Abstract
Please cite this paper as: Jules et al. (2012) Estimating age‐specific influenza‐related hospitalization rates during the pandemic (H1N1) 2009 in Davidson Co, TN. Influenza and Other Respiratory Viruses 6(3), e63–e71.
Background In April 2009, a pandemic caused by a novel influenza strain, the A(H1N1)pdm09 virus, started. Few age‐specific estimates of hospitalizations associated with the first year of circulation of the pandemic virus are available.
Objectives To estimate age‐specific hospitalization rates associated with laboratory‐confirmed A(H1N1)pdm09 virus in Davidson County, TN, from May 2009 to March 2010.
Patients/methods Two separate strategies were applied: capture–recapture and surveillance‐sampling methods. For the capture–recapture estimates, we linked data collected via two independent prospective population‐based surveillance systems: The Influenza Vaccine Effectiveness Network (Flu‐VE) tested consenting county patients hospitalized with respiratory symptoms at selected hospitals using real‐time reverse transcriptase polymerase chain reaction (rRT‐PCR); the Emerging Infections Program identified county patients with positive influenza tests in all area hospitals. For the surveillance‐sampling estimates, we applied the age‐specific proportions of influenza‐positive patients (from Flu‐VE) to the number of acute respiratory illness hospitalizations obtained from the Tennessee Hospital Discharge Data system.
Results With capture–recapture, we estimated 0·89 (95% CI, 0·72–1·49), 0·62 (0·42–1·11), 1·78 (0·99–3·63), and 0·76 (0·50–1·76) hospitalizations per 1000 residents aged <5, 5–17, 18–49, and ≥50 years, respectively. Surveillance‐sampling estimated rates were 0·78 (0·46–1·22), 0·32 (0·14–0·69), 0·99 (0·64–1·52), and 1·43 (0·80–2·48) hospitalizations per 1000 residents aged <5, 5–17, 18–49, and ≥50 years, respectively. In all age‐groups combined, we estimated approximately 1 influenza‐related hospitalization per 1000 residents.
Conclusions Two independent methods provided consistent results on the burden of pandemic virus in Davidson County and suggested that the overall incidence of A(H1N1)pdm09‐associated hospitalization was 1 per 1000 county residents.
Keywords: A(H1N1)pdm09 virus, capture–recapture, hospitalization rates, surveillance‐sampling
Introduction
A novel influenza strain, the A(H1N1)pdm09 virus, was first recognized in April 2009 leading to a pandemic that affected more than 40 million people and caused about 13 000 deaths in the United States alone. 1 , 2 , 3 , 4 However, few data are available on the age‐specific hospitalization rates of laboratory‐confirmed disease caused by the A(H1N1)pdm09 virus.
Although complete enumeration of all influenza hospitalizations would be ideal, this would be labor‐intensive, expensive, and only feasible in small populations. We used data available from surveillance systems and administrative databases to estimate hospitalization rates associated with A(H1N1)pdm09 virus in Davidson County, TN, USA, using two methods: (i) the capture–recapture analysis linked data from two independent systems, the Influenza Vaccine Effectiveness Network (Flu‐VE) and the Emerging Infections Programs (EIP), and (ii) the surveillance‐sampling method combined data from Flu‐VE and the TN State Hospital Discharge Data System (HDDS).
Methods
Population and setting
Davidson County, TN, had 635 710 residents in 2009 with similar age and gender distribution to that of the United States with the exception of a higher percentage of Black people (27·3% versus 13·6%) and a lower percentage of Hispanics (9·0% versus 15·8%). 5
Sources of data
The Davidson County population was under annual surveillance for influenza by two active independent systems both funded by the Centers for Disease Control and Prevention (CDC): Flu‐VE and EIP. In addition, all hospital discharge data in the state of TN, including admission and discharge dates and coded discharge diagnoses, and county of residence, are available from the HDDS.
Influenza Vaccine Effectiveness Network (Flu‐VE)
The Flu‐VE operated in 4 US communities including Davidson County, TN, and performed active population‐based influenza surveillance in children and adults presenting with acute respiratory illnesses (ARI) to area hospitals and emergency departments to ascertain laboratory‐confirmed influenza and to estimate annual influenza vaccine effectiveness.
During the first year of circulation of A(H1N1)pdm09 virus in Davidson County (from May 2009 to April 2010), the Flu‐VE enrolled patients 5 days a week in four adult hospitals that encompassed nearly 60% of county ARI admissions for adults. For children, Flu‐VE surveillance began in October 2009 and included the single large children’s hospital that historically admitted about 95% of all ARI admissions in the county. Surveillance data from May through September 2009 for children aged <5 years were provided by the New Vaccine Surveillance Network (NVSN) that had applied the same surveillance protocol as Flu‐VE. 6 However, there was no comparable surveillance for children aged 5–17 years during these months.
Flu‐VE enrolled Davidson County residents presenting with respiratory symptoms or with fever without other known non‐respiratory causes. Patients were approached within 48 hours of admission to assess eligibility and obtain informed consent for participation. Chronic medical conditions associated with influenza complications as defined per the Advisory Committee on Immunization Practices recommendations 7 were obtained through a standardized questionnaire and chart review.
Nasal and throat swabs were collected and transported in viral transport media to the Vanderbilt research laboratory within 2–3 hours of collection and kept at 4°C. Specimens were aliquoted into lysis buffer and stored at −80°C. Real‐time reverse transcriptase polymerase chain reaction (rRT‐PCR) was performed for influenza virus identification and influenza A subtyping using CDC‐provided primers and probes according to the CDC protocol. 8 A sample was considered positive for influenza A virus if positive on two separate runs. Subtyping was performed for seasonal H1N1 and H3N2, and A(H1N1)pdm09 viruses. Research laboratory results were not recorded in the participant’s medical record.
The Emerging Infections Program (EIP)
The EIP is also a population‐based surveillance network conducted by state and local health departments and academic health centers. Emerging Infections Program operates in 10 US states for influenza surveillance and describes clinical aspects of laboratory‐confirmed influenza hospitalizations, describes risk factors for severe disease, and provides estimates of age‐specific influenza hospitalization rates based on clinical laboratory testing. 9 , 10
During the 2009 A(H1N1)pdm09 pandemic, EIP performed active case finding at 20 hospitals located in Davidson and surrounding counties by reviewing the hospital laboratory lists and infection control practitioner logs for clinical laboratory–positive influenza cases. Patients were considered eligible if they were surveillance area residents and had a positive result from an influenza test ordered by their healthcare provider. Trained surveillance officers reviewed medical charts of all eligible patients to obtain demographic and clinical characteristics. 1 , 3 , 10 , 11 Testing for influenza and the type of clinical laboratory testing performed were at the discretion of the provider. We considered as A(H1N1)pdm09 infection any influenza‐positive test result from EIP because once the TN State laboratory started performing PCR testing for A(H1N1)pdm09, seasonal A, and seasonal B in the first week of September 2009, 99·4% of all specimens tested were A(H1N1)pdm09. 2
State Hospital Discharge Data System (HDDS)
The HDDS is a large electronic resource of all hospital‐based encounters (hospital and emergency department discharges) in the state of Tennessee. This information is collected by state mandate and it includes county of residence, date of birth, admission hospital, date of admission and discharge, and discharge diagnoses. 12
Protection of human subjects
Flu‐VE and NVSN protocols were approved by the institutional review boards (IRB) of all participating hospitals. Influenza surveillance conducted through the EIP was considered a public health response, and thus, it was declared exempt from review by the Vanderbilt University, CDC, and the Tennessee Department of Health IRBs.
Statistical analysis
Capture–recapture estimates
Capture–recapture methods combine two or more sources of data to estimate the size of a closed population. The number of cases identified by each source and the number of matched cases common to both sources are used to estimate the total number of cases in the study population assuming independence between the data sources. 13 , 14 , 15 , 16 , 17
We used data from Flu‐VE and EIP to estimate the number of A(H1N1)pdm09‐hospitalized patients applying capture–recapture. Patients with influenza‐related hospitalizations identified by both surveillance systems were defined as matched cases. To identify matched cases, we used patient’s name, date of birth, date, and place of hospitalization. After matching, all data were de‐identified for analyses.
As influenza‐related hospitalizations were rare events, we applied the capture–recapture nearly unbiased estimator correction, an analytical strategy that performs well with sparse events. 16 , 17 The calculation of total A(H1N1)pdm09‐associated hospitalizations in the surveillance hospitals (N) required the number of A(H1N1)pdm09‐associated hospitalizations that were detected by both systems or ‘matched’ (a), by Flu‐VE only (b), and by EIP only (c). Thus, N = a+b+c+y, where y = bc/(a+1), the estimated number of A(H1N1)pdm09‐associated hospitalizations missed by both surveillance systems. 16
Except for children aged <5 years, for whom there was complete surveillance, the capture–recapture estimates were weighted to account for incomplete surveillance for the three other age‐groups and the overall population. For patients aged 5–17 years for whom surveillance began in October, we assumed the same distribution of cases as reported for children aged <5 years from October 2009 to March 2010. Based on the observed distribution of laboratory‐confirmed influenza hospitalizations in children aged <5 years, we assumed that A(H1N1)pdm09‐associated hospitalizations detected from October through March in patients aged 5–17 years represented 31% of A(H1N1)pdm09 admissions for the entire period. To adjust our estimates for this incomplete surveillance, we divided the number of cases ‘captured’ by Flu‐VE only, by EIP only, and by both Flu‐VE and EIP by 0·31 prior to doing the capture–recapture calculation. For adults, for whom surveillance hospitals represented a subset of the total Davidson County hospitals, based on the HDDS data, we calculated the proportion of Davidson County residents with acute respiratory infections being admitted at the four surveillance hospitals. We adjusted the capture–recapture estimates similar to that described above for 5‐ to 17‐year‐olds.
Confidence intervals around the capture–recapture estimates were calculated by bootstrapping with 1000 samples 18 adjusted for incomplete surveillance for age‐groups 5–17, 18–49, ≥50 years, and the overall population. We reported the bias‐corrected bootstrapped intervals 19 for all age‐groups.
Age‐specific influenza‐associated hospitalization rates were defined as the estimated number of influenza‐related hospitalizations divided by Davidson County population estimates from the US Census July 2009 estimates. 5
Surveillance‐sampling estimates
The surveillance‐sampling method used data from the Flu‐VE study and HDDS. The number of influenza‐related hospitalizations was calculated by multiplying the proportion of influenza virus positives among tested samples from Flu‐VE by the total number of ARI hospitalizations identified from HDDS. Based on the clinical characteristics of A(H1N1)pdm09 virus infection and published data on previously used codes, we defined as potential ARI‐related hospitalizations those in which the discharge diagnoses included one of the following ICD‐9‐CM codes 381–382 (otitis media), 460–466 (upper respiratory infections), 480–487 (pneumonia and influenza), 490–493 (bronchitis, emphysema, asthma), 786 (dyspnea and respiratory abnormalities), and 780·6 (fever). 20 , 21 , 22 The code specific to the A(H1N1)pdm09 infection (488·1) was added in October 2009, was rarely used, and was not included.
Age‐specific influenza‐related hospitalization rates were calculated by dividing the estimated number of influenza‐related hospitalizations by Davidson County population estimates. 5 As there was complete enumeration of discharged patients from HDDS and the county population from the Census, the main source of variability was the proportion of influenza tests positive from the Flu‐VE study. We calculated the 95% CI of this proportion using the Wilson method for binomial distributions and applied the upper and lower bounds to the number of ARI admissions in county residents captured by HDDS to obtain 95% CI of the weighted number of influenza‐related hospitalizations in county residents.
We computed 95% CI around the rates for both methods by dividing the upper‐ and lower‐bound weighted number by the county population from the Census. Data concerning clinical characteristics were restricted to patients enrolled in Flu‐VE. Pearson’s chi‐square or Fisher’s exact tests were used for contingency tables when appropriate. Data analyses were performed using R version 2.12.2 and stata version 10.0.
Results
Flu‐VE identified 2221 eligible patients admitted with respiratory symptoms and approached 1975 patients (89·0%); those not approached included 125 patients discharged before enrollment was possible (50·8%) and 121 patients missed (49·2%). From the 1975 patients approached, Flu‐VE enrolled 1181 (60·0%) including 420 (35·6%), 83 (7·0%), 227 (19·2%), and 451 (38·2%) patients aged <5, 5–17, 18–49, and ≥50 years, respectively. Among patients approached but not enrolled, refusal was lower in children (121/227, 53·3%, as compared to adults, 459/567, 81·0%). Forty‐four enrolled patients had a specimen that tested positive for influenza A, negative for seasonal influenza, and positive for A(H1N1)pdm09 on two separate runs. Six patients had a specimen positive for influenza A, negative for seasonal influenza A and positive for only one A(H1N1)pdm09 subtyping assay, and one specimen was persistently positive for influenza A but negative for all subtypes tested. Thus, Flu‐VE surveillance identified 51 (4·3%) A(H1N1)pdm09‐associated hospitalizations.
Clinical characteristics
Compared with enrolled patients who tested negative, A(H1N1)pdm09‐hospitalized patients were more likely to be female (64·7% versus 47·0%), aged 18–49 (35·3% versus 18·5%), and present with cough and/or fever/feverishness (100% versus 91·6%). Sixty‐four percent of the 51 patients had at least one chronic medical condition including asthma, diabetes mellitus, and kidney diseases, which was similar in influenza‐negative patients. Compared with influenza‐negative patients who had an chest radiograph interpreted, A(H1N1)pdm09‐hospitalized patients were more likely to have a chest radiograph interpreted as pneumonia (28·9% versus 21·9%), and to be prescribed antiviral medication (29·4% versus 3·9%), but a similar percent required supplemental oxygen during their hospital stay (53·9% versus 53·2%). Of note, 9/22 (40%) patients clinically diagnosed with A(H1N1)pdm09 infection were prescribed antiviral medication. A(H1N1)pdm09‐hospitalized patients were less likely to be admitted to critical care (5·9% versus 12%); however, this difference was not statistically significant. Of note, around 50% of enrolled patients who tested negative admitted to ICU were in the two extreme age‐groups (<1 year, 26%, and 65 years or more, 22·2%). In‐hospital case fatality was 3·9% for A(H1N1)pdm09‐hospitalized patients and 0·8% for influenza‐negative patients (Table 1). The two influenza deaths were in adults aged 18–49 and ≥50 years.
Table 1.
Characteristics of patients enrolled into Flu‐VE study, Davidson Co, TN, from May 2009 to March 2010 by influenza A(H1N1)pdm09 status
| Characteristics | A(H1N1)pdm09 (+) n (%) n = 51 | A(H1N1)pdm09 (−) n (%) n = 1130 | P‐value |
|---|---|---|---|
| Gender (female) | 33 (64·7) | 531 (47) | 0·013 |
| Age‐group | |||
| <5 years | 16 (31·4) | 404 (35·8) | |
| 5–17 years | 5 (9·8) | 78 (6·9) | 0·012 |
| 18–49 years | 18 (35·3) | 209 (18·5) | |
| ≥50 years | 12 (23·5) | 439 (38·8) | |
| Signs and symptoms | |||
| Feverish | 46 (90·2) | 390 (61·1) | |
| Cough | 3 (5·9) | 312 (27·6) | 0·012 |
| Fever | 2 (3·9) | 33 (2·9) | |
| Most common conditions | |||
| Asthma | 26 (51·0) | 489 (43·3) | 0·117 |
| Diabetes mellitus | 8 (15·7) | 166 (14·7) | 0·227 |
| Kidney disorder | 4 (7·8) | 53 (4·7) | 0·490 |
| Other* | 4 (7·8) | 124 (7·8) | 0·482 |
| Any of above | 33 (64·7) | 728 (64·4) | 0·967 |
| Any influenza test ordered | 40 (78·4) | 426 (37·7) | <0·001 |
| Any negative result | 18 (45·0) | 415 (97·4) | <0·001 |
| Any positive result | 22 (55·0) | 11 (2·6) | |
| Chest X‐ray report | 38 (74·5) | 789 (69·8) | 0.475 |
| No acute abnormality | 22 (57·9) | 342 (43·4) | 0·023 |
| Pneumonia | 11 (28·9) | 173 (21·9) | |
| Other acute abnormality | 5 (13·2) | 274 (34·7) | |
| Admission to intensive care unit | 3 (5·9) | 135 (12·0) | 0·186 |
| Oxygen during hospital stay | 27 (52·9) | 601 (53·2) | 0·955 |
| Intubation | 2 (3·9) | 42 (3·7) | 0·953 |
| Died | 2 (3·9) | 9 (0·8) | 0·067 |
*Other medical conditions included cardiovascular disease, HIV infections, and cancer. The percentage does not add to 100 because one patient may have more than one underlying conditions.
Laboratory data
Forty of 51 A(H1N1)pdm09‐hospitalized patients (78·4%) had an influenza test ordered as part of their routine medical care, of which 22 (55%) had a positive result. From these 40 patients, 38 had a rapid test, primarily BinaxNOW Influenza A&B, of which 15 had a positive result yielding a sensitivity of 39% (95% CI, 26–55%) for BinaxNOW Influenza A&B using the research laboratory rRT‐PCR as reference. The age‐specific sensitivity of BinaxNOW Influenza A&B to detect A(H1N1)pdm09 viral antigen was estimated to be 75%, 67%, 20%, and 13% in patients aged <5, 5–17, 18–49, and ≥50 years, respectively. Of the 365 influenza‐negative patients enrolled through Flu‐VE who had a rapid test as the unique clinical test performed, 358 had negative results, yielding a specificity of 98% (95% CI, 96–99%) for BinaxNOW Influenza A&B compared to the research laboratory result.
Capture–recapture estimates
During the same time period that Flu‐VE identified 51 A(H1N1)pdm09 admissions in county residents at Flu‐VE surveillance hospitals, EIP surveillance identified 126 A(H1N1)pdm09 admissions in county residents.
Flu‐VE and EIP identified, respectively, 16 and 22 A(H1N1)pdm09‐positive patients aged <5 years, including eight children captured in both surveillance systems (matched), and five and four positive patients aged 5–17 years, with one matched. Among adults, in the four surveillance hospitals, Flu‐VE and EIP identified, respectively, 18 and 43 A(H1N1)pdm09‐hospitalized patients aged 18–49 years (two matched), and 12 and 21 positive patients aged ≥50 years (three matched).
Estimated rates from capture–recapture analysis were 0·89 (95% CI, 0·72–1·49), 0·62 (0. 42–1·11), 1. 78 (0·99–3·63), and 0·76 (0·50–1·76) influenza‐related hospitalizations per 1000 residents aged <5, 5–17, 18–49 and ≥50 years, respectively (Table 2). The overall population estimate was 0·92 (0·71–1·43) influenza‐related hospitalizations per 1000 Davidson County residents (Figure 1).
Table 2.
Estimated hospitalization rates associated with A(H1N1)pdm09 virus, Davidson Co, TN, from May 2009 to March 2010
| Age‐group (years) | Estimated influenza‐related hospitalizations in Davidson County | Davidson County population | Annual rate per 1000 residents (95% confidence interval [CI]) | ||
|---|---|---|---|---|---|
| Capture–recapture | Surveillance‐sampling | Capture–recapture | Surveillance‐sampling | ||
| <5 | 42 | 37 | 47 446 | 0·89 (0·72–1·49) | 0·78 (0·46–1·22) |
| 5–17 | 59 | 30 | 93 710 | 0·62 (0·42–1·11) | 0·32 (0·14–0·69) |
| 18–49 | 565 | 314 | 318 006 | 1·78 (0·99–3·63) | 0·99 (0·64–1·52) |
| ≥50 | 135 | 253 | 176 548 | 0·76 (0·50–1·76) | 1·43 (0·80–2·48) |
| Overall | 586 | 634 | 635 710 | 0·92 (0·71–1·43) | 1·00 (0·60–1·64) |
Figure 1.
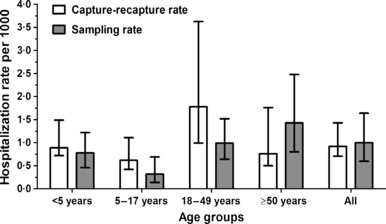
Estimated hospitalization rates associated with A(H1N1)pdm09 virus during the pandemic (H1N1) 2009 estimated by capture–recapture and surveillance‐sampling methods in Davidson County residents aged <5, 5–17, 18–49, and ≥50 years.
Reasons for the undetected influenza‐related hospitalizations by Flu‐VE included not being approached by the surveillance staff (39·4%), admission on non‐surveillance days (34·2%), refused testing (15·8%), tested falsely negative (5·3%), or admission to non‐surveillance hospitals (5·3%). Reasons for the undetected hospitalizations for EIP included not tested (35·2%), tested falsely negative (32·4%), and missed (32·4%). Of note, Flu‐VE identified all cases using rRT‐PCR, whereas for EIP, influenza‐related hospitalized patients had a rapid test alone (53·0%), rRT‐PCR and rapid test (28·0%), rRT‐PCR only (11·0%), or other tests (8%).
Surveillance‐sampling estimates
From May to August 2009 (first wave), 0·9–4·4% of patients hospitalized with respiratory symptoms were infected with the pandemic influenza virus. From September 2009 through March 2010 (second wave), influenza‐positive proportion ranged from 3·0 to 8·8% (Figure 2).
Figure 2.
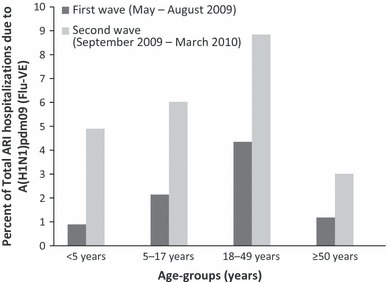
Proportion of hospitalized patients tested positive for A(H1N1)pdm09 during the pandemic (H1N1) 2009 among the total Davidson County residents aged <5, 5–17, 18–49, ≥50 years with symptoms of acute respiratory illnesses tested for A(H1N1)pdm09 through Flu‐VE from May 2009 to March 2010.
The proportions of A(H1N1)pdm09‐associated hospitalizations detected through Flu‐VE from May 2009 to March 2010 were 3·8%, 6·0%, 7·9%, and 2·7% for patients aged <5, 5–17, 18–49, and ≥50 years, respectively. We applied these age‐specific proportions to the ARI‐estimated counts from HDDS and estimated 37 (95% CI, 22–83), 30 (13–65), 314 (202–483), and 253 (142–437) A(H1N1)pdm09‐associated hospitalizations in Davidson County residents aged <5, 5–17, 18–49, and ≥50 years, respectively (Table 2).
Surveillance‐sampling rate estimates were 0·78 (95% CI, 0·46–1·22), 0·32 (0·14–0·69), 0·99 (0·64–1·52), and 1·43 (0·80–2·48) influenza‐related hospitalizations per 1000 county residents aged <5, 5–17, 18–49, and ≥50 years, respectively (Figure 1). The overall rate estimated through the surveillance‐sampling method was 1·00 (0·60–1·64) hospitalization per 1000 Davidson County residents.
The first wave comprised only 17% of all pandemic influenza‐associated hospitalizations during the study period including 8%, 13%, 19%, and 15% of hospitalizations for residents aged <5, 5–17, 18–49, and ≥50 years, respectively.
Ninety‐eight percent of A(H1N1)pdm09‐positive patients and 80·3% of A(H1N1)pdm09‐negative patients detected through Flu‐VE had at least one of the ICD‐9‐CM discharge codes used in the study to identify ARI hospitalizations in the HDDS. Among patients counted as A(H1N1)pdm09 positive, the most common discharge diagnoses were pneumonia and asthma. Only one Davidson County patient had a discharge diagnosis of A(H1N1)pdm09 infection (488·1) (Table 3).
Table 3.
Discharge diagnoses of patients enrolled in Flu‐VE from May 2009 to March 2010 stratified by whether they were included to calculate total respiratory admissions from hospital discharge data system (HDDS)
| Any discharge diagnoses (ICD9‐CM codes) | A(H1N1)pdm09 (+) total = 51 n (%) | A(H1N1)pdm09 (−) total = 1129* n (%) |
|---|---|---|
| Discharge diagnoses included in current report | 50 (98·0) | 907 (80·3) |
| Pneumonia and influenza (480–487) | 38 (76·0) | 346 (38·1) |
| Bronchitis, emphysema, asthma (490–493) | 9 (18·0) | 307 (33·9) |
| Acute respiratory infections (460–466) | 1 (2·0) | 144 (15·9) |
| Dyspnea and respiratory abnormalities (786) | 0 (0·0) | 59 (6·5) |
| Fever (780·6) | 2 (4·0) | 48 (5·3) |
| Otitis media (381–382) | 0 (0·0) | 3 (0·3) |
| Discharge diagnoses not included | 1 (2·0) | 222 (19·7) |
| Pandemic (H1N1) 2009 (488) | 0 (0·0) | 1 (0·4) |
| Other respiratory (470–478, 494–519) | 0 (0·0) | 61 (27·5) |
| Circulatory (390–459) | 0 (0·0) | 53 (23·9) |
| Dehydration (276·51) | 1 (100·0) | 16 (7·2) |
| Other | 0 (0·0) | 91 (41·0) |
*One missing discharge diagnosis.
Discussion
We applied two different methods to estimate age‐specific rates of A(H1N1)pdm09‐associated hospitalization in Davidson County, TN. Although estimates were higher from capture–recapture in adults aged 18–49 and from surveillance‐sampling in those aged ≥50 years, the confidence intervals overlapped and both methods yielded an overall rate of approximately 1 hospitalization per 1000 residents.
Our results are consistent with those obtained from a CDC probabilistic multiplier model 4 , 23 developed to account for underreporting of influenza‐related hospitalizations in the United States during the pandemic (H1N1) 2009. This CDC model recognized that only a fraction of influenza‐related hospitalizations were reported through EIP surveillance during the pandemic period because of multiple factors and attempted to correct the reported number of laboratory‐confirmed hospitalizations for several factors. These factors included the proportion of patients with specimens collected, the proportion of specimens submitted for confirmation, and the sensitivity of laboratory tests for detection of A(H1N1)pdm09 virus. The rates of laboratory‐confirmed influenza‐related hospitalizations from the 10 EIP surveillance states were extrapolated to the 50 US states taking into account regional variation in influenza activity. The CDC model estimated 274 304 (195 086–402 719) influenza‐related hospitalizations for an overall estimated rate of 0·90 (95% CI, 0·64–1·32) hospitalization per 1000 US residents (April 2009–April 2010). 23 The age‐specific estimated hospitalization rates were 1·17 (0·83–1·72), 0·83 (0·59–1·23), and 0·70 (0·50–1·03) per 1000 US residents aged <18, 18–64, and ≥65 years, respectively. 4 , 23 Although the age‐group divisions differed from those used in our study, the estimates from this CDC model provided age‐specific rates and confidence intervals that overlapped the rates and the confidence intervals estimated using two additional and complementary methods in our study. Of note, the multiplier model relies on assumptions that need to be updated annually to correct for missed diagnoses, whereas our methods may be routinely applied where surveillance for influenza is ongoing.
Two other North American studies relied on clinical testing and yielded lower rates than those we report here, especially in older age‐groups. Helferty et al. 24 reported laboratory‐confirmed A(H1N1)pdm09 infection and derived estimates for Canada of 1·65, 0·84, 0·29, 0·17, 0·24, and 0·18 hospitalization per 1000 residents aged <1, 1–4, 5–19, 20–44, 45–64, and ≥65. Kumar et al. 25 used passive surveillance based on specimens submitted for confirmation to the Midwest Respiratory Virus Program and reported A(H1N1)pdm09‐associated hospitalization rates of 0·49, 0·18, 0·13, 0·06, 0·06, and 0·03 per 1000 Milwaukee, Wisconsin, residents aged respectively <5, 5–18, 19–24, 25–49, 50–64, and ≥65 years. Rates reported from Europe were even lower, 26 , 27 likely due to reliance on passive reporting systems and underrecognition of or undertesting for influenza.
While the use of capture–recapture methods seeks to correct for missed cases, the validity of estimates made with these methods relies on the fulfillment of several assumptions: a closed population without significant migration in or out, valid tests (Flu‐VE used the highly sensitive and specific rRT‐PCR; 92% of patients identified by EIP had a positive rapid test with a specificity of 98% in the population studied as compared to the research laboratory rRT‐PCR), and independence of the two surveillance systems. We applied the unbiased estimator approach because of sparse data in some cells particularly for age‐group 5–17; this method gives more conservative estimates with less chance of overestimation. 16
Applying the surveillance‐sampling method that used the HDDS as a completely different source of data strengthened the study because it yielded similar overall estimates to those obtained from capture–recapture that used Flu‐VE and EIP. The surveillance‐sampling method represents a valuable inexpensive option to estimate disease burden. However, it relies on the accuracy of the sampling procedures assuming systematic sampling of the study population to ensure representativeness of the general population under observation. In addition, it assumes that the ARI hospitalizations identified in HDDS closely mirrored those actually sampled.
Our study has a number of limitations. First, we identified a relatively small number of influenza‐associated hospitalizations captured in the two systems because they both missed cases. Cases missed included those not approached by the recruiters in Flu‐VE, and those not tested, or tested falsely negative in EIP surveillance. In the Flu‐VE study, we enrolled only 1181/2221 (53·2%) eligible patients, raising the possibility of selection bias. Bias also could have occurred particularly in our surveillance‐sampling estimates leading to under‐ or overestimation. This problem may have limited the precision of our incidence estimates as reflected by the wide confidence intervals in our calculations. Second, we included in our analysis seven patients who were identified as influenza A but did not meet formal CDC criteria for A(H1N1)pdm09 virus detection. Inclusion of these patients would result in overestimation if they represented false positives. However, all these tests were persistently influenza A positive, negative for seasonal H1N1 and H3N2, and 6 of the 7 were positive for one of the two A(H1N1)pdm09‐specific subtyping assays. These seven were considered to be A(H1N1)pdm09 virus positives for this study, because no seasonal influenza viruses were detected through surveillance during the study period. In addition, we had incomplete surveillance for the age‐group 5–17 years, because there was no inpatient surveillance for this age‐group before October 2009. An extrapolation method based on the observed counts of influenza‐related hospitalizations in children <5 years from May to September 2009 was applied to estimate the total influenza‐related hospitalizations in children 5–17 years from May to September 2009.
In adults, our sampling included hospitals that admitted only 60% of county patients with ARI. However, the weighting procedure applied in both methods helped account for this imprecision.
We also found a large difference between influenza‐positive and influenza‐negative patients admitted to critical care (5·9% versus 12%); although not statistically significant, this difference could be explained by the age distributions of two groups. Influenza‐positive patients were more likely to be 18–49 (35·3%), whereas influenza‐negative patients were more likely to be 50 years or more (38·8%).
Another critical point for the surveillance‐sampling method is how well the diagnostic codes used to identify ARI hospitalizations match the A(H1N1)pdm09 admissions identified through Flu‐VE. To identify eligible patients for enrollment in the Flu‐VE, we used broad screening criteria, which included any respiratory symptoms and/or fever without a non‐respiratory cause. To identify patients with ARI discharge diagnoses that were similar to our target population, we used select ICD‐9‐CM codes and found that 98·0% of influenza‐positive and 80·3% of influenza‐negative patients identified through surveillance had at least one of these codes.
In summary, we estimated the rates of A(H1N1)pdm09‐associated hospitalization in Davidson County residents and found an overall rate of 1 per 1000 county residents using distinctive capture–recapture and surveillance‐sampling methods. In addition to providing new data on the pandemic burden, these methods represent inexpensive and affordable tools to estimate the completeness of case ascertainment as reported by two or more sources of data. These methods can be readily applied whenever the related assumptions are fulfilled.
Addendum
Dr. Astride Jules wrote the first draft and contributed to statistical analyses. Dr. Carlos G. Grijalva contributed to statistical analysis, and drafting and review of paper. Dr. Yuwei Zhu contributed to data management, statistical analysis, and drafting and review of paper. Dr. Keipp H. Talbot reviewed the paper. Dr. John V. Williams provided laboratory technical assistance, and drafted and reviewed the paper. Professor William D. Dupont contributed to statistical analyses. Professor Kathryn M. Edwards, Professor William Schaffner, and Dr. David K. Shay drafted and reviewed the paper. Professor Marie R. Griffin provided funding through the Flu‐VE network, and drafted and reviewed the paper.
Disclaimers
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of either the Centers for Disease Control and Prevention or the authors’ affiliations.
Financial support
This study was funded by the Centers for Disease Control and Prevention through cooperative agreements with Vanderbilt University (U01 IP000184and U50/CCU 4161 23). Internal CDC funds were used to support CDC investigators and to provide laboratory validation of RT‐PCR methods at the enrolling site laboratories. Dr. Talbot received salary support from the NIAID (1K23AI074863‐01).
Conflicts of interest
H. Keipp Talbot received research funds from VaxInnate, Sanofi Pasteur, and Wyeth (Pfizer). John V. Williams is associated with the Scientific Advisory Board of Quidel, and William Schaffner is a consultant to Novartis, GSK, Sanofi Pasteur, Dynavax, Pfizer; Member, Data Safety Monitoring Boards of Merck.
Acknowledgements
We are indebted to study coordinators Dayna Wyatt and Diane Kent from Flu‐VE, to Terri McMinn and Brenda Barnes from EIP, to other study personnel who enrolled patients and collected samples, and to all participating patients. We are indebted to the Tennessee Department of Health, Office of Health Statistics, for providing the hospital discharge data.
References
- 1. Centers for Disease Control and Prevention (CDC) . Update: influenza activity – United States, August 30, 2009–March 27, 2010, and composition of the 2010–11 influenza vaccine. MMWR Morb Mortal Wkly Rep 2010; 59:423–430. [PubMed] [Google Scholar]
- 2. Tennessee Department of Health Influenza‐Like Illness Surveillance Summary Archive. 2010.
- 3. Centers for Disease Control and Prevention (CDC) . Update: influenza activity – United States, August 30, 2009–January 9, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:38–43. [PubMed] [Google Scholar]
- 4. Reed C, Angulo FJ, Swerdlow DL et al. Estimates of the Prevalence of Pandemic (H1N1) 2009, United States, April–July 2009. Emerg Infect Dis 2009; 15:2004–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Census Bureau . Annual Estimates of the Resident Population by sex, race, age, and Hispanic Origin for the United States: April 1, 2000 to July 1, 2009 (NC‐EST 2009‐03). 2010.
- 6. Poehling KA, Edwards KM, Weinberg GA et al. The underrecognized burden of influenza in young children. N Engl J Med 2006; 355:31–40. [DOI] [PubMed] [Google Scholar]
- 7. Fiore AE, Shay DK, Broder K et al. Prevention and control of seasonal influenza with vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. Morb Mortal Wkly Rep 2009; 58:1–51. [PubMed] [Google Scholar]
- 8. WHO . The WHO Collaborating Centre for influenza at CDC Atlanta USA. CDC Realtime RTPCR (rRTPCR) Protocol for Detection and Characterization of Swine Influenza (version 2009). Atlanta, GA: WHO, 2009. [Google Scholar]
- 9. Centers for Disease Control and Prevention (CDC) . CDC Website Vaccine and Immunizations. Atlanta, GA: Centers for Disease Control and Prevention, 2010. [Google Scholar]
- 10. Grijalva CG, Craig AS, Dupont WD et al. Estimating influenza hospitalizations among children. Emerg Infect Dis 2006; 12:103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schrag SJ, Shay DK, Gershman K et al. Multistate surveillance for laboratory‐confirmed, influenza‐associated hospitalizations in children: 2003–2004. Pediatr Infect Dis J 2006; 25:395–400. [DOI] [PubMed] [Google Scholar]
- 12. Schoenman JA, Sutton JP, Elixhauser A, Love D. Understanding and enhancing the value of hospital discharge data. Med Care Res Rev 2007; 64:449–468. [DOI] [PubMed] [Google Scholar]
- 13. Arnason AN, Schwarz CJ, Gerrard JM. Estimating closed population‐size and number of marked animals from sighting data. The Journal of Wildlife Management 1991; 55:716–730. [Google Scholar]
- 14. Brookmeyer R, Stroup DF. Monitoring the Health of Populations: Statistical Principles and Methods for Public Health Surveillance. Oxford, New York: Oxford University Press, 2004. [Google Scholar]
- 15. Goldman GS. Using capture‐recapture methods to assess varicella incidence in a community under active surveillance. Vaccine 2003; 21:4250–4255. [DOI] [PubMed] [Google Scholar]
- 16. Hook EB, Regal RR. Capture‐recapture methods in epidemiology: methods and limitations. Epidemiol Rev 1995; 17:243–264. [DOI] [PubMed] [Google Scholar]
- 17. Wittes JT. On the bias and estimated variance of chapman’s two‐sample capture‐recapture population estimate. Biometrics 1972; 28:592–597. [Google Scholar]
- 18. Gjini A, Stuart JM, George RC, Nichols T, Heyderman RS. Capture‐recapture analysis and pneumococcal meningitis estimates in England. Emerg Infect Dis 2004; 10:87–93. [DOI] [PubMed] [Google Scholar]
- 19. Efron B, Tibshirani R. An Introduction to the Bootstrap. New York, NY: Chapman & Hall, 1993. [Google Scholar]
- 20. Kumar S, Havens PL, Chusid MJ, Willoughby RE Jr, Simpson P, Henrickson KJ. Clinical and epidemiologic characteristics of children hospitalized with 2009 pandemic H1N1 influenza A infection. Pediatr Infect Dis J 2010; 29:591–594. [DOI] [PubMed] [Google Scholar]
- 21. Schirmer P, Lucero C, Oda G, Lopez J, Holodniy M. Effective detection of the 2009 H1N1 influenza pandemic in U.S. Veterans Affairs medical centers using a national electronic biosurveillance system. PLoS ONE [Electronic Resource] 2010; 5:e9533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thompson WW, Shay DK, Weintraub E et al. Influenza‐associated hospitalizations in the United States. JAMA 2004; 292:1333–1340. [DOI] [PubMed] [Google Scholar]
- 23. Shrestha SS. Estimating the burden of 2009 Pandemic Influenza A (H1N1) in the United States (April 2009–April 2010). Clin Infect Dis 2011; 52:S75–S82. [DOI] [PubMed] [Google Scholar]
- 24. Helferty M, Vachon J, Tarasuk J, Rodin R, Spika J, Pelletier L. Incidence of hospital admissions and severe outcomes during the first and second waves of pandemic (H1N1) 2009. CMAJ 2010; 182:1981–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar S, Chusid MJ, Willoughby RE et al. Epidemiologic observations from passive and targeted surveillance during the first wave of the 2009 H1N1 influenza pandemic in Milwaukee, WI. Viruses 2010; 2:782–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell CN, Mytton OT, McLean EM et al. Hospitalization in two waves of pandemic influenza A(H1N1) in England. Epidemiol Infect 2011; 139:1560–1569. [DOI] [PubMed] [Google Scholar]
- 27. van‘t Klooster TM, Wielders CC, Donker T et al. Surveillance of hospitalisations for 2009 pandemic influenza A(H1N1) in the Netherlands, 5 June – 31 December 2009. Euro Surveill 2010; 15:9–16. [DOI] [PubMed] [Google Scholar]


