Abstract
Purpose
Propensity scores, a powerful bias-reduction tool, can balance treatment groups on measured covariates in non-experimental studies. We demonstrate the use of multiple propensity score estimation methods to optimize covariate balance.
Methods
We used secondary data from 1,292 adults with non-psychotic major depressive disorder in the Sequenced Treatment Alternatives to Relieve Depression trial (2001–2004). After initial citalopram treatment failed, patient preference influenced assignment to medication augmentation (n=565) or switch (n=727). To reduce selection bias, we used boosted classification and regression trees (BCART) and logistic regression iteratively to identify two potentially optimal propensity scores. We assessed and compared covariate balance.
Results
After iterative selection of interaction terms to minimize imbalance, logistic regression yielded better balance than BCART (average standardized absolute mean difference across 47 covariates: 0.03 vs. 0.08, matching; 0.02 vs. 0.05, weighting).
Conclusions
Comparing multiple propensity score estimates is a pragmatic way to optimize balance. Logistic regression remains valuable for this purpose. Simulation studies are needed to compare propensity score models under varying conditions. Such studies should consider more flexible estimation methods, such as logistic models with automated selection of interactions or hybrid models using main effects logistic regression instead of a constant log-odds as the initial model for BCART.
MeSH keywords: propensity score; statistics as topic; models, statistical; epidemiologic methods; estimation techniques
Introduction
In non-experimental studies, propensity scores (PSs) are used increasingly to address potential selection bias and confounding [1–6]. The PS is a person’s probability of receiving a particular treatment, given pre-treatment characteristics. By measuring important confounders, including them in a PS model, and balancing treatment groups on the PS, analysts can achieve balance on measured pre-treatment characteristics, and therefore a treatment effect estimate (relative risk or risk difference) that is unbiased under the assumption of no unmeasured confounding [7–11].
The research literature describes several PS estimation methods [2, 5, 6, 8, 12–15]. Logistic regression is the conventional choice, but non-parametric methods such as classification and regression trees (CART) have potential advantages: they automatically incorporate interactions, model non-linear associations easily, and may yield more accurate estimates when modeling complex relations [14]. However, studies comparing CART with logistic regression have had mixed results [14, 15]. Boosted CART (BCART) may compare more favorably to logistic regression. BCART uses machine learning to combine multiple simple classification trees, improving prediction [16, 17], yielding more stable estimates when there are many covariates [16], and reducing vulnerability to overfitting [15, 18]. BCART may also be less sensitive than logistic regression to collinearity [16].
Theoretical work and simulation studies have evaluated different PS estimation methods [14, 17] and have quantified the bias reduction due to PS matching [13, 19–21] under certain conditions. However, studies have not fully described which PS estimation methods minimize bias under which circumstances. Further, applied researchers cannot detect bias directly and therefore must seek instead to optimize covariate balance [22]. In this report, we use an empirical example from the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) trial [23] to demonstrate how covariate balance can be optimized under real-world conditions using multiple PS estimation methods. The STAR*D trial provides an appropriate example because it was a large effectiveness study with clinically relevant comparisons that were not randomized. Using the STAR*D data, we optimized between-group covariate balance with both logistic regression and BCART. Comparing these methods not only made substantive analyses possible but also generated questions and hypotheses for future simulation studies.
Methods
Sample and data
The original STAR*D study (2001–2004) enrolled 4,041 outpatients with non-psychotic major depressive disorder [23, 24]. Of these, 1,439 participants discontinued initial citalopram monotherapy because of side effects, lack of recovery after at least 9 weeks, or individual choice, and entered second-line treatment. Second-line treatment options included four treatment strategies: augmenting citalopram with another antidepressant agent (bupropion SR or buspirone), switching to an alternative antidepressant (bupropion SR, sertraline, or venlafaxine XR), augmenting with cognitive therapy, or switching to cognitive therapy. The original study’s equipoise randomization scheme allowed each participant to select acceptable treatment strategies; patients were randomized to a treatment within the categories they had selected. Using a public-release dataset available through the National Institute of Mental Health, the current study examines data from the 1,292 participants who received either medication augmentation (n=565) or an antidepressant switch (n=727).
The University’s Biomedical Institutional Review Board determined that the current study did not require its approval.
Measures
Our primary balance measure was the average standardized absolute mean (ASAM) difference across 47 covariates that we selected based on empirical evidence and theory. ASAM difference was calculated by subtracting each of the 47 comparison group means from the corresponding treatment group mean, taking the absolute value of each difference, dividing each absolute difference by the pooled standard deviation of the covariate, and then computing the mean of the standardized absolute differences [16, 25].
As auxiliary balance measures, we used statistics developed by Rubin [26]: B, the absolute standardized mean difference on the PS logit; R, the variance ratio (augment/switch) for the PS logit; the percentage of variance ratios on the 47 covariates that were in the severe range (augment variance divided by switch variance <0.5 or >2); and the percentage of variance ratios on covariates that were in the moderate range (≥0.5 but <.8, or >1.25 but ≤2). Variance ratios were based on residual variance, after regressing each covariate on the PS logit in a linear or logistic model. To calculate PS-weighted statistics, we used means x̅w = (1 / Σi wi) × Σi wixi and variances s2w = (1 / ((Σi wi) − 1)) × Σi (wi(xi − x̅w)2), where wi is the weight corresponding to observation i and xi is the covariate value or PS logit for observation i. Good covariate balance is indicated by an R value near 1 and values of the other statistics near zero.
Statistical analysis
The original STAR*D design allowed randomized comparisons of treatment options within, but not between, the augment and switch arms. We wished to estimate the effect of medication augmentation versus switching in the population of patients receiving augmentation. Because patient preference influenced assignment to these treatment categories, we sought to balance the groups on pre-treatment characteristics using PSs.
After addressing missingness, we used BCART and logistic regression in iterative procedures to identify two potentially optimal PSs. In both matched and weighted samples, we compared the BCART and logistic PSs to determine which method yielded better covariate balance and therefore would better support a comparison of medication augmentation with medication switching. We also assessed the sensitivity of treatment effect estimates (described below) to the choice of PS estimation method.
Missing data imputation
Although 82% of observations had at least one missing value, only 5% of data values were missing, making our data well-suited for imputation to avoid a biased complete-case analysis [27–30]. Assuming missingness at random, we employed the expectation-maximization method in SAS PROC MI (SAS Institute, Cary, NC), including 95 relevant variables in the imputation model and using pre-imputation diagnostics to avoid multicollinearity.
Propensity score estimation
For the logistic models we used SAS version 9.2. For PS estimation and balance checking, covariates (including interactions) should be selected based on theory and prior evidence about their relations with the outcome [31], as they were in the current study. However, Rosenbaum and Rubin recommended adding interaction and quadratic terms to optimize balance on the selected set of covariates [8]. Therefore, we employed a forward stepwise procedure to add 30 product terms to the logistic model, out of 1,101 candidates. (Quadratic terms for the 27 dichotomous covariates were excluded.) We chose 30 as the number of interaction terms because we believed that 30 would exceed the number of interaction terms that most analysts would add manually and that 30 terms would address residual imbalance sufficiently while keeping computational burden reasonable. At each step we selected the model that minimized covariate imbalance (weighted ASAM difference, described below). We automated this procedure because an automated approach can optimize balance [16] and provides a meaningful comparison with the inherently automated BCART model. Further, this approach allows the inclusion of interaction terms that improve covariate balance but might not be obvious choices for a researcher.
The BCART model was created using Ridgeway’s “gbm” package for R software, which creates a non-linear model for the log-odds of treatment assignment, starting with the constant log-odds calculated for the whole sample and iteratively improving the model by adding simple regression trees [32, 33]. Each regression tree is created by identifying an optimal combination of covariate cutpoints to predict the residuals from the previous iteration. For example, a two-level tree might first split the sample into two age groups and then split the lower age group based on a cutpoint for number of weeks of first-line treatment. The final model is the sum of the constant log-odds of treatment assignment and the incremental adjustments contributed by the series of regression trees. We specified a maximum tree depth of 2 (i.e., allowed only main effects and 2-way interactions). Based on recommendations by McCaffrey and colleagues [16], we set the shrinkage parameter to 0.0005 and used a 50% subsample of the data to fit each tree. We specified a maximum of 20,000 iterations and stopped the algorithm when weighted ASAM difference (described in the following paragraph) was minimized.
Propensity score implementation
To create the matched sample we used a 5-to-1 digit PS matching algorithm [34], which made 5 passes through the augment group in random order, starting with a caliper of ±0.000005 and widening the caliper by a factor of 10 after each pass, ending with ±0.05. During each pass, one-to-one caliper matching was performed without replacement, considering potential matches in random order. When assumptions are met, the resulting matched sample can be used to estimate the treatment effect in the treated [7, 35, 36]. To allow the same type of estimates based on weighting, we assigned a weight of 1 to each treated (augment) observation and a weight of PS / (1 − PS) to each untreated (switch) observation [16, 37]. Because matching is resource-intensive, we used these weights to calculate ASAM difference after each iteration of the logistic and BCART models.
Sensitivity of treatment effect estimates to propensity score estimation methods
We used SAS procedures FREQ and SURVEYFREQ to estimate the effect of treatment on depression remission (as measured by Quick Inventory of Depression Symptomatology-Self Report [QIDS-SR16] scores after second-line treatment). We assessed the sensitivity of the resulting risk ratios (RRs) to PS estimation method.
Results
The first 6 rows of Table 1 summarize between-group balance before propensity scoring and after using each combination of PS estimation and implementation methods. The imbalance before propensity scoring is evident in the ASAM difference of 0.20 standard deviations, B values (i.e., standardized mean differences on the PS logit) near 2, R values in or near the severe range, and the 21% of covariate variance ratios in the moderate-to-severe range.
Table 1.
Covariate balance by propensity score estimation and implementation method
| Estimation Method |
Application Method | ASAM Difference |
B* | R* | % Severe Variance Ratio† |
% Moderate Variance Ratio† |
|---|---|---|---|---|---|---|
| BCART | Before Implementation | 0.20 | 1.98 | 0.52 | 2.10 | 19.10 |
| BCART | Matching | 0.08 | 0.00 | 0.98 | 4.30 | 19.10 |
| BCART | Weighting | 0.05 | 0.41 | 0.72 | 0.00 | 8.50 |
| Logistic | Before Implementation | 0.20 | 1.68 | 0.28 | 2.10 | 19.10 |
| Logistic | Matching | 0.03 | 0.01 | 0.98 | 0.00 | 4.30 |
| Logistic | Weighting | 0.02 | 0.04 | 1.06 | 0.00 | 8.50 |
| Logistic Without Interactions | Before Implementation | 0.20 | 1.67 | 0.38 | 2.10 | 19.10 |
| Logistic Without Interactions | Matching | 0.04 | 0.01 | 0.98 | 0.00 | 6.40 |
| Logistic Without Interactions | Weighting | 0.06 | −0.12 | 0.82 | 0.00 | 10.60 |
ASAM difference = average standardized absolute mean difference across covariates; B = standardized difference between treatment conditions on mean propensity score logit; BCART = boosted classification and regression trees; R = variance ratio (treatment/comparison) for propensity score logit
B and R vary between estimation methods even before application because they are conditioned on the estimated propensity score.
Severe range is <.5 or >2; moderate range is >=.5 but <.8, or >1.25 but <=2. Covariates are conditioned on the propensity score logit before variance ratios are calculated (26).
Every permutation of propensity scoring methods improved covariate balance. However, using weights to apply the BCART PS resulted in relatively poor balance according to the B statistic and moderately severe imbalance according to the R statistic. Regardless of PS implementation method (matching or weighting), the balance statistics obtained under the logistic model were equal or superior to those obtained under the BCART model. In a sensitivity analysis using a main effects logistic model, this pattern generally persisted (last 3 rows of Table 1), though the results for weighting were mixed. Compared with BCART, logistic regression resulted in a lower c-statistic (i.e., area under the receiver operating characteristic curve [38]; 0.88 vs. 0.90) and a larger matched sample (n=538 vs. 470).
Table 2 reports balance on selected characteristics before and after implementing the logistic PS. Before propensity scoring, the augment and switch groups resembled each other closely on sociodemographic variables (e.g., age 41.6 [12.7] and 42.4 [12.8] respectively) and somewhat closely on depression history variables (e.g., number of lifetime major depressive episodes 5.3 [8.0] and 6.4 [10.3] respectively) but differed markedly on characteristics at the end of first-line treatment (e.g., treatment duration 11.9 [2.9] vs. 8.0 [4.2] weeks; citalopram dose 55.1 [10.9] vs. 41.5 [17.7] mg/day; QIDS-SR16 depressive severity 11.4 [4.9] vs. 13.2 [4.9]; percentage discontinuing first-line treatment due to side effects 10.2% vs. 62.7%). After PS implementation, minimal differences remained.
Table 2.
Participant characteristics before and after implementing logistic propensity scores, United States, 2001–2004
| Pre-Propensity Score | After Propensity Score Matching | After Propensity Score Weighting | ||||
|---|---|---|---|---|---|---|
| Augment N = 565 |
Switch N = 727 |
Augment N = 269 |
Switch N = 269 |
Augment N = 565 |
Switch N = 727 |
|
| Age (yr) | 41.6 (12.7) | 42.4 (12.8) | 42.0 (12.6) | 42.6 (12.8) | 41.6 (12.7) | 40.9 (13.2) |
| Female sex (%) | 58.8 | 58.7 | 58.0 | 59.1 | 58.8 | 61.1 |
| Age at first major depressive episode (yr) | 25.1 (14.0) | 24.9 (13.9) | 25.4 (14.2) | 25.7 (14.6) | 25.1 (14.0) | 24.6 (14.2) |
| No. of major depressive episodes | 5.3 (8.0) | 6.4 (10.3) | 6.4 (9.6) | 6.1 (9.5) | 5.3 (8.0) | 5.3 (8.0) |
| Duration of first-line treatment (wk) | 11.9 (2.9) | 8.0 (4.2) | 10.8 (3.2) | 11.0 (2.8) | 11.9 (2.9) | 11.9 (3.0) |
| Citalopram dose during first-line treatment (mg/day) | 55.1 (10.9) | 41.5 (17.7) | 53.1 (12.7) | 53.1 (12.4) | 55.1 (10.9) | 54.0 (11.3) |
| QIDS-SR16 score at end of first-line treatment | 11.4 (4.9) | 13.2 (4.9) | 12.5 (5.1) | 12.5 (4.8) | 11.4 (4.9) | 11.5 (4.7) |
| Exited first-line treatment due to side effects (%) | 10.2 | 62.7 | 19.0 | 19.9 | 10.2 | 10.6 |
QIDS-SR16 = the 16-item Quick Inventory of Depressive Symptomatology, Self-Report (scores can range from 0 to 27; higher scores indicate increased severity of depressive symptoms)
Statistics reported are Mean (SD) unless otherwise indicated.
The crude RR for remission favored augmentation (RR: 1.41, 95% confidence interval [CI]: 1.19 to 1.67). In the PS-matched sample, treatment effect estimates were identical between PS estimation methods (Table 3), but in the weighted sample, the logistic PSs resulted in a 3% larger treatment effect estimate (ratio 1.28/1.24=1.03).
Table 3.
Treatment effect estimates for augmentation versus switching by propensity score estimation and implementation method
| Risk Ratio (95% CI) | ||
|---|---|---|
| Estimation Method |
Matched Sample |
Weighted Sample |
| BCART | 1.00 (0.73, 1.37) | 1.24 (0.94, 1.65) |
| Logistic | 1.00 (0.75, 1.34) | 1.28 (0.97, 1.69) |
BCART = boosted classification and regression trees; CI = confidence interval. Crude risk ratio was 1.41 (95% CI: 1.19 to 1.67).
To explore whether the differences in residual imbalance between BCART and logistic regression resulted from grossly different treatment of covariates in the two estimation models, we described the BCART model in more detail and compared the balance results for individual covariates. Of the 19,002 trees included in the final model, 999 (5.3%) were “main effect” trees with splits on only one variable. Although all 47 covariates were used in the BCART model, only 18 (38.3%) were used in main effect trees. The gbm package reports relative influence, a measure of the reduction in sum of squared error attributable to each covariate [32, 39], normalized to sum to 100. Relative influence ranged from 0.02 to 45.7 with a median of 0.34 (mean, 2.13; standard deviation, 6.85), indicating that some covariates influenced the BCART model more strongly than others. Although the covariates used in main effect trees accounted for 94.2% of the total reduction in sum of squared error, the main effect trees themselves accounted for only 2.3%. Compared with logistic regression, BCART achieved a very similar pattern of results across the 47 covariates (r=.98 for both absolute and relative changes in ASAM difference with matching, r=.99 for absolute and relative changes with weighting). Absolute change in ASAM difference using the BCART PSs was correlated with relative influence in the BCART model (r=−0.70 and r=−0.72 for matching and weighting respectively) and also with the proportion of trees in which each covariate was included at level 1 (r=−0.76 and r=−0.78 respectively) (P<.0001).
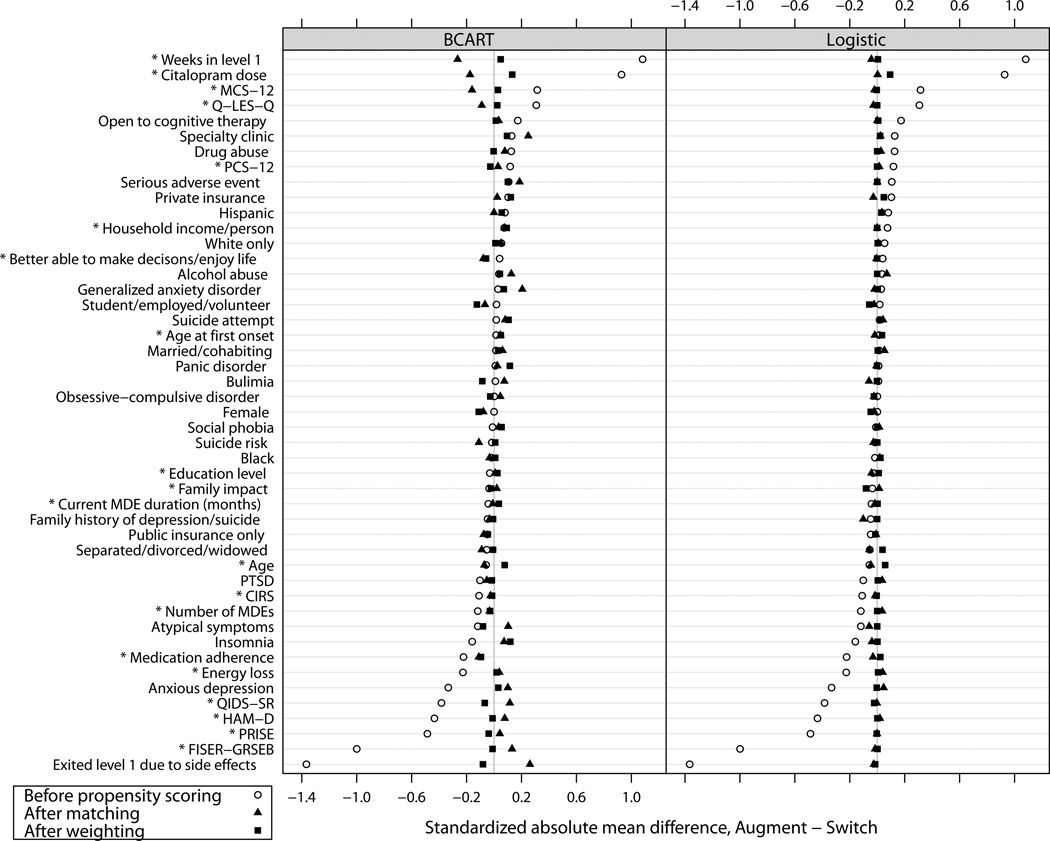
Figure 1 shows the standardized differences on individual covariates before and after PS implementation. Despite the similarity between the BCART and logistic PSs in patterns of absolute and relative change on individual covariates, generally the PSs from BCART resulted in slightly greater residual imbalance. For the covariates that had the largest imbalances before propensity scoring, matching on the BCART PSs appeared to result in over-correction (i.e., slight imbalance in the opposite direction). We examined dichotomous and continuous covariates separately (the latter are marked with asterisks in Figure 1). Although the covariates with the largest initial imbalances tended to be continuous, covariate type did not appear to explain the differences between the BCART results and the logistic regression results.
Figure 1. Balance on individual covariates before and after propensity score implementation.
* Continuous (versus dichotomous) covariate.
BCART=boosted classification and regression trees; CIRS=Cumulative Illness Rating Scale; FISER-GRSEB=Frequency and Intensity of Side Effects Rating/Global Rating of Side Effects Burden; HAM-D=Hamilton Depression Scale; MCS-12=Mental Component Summary (of the SF-12 Short-Form Health Survey); PCS-12=Physical Component Summary (of the SF-12 Short-Form Health Survey); PRISE=Patient-Rated Inventory of Side Effects; PTSD=post-traumatic stress disorder; QIDS-SR=Quick Inventory of Depressive Symptomatology-Self Report; Q-LES-Q= Quality of Life Enjoyment and Satisfaction Questionnaire
Discussion
We examined the effects of PS estimation methods on covariate balance in order to select a PS model for treatment effect estimation. In our STAR*D subsample, logistic regression provided better balance than BCART regardless of the PS implementation method used (matching or weighting). Logistic regression also resulted in a larger matched sample and therefore a more efficient matched comparison.
The better balance results from the logistic model were surprising given the perceived flexibility of BCART models. However, in a simulation study, Lee and colleagues found that in most scenarios main effects logistic regression yielded better covariate balance (i.e., lower ASAM differences) than BCART, on average [17]. Our findings are consistent with those simulation results. Importantly, Lee and colleagues also observed that main effects logistic regression yielded a skewed distribution of ASAM differences, in which outliers had large residual imbalance. Further, in their study, BCART generally resulted in similar average levels of balance with a more symmetric distribution, lower bias, lower standard errors, and higher 95% CI coverage. Their findings called into question the use of main effects logistic regression to estimate PSs and the use of ASAM difference as the sole measure of covariate balance. They noted that logistic regression might yield better results with more model calibration and/or a different measure of covariate balance. Our logistic model included multiple interaction terms designed to reduce residual imbalance, and we assessed balance using four measures other than ASAM difference.
It occurred to us that our findings might be explained in part by the different handling of main effects and interactions in the two methods. BCART automatically incorporated interactions, but not necessarily main effects or even all of the covariates. The logistic model, on the other hand, included all main effects by default, as well as 30 selected product terms. As it turned out, BCART put relatively little weight on main effects. However, by using key variables in many trees, BCART generated a pattern of balance improvements on individual covariates that was similar to the pattern generated by the logistic regression procedure. Despite the similar patterns of results, BCART generally resulted in slightly greater residual imbalance on individual covariates. On average, the differences in residual imbalance were small.
The stepwise procedure we used to select interactions for the logistic model was more complicated than the manual procedures that might typically be used for logistic PS models. Interestingly, when we ran the logistic model without interactions as a sensitivity analysis, it was inferior to the full logistic model but generally equivalent to or slightly better than the BCART model in terms of covariate balance.
Despite the better covariate balance achieved by logistic regression as compared with BCART, our treatment effect estimates were not sensitive to PS estimation method. Possibly the difference in balance (.03 to .05 standard deviations depending on implementation method) was too small to have a meaningful impact. Alternatively, the additional imbalance associated with BCART may have involved covariates unrelated to depression remission (unlikely given the careful selection of covariates) or covariates with offsetting effects on remission. The weighted treatment effect estimates did differ from the matched estimates, because there was some treatment effect heterogeneity and these two samples represented different populations. Even though we found little difference between PS estimation methods in treatment effect estimates, optimizing balance remains important because even a small imbalance on a strong risk factor can bias treatment effect estimates substantially.
This study is subject to certain limitations. First, it is based on a single empirical sample, so its primary contributions are demonstrating a pragmatic approach to PS estimation and identifying questions (below) for future simulation studies, rather than establishing differences among methods. Second, our selection of balance statistics was somewhat arbitrary, as standards for assessing balance have not yet been established. We addressed this limitation by using several balance statistics with face validity. In fact, we obtained similar results with 3 measures not reported here: ASAM difference with an arcsine transformation for dummy variables; ASAM difference with each covariate weighted in proportion to its association with the outcome; and distribution of the percent change in the treatment effect estimate after additionally adjusting for each covariate, one at a time, in the analysis model (a measure of residual confounding) [40]. Third, we included an arbitrary degree of flexibility in the logistic regression approach. We could have included more or fewer interaction terms, greater interaction depth, or splines. We anticipated that 30 product terms would suffice to address the residual imbalance associated with the main effects model. Fourth, and similarly, in the BCART procedure we allowed only main effects and two-way interactions. Some authors have recommended allowing higher-level interactions; for example, McCaffrey and colleagues recommended allowing up to four-way interactions [16]. However, when we did so (not shown), it did not improve covariate balance compared with our primary BCART model, based on ASAM difference, the B and R statistics, and variance ratios on individual covariates. Fifth, although careful covariate selection and balance optimization lead to better control for measured confounders, we were unable to measure bias in treatment effect estimates because the true treatment effect is unknown. ASAM difference has been shown to be only moderately correlated with bias under a variety of data generating models [17]. Although we employed a variety of balance measures, these measures may be imperfect proxies for bias. Finally, we imputed missing values under the plausible assumption of missingness at random. In settings where this assumption is implausible, more sophisticated methods for imputation should be considered [41].
Logistic regression may not always yield better covariate balance than BCART. Future studies should use both simulated and empirical data to explore further why and under what circumstances this is the case. Because the two methods handle main effects and interactions differently, their relative performance may depend on conditions such as covariate distributions, the functional forms of relations between covariates and treatment (or outcome), and the presence or absence of interactions in the true treatment or outcome model. Along with the main effects logistic regression used in previous simulations [14, 17], it would be worth testing more complex models such as the automated model presented here, or perhaps a hybrid model that uses main effects logistic regression instead of a constant log-odds as the initial model for BCART. Researchers who use propensity scoring and consumers of research findings, including clinicians, would benefit from these investigations and also from further standardization of PS estimation methods and balance statistics.
Conclusion
Using data from the STAR*D study, including many continuous covariates carefully selected to capture risk for the outcome of interest, we compared a logistic model with forward stepwise selection of interactions to a boosted CART model. Although both were designed to optimize covariate balance, the logistic model achieved better balance in both PS-matched and PS-weighted samples. Until more is known about which models perform best under which circumstances, a pragmatic approach is to compare multiple PS estimates in order to optimize between-group balance on risk factors affecting treatment assignment.
Acknowledgments
This work was supported by a contract from the Agency for Healthcare Research and Quality to The University of North Carolina Developing Evidence to Inform Decisions about Effectiveness Center (contract no. HHSA290200500401). At the time of this work, Dr. Dusetzina received funding from a National Research Service Award Pre-Doctoral Traineeship from the AHRQ, sponsored by the Cecil G. Sheps Center for Health Services Research, University of North Carolina at Chapel Hill, Grant No. 5-T-32 HS000032-20. Dr. Til Stürmer’s work on this project was funded in part by R01 AG023178 from the National Institute on Aging at the National Institutes of Health.
The authors of this report are responsible for its content. No statement in the report should be construed as endorsement by AHRQ, whose role in this work was limited to funding, review and approval of the research protocol, and review and approval of the final report.
List of abbreviations and acronyms
- ASAM difference
average standardized absolute mean difference
- BCART
boosted classification and regression trees
- CART
classification and regression trees
- CI
confidence interval
- PS
propensity score
- RR
risk ratio
- STAR*D
Sequenced Treatment Alternatives to Relieve Depression
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shadish W, Cook T, Campbell D. Experimental and quasi-experimental designs for generalized causal inference. Boston: Houghton-Mifflin; 2002. [Google Scholar]
- 2.Stürmer T, Joshi M, Glynn R, Avorn J, Rothman K, Schneeweiss S. A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol. 2006;59(5):437–447. doi: 10.1016/j.jclinepi.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eklind-Cervenka M, Benson L, Dahlström U, Edner M, Rosenqvist M, Lund L. Association of candesartan vs losartan with all-cause mortality in patients with heart failure. JAMA. 2011 Jan 12;305(2):175–182. doi: 10.1001/jama.2010.1949. [DOI] [PubMed] [Google Scholar]
- 4.Tarakji K, Sabik, Bhudia S, Batizy L, Blackstone E. Temporal onset, risk factors, and outcomes associated with stroke after coronary artery bypass grafting. JAMA. 2011 Jan 26;305(4):381–390. doi: 10.1001/jama.2011.37. [DOI] [PubMed] [Google Scholar]
- 5.Weitzen S, Lapane K, Toledano A, Hume A, Mor V. Principles for modeling propensity scores in medical research: a systematic literature review. Pharmacoepidemiol Drug Saf. 2004;13(12):841–853. doi: 10.1002/pds.969. [DOI] [PubMed] [Google Scholar]
- 6.Perkins S, Tu W, Underhill M, Zhou X, Murray M. The use of propensity scores in pharmacoepidemiologic research. Pharmacoepidemiol Drug Saf. 2000;9(2):93–101. doi: 10.1002/(SICI)1099-1557(200003/04)9:2<93::AID-PDS474>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983 Apr 1;70(1):41–55. [Google Scholar]
- 8.Rosenbaum PR, Rubin DB. Reducing bias in observational studies using subclassification on the propensity score. J Am Stat Assoc. 1984;79(387):516–524. [Google Scholar]
- 9.Greenland S, Robins J. Identifiability, exchangeability, and epidemiological confounding. Int J Epidemiol. 1986;15(3):413–419. doi: 10.1093/ije/15.3.413. [DOI] [PubMed] [Google Scholar]
- 10.Greenland S, Robins J. Identifiability, exchangeability and confounding revisited. Epidemiol Perspect Innov. 2009;6(4) doi: 10.1186/1742-5573-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maldonado G. Update: Greenland and Robins (1986). Identifiability, exchangeability and epidemiological confounding. Epidemiol Perspect Innov. 2009;6(3) doi: 10.1186/1742-5573-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drake C. Effects of misspecification of the propensity score on estimators of treatment effect. Biometrics. 1993;49(4):1231–1236. [Google Scholar]
- 13.Rubin DB, Thomas N. Matching using estimated propensity scores: relating theory to practice. Biometrics. 1996;52(1):249–264. [PubMed] [Google Scholar]
- 14.Setoguchi S, Schneeweiss S, Brookhart M, Glynn R, Cook E. Evaluating uses of data mining techniques in propensity score estimation: a simulation study. Pharmacoepidemiol Drug Saf. 2008;17(6):546–555. doi: 10.1002/pds.1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luellen J, Shadish W, Clark M. Propensity scores: an introduction and experimental test. Eval Rev. 2005;29(6):530–558. doi: 10.1177/0193841X05275596. [DOI] [PubMed] [Google Scholar]
- 16.McCaffrey D, Ridgeway G, Morral A. Propensity score estimation with boosted regression for evaluating causal effects in observational studies. Psychol Methods. 2004;9(4):403–425. doi: 10.1037/1082-989X.9.4.403. [DOI] [PubMed] [Google Scholar]
- 17.Lee B, Lessler J, Stuart E. Improving propensity score weighting using machine learning. Statist Med. 2010;29(3):337–346. doi: 10.1002/sim.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westreich D, Lessler J, Jonsson Funk M. Propensity score estimation: neural networks, support vector machines, decision trees (CART), and meta-classifiers as alternatives to logistic regression. J Clin Epidemiol. 2010;63(8):826–833. doi: 10.1016/j.jclinepi.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rubin DB, Thomas N. Characterizing the effect of matching using linear propensity score methods with normal distributions. Biometrika. 1992;79(4):797–809. [Google Scholar]
- 20.Rubin DB, Thomas N. Affinely invariant matching methods with ellipsoidal distributions. Annals of Statistics. 1992;20(2):1079–1093. [Google Scholar]
- 21.Rubin DB, Thomas N. Combining propensity score matching with additional adjustments for prognostic covariates. Journal of the American Statistical Association. 2000;95(450):573–585. [Google Scholar]
- 22.Rubin DB. The design versus the analysis of observational studies for causal effects: Parallels with the design of randomized trials. Statistics in Medicine. 2007;26:20–36. doi: 10.1002/sim.2739. [DOI] [PubMed] [Google Scholar]
- 23.Rush A, Fava M, Wisniewski S, Lavori P, Trivedi M, Sackheim H, et al. Sequenced treatment alternatives to relieve depression (STAR*D): rationale and design. Control Clin Trials. 2004;25(1):119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 24.Wisniewski SR, Fava M, Trivedi MH, Thase ME, Warden D, Niederehe G, et al. Acceptability of second-step treatments to depressed outpatients: a STAR*D report. Am J Psychiatry. 2007 May;164(5):753–760. doi: 10.1176/ajp.2007.164.5.753. [DOI] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics. 1985;41(1):103–116. [PubMed] [Google Scholar]
- 26.Rubin DB. Using propensity scores to help design observational studies: application to the tobacco litigation. Health Serv Outcomes Res Methodol. 2001;2(3):169–188. [Google Scholar]
- 27.Rubin D. Multiple imputation for nonresponse in surveys. New York: John Wiley & sons; 1987. [Google Scholar]
- 28.Little R. Regression with missing X's: a review. J Am Stat Assoc. 1992;87(420):1227–1237. [Google Scholar]
- 29.Allison P. Missing data. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- 30.Graham J. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;6(1–6.28) doi: 10.1146/annurev.psych.58.110405.085530. 60. [DOI] [PubMed] [Google Scholar]
- 31.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006 Jun 15;163(12):1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ridgeway G. A guide to the gbm package. [accessed 6.2.13];The Comprehensive R Archive Network. 2012 Web Site, http://cran.r-project.org/web/packages/gbm/index.html. [Google Scholar]
- 33.R Development Core Team. a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2004. [Google Scholar]
- 34.Parsons L. Reducing bias in a propensity score matched-pair sample using greedy matching techniques. Twenty-Sixth Annual SAS Users Group International Conference; 2001; Long Beach, CA. [Google Scholar]
- 35.Stürmer T, Rothman K, Glynn R. Insights into different results from different causal contrasts in the presence of effect-measure modification. Pharmacoepidemiol Drug Saf. 2006;15(10):698–709. doi: 10.1002/pds.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 37.Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology. 2003;14(6):680–686. doi: 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 38.Westreich D, Cole S, Jonsson-Funk M, Brookhart M, Stürmer T. The role of the c-statistic in variable selection for propensity score models. Pharmacoepidemiology and Drug Safety. 2011;20(3):317–320. doi: 10.1002/pds.2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman J. Greedy function approximation: a gradient boosting machine. Ann Stat. 2001;29(5):1189–1232. [Google Scholar]
- 40.Lunt M, Solomon D, Rothman K, Glynn R, Hyrich K, Symmons D, et al. Different methods of balancing covariates leading to different effect estimates in the presence of effect modification. Am J Epidemiol. 2009;169(7):909–917. doi: 10.1093/aje/kwn391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.D'Agostino RB, Jr, Rubin DB. Estimating and using propensity scores with partially missing data. Journal of the American Statistical Association. 2000;95(451):749–759. [Google Scholar]