Abstract
The World Health Organization describes calcifying fibrous tumors (CFTs) as rare, benign lesions characterized by hypocellular, densely hyalinized collagenization with lymphoplasmacytic infiltration. These tumors rarely involve the gastrointestinal (GI) tract. A routine endoscopic upper gastrointestinal screen detected a 10-mm submucosal tumor (SMT) in the lesser curvature of the lower corpus of the stomach of an apparently healthy, 37-year-old woman with no history of Helicobacter pylori infection. Endoscopic ultrasonography (EUS) localized the internally isoechoic, homogeneous SMT mainly within the submucosa. Malignancy was ruled out using endoscopic submucosal dissection (ESD). A pathological examination confirmed complete resection of the SMT, and defined a hypocellular, spindle-cell tumor with a densely hyalinized, collagenous matrix, scattered lymphoplasmacytic aggregates as well as a few psammomatous, dystrophic calcified foci. The mass was immunohistochemically positive for vimentin and negative for CD117 (c-kit protein), CD34, desmin, smooth muscle actin (SMA) and S100. Therefore, the histological findings were characteristic of a CFT. To date, CFT resection by ESD has not been described. This is the first case report of a gastric calcifying fibrous tumor being completely resected by ESD after endoscopic ultrasonography.
Keywords: Calcifying fibrous tumor, Endoscopic submucosal dissection, Submucosal tumor, Endoscopic ultrasonography
Core tip: Calcifying fibrous tumors (CFTs) rarely involve the gastrointestinal tract. Resection of CFT by endoscopic submucosal dissection (ESD) has not been reported. This is the first case report of a gastric calcifying fibrous tumor being completely resected by ESD after endoscopic ultrasonography.
INTRODUCTION
Rosenthal originally identified benign, fibrous, calcifying fibrous tumors (CFTs) in soft tissues of the extremities in children[1]. These tumors comprised hyalinized fibrous tissue interspersed with bland fibroblastic spindle cells, scattered psammomatous, and/or dystrophic calcifications and variably prominent mononuclear inflammatory infiltrates. CFTs have recently been identified in the mesentery and peritoneum[2-4], mediastinum[5], pleura[6], lung[7], adrenal glands[8] and in the paratesticular and spermatic cord[4]. Although CFTs can involve various organ systems, the gastrointestinal (GI) tract is rarely involved[9]. Calcifying fibrous submucosal tumors (SMTs) are difficult to differentiate from other SMTs such as small lipomas, neuroendocrine and gastrointestinal stromal tumors (GISTs) cell tumors. Only a few case reports have described CFTs occurring in the stomach[9-11]. The CFTs described in these reports were relatively large when discovered and required surgical resection. Here, we describe a gastric CFT that was completely removed by endoscopic submucosal dissection (ESD) after a thorough assessment by endoscopic ultrasonography (EUS).
CASE REPORT
A routine health screen using upper gastrointestinal endoscopy revealed a submucosal tumor in a 37-year-old apparently healthy woman with no known family history of gastrointestinal disorders or malignant diseases. She had no abdominal discomfort or stomach and intestinal symptoms. Physical findings were unremarkable and all initial biochemical and hematological parameters were within normal limits. Narrow-band imaging endoscopy (GIF-H260Z; Olympus, Tokyo, Japan) indicated a 10 mm diameter SMT with normal overlying mucosa in the lesser curvature of the lower corpus of the stomach (Figure 1A and B). The mucosa of the whole stomach was normal without chronic gastritis. Mucosal biopsies of both the middle portion and antrum of stomach confirmed the absence of Helicobacter pylori infection. Computed tomography (CT) did not detect any submucosal tumors or abnormal findings in any other organs, and no swollen lymph nodes. EUS visualized the SMT mainly within the second and third layers of the gastric wall, and the first layer was preserved (Figure 1E). The homogeneous tumor was internally isoechoic (Figure 1E). Hyperechoic foci with acoustic shadowing within the mass were consistent with calcifications (Figure 1F). The fourth layer of the gastric wall was obvious (Figure 1E and F), and therefore, the SMT was considered not to have invaded the muscularis propria. The endoscopy and EUS findings indicated that the SMT was localized within the submucosal propria, but it was too small to perform fine needle aspiration biopsy (FNA) under EUS. A biopsy specimen obtained from SMT also did not include the tumor contents and a definitive pathological diagnosis of the tumor could not be achieved. However, a precise diagnosis was required to rule out malignancy. The patient refused to undergo surgery, but consented to undergo endoscopic treatment. To completely resect the SMT using only endoscopic mucosal resection (EMR) was considered very difficult. Therefore, SMT was removed by ESD and not EMR to avoid SMT retention and comprehensively diagnose the SMT (Figure 1C and D). Pathological assessment of the resected SMT (Figure 2A) revealed a hypocellular, spindle-cell mass with a densely hyalinized, collagenous matrix, scattered lymphoplasmacytic aggregates and a few foci comprising psammomatous and dystrophic calcifications (Figure 2B). The spindle cells in the tumor harbored no mitotic activity or atypia. Immunohistochemical staining was positive for vimentin (Figure 2C), but negative for CD117 (c-kit protein), CD34, desmin, smooth muscle actin (SMA) and S100. Therefore, the histopathological findings concurred with a diagnosis of a CFT.
Figure 1.
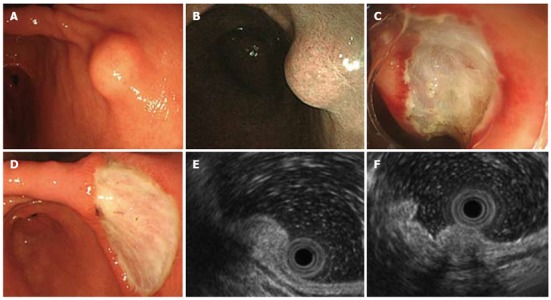
Endoscopic ultrasonography findings of visualized submucosal tumor. A: Endoscopy shows 10-mm shows submucosal tumor (SMT) in lesser curvature of lower corpus of stomach; B: Narrow-band endoscopic imaging SMT covered by normal gastric mucosa; C: Endoscopic submucosal dissection (ESD) for SMT; D: Stomach ulceration five days after ESD; E: Endoscopic ultrasonography (EUS) findings show internally isoechoic, homogeneous sub-mucosal tumor mainly localized within second and third layers, whereas first and fourth layers are preserved; F: Acoustic shadowing of hyperechoic foci inside lesion is consistent with calcifications. Fourth layer is obvious.
Figure 2.
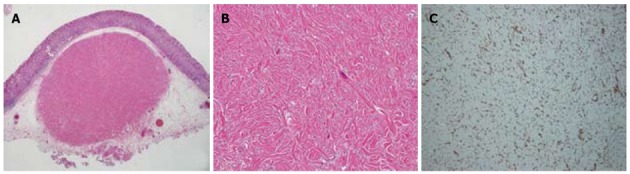
Pathological findings of resected small mucosal tumor. A: Complete submucosal tumor resection was confirmed; B: Hypocellular, spindle-cell tumor has densely hyalinized, collagenous matrix, scattered lymphoplasmacytic aggregates, some psammomatous foci and dystrophic calcification, Spindle-cells harbor no mitotic activity or atypia; C: Positive immunohistochemical staining for vimentin. Original magnification × 10 (A), × 200 (B), × 100 (C).
DISCUSSION
Rosenthal originally described CFTs as benign, soft, fibrous masses with psammoma bodies in two girls aged 2 and 11 years[1]. The histopathology of CFTs is that of a heavily collagenized paucicellular fibrous lesion composed of bland spindled cells, scattered psammomatous and/or dystrophic calcifications and variably prominent mononuclear inflammatory infiltrates. Because they were initially thought to represent a reactive process resulting from abnormally healing tissue, CFTs were originally described as calcifying fibrous pseudotumors[12]. However, later studies indicated that they are true neoplasms with a tendency towards non-destructive local recurrence[4]. Later reports described finding CFTs in ubiquitous anatomical sites including the pleura[6], abdominal cavity and peritoneum[3] and elsewhere[4,12]. The etiology and pathogenesis of CFTs remain unknown, although location, immunohistochemical and pathological features suggest a mesenchymal sub-mesothelial origin[3].
Small SMTs are usually asymptomatic and incidentally detected during endoscopic or radiological examinations. One retrospective study has suggested that the incidence of gastric submucosal lesions is 0.36%[13]. Submucosal tumors are very difficult to accurately diagnose by endoscopic or radiological means. The most common SMTs of the alimentary tract are GISTs that originate from interstitial cells of Cajal[14,15]. Other differential diagnoses of SMTs include fibromatosis, inflammatory myofibroblastic tumors, neuroendocrine cell tumors, schwannomas, heterotopic pancreas, lipomas, cystic lesions, lymphomas and leiomyomas. Differentially diagnosing gastric mesenchymal tumors using only endoscopic imaging is also challenging. Gastric CFTs include SMTs that are endoscopically difficult to differentiate from other SMTs such as those described above, especially when they are very small. Although EUS and EUS-guided FNA are considered useful for diagnosing SMTs, these modalities cannot perfectly diagnose whole SMTs, when EUS findings are non-specific, or when SMTs are too small to be treated by FNA. The SMT was located in the second and third layers of the gastric wall in our patient and it had the same homogeneous, isoechoic features as the third layer. These findings excluded GIST, leiomyoma, cystic lesion, schwannoma, and lipoma from the differential diagnosis, but a more precise diagnosis by EUS remained impossible. The findings indicated that the mass was most likely a neuroendocrine cell tumor. However, the calcification detected by EUS in the SMT is uncommon among neuroendocrine cell tumors. Moreover, it was only 10 mm in diameter, which was too small to treat using EUS-guided FNA. Since endoscopy and EUS could not conclude a diagnosis, the SMT was resected by ESD.
Small mucosal tumors that are not diagnosed beforehand are always diagnosed by immunohistochemistry after surgical resection when FNA is not performed. Common SMTs are diagnosed as follows based on immunohistochemical positivity for CD117 (c-kit protein; GIST), CD34 (almost all mesenchymal neoplasms), smooth muscle actin (SMA), desmin (leiomyoma) and S100 (schwannoma derived from nerves)[15]. The SMT in our patient did not express any of these immunohistochemical markers, which is a characteristic of CFT. Almost all reported CFTs were quite large when they were discovered, and thus to estimate the initial pathogenesis of CFTs difficult. The CFT in our patient was extremely small, and thus might represent the initial status of CFTs. Therefore, further examination was required to analyze this CFT in more detail.
A search of the Pub-Med database did not uncover any reports describing complete resection of a CFT using ESD. We completely resected an extremely small gastric CFT by ESD after the patient had undergone a detailed examination using EUS. The calcification indicated by EUS is considered a useful feature for detecting CFTs and for narrowing down the differential diagnoses of SMTs. We believe that this manuscript is the first report to describe a calcified gastric CFT detected by EUS. The size of this CFT might indicate the initial status of such tumors, clarify one pathogenetic mechanism of development in the GI tract and provide an informative clue to the pathogenesis and development of CFTs in general.
Footnotes
P- Reviewers Li XL, Tham TCK S- Editor Zhai HH L- Editor A E- Editor Wang CH
References
- 1.Rosenthal NS, Abdul-Karim FW. Childhood fibrous tumor with psammoma bodies. Clinicopathologic features in two cases. Arch Pathol Lab Med. 1988;112:798–800. [PubMed] [Google Scholar]
- 2.Ben-Izhak O, Itin L, Feuchtwanger Z, Lifschitz-Mercer B, Czernobilsky B. Calcifying fibrous pseudotumor of mesentery presenting with acute peritonitis: case report with immunohistochemical study and review of literature. Int J Surg Pathol. 2001;9:249–253. doi: 10.1177/106689690100900314. [DOI] [PubMed] [Google Scholar]
- 3.Kocova L, Michal M, Sulc M, Zamecnik M. Calcifying fibrous pseudotumour of visceral peritoneum. Histopathology. 1997;31:182–184. doi: 10.1046/j.1365-2559.1997.5860817.x. [DOI] [PubMed] [Google Scholar]
- 4.Nascimento AF, Ruiz R, Hornick JL, Fletcher CD. Calcifying fibrous ‘pseudotumor’: clinicopathologic study of 15 cases and analysis of its relationship to inflammatory myofibroblastic tumor. Int J Surg Pathol. 2002;10:189–196. doi: 10.1177/106689690201000304. [DOI] [PubMed] [Google Scholar]
- 5.Dumont P, de Muret A, Skrobala D, Robin P, Toumieux B. Calcifying fibrous pseudotumor of the mediastinum. Ann Thorac Surg. 1997;63:543–544. doi: 10.1016/s0003-4975(96)01022-3. [DOI] [PubMed] [Google Scholar]
- 6.Mito K, Kashima K, Daa T, Kondoh Y, Miura T, Kawahara K, Nakayama I, Yokoyama S. Multiple calcifying fibrous tumors of the pleura. Virchows Arch. 2005;446:78–81. doi: 10.1007/s00428-004-1148-4. [DOI] [PubMed] [Google Scholar]
- 7.Soyer T, Ciftci AO, Güçer S, Orhan D, Senocak ME. Calcifying fibrous pseudotumor of lung: a previously unreported entity. J Pediatr Surg. 2004;39:1729–1730. doi: 10.1016/j.jpedsurg.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Eftekhari F, Ater JL, Ayala AG, Czerniak BA. Case report: Calcifying fibrous pseudotumour of the adrenal gland. Br J Radiol. 2001;74:452–454. doi: 10.1259/bjr.74.881.740452. [DOI] [PubMed] [Google Scholar]
- 9.Attila T, Chen D, Gardiner GW, Ptak TW, Marcon NE. Gastric calcifying fibrous tumor. Can J Gastroenterol. 2006;20:487–489. doi: 10.1155/2006/378532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delbecque K, Legrand M, Boniver J, Lauwers GY, de Leval L. Calcifying fibrous tumour of the gastric wall. Histopathology. 2004;44:399–400. doi: 10.1111/j.1365-2559.2004.01779.x. [DOI] [PubMed] [Google Scholar]
- 11.Jang KY, Park HS, Moon WS, Lee H, Kim CY. Calcifying fibrous tumor of the stomach: a case report. J Korean Surg Soc. 2012;83:56–59. doi: 10.4174/jkss.2012.83.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fetsch JF, Montgomery EA, Meis JM. Calcifying fibrous pseudotumor. Am J Surg Pathol. 1993;17:502–508. doi: 10.1097/00000478-199305000-00010. [DOI] [PubMed] [Google Scholar]
- 13.Hedenbro JL, Ekelund M, Wetterberg P. Endoscopic diagnosis of submucosal gastric lesions. The results after routine endoscopy. Surg Endosc. 1991;5:20–23. doi: 10.1007/BF00591381. [DOI] [PubMed] [Google Scholar]
- 14.Polkowski M, Butruk E. Submucosal lesions. Gastrointest Endosc Clin N Am. 2005;15:33–54, viii. doi: 10.1016/j.giec.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Sugár I, Forgács B, István G, Bognár G, Sápy Z, Ondrejka P. Gastrointestinal stromal tumors (GIST) Hepatogastroenterology. 2005;52:409–413. [PubMed] [Google Scholar]


