibition. Genes Dev. 2005;19(4):445–452. doi: 10.1101/gad.1267905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Friedman A, Perrimon N. A functional RNAi screen for regulators of receptor tyrosine kinase and ERK signalling. Nature. 2006;444(7116):230–234. doi: 10.1038/nature05280. [DOI] [PubMed] [Google Scholar]
- 55.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439(7075):470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.