Abstract
Our data demonstrate that estrogens, estrogen receptor-α (ERα), and estrogen receptor-β (ERβ) regulate adipose tissue distribution, inflammation, fibrosis, and glucose homeostasis, by determining that αERKO mice have increased adipose tissue inflammation and fibrosis prior to obesity onset. Selective deletion of adipose tissue ERα in adult mice using a novel viral vector technology recapitulated the findings in the total body ERα null mice. Generation of a novel mouse model, lacking ERα specifically from adipocytes (AdipoERα), demonstrated increased markers of fibrosis and inflammation, especially in the males. Additionally, we found that the beneficial effects of estrogens on adipose tissue require adipocyte ERα. Lastly, we determined the role of ERβ in regulating inflammation and fibrosis, by breeding the AdipoERα into the βERKO background and found that in the absence of adipocyte ERα, ERβ has a protective role. These data suggest that adipose tissue and adipocyte ERα protects against adiposity, inflammation, and fibrosis in both males and females.
Abbreviations: ERα, estrogen receptor alpha; ERβ, estrogen receptor beta; WAT, white adipose tissue; BAT, brown adipose tissue
Keywords: Estrogen receptor alpha (ERα), White adipose tissue (WAT), Fibrosis, Inflammation
1. Introduction
Intra-abdominal white adipose tissue has been strongly correlated with insulin resistance, inflammation, cardiovascular disease, and the Metabolic Syndrome in animal models and humans [1–3]. Men, on average, have less total body fat but more intra-abdominal adipose tissue than women, whereas women, on average, have more total fat and subcutaneous adipose tissue [4–8]. Male and female adipose tissues are sexually dimorphic; female adipose tissue is more insulin sensitive, less susceptible to inflammation, and has higher expression of estrogen receptors (ERs) [9–11].
Estrogens are produced in the ovary and testes, as well as in adipocytes (by the action of aromatase on androgens), and are increased in proportion to total body adiposity [12,13]. Reduced circulating estrogens, as seen in post-menopausal females, result in the development of increased intra-abdominal adiposity and increased susceptibility to diseases associated with the Metabolic Syndrome. Importantly, women who receive estrogen replacement therapy are less likely to deposit adipose tissue in the intra-abdominal depot [14–16], and are relatively more protected from the Metabolic Syndrome. To further demonstrate that ovarian hormones are responsible for the effects on adipose tissue distribution, we and others have data demonstrating removal of ovaries (ovariectomy (OVX)) results in body weight gain primarily as adipose tissue [17–19], which is deposited primarily in the intra-abdominal adipose depot and results in impaired glucose homeostasis, whereas, administration of estrogens reduces body weight, body adiposity, changes body fat distribution, and improves glucose homeostasis [18,20,21].
Increased adiposity and adipose tissue inflammation are now recognized as underlying mechanisms in the pathogenesis of dysregulated glucose homeostasis and the Metabolic Syndrome. As previously mentioned, females are protected from the Metabolic Syndrome, which may likely be due to the potent anti-inflammatory effects of estrogens [22,23]. Estrogens bind to two ‘classical’ estrogen receptor subtypes, estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), with similar affinity; however these two receptors are believed to differ in their translational properties. Importantly, the relative direct importance of ERα and/or ERβ in modulating adipose tissue inflammation is not known. Previous studies have described ERα protein [24,25], as well as specific estrogen binding and ERα mRNA to be present in human subcutaneous adipose tissue [25]. However, others have not been able to detect estrogen receptors in human adipose tissue [26,27]. More recently, ERβ mRNA has been detected in human subcutaneous adipose tissue, suggesting that direct effects of estrogen may involve both receptor subtypes [20].
The effects of estrogens on energy homeostasis are primarily mediated by ERα, as indicated by findings that women or female mice with mutations in the ERα gene display hyper-adiposity [28,29]. We recently reported that site-specific knockout of ERα in certain brain regions results in increased adiposity, changes in body fat distribution, reductions in energy expenditure, and alterations in fertility. Furthermore, global deletion of the ERα gene (αERKO) results in increased adiposity in both males and females, with a near doubling of the intra-abdominal adipose tissues when compared to age-matched wild type (WT) mice [29,30]. However, mice with a deletion in ERβ (βERKO) do not have increased adiposity or metabolic derangements. These data suggest an important role for ERα in regulating adipose tissue distribution and potentially inflammation. Therefore, we sought to determine the role of ERα in regulating adipose tissue distribution and ‘function’ as measured by levels of inflammation, and fibrosis. To do this, we first compared weight-matched wild type (WT) and αERKO mice with respect to adipose tissue distribution, fibrosis, and inflammation. Our data suggest that αERKO males and females have increased adipose tissue that has elevated levels of markers of inflammation and fibrosis. Since the αERKO is a total body deletion of ERα and we were specifically interested in selective effects of ERα in adipose tissue we developed a novel approach to knock down ERα expression in an adipose tissue depot-specific manner using viral technology. We found that knockdown of ERα selectively in intra-abdominal adipose tissue results in increased adipose tissue mass, increased adipocyte size, and is associated with elevated adipose tissue inflammation and fibrosis in both males and females. Importantly, our novel viral-mediated knockdown of ERα expression reduces ERα in all adipose tissue cell types, including adipocytes, preadipocytes, immune cells and vascular cells. Therefore, in order to assess the role of ERα specifically in adipocytes, we created a mouse model in which we can knock down ERα selectively in adipocytes by generating a unique and novel animal model using the Cre-loxP system where we bred the ERαlox/lox mouse [31] combined with the adipocyte-specific Adiponectin-Cre transgenic model [32,33] to produce AdipoERα mice, or mice that specifically lack ERα only in adipocytes. Our data suggest that adipocyte ERα regulates adipocyte size, adipose tissue inflammation and fibrosis in both males and females. Finally, to rule out any contribution ERβ may have with respect to the metabolic phenotypes observed, we bred the AdipoERα mouse into the total body βERKO mouse resulting in a mouse model that lacks both estrogen receptors on adipocytes. Our data suggest that knockdown of adipocyte ERα in the context of the βERKO mouse increases inflammation and fibrosis indicating a role for ERβ in the absence of adipocyte ERα.
2. Material and methods
2.1. αERKO mice
Wild type (WT) and αERKO male and female littermates were housed in a temperature-controlled environment in groups of two to five at 22–24 °C using a 12 h light/12 h dark cycle (singly housed to measure food intake). The mice were fed standard chow (♯2916, Harlan-Teklad, Madison, WI) and ad libitum water. Body weight was monitored weekly, mice (n=8) were sacrificed at 6 weeks of age, a time in which body weight did not differ, and a time in which the WT mice had gone through puberty as confirmed by observation of vaginal opening in the females. Care of all animals and procedures were approved by the UT Southwestern Medical Center in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. All WT females were sacrificed in proestrus cycle.
2.2. Gonadal fat depot-specific viral knockdown of ERα
In vivo fat pad-specific injections were made in WT C57Bl6 male and female mice (n=9) at 8 weeks of age. Mice were anesthetized with isoflurane (Aerrane, Baxter, IL), the ventral surface shaved and treated with betadine and alcohol 3×. A bilateral ventral abdominal incision was made and the gonadal fat pads (peri-ovarian for females, and epididymal for males) were injected with an adeno-associated (AAV) ERα siRNA virus (on the right side) and with a control (scrambled sequence) siRNA (on the left side). Each virus was diluted using sterile saline and was injected in equal volumes of 4×1011 particles per fat pad. Approximately 5–7 injections were performed to dispense the virus across the pad. The peritoneum was closed with Vicryl suture (Ethicon) and the skin was closed with nylon suture and staples. Post-operative pain was managed using 0.5 mg/kg buprenorphine injection post-surgery and 2 mg/kg rimadyl tablets for the following 48 h. After the surgery the mice were monitored daily for body weight and food intake to assess recovery. The tissues were harvested at 3 weeks post-injection (sacrificed at 11 weeks of age). To illustrate the validity and specificity of the technique, the AAV ERα siRNA virus was tagged with green fluorescent protein (GFP) and viewed under GFP fluorescence.
2.3. Adipocyte-specific knockdown of ERα
In order to generate mice lacking ERα specifically in adipocytes, we used Adiponectin promoter driven-Cre transgenic mice [33], and crossed them with mice carrying the loxP-flanked ERα allele (ERαlox/lox) [31] producing ERαlox/lox/Adiponectin-Cre (AdipoERα) mice and their ERαlox/lox (WT) littermates on a C57Bl6 background. Mice were housed in a temperature-controlled environment in groups of two to five at 22–24 °C using a 12 h light/12 h dark cycle (singly housed to measure food intake). The mice were fed standard chow (♯2916, Harlan-Teklad, Madison, WI) and ad libitum water. Care of all animals and procedures were approved by the UT Southwestern Medical Center in accordance with the Guide for Care and Use of Laboratory Animals of the National Institutes of Health. Body weight was measured weekly from group housed mice (n=10). Food intake was measured daily, and average daily food intake was calculated using data from at least 10 continuous days. Body composition was determined using quantitative magnetic resonance (QMR) (Bruker's Minispec MQ10, Houston, TX). Mice were sacrificed at 18 weeks of age.
2.4. Adipocyte-specific knockdown of ERα in ERβ null mice (AdipoERα/βERKO)
In order to determine the potential role of ERβ in mediating the AdipoERα phenotype, we generated mice lacking ERα specifically in adipocytes (ERαlox/lox/Adiponectin-Cre (AdipoERα)) mice and bred them to βERKO mice on a C57Bl6 background (n=9). Care of mice, food intake, body composition, fat distribution, tissue analysis, gene expression and histology were carried out as previously described.
2.5. Tissue mRNA analyses
Mice (number of mice and age for each group are noted in figure legends where appropriate) were euthanized with isoflurane (Aerrane, Baxter, IL), and the gonadal white adipose tissue (WAT or Gonadal–the fat pad surrounding the ovaries and uterus for the females, and the epididymal for the males) was isolated, weighed, and snap-frozen in liquid nitrogen. Total RNA was isolated following tissue homogenization in Trizol (Invitrogen, Carlsbad, CA) using a TissueLyser (Qiagen, Valencia, CA) and isolated using the RNeasy RNA extraction kit (Qiagen). The quality and quantity of the RNA were determined by absorbance at 260/280 nm. cDNA was prepared by reverse transcribing 1.5 μg RNA with SuperScript III reverse transcriptase (Invitrogen) and oligo(dT)20 (Invitrogen). Quantitative PCR (qPCR) was performed using TaqMan primers for tumor necrosis factor-α (TNFα), toll-like receptor-4 (TLR-4), serum amyloid A-3 (SAA3), lysyl oxidase (LOX), collagen-6 (COL6), and EGF-like module-containing mucin-like hormone receptor-like 1 (F4/80). Beta-2-microglobulin was used as the housekeeping gene.
2.6. Hematoxylin and eosin staining
As above, gonadal WAT was isolated, weighed, and fixed with 10% formalin for overnight and then stored in 50% ethanol. The fixed fat pads were sent to Richardson Molecular Pathology Core at UT Southwestern Medical Center, where the tissues were embedded with paraffin, sectioned and stained with haematoxylin and eosin (H&E). Images were viewed under rhodamine fluorescence and imaged using Leica DM2000 compound epifluorescence microscope equipped with an Optronics Microfire Color CCD Camera and analyzed for adipocyte area using NIH ImageJ software. Eight hundred to 1000 cells from each sample were included in the analysis. (See figure legends for details of each cohort).
2.7. ERα immunohistochemistry
To determine presence of ERα, sections (n=4) were de-paraffinized in xylene followed by decreasing concentrations of ethanol. Sections were microwaved in citric acid buffer for antigen retrieval. They were then blocked for endogenous peroxidases using 3% H2O2 in PBS. Tissues were also blocked for avidin and biotin (Vector SP-2001) as well as protein (10% NGS S-1000, Vector). After blocking, sections were stained with rabbit polyclonal anti-ERα primary antibody (1:400) (Santa Cruz) followed by biotinylated anti-rabbit antibody (Vector). Following incubation in HRP polymer (PK-6101) the secondary antibody (1:800) was detected using Vector Immpact DAB kit (SK-4105) and counterstained with hematoxylin. All images were obtained using Nikon Coolscope.
2.8. Adipocyte/SV fractionation and isolation of nuclei
To confirm knockdown of ERα specifically from adipocytes, adipose tissue was excised as previously described and adipocytes were isolated from the stromal vascular tissue (SV) using the collagenase fractionation method [34]. Specifically, isolated adipocytes were resuspended in cold nuclei extraction buffer (320 mM sucrose, 5 mM MgCl2, 10 mM HEPES, 1% Triton-X at pH7.4) at 106 cells/ml. The cells were vortexed gently for 10 s and incubated on ice for 10 min. Nuclei were pelleted at 2000×g, and washed twice with nuclei wash buffer (320 mM sucrose, 5 mM MgCl2, 10 mM HEPES at pH7.4). Nuclei in nuclei wash buffer was allowed to attach to silicone-coated slides for 1 h at 4 °C. Then nuclei were fixed in 3.7% formaldehyde in nuclei wash buffer for 10 min and subjected to BrdU analyses using BrdU Flow Kit (BD Biosciences). (See figure legends for details of each cohort).
2.9. Ovariectomy and estrogen replacement
Ovariectomy (OVX) and sham surgeries were performed in 12-week-old female mice as previously described [10]. In each study animal, a pellet of estradiol-17β (0.03 mg per pellet for a 60 day release, (0.5 μg/day), a dose in which plasma levels of estrogens mirror those of intact females in proestrus [35]), or placebo control was administered subcutaneously. Food intake, body weight, and body adiposity were tracked as previously described. Mice were sacrificed 4 weeks post-surgery.
2.10. Oral glucose tolerance test (OGTT)
Mice were fasted for 3 h (starting at 8 a.m.) prior to administration of glucose (2.5 g/kg body weight) by gavage. At the indicated time points, venous blood samples were collected in heparin-coated capillary tubes from the tail vein. Mice did not have access to food throughout the experiment. (See figure legends for details of each cohort).
2.11. Hormone measurements
For all measurements, plasma was obtained from trunk blood from mice fasted 3–4 h. Estradiol-17β (Invitrogen) was assayed by enzyme-linked immunosorbent assay kits. (See figure legends for details of each cohort).
2.12. Statistics
The data are presented as mean±SEM. After confirming normal distribution of data, comparisons between two genotypes were made by the unpaired two-tailed Student's t-test; repeated-measures ANOVA was used to compare changes over time between two genotypes. P<0.05 is considered to be statistically significant. Significance between WT and AdipoERα measurements is denoted by a (⁎) and significance between male and female, or VEH and estradiol-17β within a genotype, is denoted by a (♯).
3. Results
3.1. Adipose tissue function (inflammation/fibrosis) in αERKO mice
To determine the role of ERα in modulating adipocyte size, inflammation, and fibrosis, WT and littermate αERKO weight-matched female and male mice (Figure 1A and B) were compared with respect to adipose tissue histology, inflammation, and fibrosis. Despite body weights being equivalent, αERKO males and females have increased body fat as measured by NMR (Figure 1C and D). αERKO mice have significantly larger adipocytes when compared to WT mice, as shown by representative histological images (Figure 1E) and adipocyte size calculations (Figure 1F), consistent with previous reports from Cooke et al. [30]. Enlarged adipocytes can be associated with increased macrophage infiltration into the fat pad [36]. To investigate if ERα in gonadal adipose tissue directly regulates macrophage infiltration, a marker for macrophage expression, F4/80, was measured. Figure 1G shows that in αERKO mice there is an increase in F4/80 expression in both males and females over WT mice that reached statistical significance in the males. To test for adipose tissue inflammation in αERKO mice, gene expression for the inflammatory markers serum amyloid A3 (SAA3), Toll-like receptor 4 (TLR4) and tumor necrosis factor-α (TNFα) was measured and compared between αERKO and WT mice. Figure 1H demonstrates that αERKO results in an up-regulation of SAA3 and TLR4 in both sexes and TNFα in males. Adipose tissue inflammation has often been correlated with enhanced adipose tissue fibrosis; therefore, to test the effects of αERKO on adipose tissue fibrosis, we analyzed gene expression levels of collagen VI (COL6) and lysyl oxidase (LOX). Data demonstrate a significant increase in the expression of LOX in both the female and male αERKO adipose tissues when compared to WT control mice (Figure 1I). Further, female αERKO mice have upregulated COL6 as compared to WT controls. Together these data suggest αERKO results in fibrotic and inflamed adipose tissue associated with elevated macrophage infiltration, consistent with unhealthy adipose tissue, in both males and females, indicating that ERα is critical in maintaining adipocyte size, adipose tissue inflammation, and fibrosis in both males and females.
Figure 1.
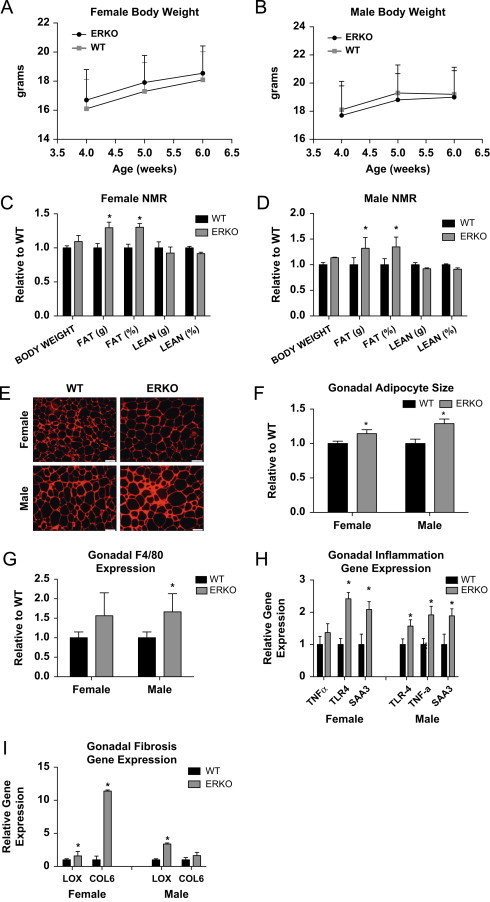
Wild type vs αERKO adipose tissue inflammation and fibrosis. (A and B) Weekly body weight was measured in singly housed female and male mice on normal chow (n=8/genotype). (C and D) Body composition was measured by NMR in 6-week-old female and male mice (n=8/genotype). (E) Representative photomicrographs of H&E staining of gonadal WAT from 6-week-old male and female mice. (F) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue from 6-week-old mice (n=8/genotype). (G) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 6-week-old chow-fed females and males (n=8/genotype). (H and I) Messenger RNA levels were quantified using qPCR of whole adipose tissue from 6-week-old chow-fed females and males (n=8/genotype). Data are presented as mean±SEM, and *P<0.05 between WT and αERKO.
3.2. Gonadal fat depot-specific viral knockdown of ERα
To begin to determine the adipose tissue-specific role of ERα in adult animals, we generated a novel technique that allows us to reduce the expression of ERα in a fat pad specific method. Importantly, this technique allows us to compare, within the same animal with the same hormonal milieu, the influence of adipose tissue ERα. To illustrate the selectivity of the viral technique, Figure 2A demonstrates that only in the AAVsiRNA ERα GFP-injected pad (L Gon) is GFP visible (the tissue from each view has been outlined for reference), whereas the fat pad injected with the control AAVsiRNA/non-GFP virus is not fluroescent. Importantly, only trace amounts of signal are detected in the contralateral pad (R Gon), adjacent retroperitoneal pad (L Ret) and liver (Liv), which is most likely the result of auto-fluroescence. The effectiveness of the siRNA in gonadal adipose tissue was further determined using qPCR for ERα following injections of AAV-ERα siRNA (ERα siRNA) in the left gonadal pad or the control AAV-siRNA-scrambled sequence (control) in the right gonadal pad. These data suggest a ∼50% reduction in ERα expression, as compared to control/contralateral fat pad (which is normalized to 1), in both males and females (Figure 2B). Fat pads that received the ERα siRNA are significantly heavier at 3 weeks post-injection than the pads injected with the control virus (regardless of sex) (Figure 2C). Consistent with αERKO adipose tissue, adipocyte morphology analyses demonstrate the pad with reduced ERα has enlarged adipocytes (Figure 2D and E). Additionally, Figure 2F shows that reduced adipose tissue levels of ERα result in a 4–6 fold increase in F4/80 expression in both males and females over control injected pads, and this is associated with an up-regulation of SAA3, TNFα, and TLR4 (Figure 2G). To test the effects of reduced adipose tissue ERα on fibrosis, we analyzed gene expression levels of COL6 and LOX. Consistent with αERKO, adipose tissue specific reductions in ERα increase expression of LOX; however only a slight trend was observed in COL6 levels (Figure 2H). Together these data suggest that decreased adipose tissue ERα results in fibrotic and inflamed adipose tissue associated with elevated macrophage expression, consistent with unhealthy adipose tissue, in both males and females.
Figure 2.
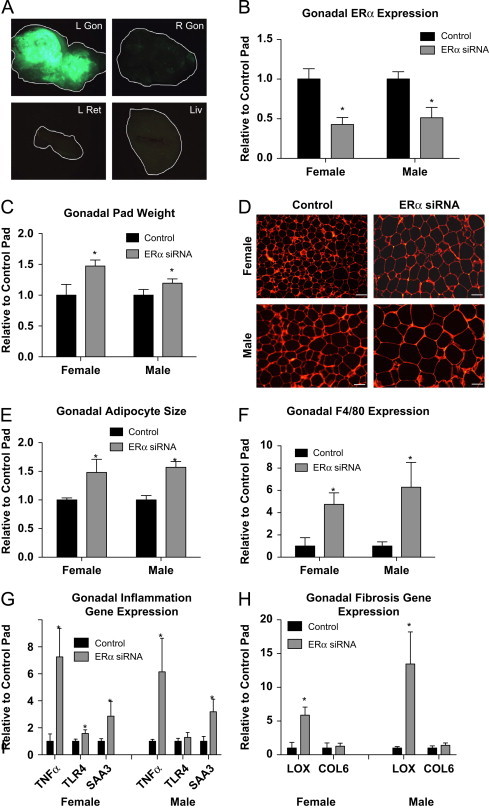
Viral mediated knockdown of ERα in visceral adipose tissue regulates adipocyte size and gene function. Injection methods were determined by using the average surface area and shape of the gonadal adipose tissue. The right gonadal pad that was injected with the control virus is set to 1, and data are normalized to the control-injected pad. (A) Representative images of green fluorescent protein (GFP) in the pad injected with the AAVsiRNA ERα demonstrate that only in the AAVsiRNA ERα GFP-injected pad (L Gon) is GFP visible (the tissue from each view has been outlined for reference), whereas the fat pad injected with the control AAVsiRNA/non-GFP virus is not. Representative photomicrographs of H&E staining of the Gonadal control and siRNA pad from both the males and females at 3 weeks post-injection (n=9). (B) Messenger RNA levels of ERα were quantified using qPCR in whole adipose tissue at 3 weeks post-injection of both males and females (n=9). (C) Gonadal pad weights of both the control and the ERα siRNA of both males and females were weighed at the time of sacrifice at 3 weeks post-injection (n=9). (D) Representative photomicrographs of H&E staining of gonadal WAT from male and female mice. (E) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue (n=9). (F) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue at 3 weeks post-injection of both male and female mice (n=9). (G and H) Messenger RNA levels of indicated genes were quantified using qPCR in whole adipose tissue at 3 weeks post-injection of both male and female mice (n=9). Data are presented as mean±SEM, and *P<0.05 between the ERα siRNA injected pad and the pad injected with the control virus.
3.3. Adipocyte specific knockdown of ERα by generation of an ERα/Adiponectin Cre mouse
To determine the specific effect of ERα on adipocytes, we generated mice with adipocyte-specific knockdown of ERα (AdipoERα−ERαlox/lox+AdipoCRE) and control littermates (WT-ERαlox/lox). With this model, we demonstrate about ∼60% knockdown of ERα in both male and female AdipoERα mice as compared to their WT littermates (Figure 3A). As previously indicated, females have higher ERα expression in the visceral depot than do males [10], and based on our previous work we know that knockdown of ERα from adipose tissue in females results in adipose tissue that is more ‘male-like’. Here, we specifically chose an AdipoCRE line that results in downregulation of ERα expression in females to levels similar to those measured in males (Figure 3A–shown by comparing AdipoERα female to male WT levels). Adipocyte-specific ERα gene expression was determined by RNA expression of collagenase-mediated isolation of adipocytes from the stromal vascular fraction. Consistently, representative gonadal adipose tissue sections stained for ERα demonstrate significantly fewer ERα immunoreactive adipocytes in the AdipoERα when compared to their littermate WT controls (Figure 3B). By generating mice with a ‘physiological’ knockdown of ERα we are able to metabolically determine the functional role of ERα specifically in adipocytes.
Figure 3.
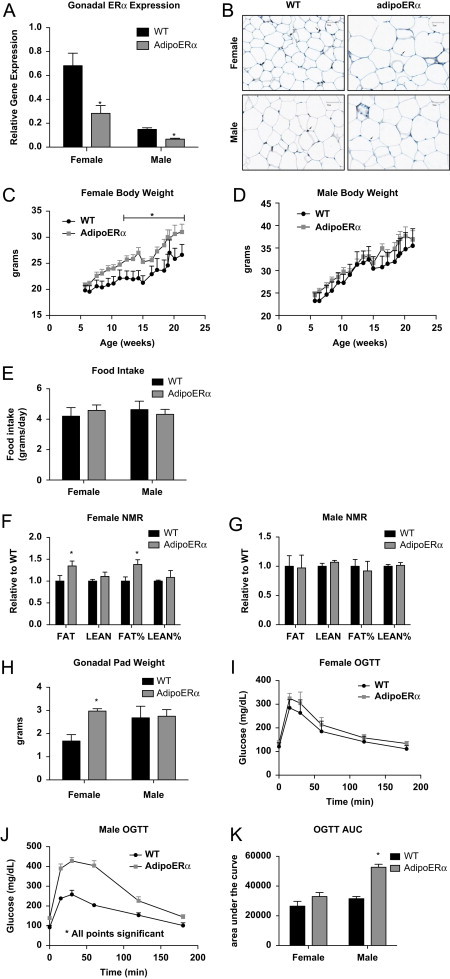
Adipocyte ERα regulates body weight adipocyte distribution. (A) Messenger RNA levels of ERα were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow fed females and males (n=10/genotype). (B) Immunohistochemistry for ERα from male and female gonadal adipose tissue from WT and AdipoERα mice demonstrating significantly fewer ERα immunoreactive positive adipocytes in the AdipoERα mice relative to WT. Arrows point to ERα positive adipocyte nuclei, whereas the arrowhead demonstrates non-adipocyte nuclei positively stained for ERα demonstrating efficacy of the staining. (C and D) Weekly body weight was measured in singly housed female and male mice on normal chow (n=10/genotype). (E) Weekly food intake was measured in singly housed female and male mice on normal chow (n=6/genotype). (F and G) Body composition was measured by NMR in 15-week-old male and female mice (n=10/genotype). (H) Gonadal fat pad weights in 18 week old females and males (n=10/genotype). (I and J) Oral glucose tolerance tests (OGTT) were performed in 15-week-old females and males (n=10/genotype). (K) Area under the curve (AUC) from the OGTT's was calculated. Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice.
Male and female AdipoERα are born in normal Mendelian ratio and are viable. At weaning, WT and AdipoERα males and females do not differ in body weight or circulating estradiol 17β levels (mice were sacrificed at 4 weeks of age in the proestrus stage of their cycle for comparison; WT 52.7±8.2 vs AdipoERα 48.67±5.9 pg/mL estradiol 17β however, at about 12 weeks of age, AdipoERα females begin to gain more weight when compared to WT females (Figure 3C). Male WT and AdipoERα mice do not differ in body weight through 25+ weeks of age (Figure 3D), or testosterone levels (WT 55.4±12.8 vs AdipoERα 57.49±14.9 pg/mL). Despite the findings that female AdipoERα mice weigh more, food intake does not differ between genotypes of either sex (Figure 3E). Nuclear magnetic resonance (NMR) imaging demonstrates that the increased body weight in AdipoERα females is due to increased fat mass, observed both in measurements of total grams of fat mass as well as percent of body weight (Figure 3F) while AdipoERα males do not differ significantly in any measure of body composition (Figure 3G). Moreover, female AdipoERα mice have an almost 2-fold increase in gonadal adipose tissue weight as compared to WT littermates (Figure 3H), equaling gonadal adipose tissue weights observed in males. These data suggest that adipocyte-specific knockdown of ERα does not alter body adiposity in males, dissimilar to knocking down ERα in the whole body or selectively in the adipose tissue depot (Figures 1D and 2C). In females however, reduced adipocyte ERα results in an increased fat mass, an increase that is largely due to increased gonadal adipose tissue, correlating to whole adipose body and tissue-selective knock down of ERα (Figures 1C and 2C).
In order to determine the metabolic consequences of reduced adipocyte ERα, oral glucose tolerance tests (OGTT) were performed on male and female WT and AdipoERα mice. In the females, we observe no statistical difference in glucose clearance with an OGTT (Figure 3I). This was surprising given their substantial increase in gonadal adipose tissue mass. The area-under-the-curve calculations demonstrate a slight impairment in glucose disposal in the female AdipoERα mice (Figure 3K); however, these differences do not attain statistical significance. More surprisingly the male AdipoERα mice, which do not differ in body weight or adiposity, show a profound compromise in glucose clearance (Figure 3J), as demonstrated by a near doubling in the area-under-the-curve measurement for AdipoERα males as compared to WT males (Figure 3K). These data suggest that, despite no changes in adiposity, reduced adipocyte ERα in males results in a profound metabolic compromise, further suggesting that reductions of ERα to near ablation levels result in unhealthy adipose tissue. AdipoERα females are able to maintain glucose clearance even though they have male-patterned adiposity.
Expansion of adipose tissues can occur through increased triglyceride storage as previously discussed, thus we analyzed adipocyte size with H&E staining. Our data demonstrate that both male and female AdipoERα mice have enlarged gonadal adipocytes as compared to their WT littermates (Figure 4A) and calculation of average adipocyte area further demonstrates this (Figure 4B). AdipoERα males have an approximate doubling in average adipocyte size, while female AdipoERα mice have 3 times larger gonadal adipocytes as compared to their WT littermates. Males have significantly larger gonadal adipocytes than females; however, our data demonstrate that when ERα levels in a female are reduced to male levels, the resulting adipocyte size is far larger than that observed in WT males.
Figure 4.
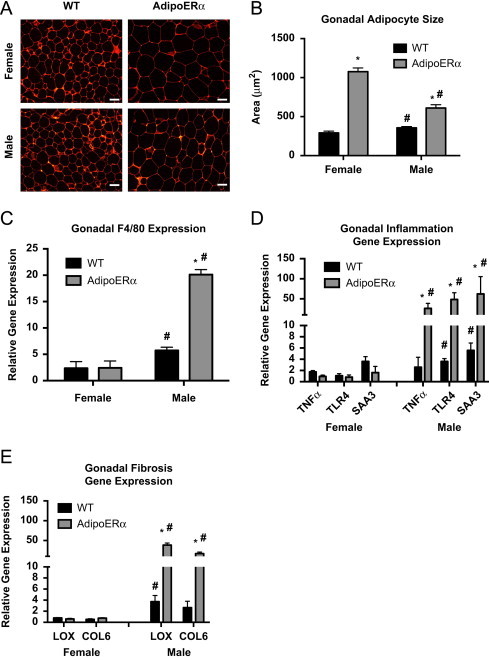
Adipocyte ERα regulates adipocyte size and gene function. (A) Representative photomicrographs of H&E staining of gonadal WAT from 18-week-old male and female mice. (B) Analysis of adipocyte cell size in both male and female of gonadal adipose tissue from 18-week-old mice (n=10/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 18-week-old chow-fed females and males (n=10/genotype). (D) Messenger RNA levels of inflammatory genes were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow-fed females and males (n=10/genotype). (E) Messenger RNA levels of fibrosis genes were quantified using qPCR in collagenase-isolated adipocytes from 18-week-old chow-fed females and males (n=10/genotype). Data are presented as mean±SEM, *P<0.05 between WT and AdipoERα mice, and ♯P<0.05 between male and female mice of like genotype.
To assess the macrophage content of adipose tissues in male and female WT and AdipoERα mice, F4/80 was measured (Figure 4C). These data demonstrate that even though the female AdipoERα mice have enlarged adipocytes, the macrophage content of their gonadal adipose tissue does not differ from their WT littermates. In contrast, AdipoERα males have much higher levels of macrophage infiltration, which correlates with enlarged unhealthy adipocytes.
To further measure the level of adipocyte inflammation, we isolated adipocytes, extracted RNA and performed qPCR for known inflammatory genes. Similar to the F4/80 data, there is no marked increase in inflammatory gene expression in female AdipoERα mice as compared to WT (Figure 4D). However, AdipoERα males show a substantial increase in inflammatory gene expression as compared to male WT levels. Similar to the inflammatory data, AdipoERα males show significant increases in expression of both LOX and COL6, while females show no apparent increase (Figure 4E).
Thus, these data together suggest that reduction of ERα levels in females to that of males results in male-like patterning in adiposity, without the metabolic complications of visceral adipose tissue deposition. By contrast, males do not have any alterations in adipose tissue distribution when adipocyte ERα levels are reduced; however there are derangements in glucose homeostasis accompanied by elevated adipose tissue inflammation and fibrosis. These data demonstrate a sexual dimorphism with respect to the metabolic effects of gonadal adiposity and adipocyte ERα expression.
3.4. Adipocyte ERα is critical for the beneficial estrogenic effects on adipose tissue and metabolism in females
Despite having similar expression levels of ERα, AdipoERα females do not display the same metabolic consequences as WT males. AdipoERα females maintain normal ovarian production of estrogens and this may contribute to metabolic stability even in the absence of adipocyte ERα and the associated increase in gonadal adiposity. AdipoERα females cycle normally (data not shown); therefore, it could be hypothesized that the differential effects of adipocyte ERα in males and females could be levels of circulating estrogens. In order to determine the role of adipocyte ERα in the capacity of circulating estrogens to regulate body adiposity and metabolic homeostasis, female WT and AdipoERα mice were ovariectomized (OVX) at 15 weeks of age and implanted with a subcutaneous pellet of estradiol-17β or vehicle control (VEH). Post-surgical body weight demonstrates that OVX without supplementation of exogenous estrogens results in significant body weight gain in WT mice and with the addition of estradiol-17β, the OVX-induced weight gain does not occur (Figure 5A). In the AdipoERα group, there is little to no observed estradiol-17β induced reduction in body weight gain (Figure 5B). Even more striking, the AdipoERα group that received VEH does not show the OVX-induced body weight gain, as seen in the WT group. Food intake data demonstrate that OVX WT VEH females eat significantly more as a per day average than WT mice receiving estradiol-17β (Figure 5C); however this estrogenic reduction in food intake is not observed in the AdipoERα cohort. Further, OVX AdipoERα females eat significantly less than OVX WT females. OVX WT females treated with estradiol-17β have decreased fat mass as compared to those treated with VEH (Figure 5D), while AdipoERα females show no effect of exogenous estrogens on body composition (Figure 5E). To this end, the weight of gonadal adipose tissues is decreased with estradiol-17β administration in WT mice; however no such decrease is observed in AdipoERα mice when treated with exogenous estrogens (Figure 5F). Furthermore, our data demonstrate that the estrogenic reduction on body adiposity that occurs in WT females is dependent on adipocyte ERα. Moreover, the OVX-induced weight gain is also dependent on adipocyte ERα, as OVX does not induce weight gain in AdipoERα females, thus suggesting that in the absence of circulating estrogens, adipocyte ERα may contribute to adipose tissue expansion.
Figure 5.
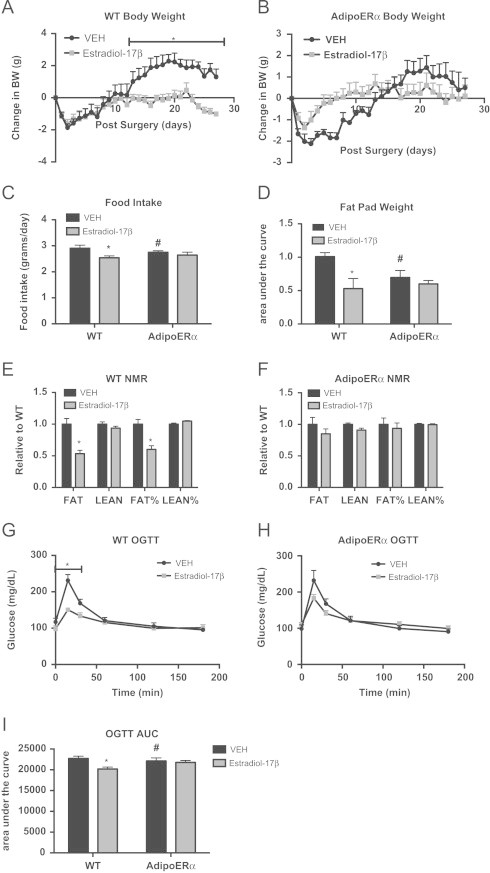
Adipocyte ERα regulates the capacity of estradiol-17β to modulate body weight and adipose tissue distribution. All females were OVX and administered VEH or estradiol-17β at 12 weeks and all post-sacrifice measurements were taken in 18-week-old mice, 42 days post-OVX (n=8/genotype). (A and B) Daily body weight was measured in singly housed female mice post-OVX on normal chow (n=8/genotype). (C) Daily food intake was measured in singly housed female mice post-OVX on normal chow (n=8/genotype). (D and E) Body composition was in 30-day post-OVX chow fed females (n=8/genotype). (F) At the time of sacrifice (42 days post-OVX), bilateral gonadal fat pads were weighed. Data presented as a fold change over VEH of pad weight/mouse body weight. (G and H) Oral glucose tolerance test (OGTT) was performed in 16-week-old females (n=8/genotype), 30 days post-OVX. (I) Area under the curve (AUC) was calculated for the OGTTs. Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice and # P<0.05 between VEH and 17β-estradiol.
In order to better understand the role of adipocyte ERα in the estrogenic regulation of metabolic homeostasis, WT and AdipoERα mice treated with VEH or estradiol-17β were administered an OGTT. Data show that while exogenous estrogen improves glucose tolerance in WT OVX females (Figure 5G), it has little discernible effect in AdipoERα OVX females (Figure 5H). This is further demonstrated with area under the curve calculations; estradiol-17β administration significantly reduces the area under the curve in WT OVX mice, however has no significant effect on the glucose curves in AdipoERα OVX females (Figure 5I).
Other data presented here demonstrate that in males and females, adipocyte ERα functions to maintain adipocyte size and lower levels of ERα results in enlarged adipocytes. To this end, OVX of WT female mice results in enlarged adipocytes and addition of exogenous estrogens results in a significant reduction in adipocyte area (Figure 6A and B), which is consistent with previous reports [37]. While there is a slight trend for a reduction in adipocyte area with estradiol-17β treatment of AdipoERα OVX females, it does not attain significance. Interestingly, gonadal adipocytes from AdipoERα OVX mice treated with VEH are significantly smaller than those from WT OVX treated with VEH, thus suggesting that in the absence of exogenous estrogens, adipocyte ERα acts adversely and enhances adipocyte size. Similar to trends in previous data, F4/80 expression is drastically decreased in AdipoERα OVX treated with VEH as compared to WT OVX treated with VEH, again suggesting that ERα, in the absence of circulating estrogens, may have unfavorable effects on adipose tissue macrophage infiltration (Figure 6C). Administration of exogenous estradiol-17β to WT OVX results in a decrease in the expression of the pro-inflammatory genes TNFα, TLR4 and SAA3 (Figure 6D); however, in AdipoERα OVX the addition of estradiol-17β actually enhances expression of both TLR4 and SAA3 (there is no observable effect on TNFα expression). Markers of adipose tissue fibrosis, LOX and COL6, both decrease with administration of estradiol-17β in WT OVX females; however no decrease is observed in AdipoERα females (Figure 6E). Interestingly, there is no improvement in fibrotic gene expression in AdipoERα OVX females over WT OVX females treated with VEH, as observed in other measures of adipocyte dysfunction. Taken together, these data suggest that adipocyte ERα is critical for the estrogen-induced improvements in adipocyte metabolism. In the absence of circulating estrogens, our data suggest that reduced adipocyte ERα expression helps counter the OVX-induced adipose tissue inflammation and fibrosis, thus improving metabolic homeostasis.
Figure 6.
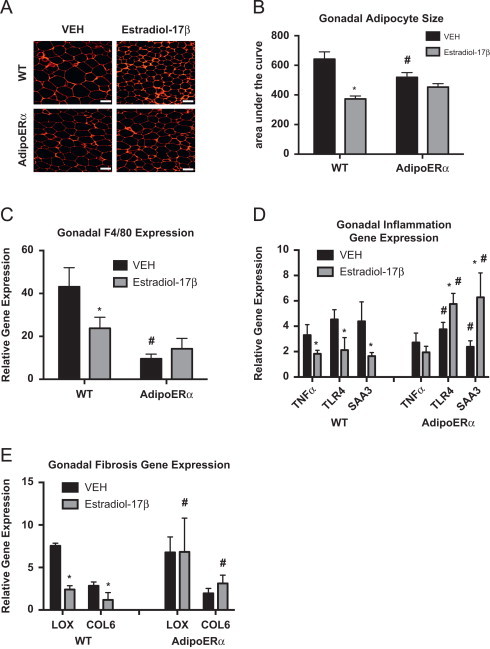
Adipocyte ERα regulates the capacity of estradiol-17β to modulate adipose tissue function, adipocyte size and glucose homeostasis. All females were OVX and administered VEH or estradiol-17β at 12 weeks and all post-sacrifice measurements were taken in 18-week-old mice, 42 days post-OVX (n=8/genotype). (A) Representative photomicrographs of H&E staining of gonadal WAT. (B) Analysis of adipocyte cell size in gonadal WAT from 18-week-old mice, 42 days post-OVX (n=8/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR in whole adipose tissue from 18-week-old chow-fed females (n=8/genotype). (D and E) Messenger RNA levels of indicated genes were quantified using qPCR in Collagenase-isolated from 18-week-old chow fed females (n=8/genotype). Data are presented as mean±SEM, and *P<0.05 between WT and AdipoERα mice and ♯P<0.05 between VEH and 17β-estradiol.
3.5. Adipocyte-specific knockdown of ERα in ERβ null mice (AdipoERα/βERKO)
Estrogen receptor beta (ERβ) is also expressed in adipose tissue [20] and following adipocyte specific knockdown of ERα there is a significant increase in the expression of ERβ in both females and males (Figure 7A). Therefore, to understand the contribution of ERβ on the outcomes of adipocyte-specific ERα knockdown, we generated mice with adipocyte-specific knockdown of ERα (AdipoERα) in the βERKO background (AdipoERα/βERKO). Similar to our previous results, we demonstrate about ∼50% knockdown of ERα (Figure 7B) and 100% knockdown of ERβ (Figure 7C) by RNA expression of collagenase-mediated isolation of adipocytes in both male and female AdipoERα/βERKO mice as compared to their WT littermates.
Figure 7.
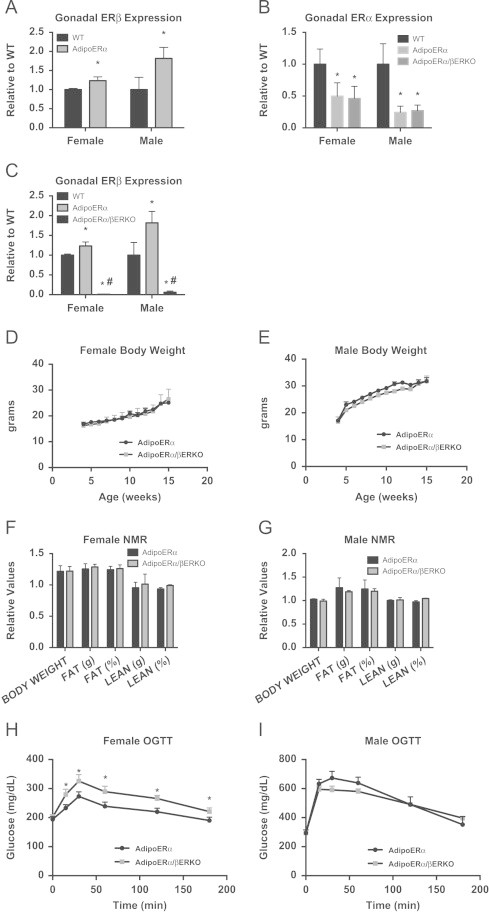
Contribution of ERβ to the AdipoERα phenotype. (A) ERβ is upregulated in gonadal adipose tissue in male and female AdipoERα mice (n=9/genotype, 18-week old at sacrifice). (B) ERα mRNA from isolated adipocytes is reduced to similar levels in AdipoERα and AdipoERα/βERKO mice (n=9/genotype). (C) ERβ mRNA from isolated adipocytes is reduced to similar levels in AdipoERα and AdipoERα/βERKO mice (n=9–10/genotype). (D and E) Weekly body weight was measured in AdipoERα and AdipoERα/βERKO singly housed female and male mice on normal chow (n=6/genotype). (F and G) Body composition was measured by NMR in 15-week-old AdipoERα and AdipoERα/βERKO male and female mice (n=9–10/genotype). (H and I) Oral glucose tolerance tests (OGTT) were performed in 15-week-old AdipoERα and AdipoERα/βERKO females and males (n=9–10/genotype).
Male and female AdipoERα/βERKO are born in normal Mendelian ratio and are viable. At weaning, AdipoERα and AdipoERα/βERKO males and females do not differ in body weight (Figure 7D and E) or Estradiol 17β levels (mice sacrificed at 12 weeks of age in the proestrus stage of their cycle for comparison; AdipoERα 49.6±6.9 pg/mL vs AdipoERα/βERKO 54.5±5.9). NMR imaging demonstrates that both AdipoERα/βERKO males and females do not differ from AdipoERα of comparable sex on any measure of body composition (Figure 7F and G). These data suggest that lack of both ERβ and adipocyte ERα does not enhance the already existing body weight phenotype of the AdipoERα mice.
In order to determine if there are additional metabolic consequences of reduced adipocyte ERα and ERβ, oral glucose tolerance tests (OGTT) were performed on male and female WT and AdipoERα/βERKO mice (cycle day was determined at the time of the test, but did not factor into the results). Here, we find AdipoERα/βERKO females had reduced glucose clearance when compared to the AdipoERα females (Figure 7H). For the males, we do not see any difference from the already impaired glucose clearance previously observed in the AdipoERα (Figure 7I). We found that the gonadal adipocytes in both male and female AdipoERα/βERKO mice are similar to those of AdipoERα mice (Figure 8A and B). Consistent with the changes in glucose clearance, we found increases in F4/80 (Figure 8C), markers of inflammation (Figure 8D), and fibrosis (Figure 8E) in both sexes when compared to the AdipoERα indicating that in the absence of adipocyte ERα, ERβ plays a protective role in adipocyte fibrosis and inflammation.
Figure 8.
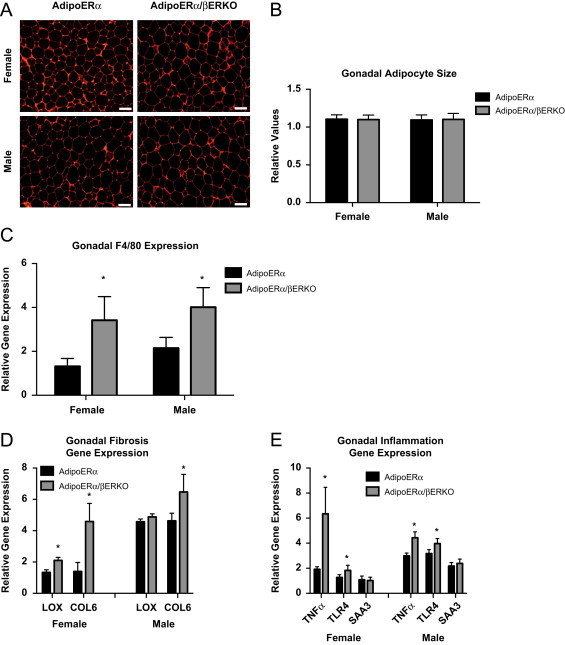
(A) Representative photomicrographs of H&E staining of VISC WAT from 18-week-old male and female mice. (B) Analysis of adipocyte cell size in both AdipoERα and AdipoERα/βERKO male and female of gonadal adipose tissue from 18-week-old mice (n=9–10/genotype). (C) Messenger RNA levels of F4/80 were quantified using qPCR whole adipose tissue from 18-week-old AdipoERα and AdipoERα/βERKO chow-fed females and males (n=9–10/genotype). (D and E) Messenger RNA levels were quantified using qPCR of whole adipose tissue from 18-week-old AdipoERα and AdipoERα/βERKO chow-fed females and males (n=9–10/genotype). Data are presented as mean±SEM, and *P<0.05 between AdipoERα and AdipoERα/βERKO.
4. Discussion
Here we demonstrate the critical role of adipose tissue and adipocyte ERα in regulating adipocyte size, fibrosis, and inflammation. ERα is well established to regulate metabolic homeostasis and the ERα whole body knockout displays obesity and metabolic compromise [29]. Here we extend those findings to indicate they also have inflamed and fibrotic adipose tissue independent of differences in body weight. Our data demonstrate that knockdown of ERα in gonadal adipose tissues results in an enlarged depot with larger adipocytes and with increased expression of a marker of macrophages, local inflammation, and fibrosis. Altering ERα levels in total gonadal adipose tissues has similar effects in males and females. These data demonstrate that many adipose tissue effects observed in the total body ERα knockout can be attributed to lack of ERα selectively in adipose tissues. We further demonstrate that adipocyte ERα per se appears to be critical in regulating adipocyte size, inflammation, and fibrosis. We demonstrate this by using mice with selective knockdown of ERα in adipocytes, which results in significantly increased fibrosis and inflammation selectively in the males. Lastly, our data suggest that in the absence of adipocyte ERα, ERβ provides a protective role in adipose tissue inflammation and fibrosis. Our data for the first time begin to delineate the roles of ERα and ERβ in modulating adipose tissues.
4.1. αERKO mice have enlarged adipocytes with increased inflammation and fibrosis in both males and females
It has previously been demonstrated by Cooke et al. [30] that αERKO mice have enlarged fat cells relative to WT/control mice. Here we confirm these data in weight-matched male and female mice, and extend these findings to demonstrate increased levels of inflammation and fibrosis in adipose tissue.
4.2. Viral-mediated knockdown of ERα in visceral adipose tissue results in enlarged adipocytes with increased inflammation and fibrosis in males and females
In order to address the role of ERα in individual adipose depots, without the complications of changing the metabolic or hormonal profile in vivo, our lab has developed a novel technique with which we can site specifically modulate gene expression in a fat pad specific way using adeno-associated viral (AAV) technology. We demonstrate for the first time that gonadal fat pad specific knockdown of ERα results in increased adipose tissue weight, adipocyte size, and increased markers of inflammation and fibrosis. The purpose of this experiment is to demonstrate, regardless of the hormonal milieu, that fat pad specific knockdown of ERα has a profound effect. This experimental paradigm was not conducted to determine the metabolic perturbations associated with fat pad knockdown of ERα, but rather to probe/interrogate the importance of gonadal adipose tissue ERα per se.
4.3. Adipocyte-specific ERα regulates body adiposity, adipocyte inflammation, fibrosis and systemic glucose tolerance in a sexually dimorphic way
Adipose tissues are comprised of many different cell types, including adipocytes, inflammatory cells and preadipocytes. In order to understand the specific role of adipocyte ERα we used the adipocyte-specific Adiponectin-CRE crossed to the ERα-floxed mouse, thus altering ERα only in adipocytes. We chose to work with mouse lines where adipocyte ERα in female mice is reduced to levels seen in males. Our data demonstrate that reductions in adipocyte ERα levels result in increased body weight, adipose tissue mass, and adipocyte size in females. This increased adiposity, however, does not result in altered metabolic homeostasis, adipose tissue inflammation or fibrosis. Contrary to females, reduced adipocyte ERα in males does not alter body weight or adiposity; however, adipocyte size is enlarged and accompanied by increased adipose tissue inflammation and fibrosis. Furthermore, glucose tolerance is blunted in AdipoERα males, but not in females.
Despite enlarged adipose tissues, AdipoERα females remain protected from the obesity-associated co-morbidities seen in males. While males have increased levels of macrophage infiltration and elevated adipocyte inflammation and fibrosis, AdipoERα females display none of these characteristics. Interestingly, knockdown of whole adipose depot ERα in females results in an upregulation of macrophage infiltration, adipocyte inflammation and fibrosis, similar to male levels. Together these data suggest other adipose tissue cell types, and the ERα levels within those cell types regulate the obesity-associated co-morbidities in females. A recently published study demonstrates that knockout of macrophage ERα expression in females results in obesity and metabolic complications [38]. The discontinuity between the data in females with total depot knockdown and adipocyte-specific knockdown could be the fact that, unlike in the depot-specific knockdown, the macrophages in the AdipoERα females retain their ERα levels, thus reducing the inflammatory state. In addition, AdipoERα females do not have altered glucose clearance, while reducing adipocyte ERα in males results in profound metabolic compromise. This could be in part due to changes in adipocyte metabolism and inflammatory state. Alternatively, estrogen signaling in the brain and other peripheral tissues may compensate to maintain systemic metabolic homeostasis in females.
Given this possibility, we investigated the role of adipocyte ERα in the ability of estrogens to regulate both adipose tissue and systemic metabolism. Circulating estrogens are known to influence adipose tissue deposition and whole body metabolic homeostasis [19,22]. Additionally, the contribution of the several-fold increase in circulating E2 levels in αERKO females [39] has been questioned as to its contribution of obesity/adiposity phenotype. Specifically, circulating estrogens in the absence of functional ERα could have adverse effects on metabolic homeostasis. Our data demonstrate that OVX WT females respond metabolically to exogenous estrogens in a beneficial way, to include decreased adiposity and adipose tissue inflammation and fibrosis and improved glucose tolerance which may be mediated peripherally as well as centrally. OVX AdipoERα mice, however, seem largely unresponsive to estrogens with respect to body weight and adiposity. Moreover, the addition of estradiol-17β to AdipoERα females results in increased fibrosis and inflammation, thus suggesting that estrogens can have a negative impact locally on adipose tissue with reduced levels of adipoctye ERα expression. As the OVX AdipoERα females express ERα to levels seen in males, we compared levels of adiposity and adipocyte gene expression between the two groups. Even with little circulating estrogens and low adipocyte ERα expression, OVX AdipoERα females retain better glucose clearance than did males. Similar to results described above, OVX AdipoERα females have far lower adipose tissue macrophage infiltration than WT males. Measures of adipocyte size as well as inflammation and fibrosis, however, seem on par with levels in male adipocytes. These data provide further support that the levels of adipocyte ERα are critical in determining adipose tissue deposition and adipocyte size. However, once circulating estrogens (as seen in the intact AdipoERα females) are removed, adipocyte dysfunction appears.
Another intriguing observation is the apparent improvement of the OVX AdipoERα treated with VEH over the OVX WT treated with VEH. These data suggest that in the absence of circulating estrogens, adipocyte ERα has antagonistic actions with respect to adiposity and adipocyte function. Even more, this is also seen in the OGTT AUC, where there is a slight but significant improvement in the OVX AdipoERα VEH over OVX WT VEH. The estrogen-induced pathways that regulate metabolic function are not completely understood and it is likely that, in the absence of sufficient ligand, ERα may bind to alternative estrogen response elements (EREs) or other factors critical to adipocyte function and induce metabolically detrimental effects. In addition, ERα could also associate with an alternative group of signaling factors in the absence of ligand, thus inducing a differential signaling program. Either way these data demonstrate that the presence of ligand or receptor alone can be detrimental to metabolic function.
4.4. Adipocyte-specific ERα/βERKO regulates body adiposity, adipocyte inflammation, fibrosis and systemic glucose tolerance in a sexually dimorphic way
The specific function of ERα in adipose tissue has previously been unknown. Additionally, the ratio of ERα/ERβ could be important with respect to adipose tissue function. The absence of heterodimer formation by ERα and ERβ [40] or other putative estrogen receptors [41] in total body αERKO mice could play a role in the development of the obese/adipose tissue phenotype. Interestingly, the double knockout of ERα and ERβ does not seem to have metabolic effects over that of the ERα knockout alone [42], suggesting that ERβ functions may require the presence of ERα. In our AdipoERα model, adipocyte ERα is only downregulated ∼60% and metabolically does not have a huge impact in females. It is possible that ERβ expression plays a protective role in the state of reduced ERα through enhanced estrogen signaling through ERβ or other estrogen receptors [41] thereby mediating the effects on WAT. Therefore, to test the contribution of ERβ to the AdipoERα phenotype, we crossed the AdipoERα mouse to the ERβ total body knockout, creating a mouse that lacks ERβ in adipose tissue with reduced levels of adipocyte ERα. Our findings suggest that in the absence of adipocyte ERα, ERβ plays a protective role in suppressing inflammation and fibrosis in the females. However, in the absence of both ERα and ERβ there is a significant increase in adipose tissue markers of fibrosis, inflammation, macrophage infiltration in both sexes. Interestingly, knocking out ERβ in female AdipoERα mice reduces glucose clearance while in males this effect is not above and beyond that of the AdipoERα male mice. Our data presented here begin to define the roles of ERα and ERβ in modulating adipose tissue function. Additionally, our data suggest that ERβ may serve a protective role in states of reduced ERα expression.
5. Conclusions
We demonstrate for the first time that adipose tissue ERα regulates body fat distribution, adipose tissue inflammation, and fibrosis. Our data further suggest that adipocyte ERα is required for many of the metabolically beneficial effects of estrogens on metabolism. In males, extremely low levels of adipocyte ERα result in significant increases in adipose tissue fibrosis, with systemic (data not shown) as well as adipose tissue inflammation, which results in metabolic dysfunction (Figure 9). AdipoERα females retain modest levels of both adipose tissue ERα and ERβ which appears to facilitate adipose tissue expansion (as indicated by increased adipocyte size), reductions in markers of fibrosis, reductions in markers of systemic (data not shown) as well as adipose tissue inflammation, and increased adiposity. Therefore, our data are consistent with recent research focusing on inflammation and the metabolic syndrome. Importantly, our data suggest that the expandability of the fat cell suppresses adipose tissue and systemic inflammation and is ‘protective’ for the metabolic syndrome and that both ERα and ERβ play critical roles in modulating the expandability of adipocytes. Together, our studies demonstrate for the first time the effects of adipocyte-specific ERα in males and females, and the requirement of adipocyte ERα for estrogen-mediated metabolic effects.
Figure 9.
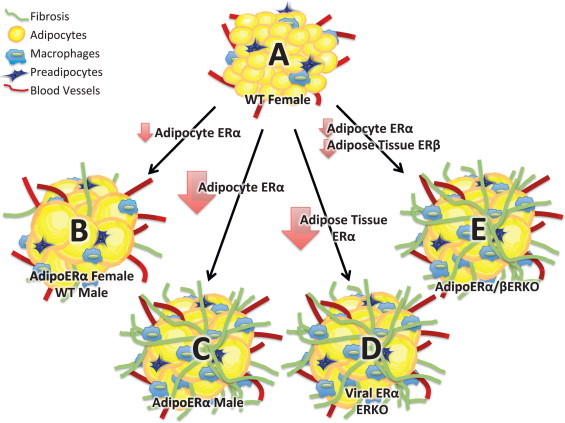
Cartoon depiction of our results. (A) ‘Healthy’ female adipose tissue represented by small (expandable) adipocytes (that contain ERα), low levels of inflammation and fibrosis. (B) Reductions in adipocyte ERα as seen in males (relative to females) and AdipoERα females result in larger (expandable) adipocytes with low levels of inflammation and fibrosis. (C) Knockout of ERα (as seen in AdipoERα males) results in enlarged adipocytes that lack the ability to expand further due to increased levels of inflammation and fibrosis. (D) Knockout of ERα from total adipose tissues results in enlarged adipocytes that lack the ability to expand further due to increased levels of inflammation and fibrosis. (E) Reductions in adipocyte ERα and knockout of adipose tissue ERβ result in adipocytes that lack the ability to expand due to increased levels of inflammation and fibrosis.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Despres J.P. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9(5):452–459. [PubMed] [Google Scholar]
- 2.Kannel W.B. Regional obesity and risk of cardiovascular disease; the Framingham Study. Journal of Clinical Epidemiology. 1991;44(2):183–190. doi: 10.1016/0895-4356(91)90265-b. [DOI] [PubMed] [Google Scholar]
- 3.Lee C.G. Adipokines, inflammation, and visceral adiposity across the menopausal transition: a prospective study. Journal of Clinical Endocrinology and Metabolism. 2009;94(4):1104–1110. doi: 10.1210/jc.2008-0701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dua A. Leptin: a significant indicator of total body fat but not of visceral fat and insulin insensitivity in African-American women. Diabetes. 1996;45(11):1635–1637. doi: 10.2337/diab.45.11.1635. [DOI] [PubMed] [Google Scholar]
- 5.Kotani K. Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. International Journal of Obesity and Related Metabolic Disorders. 1994;18(4) 207–2. [PubMed] [Google Scholar]
- 6.Legato M.J. Gender-specific aspects of obesity. International Journal of Fertility and Women's Medicine. 1997;42(3):184–197. [PubMed] [Google Scholar]
- 7.Legato M.J. Gender-specific physiology: how real is it? How important is it? International Journal of Fertility and Women's Medicine. 1997;42(1):19–29. [PubMed] [Google Scholar]
- 8.Havel P.J. Gender differences in plasma leptin concentrations. Nature Medicine. 1996;2(9):949–950. doi: 10.1038/nm0996-949b. [DOI] [PubMed] [Google Scholar]
- 9.Payette C. Sex differences in postprandial plasma tumor necrosis factor-alpha, interleukin-6, and C-reactive protein concentrations. Metabolism. 2009;58(11):1593–1601. doi: 10.1016/j.metabol.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Grove K.L. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. International Journal of Obesity (London) 2010;34(6):989–1000. doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Macotela Y. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4):803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider G. Increased estrogen production in obese men. Journal of Clinical Endocrinology and Metabolism. 1979;48(4):633–638. doi: 10.1210/jcem-48-4-633. [DOI] [PubMed] [Google Scholar]
- 13.Tchernof A. Relation of steroid hormones to glucose tolerance and plasma insulin levels in men. Importance of visceral adipose tissue. Diabetes Care. 1995;18(3):292–299. doi: 10.2337/diacare.18.3.292. [DOI] [PubMed] [Google Scholar]
- 14.Gambacciani M. Body weight, body fat distribution, and hormonal replacement therapy in early postmenopausal women. Journal of Clinical Endocrinology and Metabolism. 1997;82(2):414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- 15.Haarbo J., Hansen B.F., Christiansen C. Hormone replacement therapy prevents coronary artery disease in ovariectomized cholesterol-fed rabbits. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1991;99(8):721–727. doi: 10.1111/j.1699-0463.1991.tb01250.x. [DOI] [PubMed] [Google Scholar]
- 16.Haarbo J. Postmenopausal hormone replacement therapy prevents central distribution of body fat after menopause. Metabolism. 1991;40(12):1323–1326. doi: 10.1016/0026-0495(91)90037-w. [DOI] [PubMed] [Google Scholar]
- 17.D'Eon T.M. Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. Journal of Biological Chemistry. 2005;280(43):35983–35991. doi: 10.1074/jbc.M507339200. [DOI] [PubMed] [Google Scholar]
- 18.Matelski H. Randomized trial of estrogen vs. tamoxifen therapy for advanced breast cancer. American Journal of Clinical Oncology. 1985;8(2):128–133. doi: 10.1097/00000421-198504000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Clegg D.J. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55(4):978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 20.Crandall D.L. Identification of estrogen receptor beta RNA in human breast and abdominal subcutaneous adipose tissue. Biochemical and Biophysical Research Communications. 1998;248(3):523–526. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- 21.Kuiper G.G. Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(12):5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribas V. Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. American Journal of Physiology–Endocrinology and Metabolism. 2010;298(2):E304–E319. doi: 10.1152/ajpendo.00504.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rogers N.H. Reduced energy expenditure and increased inflammation are early events in the development of ovariectomy-induced obesity. Endocrinology. 2009;150(5):2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mizutani T. Identification of estrogen receptor in human adipose tissue and adipocytes. Journal of Clinical Endocrinology and Metabolism. 1994;78(4):950–954. doi: 10.1210/jcem.78.4.8157726. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen S.B. Identification of steroid receptors in human adipose tissue. European Journal of Clinical Investigation. 1996;26(12):1051–1056. doi: 10.1046/j.1365-2362.1996.380603.x. [DOI] [PubMed] [Google Scholar]
- 26.Bronnegard M. Lack of evidence for estrogen and progesterone receptors in human adipose tissue. Journal of Steroid Biochemistry and Molecular Biology. 1994;51(5–6):275–281. doi: 10.1016/0960-0760(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 27.Rebuffe-Scrive M. Steroid hormone receptors in human adipose tissues. Journal of Clinical Endocrinology and Metabolism. 1990;71(5):1215–1219. doi: 10.1210/jcem-71-5-1215. [DOI] [PubMed] [Google Scholar]
- 28.Okura T. Association of polymorphisms in the estrogen receptor alpha gene with body fat distribution. International Journal of Obesity and Related Metabolic Disorders. 2003;27(9):1020–1027. doi: 10.1038/sj.ijo.0802378. [DOI] [PubMed] [Google Scholar]
- 29.Heine P.A. Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(23):12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooke P.S. The role of estrogen and estrogen receptor-alpha in male adipose tissue. Molecular and Cellular Endocrinology. 2001;178(1–2):147–154. doi: 10.1016/s0303-7207(01)00414-2. [DOI] [PubMed] [Google Scholar]
- 31.Feng Y. Estrogen receptor-alpha expression in the mammary epithelium is required for ductal and alveolar morphogenesis in mice. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(37):14718–14723. doi: 10.1073/pnas.0706933104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y. GSK-3beta mediates in the progesterone inhibition of estrogen induced cyclin D2 nuclear localization and cell proliferation in cyclin D1−/− mouse uterine epithelium. FEBS Letters. 2007;581(16):3069–3075. doi: 10.1016/j.febslet.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 33.Wang Z.V. Identification and characterization of a promoter cassette conferring adipocyte-specific gene expression. Endocrinology. 2010;151(6):2933–2939. doi: 10.1210/en.2010-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang W. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karas R.H. Effects of estrogen on the vascular injury response in estrogen receptor alpha, beta (double) knockout mice. Circulation Research. 2001;89(6):534–539. doi: 10.1161/hh1801.097239. [DOI] [PubMed] [Google Scholar]
- 36.Strissel K.J. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 37.Stubbins R.E. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European Journal of Nutrition. 2012;51(7):861–870. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 38.Ribas V. Myeloid-specific estrogen receptor alpha deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(39):16457–16462. doi: 10.1073/pnas.1104533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Couse J.F., Korach K.S. Estrogen receptor null mice: what have we learned and where will they lead us? Endocrine Reviews. 1999;20(3):358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 40.Cowley S.M. Estrogen receptors alpha and beta form heterodimers on DNA. Journal of Biological Chemistry. 1997;272(32):19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 41.Das S.K. Estrogenic responses in estrogen receptor-alpha deficient mice reveal a distinct estrogen signaling pathway. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohlsson C. Obesity and disturbed lipoprotein profile in estrogen receptor-alpha-deficient male mice. Biochemical and Biophysical Research Communications. 2000;278(3):640–645. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]


