Abstract
Suicide is among the top ten leading causes of death in individuals of all ages. An explanatory model for suicidal behavior that links clinical and psychological risk factors or endophenotypes, to the underlying neurobiological abnormalities associated with suicidal behavior may enhance prediction, help identify treatment options and have heuristic value. Our explanatory model proposes that developmental factors that are biological (genetics) and psychological or clinical (early childhood adversity) may have causal relevance to the disturbances found in subjects with suicidal behavior. In this way, our model integrates findings from several perspectives in suicidology and attempts to explain the relationship between various neurobiological, genetic, and clinical observations in suicide research, offering a comprehensive hypothesis to facilitate understanding of this complex outcome.
Keywords: child abuse, neurobiology, risk factors, suicide
INTRODUCTION
On a global scale, suicide accounts for about one million deaths with devastating socioeconomic costs and consequences (Mann, 2003). It is the fifth leading cause of years of potential life lost before the age of 65 in the US (http://webappa.cdc.gov/sasweb/ncipc/yp1110.html). Risk factors for suicide have been generally classified into psychiatric, biological, and environmental factors. Over 90% of suicide attempters or suicide victims have a psychiatric illness with 60% of all suicides occurring in relation to mood disorders (Beautrais, Joyce, Mulder et al., 1996; Shaffer, Gould, Fisher et al., 1996).
Multiple risk factors are associated with suicidal behavior, which has been conceptualized as the outcome of the interaction between an individual’s diathesis for suicidal acts and triggers for suicidal behavior (Mann & Arango, 1992). The diathesis refers to the propensity for manifesting suicidal behavior and may be considered trait-related and independent of psychiatric diagnosis (Mann, 2003). In contrast, triggers are precipitants or stressors that determine the timing and probability of suicidal acts. Thus, triggers may be considered state-related. In this regard, risk factors for suicidal behavior may be categorized according to whether they affect the diathesis or the triggers (Oquendo, Malone, & Mann, 1997). This model helps to explain why, in the face of a stressor, one individual would commit suicide while another would not. Thus, suicide and suicidal behavior are not merely reactions to extreme stress, nor do they merely correlate with the severity of a potential stressor such as a psychiatric disorder (van Heeringen, Audenaert, van de Wiele et al., 2000).
Although little is known about the state of mind of suicidal individuals, cognitive psychology has contributed to our understanding and offers new possibilities for interventions to prevent suicidal behavior (Williams & Pollock, 2001). Williams and Pollock (2001) indicated three characteristics that differentiate suicidal depressed individuals from non-suicidal depressed individuals: 1) a sensitivity to specific life experiences that are considered to be a sign of defeat or “loser status” through an attentional bias; 2) the sense of being trapped, as a result of overgeneral memories that prevent both adequate problem identification and problem solving; 3) the lack of rescue factors, possibly based on the absence of generating positive events, leading to hopelessness. Van Heeringen and Marusic (2003) suggested that these characteristics may be useful for classification of biological and neuropsychological findings in suicide research.
This review presents a model explaining the biological substrates of suicide behavior based on the stress-diathesis model of suicidal behavior initially proposed by Mann and Arango (1992) (see Table 1). We will describe our model (see Figure 1) working back from the clinical to the neurobiological substrates. First, we will describe three discrete types of endophenotypes: clinical, neurochemical, and neuroendocrine, and illustrate the complex relationships among them. Then, we will examine the effects of genes and childhood history of abuse on clinical, neurochemical and neuroendocrine endophenotypes. Finally an integrated model will be presented. Such a model, that integrates multiple risk factors, may have predictive capacity.
TABLE 1.
Risk Factors for Suicidal Behavior
| Affecting the diathesis | Acting as triggers |
|---|---|
| Family history of suicide | Major depressive episode |
| Low 5-HIAA in CSF | Acute substance intoxication |
| Alcohol abuse | Social crisis |
| Substance abuse | Financial crisis |
| Cluster B personality disorder |
Family crisis |
| Chronic physical illness | Contagion (Werther’s effect) |
| Marital isolation | |
| Parental loss before age 11 | |
| Childhood history of physical and sexual Abuse |
|
| Hopelessness | |
| Low self-esteem | |
| Not living with a child under age 18 |
FIGURE 1.
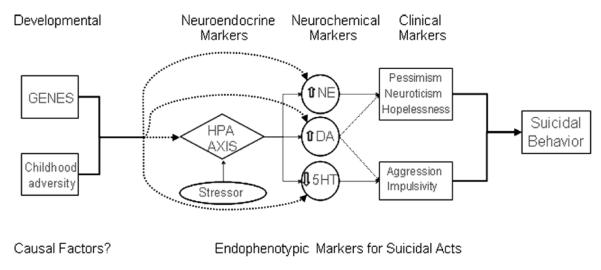
Explanatory model for suicidal acts.
EXPLANATORY MODEL FOR SUICIDAL ACTS
Endophenotypes
An endophenotype has been defined as a “measurable component along the pathway between disease and distal genotype” (Gottesman & Gould, 2003). Abnormal neurophysiological, biochemical, endocrinological, neuroanatomical, cognitive, and neuropsychological findings are characteristics that often accompany psychiatric illness (Gottesman & Gould, 2003). Thus, these measures of neuropsychiatric functioning may be useful in studies pursuing the biological and genetics components of psychiatric disorders (Gottesman & Gould, 2003). In our model, endophenotypic markers for suicidal behavior are clinical, neurochemical, and neuroendocrine.
Clinical Endophenotypes
Clinical endophenotypes are less complex than psychiatric disorders, although these constructs can have multiple components. For example, impulsivity, aggression, neuroticism, and hopelessness may be regarded as intermediary phenotypes, possibly predisposing individuals to suicidal behavior (Baud, 2005), but are often complex themselves. The heterogeneity of the impulsivity construct illustrates this point. Cognitive, motor, and non-planning impulsiveness are major factors found across several self-report impulsiveness scales (Keilp, Sackeim, & Mann, 2005) and self-report and performance measures of impulsiveness may be addressing independent and complementary traits (Fuentes, Tavares, Artes et al., 2006).
Impulsivity/Aggression
The term impulsivity has been defined as a personality trait or cognitive style characterized by disinhibition and a tendency to act quickly on urges or in response to stimuli (Brodsky, Oquendo, Ellis et al., 2001). An association between impulsivity and suicidal/self-destructive behaviors is reported in various adult psychiatric populations (Brodsky, Oquendo, Ellis et al., 2001; Herpertz, Sass, & Favazza, 1997). Aggression and anger-related traits have also been implicated in the diathesis for suicidal behavior. Studies conducted on suicidal and non-suicidal patients suggest that anger and impulsivity synergistically contribute to an increase in suicide risk (Horesh, Rolnick, Iancu et al., 1997).
The role of impulsivity in suicidal behavior has been established as a predisposing trait in at least a subgroup of suicidal patients. However, there is ongoing debate as to whether impulsivity increases the risk of suicidal behavior independently of aggressive traits or if impulsivity is more closely related to suicide attempts than suicide completions (Baud, 2005).
Pessimism, Neuroticism, and Hopelessness
Pessimism
The tendency toward pessimism has also been implicated in suicidal behavior. Studies have shown that compared to non-attempters, suicide attempters tend to experience more pessimism as reflected in more suicidal ideation, fewer perceived reasons for living in response to illness or social adversity, and higher subjective ratings of the severity of depression and hopelessness (Oquendo, Galfalvy, Russo et al., 2004).
Neuroticism
Neuroticism is a personality trait that has been linked to negative affect (Costa & McCrae, 1980; Larsen & Ketelaar, 1991). Reports from family studies suggest that elevated neuroticism may constitute a vulnerability factor for suicidal behavior (Duggan, Sham, Lee et al., 1995). Differences in neuroticism scores between those with and without a family history of suicide were significant when personal histories of suicide attempts were taken into account, with patients with a positive family history for suicide and who had themselves attempted suicide having higher neuroticism scores (Roy, 2002).
Hopelessness
Hopelessness may also be a clinical endophenotype associated with suicidal behavior. Pollock and Williams (2004) propose that suicidal behavior is associated more with hopelessness than with the severity of depression. Hopelessness was the principal predictor of suicidality in a study conducted among schizophrenic patients (Kim, Jayathilake, & Meltzer, 2003). In elderly patients, elevated hopelessness following remission of depression was associated with a history of suicidal behavior and may predict future attempts and completion (Rifai, George, Stack et al., 1994).
Neurochemical Endophenotypes
Serotonin
Serotonin (5-HT) has long been known to be involved in emotional and cognitive functions including suicidal behavior. Of note, cerebrospinal fluid (CSF) concentrations of the major serotonin metabolite 5 hydroxyindole acetic acid (5-HIAA) are reported to reflect central nervous system serotonergic function (Stanley, Traskman-Bendz, & Dorovini-Zis, 1985). Studies of CSF 5-HIAA in suicide attempters and postmortem studies of 5-HT receptors subtypes and the 5-HT transporter in the brains of suicide victims also support a role for 5HT in suicide.
Serotonin and Clinical Endophenotypes
Low CSF 5-HIAA has been associated with impulsive, externally directed aggression has been observed in impulsive murderers and arsonists compared to non-impulsive murderers and control subjects (Brown, Ebert, Goyer et al., 1982; Linnoila, Virkkunen, Scheinin et al., 1983). This relationship between impulsivity and reduced serotonergic function led to the hypothesis that serotonergic function supports a restraint mechanism and a deficiency of serotonergic function results in greater impulsivity and aggression including the self-directed aggression of suicidal behavior (Mann, 2003). Of note, a large clinical study by Baca-Garcia, Diaz-Sastre, Basurte et al. (2001) showed an inverse relationship between impulsivity and lethality of suicide attempts, perhaps due to poorer planning capacity. Impulsivity may relate to the likelihood of suicidal behavior but not necessarily its lethality.
Only one study has focused on the association between the serotonergic system and neuroticism. A short (s) variant of the serotonin transporter linked polymorphic region (5-HTTLPR) is associated with higher neuroticism scores across individuals and families (Greenberg, Li, Lucas et al., 2000). However, further studies are needed to determine causal relationships between central serotonergic function and neuroticism.
Similarly, little is known about the role for the serotonergic system in hopelessness. A study conducted by van Heeringen, Audenaert, Van Laere et al. (2003), reported a negative correlation between prefrontal 5-HT2A receptor binding and hopelessness. Moreover, higher levels of hopelessness were reported in suicide attempters compared to normal controls. However, Meyer, McCain, Kennedy et al. (2003) noted a decrease of dysfunctional attitudes after administration of d-fenfluramine in healthy volunteers suggesting that increasing 5-HT agonism can improve negatively biased views. Bhagwager, Hinz, Taylor et al. (2006) similarly found that 5HT2A binding potential was positively associated with dysfunctional attitudes in patients with a history of depression who were euthymic at the time of study. Thus, hopelessness may be mediated through serotonergic dysfunction.
Norepinephrine
Catecolaminergic dysfunction has been hypothesized to play a role in suicide based on several observations (De Lucas, Tharmalingam, Sicard et al., 2005). A high concentration of norepinephrine (NE) with decreased alpha2-adrenergic bindings has been observed in the prefrontal cortex of suicide victims (Arango, Ernsberger, Sved et al., 1993).
Norepinephrine and Clinical Endophenotypes
Higher norepinephrine concentrations have been reported to be associated with higher levels of aggression (Mann, 2003). Similarly, lower norepinephrine concentrations appear to protect against the effects of childhood abuse on the development of aggressive behaviors or impulsivity in adulthood in males (Caspi, McClay, Moffitt et al., 2002; Huang, Cate, Buttistuziea et al., 2004).
Studies of learned helplessness have shown that rats exposed to a stressor have no significant changes in the norepinephrine system if they do not develop helplessness. However, in rats exposed to stressors who do become helpless, changes are found in the norepinephrine system (Henn & Vollmayr, 2005). These preliminary results from preclinical studies suggest there may be a role of noradrenaline in the development of pessimism or hopelessness, although human data are not yet available to test this hypothesis.
Dopamine
The dopaminergic system is abnormal in depression but the role of dopamine in suicide is still relatively uncertain (Mann, 2003). Traskman and colleagues (1981) found no difference between suicide attempters with other psychiatric illnesses compared to controls suggesting that an association between HVA and depressive illness, rather than suicidal behavior, might explain inconsistencies in HVA findings in suicidal behavior (Stoff & Mann, 1997).
Few brain-imaging studies have focused on the role of dopamine in suicidal behavior. Using single photon emission computed tomography and beta-CIT, a radioligand that tags both the dopamine and serotonin transporter, correlations between beta-CIT binding potential in the basal ganglia and mental energy as measured by the Marke-Nyman Temperament scale among suicide attempters were found (Ryding, Ahnlide, Lindström et al., 2006). This association was not noted in control subjects. Although these results were interpreted to indicate a role for the dopamine transporter in regulatory deficiency in suicide attempters, clearly, further studies are needed to delineate the precise involvement of the dopaminergic system in suicidal behavior.
Dopamine and Clinical Endophenotypes
Aggression
Increased dopamine concentrations have been associated with aggressive behavior (Rujescu, Giegling, Gietl et al., 2003) and some suggest that increased dopamine concentrations may be related to violent suicide attempts or completions (Rujescu, Giegling, Guti et al., 2003).
Neuroticism
Studies have shown associations between the dopaminergic system and neuroticism. Striatal dopamine D2 receptor may be a significant predictor of neuroticism scores (Lee, Cheng, Yang et al., 2005). Further studies are needed to determine causal relationships between neuroticism and central dopaminergic function.
Neuroendocrine Endophenotypes
Hypothalamic-Pituitary-Adrenal Axis
The hypothalamic-pituitary-adrenal (HPA) axis is the neuroendocrine system that regulates the body’s response to stress and has complex interactions with brain serotonergic, noradrenergic, and dopaminergic systems. Stress results in the release of the corticotrophin releasing hormone (CRH). CRH activates the HPA axis by stimulating the release of adrenocorticotropin (ACTH) from the pituitary. These events lead to the release of corticosteroids from adrenal glands and subsequent behavioral changes (see Chrousos & Gold, 1992). Some, but not all, studies indicate that suicidal behavior may be associated with hyperactivity of the HPA axis. These studies indicate higher cortisol levels after dexamethasone suppression (a clinical measure of HPA axis hyperactivity) and HPA axis hyperactivity at baseline levels may increase the risk of eventual suicide by as much as 14-fold (Brown, Ebert, Goyer et al., 1988; Coryell & Schlesser, 2001). Further evidence for the role of the HPA axis in suicide is provided by the association of suicide with larger adrenal glands and less prefrontal cortical CRH binding (Mann, 2003). Thus, suicidal behavior may be associated with an abnormal physiological stress response. To outline the role of stress in modulating suicidal behavior, the relationship between the HPA axis and the serotonergic, noradrenergic, and dopaminergic systems will be explored.
HPA Axis and Serotonin
The HPA axis has a bidirectional relationship with the serotonergic system (see Meijer & de Kloet, 1998). CRH neurons of the central amygdala are directly and indirectly connected to brain-stem nuclei including raphe nuclei and the locus coeruleus (LC) (Heim & Nemeroff, 2001). The raphe nuclei are the major source of serotonergic projections to the forebrain and send projections to various brain regions that contain CRH and are involved in the stress response (see also Owens & Nemeroff, 1991). The hippocampus is important for HPA feedback mechanisms as has been demonstrated in rats, where hippocampal lesions result in increased circulating corticosterone (Herman et al., 1989). In a review article, Lopez, Vasquez, Chalmers et al. (1997) considered the hippocampus as an ideal anatomical substrate to study the HPA axis, the serotonergic system and their relationship and involment with regard to suicidal behavior.
HPA hyperactivity observed in suicide appears to be not merely an epiphenomenon of the suicidal state but might be responsible for, or worsen, some of the serotonin abnormalities found in suicide (Lopez, Vasquez, Chalmers et al., 1997). For example, the removal of circulating corticosteroids by adrenalectomy results in anatomically specific decreases in indices of serotonin metabolism whereas stressful conditions, which raise levels of corticosteroids, lead to corresponding increases in serotonin turnover (reviewed by Lopez, Vasquez, Chalmers et al., 1997). Further, corticosteroids may also modulate serotonergic neurotransmission by directly regulating 5-HT receptors as indicated by increased 5-HT1 receptor binding in the rat hippocampal formation following bilateral adrenalectomy (Biegon, Rainbow, & McEwen, 1985). Thus, corticosteroids through their interaction with the 5-HT1A receptor, may play a role in the relationship between stress, mood changes, and perhaps suicide (Stoff & Mann, 1997). Other studies have examined the effects of stress and corticosteroids on 5-HT1B and 5-HT2A receptors (reviewed by Lopez, Vasquez, Chalmers et al., 1997). These studies suggest that corticosteroid modulation of 5-HT receptors may have important implications for the pathophysiology and treatment of both mood disorders and suicide.
HPA Axis and Norepinephrine
Corticotropin-releasing hormone (CRH)- and norepinephrine (NE)-containing neurons in the brain are activated during stress, and both have been implicated in behavioral responses to stress (Dunn, Swiergiel, & Palamarchouk, 2004). Animal studies show that stress activates the locus ceruleus (LC), the major norepinephrine containing nucleus in the brain, leading to the classic biological changes associated with the “fight-or-flight” reaction (Aston–Jones, Shipley, Chouvet et al., 1991). This reaction is associated with increased circulating catecholamines including NE and dopamine (see also De Bellis, Baum, Birmaher et al., 1999). LC neurons influence the neuroendocrine stress response system through their broad innervation of the paraventricular nucleus (PVN) projection pathways and it has been suggested that the reciprocal interactions connecting cerebral NE and CRH systems generate a “feed-forward” loop (Dunn, Swiergiel, Palamarchouk et al., 2004).
Studies in rats indicate that stressors, such as tail-pinch or foot-shock, elevate extracellular concentrations of NE in rat prefrontal cortex, hippocampus and amygdala (Galvez, Mesches, & McGaugh, 1996). In addition, changes in the expression of glucocorticoid receptors on LC neurons indicate a capacity to respond to stress-induced fluctuations in circulating corticosterone (Ziegler, Cass, & Herman, 1999).
Human studies have shown that although there may be fewer noradrenergic neurons in the LC of depressed suicide victims (Arango, Underwood, & Mann, 1996), the number of α2-adrenergic receptors may be higher in the LC of suicide victims (Ordway, Widdowson, Smith et al., 1994), perhaps upregulated secondary to lower noradrenaline levels. Similarly, in a study conducted by Arango, Ernsberger, Sved et al. (1993), NE levels in the prefrontal cortex of suicide victims were higher and α-adrenergic binding was lower, suggesting cortical NE overactivity. Such overactivity may be attributed to NE depletion from the smaller population of NE neurons found in suicide victims.
Individuals with adverse childhood experience exhibit exaggerated sympathetic responses to stress (Heim & Nemeroff, 2001), which can further deplete noradrenergic function (Weiss et al., 1994). Therefore, severe anxiety or agitation in response to stress might be associated with noradrenergic overactivity, overactivity of the HPA axis, and higher suicide risk (Brown, Stoll, Stokes et al., 1988).
HPA Axis and Dopamine
In addition to NE, another catecholamine, dopamine, interacts with the HPA axis. The medial prefrontal cortex (mPFC) has been implicated in the modulation of subcortical pathways that contribute to endocrine responses to stress, and dopamine is a key neurotransmitter involved in the function of the medial prefrontal cortex (Spencer, Ebner, & Day, 2004). Studies involving interruption of mPFC function have shown that this structure can operate to suppress HPA axis responses to stress (Brake, Flores, Frances et al., 2000; Buijs & Van Eden, 2000). Pharmacological antagonism of either mPFC dopamine type 1 (D1) receptors or dopamine type 2 (D2) receptors have been reported to strongly attenuate Fos expression in the CRH cells of the medial parvocellular zone of the hypothalamic paraventricular nucleus (mpPVN), a key modulator of the HPA axis, as well as blunting plasma ACTH response to a stressor (Spencer, Ebner, Day et al., 2004). These findings suggest an important role for dopamine in the regulation of HPA axis responses to stress. Indeed, the dopamine system is particularly vulnerable to stress, with low intensity stressors that normally do not produce detectable effects in most ascending catecholaminergic systems, activating mesoprefrontal dopamine neurons (Horger & Roth, 1996). Moreover, preclinical studies suggest that both acute and chronic stress may have a negative impact on the normal functioning of the dopamine system. For example, low intensity stress or brief exposure to stress increases dopamine metabolism and release in the prefrontal cortex (Vermetten & Bremner, 2002). However, despite these promising results from studies in rodents, no human data are yet available and clearly more studies are needed to evaluate these hypothesis.
In summary, the depletion of noradrenaline and the decrease in serotonergic function reported to occur in suicide and the increase in dopamine metabolism after exposure to stress, might have important physiological consequences and relate to the observed clinical endophenotypes associated with suicidal behavior.
PUTATIVE CAUSAL FACTORS
Genetics
The role of genetics in suicidal behavior has been established by twin, adoption and family studies (see also Brent & Mann, 2005). In addition, these family studies strongly support a role for genetics in the transmission of suicidal acts independently of the transmission of psychiatric disorders and suicidal ideation.
Candidate Genes
It is unlikely that a complex behavior such as suicide is dependent on a single dominant gene. Rather, a polygenic mode of inheritance has been proposed (Papadimitriou, Linkowski, Delarbre et al., 1991). One approach to identifying potential genetic markers is to study candidate genes that may be involved in the predisposition to suicidal behavior. The candidate genes for suicide include genes involved in the serotonergic, noradrenergic and dopaminergic systems such as those coding for serotonin (5-HT) receptors, 5-HT transporter (SERT), tryptophan hydroxylase (TPH), monoamine oxidase-A (MAO-A), cathecol-O-methyltransferase (COMT), and dopamine receptors.
Serotonergic System
5-HT2A Receptor Gene
Most studies have found increased prefrontal cortical 5-HT2A binding in the brain of suicide victims (Arango, Emsberger, Marzuk et al., 1990; Hrdina, Demeter, Vu et al., 1993; Mann, Stanley, McBride et al., 1986; Stanley & Mann, 1983; Turecki, Brier, Dewar et al., 1999). These findings may be the consequence of adaptive or compensatory mechanisms that up-regulate 5-HT2A receptors secondary to defective serotonin neurotransmission or genetic mediation (Mann, Arango, & Underwood, 1990). Thus, functional polymorphisms involving the promoter and changes in 5-HTA2 gene expression might play a role (Mann, Brent, & Arango, 2001). Moreover, 5HT2A binding in suicide appears to be associated with aggression (Oquendo, Russo, Underwood et al., 2006). Zhang, Ishaigaki, Taki et al. (1997) examined a polymorphism (102 T/C) in the 5-HT2A receptor (5-HTR2A) gene and found a weak association between the TT genotype and suicide attempts in patients with mood disorders. Turecki, Briere, Dewar et al. (1999) studied the effect of 102 T/C and 1438 A/G polymorphisms on 5-HT2Areceptor binding. They found a relatively large effect size for suicide victims with haplotype 102T-1438A as opposed to non-suicide victims with haplotype 102C/-1438G. A postmortem study of teenage suicide victims noted that the increased number of 5-HT2A receptor binding sites was not a consequence of more binding sites alone (Pandey, Divendi, Rizavi et al., 2002). Rather, gene expression contributed to this increased number as indicated by higher gene expression of 5-HT2A receptors and the cognate protein in the prefrontal cortex and the hippocampus.
However, not all studies agree that there are altered numbers of 5-HT2A receptors in the prefrontal cortex of suicide victims (reviewed by Stockmeier, Dilley, Shapiro et al., 1997). For example, we (Oquendo, Russo, Underwood et al., 2006) recently have reported no differences in 5-HT2A binding in most BA regions of the prefrontal cortex in suicide victims using a similar methodology to Turecki, Briere, Dewar et al.’s (1999) study in a larger sample. Methodological differences related to the psychiatric diagnosis of the suicide victims, the diagnostic criteria used, the precise defined area of the PFC studied, the use of different ligands, (e.g., agonists compared to antagonists), the inclusion and exclusion criteria, the presence of subjects with antidepressant treatment prior to or at the time of the death, etc. may explain the lack of agreement on the status of the 5-HT2A receptor in suicide (Stockmeier, Dilley, Shapiro et al., 1997).
Serotonin Transporter Gene
The serotonin transporter (5-HTT) binding site is found on serotonin nerve terminals and platelets and can be regarded as an index of serotonin nerve terminal number or integrity (Kovachich, Aronson, Brunswick et al., 1988). The human serotonin transporter gene is located on chromosome 17 and a 44 base pair deletion/insertion polymorphism (5-HTTLPR) results in differential expression of the gene product and Vmax of serotonin reuptake in transformed lymphoblastoid cell lines (Lesch, Bengal, Heils et al., 1996). This relatively common 5-HTTLPR polymorphism results in the expression of short [homozygous (SS) and heterozygous (SL)] and long [homozygous (LL)] variants of the 5-HTTLPR locus (see Arango, Huang, Underwood, et al., 2003). In transformed lymphoblast cell lines, the short variant of the 5-HTTLPR locus is associated with fewer binding sites than the long variants (Lesch, Bengal, Heils et al., 1996). Various lines of evidence indicate decreased serotonin function associated with the low expressing 5-HTTLPR allele (SS or SL alleles) including; blunted neuroendocrine response to fenfluramine (the serotonin reuptake inhibitor and releasing agent), lower platelet serotonin uptake, and lower cerebrospinal fluid concentrations of serotonin metabolites (5-hydroxyindoleacetic acid, 5-HIAA) in women. Smith, Lotrich, Malhorta et al. (2004) report these alleles are associated with blunted prolactin and cortisol response and greater decreases in left frontal, precentral and middle temporal gyri compared to the higher expressing 5-HTTLPR genotype (LL allele) in response to the SSRI citalopram in healthy volunteers. The latter allele carriers show greater decreases in right frontal, insula and superior temporal gyrus compared to SS genotype. Some studies have found an association between 5-HTTLPR polymorphisms and suicidality (Baca-Garcia, Vaquero, Diaz-Sastre et al., 2002 ; Bondy, Erfurth, de Jonge et al., 2000) while other studies have not (Bellivier, Szoke, Henry et al., 2000; Mann, Huang, Underwood et al., 2000; Zalsman, Frisch, Lewis et al., 2001). Although alterations in serotonin transporter binding specifically related to the risk for suicide appear to be concentrated in the ventral prefrontal cortex (Arango, Underwood, Gubbi et al., 1995; Mann, Huang, Underwood et al., 2000) a brain region known to play a role in mediating inhibition/restraint, some studies find that the 5-HTTLPR genotype is not clearly associated with impulsive traits (Ball, Hill, Freeman et al., 1997; Lesch, Bengal, Heils et al., 1996).
Tryptophan Hydroxylase Gene
Tryptophan hydroxylase (TPH) is the rate limiting biosynthetic enzyme for serotonin. The recently discovered TPH2 variant is largely expressed in the human brain, and has been the subject of few studies to date (Zill, Buttner, Eisenmenger et al., 2004). While one study did not find any difference in the mRNA levels of the TPH2 gene in the dorsolateral prefrontal cortex between suicide and non-suicide subjects, another recent study provides evidence for the involvement of genetic variants of the TPH2 gene in suicidal behavior (De Lucas, Mueller, Tharmalingen et al., 2004; Zill, Buttner, Eisenmenger et al., 2004). However, given that most forebrain serotonin is synthesized by neurons in the dorsal and median raphe nuclei (DRN and MRN, respectively) in the brainstem (Tork, 1990) and the cortex only contains serotonin terminals, the relevance of measuring mRNA levels of TPH2 in the cortex is controversial. Moreover, polymerase chain reaction (PCR) can magnify the amount of transcript, and thus, verification with other methods, such as in situ hybridization, is key. Interestingly, Bach-Mizrachi, Underwood, Kassir et al. (2006) recently measured TPH2 mRNA in DRN and MRN using in situ hybridization. They found 33% more TPH2 mRNA expression in suicides compared with matched controls in the DRN and 17% more in the MRN. These results were interpreted as a homeostatic response to chronically deficient serotonin levels in depressed suicide victims. Further studies are needed to investigate the role of genetic variations in TPH2 activity and their impact suicidal behavior.
Noradrenergic System
To address the role of NE in suicidal behavior, data regarding two enzymes, monoamine oxidase A (MAOA) and COMT, associated with NE will be presented.
Monoamine Oxidase A
MAOA is a mitochondrial enzyme that preferentially deaminates NE and serotonin. Note that our consideration of MAOA under the section on the noradrenergic system is not intended to deemphasize its role in the metabolism of serotonin. The MAOA gene has been mapped to the short arm of the X chromosomes and a number of polymorphisms have been described within the gene. Because the gene for MAOA is sex linked, it has been suggested that the higher rate of suicides among males could be due to greater impulsivity and aggression, secondary to MAOA polymorphisms (Du et al., 2002). Of note, an animal study showed that deletion of the MAOA gene in mice resulted in a more aggressive phenotype (Cases, Seif, Grimsby et al., 1995) and a study of an extended Dutch pedigree suggested that failure to express the gene for MAOA was associated with pathological aggressive behavior in males (Brunner, Nelen, Breakefield et al., 1993). The lower expressing allele of the MAOA-uVNTR polymorphism has also been associated with a history of early abuse in males and with higher impulsivity in males but not females (Huang, Cate, Battistuzzi et al., 2004). These results suggest that the lower expression of the MAOA-uVNTR polymorphism is related to a history of early abuse and may sensitize males to the effects of early abuse experiences on impulsive traits in adulthood (Huang, Cate, Battistuzzi et al., 2004).
COMT Gene
The cathecol-O-methyl transferase (COMT) enzyme is a major enzyme in norepinephrine inactivation and several polymorphisms have been identified in this gene. A Val158Met polymorphism in the COMT gene results in the allele encoding valine having relatively high activity compared to that encoding methionine (De Lucas, Tharmalingam, Sicard et al., 2005). The methionine allele (Met allele) is reportedly associated with violent suicide attempt, specifically in male schizophrenics (Nolan, Volavka, Czobar et al., 2000). The Met allele for COMT may be associated with suicide attempts but it has been suggested that in order to increase its penetrance it needs to interact with the high expression allele in the MAOA gene or male gender (De Lucas, Tharmalingam, Sicard et al., 2005). A low enzyme activity COMT variant (L allele) was found to be associated with suicide, especially by violent means (Nolan, Volavka, Czobar et al., 2000; Rujescu, Giegling, Gietl et al., 2003). Some authors suggest, however, that this polymorphism might modify the phenotype of suicidal behavior rather than susceptibility to suicidal behavior itself (Rujescu, Giegling, Gietl et al., 2003). Further, in a comparison of male suicide completers and controls, the Val/Val (high activity) genotype of the COMT 158Val/Met polymorphism was reported to have a protective role against suicide (Ono, Shirakawa, Nushida et al., 2004). However, another study did not discover any differences in COMT genotype frequencies between suicidal inpatients and controls (Russ, Lachman, Kashdan et al., 2000). In that study, not all suicidal inpatients had made a suicide attempt, and as noted earlier, suicidal ideation may have a genetic component that differs from that for suicidal acts. The involvement of COMT in the genetics of aggression suggests a role for the catecholamines (dopamine and NA) in aggression.
Dopaminergic System
Studies on the relationship between genetic variants of dopamine receptors and suicide have yielded mixed results. No alteration of mRNA has been found for the dopamine D1 and D2 receptors in the caudate nucleus of suicide victims (Hurd, Herman, Hyde et al., 1997). On the other hand, studies assessing growth hormone response to apomorphine, a dopamine agonist, in depressed and non-depressed patients with histories of suicide attempt, suggest involvement of D2-dopaminergic function in the expression of suicidal behavior (Pitchot, Hansenne, & Ansseau, 2001; Pitchot, Hansenne, Moreno et al., 1992). A study by Persson, Geijer, Wasserman et al. (1999) of a 48 base pair polymorphism in the gene coding for dopamine D4 receptor did not find any association with suicide attempt. This lack of association persisted even when the suicide attempters were grouped into different diagnostic groups. Similarly, a study of Israeli suicidal adolescent inpatients did not discover any significant association between a polymorphism in dopamine receptor subtype 4 gene (DRD4) and suicidal behavior (Zalsman, Frisch, Bromberg et al., 2004). Thus, genetic evidence linking DA related genes and suicidal acts is minimal. However, research focusing on the role of cholecystokinin, a neurotransmitter related to dopamine, in suicidal behavior has recently yielded interesting results (Bachus, Hyde, Herman et al., 1997; Lofberg, Agren, Harro et al., 1998).
Inconsistencies in candidate gene studies may result from methodological difficulties, which are to be expected when studying a complex phenomenon such as suicide. Future research focused on the relationship of genetic variants to intermediate phenotypes such as impulsivity and aggression may be of utility, as the relationship between these phenotypes and vulnerability genes may be easier to demonstrate than those for complex syndromes like suicide (Terwilliger & Goring, 2000). Another reason for such inconsistencies may be the influence of environmental factors.
Childhood Adversity
The second causal factor in our model for suicide concerns the impact of early childhood adversity on risk for suicidal behavior. Adverse childhood events may include sexual abuse, physical abuse, child neglect, parental loss, and severe family discord, however, to date, physical and sexual abuse have been the focus of most studies (Mann, 2003). Initial findings suggest sexual and physical abuse independently contribute to repeated suicide attempts after controlling for a range of childhood adversities such as parental loss and neglect (Ystgaard, Hestetun, Loeb et al., 2004). Moreover, the risk of suicide attempt in individuals with a childhood history of sexual abuse is reported to be over ten times that of those who have never been sexually abused (Soloff, Lynch, & Kelly, 2002). There is also evidence that childhood abuse might contribute to earlier age of onset of suicidal behavior (Brodsky, Oquendo, & Ellis, 2001) and that the presence of sexual abuse in parent and child increases the risk for transmission of suicidal behavior (Brent & Mann, 2005). The relationship between childhood trauma and self-destructive behavior in adulthood may be mediated in part by a relationship between abuse history and the development of the biological features and psychological aspects of the impulsivity trait (Brodsky, Oquendo, Ellis et al., 2001). This implies that impulsivity and aggression may be personality traits that are heavily influenced and modulated by early childhood experiences including childhood adversity. However, after adjusting for impulsivity and aggression, abuse history was still significantly associated with suicide attempt status illustrating that the presence of impulsivity and aggression do not fully explain the relationship between childhood abuse history and suicidal behavior (Brodsky, Oquendo, Ellis et al., 2001).
Although some have postulated that childhood adversity is transmitted in families through a shared environment rather than genetic mechanisms, the liability for suicide attempts may in fact arise from both environmental and genetic manifestations of a common diathesis (Brent & Mann, 2005). Stressful life events and a reported history of abuse moderate the expression of the genetic liability for violence and mood disorders (Caspi, McClay, Moffitt et al., 2002; Caspi, Sugden, Moffitt et al., 2003). This is exemplified by the notion that parents who abuse their children are also more likely to be suicide attempters as well as have mood and substance abuse disorders (Chaffin, Kelleher, Hollenberg et al., 1996). In addition, studies by Caspi, McClay Moffitt et al. (2002) and Caspi, Sugden, Moffitt et al. (2003), support a gene-environment interaction. In their first study, a large sample of males were studied from birth to adulthood to determine why some maltreated children develop antisocial behavior (and also impulsivity and aggression), whereas others do not. The authors suggested that an individual’s response to environmental insult is moderated by their genetic makeup.
A review of preclinical and clinical studies found considerable evidence to suggest that adverse early life experiences have a profound effect on the developing brain. Repeated early life stress leads to alterations in central neurobiological systems, particularly in the corticotropin-releasing hormone system, leading to increased responsiveness to stress. Children who are exposed to sexual or physical abuse, or the death of a parent are at higher risk for developing depressive and anxiety disorders later in life (Nemeroff, 2004). Therefore, persistent sensitization of central nervous system (CNS) circuits as a consequence of early life stress may represent an underlying biological substrate of the development of depression and anxiety (Heim & Nemeroff, 2002) as well as suicidal behavior.
In terms of neurochemical endophenotypic markers, there appears to be a significant interaction between genes and the environment in relation to the functioning of the serotonergic, noradrenergic, and dopaminergic systems (Mann, 2003). For example, studies in rhesus monkeys demonstrate that peer reared monkeys have lower serotonergic activity compared to maternally raised monkeys (Higley, Thompson, Champoux et al., 1993). Furthermore, this lower activity persists into adulthood and is manifested in greater impulsivity and aggression. This phenomenon may be due the effects of adverse rearing (childhood adversity) on serotonergic function, setting it at lower levels, and the persistence of these low serotonergic levels into adulthood might contribute to the diathesis to suicide (Mann, 2003). Consistent with this, Roy et al. (2002) found a negative correlation between childhood emotional neglect scores and CSF 5-HIAA and HVA concentration in adult individuals. Therefore, it appears that childhood adversity in these patients is associated with lower serotonergic and dopaminergic function in adulthood.
SUMMARY AND CONCLUSIONS
Suicide is among the top ten leading causes of death in individuals of all ages. Most prior research efforts have focused on the role of psychological and sociocultural factors in determining suicide risk. However, to date, these risk factors offer only weak prediction capacity hindering their clinical relevance. An explanatory model for suicidal behavior that links clinical and psychological risk factors or endophenotypes, to the underlying neurobiological abnormalities associated with suicidal behavior may enhance prediction, help identify treatment options and may have heuristic value.
Integrating clinical and neurobiological data, our explanatory model proposes that developmental factors that are biological (genetics) and psychological or clinical (early childhood adversity) may have causal relevance to the disturbances found in subjects with suicidal behavior. Specifically, genotype and early childhood experiences may influence the manifestation of neurobiological and clinical factors associated with suicidal behavior, namely neuroendocrine (hypothalamic-pituitaryadrenal axis-HPA axis), neurochemical (serotonin, norepinephrine and dopamine), and clinical (aggression/impulsivity, pessimism, neuroticism, and hopelessness) endophenotypes. We provide data to illustrate the close interaction between serotonin, norepinephrine and dopamine; and the hypothalamic-pituitary-adrenal axis; as well as with aggression/impulsivity, pessimism, neuroticism, and hopelessness endophenotypes. In essence, we hypothesize that genetics and early environmental challenges influence HPA axis function, which in turn interacts with brain neurochemistry. These effects of HPA axis function on neurochemistry and HPA function itself have been shown to be related to several clinical endophenotypes relevant to suicidal acts and may provide the link between suicidal behavior and these diverse clinical and neurobiological risk factors. In this way, our model integrates findings from several perspectives in suicidology and attempts to explain the relationship between various neurobiological, genetic, and clinical observations in suicide research, offering a comprehensive hypothesis to facilitate understanding of this complex outcome.
Acknowledgments
This study was supported by PHS grants MH059710, MH062185 and MH056390. Dr Carballo’s work was supported by the Alicia Koplowitz Foundation via Fellowship in Child and Adolescent Psychiatry.
REFERENCES
- Arango V, Ernsberger P, Marzuk PM, et al. Autoradiographic demonstration of increased serotonin 5-HT2 and beta-adrenergic receptor binding sites in the brain of suicide victims. Archives of General Psychiatry. 1990;47:1038–1047. doi: 10.1001/archpsyc.1990.01810230054009. [DOI] [PubMed] [Google Scholar]
- Arango V, Ernsberger P, Sved AF, et al. Quantitative autoradiography of alpha 1- and alpha 2-adrenergic receptors in the cerebral cortex of controls and suicide victims. Brain Research. 1993;630:271–282. doi: 10.1016/0006-8993(93)90666-b. [DOI] [PubMed] [Google Scholar]
- Arango V, Huang YY, Underwood MD, et al. Genetics of the serotonergic system in suicidal behavior. Journal of Psychiatirc Research. 2003;37:375–386. doi: 10.1016/s0022-3956(03)00048-7. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Gubbi AV, et al. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontal cortex of suicide victims. Brain Research. 1995;7:121–33. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Fewer pigmented locus coeruleus neurons in suicide victims: preliminary results. Biological Psychiatry. 1996;39:112–120. doi: 10.1016/0006-3223(95)00107-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Shipley MT, Chouvet G, et al. Afferent regulation of locus coeruleus neurons: Anatomy, physiology and pharmacology. Progress in Brain Research. 1991;88:47–75. doi: 10.1016/s0079-6123(08)63799-1. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Diaz-Sastre C, Basurte E, Prieto R, et al. A prospective study of the paradoxical relationship between impulsivity and lethality of suicide attempts. Journal of Clinical Psychiatry. 2001;62:560–564. doi: 10.4088/jcp.v62n07a11. [DOI] [PubMed] [Google Scholar]
- Baca-Garcia E, Vaquero C, Diaz-Sastre C, et al. A gender-specific association between the serotonin transporter gene and suicide attempts. Neuropsychopharmacology. 2002;26:692–695. doi: 10.1016/S0893-133X(01)00394-3. [DOI] [PubMed] [Google Scholar]
- Bach-Mizrachi H, Underwood MD, Kassir SA, et al. Neuronal tryptophan hydroxylase mRNA expression in the human dorsal and median raphe nuclei: Major depression and suicide. Neuropsychopharmacology. 2006;31:814–824. doi: 10.1038/sj.npp.1300897. [DOI] [PubMed] [Google Scholar]
- Bachus SE, Hyde TM, Herman MM, et al. Abnormal cholecystokinin mRNA levels in entorhinal cortex of schizophrenics. Journal of Psychiatric Research. 1997;31:233–256. doi: 10.1016/s0022-3956(96)00041-6. [DOI] [PubMed] [Google Scholar]
- Ball D, Hill L, Freeman B, Eley TC, et al. The serotonin transporter gene and peer-rated neuroticism. Neuroreport. 1997;8:1301–1304. doi: 10.1097/00001756-199703240-00048. [DOI] [PubMed] [Google Scholar]
- Baud P. Personality traits as intermediary phenotypes in suicidal behavior: Genetic issues. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2005;133:34–42. doi: 10.1002/ajmg.c.30044. [DOI] [PubMed] [Google Scholar]
- Beautrais AL, Joyce PR, Mulder RT, et al. Prevalence and comorbidity of mental disorders in persons making serious suicide attempts: A case-control study. American Journal of Psychiatry. 1996;153:1009–1014. doi: 10.1176/ajp.153.8.1009. [DOI] [PubMed] [Google Scholar]
- Bellivier F, Szoke A, Henry C, et al. Possible association between serotonin transporter gene polymorphism and violent suicidal behavior in mood disorders. Biological Psychiatry. 2000;48:319–322. doi: 10.1016/s0006-3223(00)00891-x. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Hinz R, Taylor M, et al. Increased 5-HT(2A) receptor binding in euthymic, medication-free patients recovered from depression: a positron emission study with [(11)C]MDL 100,907. American Journal of Psychiatry. 2006;163:1580–1587. doi: 10.1176/ajp.2006.163.9.1580. [DOI] [PubMed] [Google Scholar]
- Biegon A, Rainbow TC, McEwen BS. Corticosterone modulation of neurotransmitter receptors in rat hippocampus: A quantitative autoradiographic study. Brain Research. 1985;332:309–314. doi: 10.1016/0006-8993(85)90599-2. [DOI] [PubMed] [Google Scholar]
- Bondy B, Erfurth A, de Jonge S, et al. Possible association of the short allele of the serotonin transporter promoter gene polymorphism (5-HTTLPR) with violent suicide. Molecular Psychiatry. 2000;5:193–195. doi: 10.1038/sj.mp.4000678. [DOI] [PubMed] [Google Scholar]
- Brake WG, Flores G, Francis D, et al. Enhanced nucleus accumbens dopamine and plasma corticosterone stress responses in adult rats with neonatal excitotoxic lesions to the medial prefrontal cortex. Neuroscience. 2000;96:687–695. doi: 10.1016/s0306-4522(00)00002-6. [DOI] [PubMed] [Google Scholar]
- Breidenthal SE, White DJ, Glatt CE. Identification of genetic variants in the neuronal form of tryptophan hydroxylase (TPH2) Psychiatric Genetics. 2004;14:69–72. doi: 10.1097/01.ypg.0000107929.32051.58. [DOI] [PubMed] [Google Scholar]
- Brent DA, Mann JJ. Family genetic studies, suicide, and suicidal behavior. American Journal of Medical Genetics Part C: Seminars in Medical Geneticsv. 2005;133:13–24. doi: 10.1002/ajmg.c.30042. [DOI] [PubMed] [Google Scholar]
- Brodsky BS, Oquendo M, Ellis SP, et al. The relationship of childhood abuse to impulsivity and suicidal behavior in adults with major depression. American Journal of Psychiatry. 2001;158:1871–1877. doi: 10.1176/appi.ajp.158.11.1871. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, et al. Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. American Journal of Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Brown RP, Stoll PM, Stokes PE, et al. Adrenocortical hyperactivity in depression: Effects of agitation, delusions, melancholia, and other illness variables. Psychiatry Research. 1988;23:167–178. doi: 10.1016/0165-1781(88)90007-8. [DOI] [PubMed] [Google Scholar]
- Brunner HG, Nelen M, Breakefield XO, et al. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science. 1993;262:578–580. doi: 10.1126/science.8211186. [DOI] [PubMed] [Google Scholar]
- Buijs RM, Van Eden CG. The integration of stress by the hypothalamus, amygdala and prefrontal cortex: Balance between the autonomic nervous system and the neuroendocrine system. Progress in Brain Research. 2000;126:117–132. doi: 10.1016/S0079-6123(00)26011-1. [DOI] [PubMed] [Google Scholar]
- Cases O, Seif I, Grimsby J, et al. Aggressive behavior and altered amounts of brain serotonin and norepinephrine in mice lacking MAOA. Science. 1995;268:1763–1766. doi: 10.1126/science.7792602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaffin M, Kelleher K, et al. Onset of physical abuse and neglect: Psychiatric, substance abuse, and social risk factors from prospective community data. Child Abuse & Neglect. 1996;20:191–203. doi: 10.1016/s0145-2134(95)00144-1. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Journal of the American Medical Association. 1992;267:1244–1252. [PubMed] [Google Scholar]
- Coryell W, Schlesser M. The dexamethasone suppression test and suicide prediction. American Journal of Psychiatry. 2001;158:748–753. doi: 10.1176/appi.ajp.158.5.748. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr., McCrae RR. Influence of extraversion and neuroticism on subjective well-being: Happy and unhappy people. Journal of Personality and Social Psychology. 1980;38:668–678. doi: 10.1037//0022-3514.38.4.668. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, et al. A.E. Bennett Research Award. Developmental traumatology. Part I: Biological stress systems. Biological Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- De Lucas V, Mueller DJ, Tharmalingam S, et al. Analysis of the novel TPH2 gene in bipolar disorder and suicidality. Molecular Psychiatry. 2004;9:896–897. doi: 10.1038/sj.mp.4001531. [DOI] [PubMed] [Google Scholar]
- De Lucas V, Tharmalingam S, Sicard T, et al. Gene-gene interaction between MAOA and COMT in suicidal behavior. Neuroscience Letters. 2005;383:151–154. doi: 10.1016/j.neulet.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Du L, Faludi G, Palkovits M, et al. High activity-related allele of MAO-A gene associated with depressed suicide in males. Neuroreport. 2002;13:1195–1198. doi: 10.1097/00001756-200207020-00025. [DOI] [PubMed] [Google Scholar]
- Duggan C, Sham P, Lee A, et al. Neuroticism: A vulnerability marker for depression evidence from a family study. Journal of Affective Disorders. 1995;35:139–143. doi: 10.1016/0165-0327(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH, Palamarchouk V. Brain circuits involved in corticotropin-releasing factor-norepinephrine interactions during stress. Annals of the New York Academy of Sciences. 2004;1018:25–34. doi: 10.1196/annals.1296.003. [DOI] [PubMed] [Google Scholar]
- Fuentes D, Tavares H, Artes R, et al. Self-reported and neuropsychological measures of impulsivity in pathological gambling. Journal of the International Neuropsychological Society. 2006;12:907–912. doi: 10.1017/S1355617706061091. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiology of Learning and Memory. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. American Journal of Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Greenberg BD, Li Q, Lucas FR, et al. Association between the serotonin transporter promoter polymorphism and personality traits in a primarily female population sample. American Journal of Medical Genetics. 2000;96:202–216. doi: 10.1002/(sici)1096-8628(20000403)96:2<202::aid-ajmg16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biological Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Henn FA, Vollmayr B. Stress models of depression: Forming genetically vulnerable strains. Neuroscience and Biobehavioral Reviews. 2005;29:799–804. doi: 10.1016/j.neubiorev.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Herman JP, Schäfer MK, Young EA, et al. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo-pituitary-adrenocortical axis. The Journal of Neuroscience. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herpertz S, Sass H, Favazza A. Impulsivity in self-mutilative behavior: Psychometric and biological findings. Journal of Psychiatric Research. 1997;31:451–465. doi: 10.1016/s0022-3956(97)00004-6. [DOI] [PubMed] [Google Scholar]
- Higley JD, Thompson WW, Champoux M, et al. Paternal and maternal genetic and environmental contributions to cerebrospinal fluid monoamine metabolites in rhesus monkeys (Macaca mulatta) Archives of General Psychiatry. 1993;50:615–623. doi: 10.1001/archpsyc.1993.01820200025003. [DOI] [PubMed] [Google Scholar]
- Horesh N, Rolnick T, Iancu I, et al. Anger, impulsivity and suicide risk. Psychotherapy and Psychosomatics. 1997;66:92–96. doi: 10.1159/000289115. [DOI] [PubMed] [Google Scholar]
- Horger BA, Roth RH. The role of mesoprefrontal dopamine neurons in stress. Critical Reviews in Neurobiology. 1996;10:395–418. doi: 10.1615/critrevneurobiol.v10.i3-4.60. [DOI] [PubMed] [Google Scholar]
- Hrdina PD, Demeter E, Vu T, et al. 5-HT uptake sites and 5-HT2 receptors in brain of antidepressant-free suicide victims/depressives: sives: Iincrease in 5-HT2 sites in cortex and amygdala. Brain Research. 1993;614:37–44. doi: 10.1016/0006-8993(93)91015-k. [DOI] [PubMed] [Google Scholar]
- Huang YY, Cate SP, Battistuzzi C, et al. An association between a functional polymorphism in the monoamine oxidase a gene promoter, impulsive traits and early abuse experiences. Neuropsychopharmacology. 2004;29:1498–1505. doi: 10.1038/sj.npp.1300455. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herman MM, Hyde TM, et al. Prodynorphin mRNA expression is increased in the patch vs. matrix compartment of the caudate nucleus in suicide subjects. Molecular Psychiatry. 1997;2:495–500. doi: 10.1038/sj.mp.4000319. [DOI] [PubMed] [Google Scholar]
- Keilp JG, Sackeim HA, Mann JJ. Correlates of trait impulsiveness in performance measures and neuropsychological tests. Psychiatry Research. 2005;30:191–201. doi: 10.1016/j.psychres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kim CH, Jayathilake K, Meltzer HY. Hopelessness, neurocognitive function, and insight in schizophrenia: Relationship to suicidal behavior. Schizophrenia Research. 2003;60:71–80. doi: 10.1016/s0920-9964(02)00310-9. [DOI] [PubMed] [Google Scholar]
- Kovachich GB, Aronson CE, Brunswick, et al. Quantitative autoradiography of serotonin uptake sites in rat brain using [3H]cyanoimipramine. Brain Research. 1988;28:78–88. doi: 10.1016/0006-8993(88)90805-0. [DOI] [PubMed] [Google Scholar]
- Larsen RJ, Ketelaar T. Personality and susceptibility to positive and negative emotional states. Journal of Personality and Social Psychology. 1991;61:132–140. doi: 10.1037//0022-3514.61.1.132. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cheng CC, Yang YK, et al. Correlation between striatal dopamine D2 receptor density and neuroticism in community volunteers. Psychiatry Research. 2005;138:259–264. doi: 10.1016/j.pscychresns.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Lesch KP, Bengel D, Heils A, et al. Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science. 1996;274:1527–1531. doi: 10.1126/science.274.5292.1527. 1996. [DOI] [PubMed] [Google Scholar]
- Linnoila M, Virkkunen M, Scheinin M, et al. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentration differentiates impulsive from nonimpulsive violent behavior. Life Science. 1983;33:2609–2614. doi: 10.1016/0024-3205(83)90344-2. 1983. [DOI] [PubMed] [Google Scholar]
- Lofberg C, Agren H, Harro J, et al. Cholecystokinin in CSF from depressed patients: Possible relations to severity of depression and suicidal behaviour. European Neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1998;8:153–157. doi: 10.1016/s0924-977x(97)00046-1. [DOI] [PubMed] [Google Scholar]
- Lopez JF, Vazquez DM, Chalmers DT, et al. Regulation of 5-HT receptors and the hypothalamic-pituitary-adrenal axis. Implications for the neurobiology of suicide. Annals of the New York Academy of Sciences. 1997;29:106–134. doi: 10.1111/j.1749-6632.1997.tb52357.x. [DOI] [PubMed] [Google Scholar]
- Mann JJ. Neurobiology of suicidal behaviour. Nature Reviews. Neuroscience. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Arango V. Integration of neurobiology and psychopathology in a unified model of suicidal behavior. Journal of Clinical Psychopharmacology. 1992;12:2S–7S. [PubMed] [Google Scholar]
- Mann JJ, Arango V, Underwood MD. Serotonin and suicidal behavior. Annals of the New York Academy of Sciences. 1990;600:476–484. doi: 10.1111/j.1749-6632.1990.tb16903.x. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Brent DA, Arango V. The neurobiology and genetics of suicide and attempted suicide: A focus on the serotonergic system. Neuropsychopharmacology. 2001;24:467–477. doi: 10.1016/S0893-133X(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Huang YY, Underwood MD, et al. A serotonin transporter gene promoter polymorphism (5-HTTLPR) and prefrontal cortical binding in major depression and suicide. Archives of General Psychiatry. 2000;57:729–738. doi: 10.1001/archpsyc.57.8.729. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Stanley M, McBride PA, et al. Increased serotonin2 and beta-adrenergic receptor binding in the frontal cortices of suicide victims. Archives of General Psychiatry. 1986;43:954–959. doi: 10.1001/archpsyc.1986.01800100048007. [DOI] [PubMed] [Google Scholar]
- Meijer OC, De Kloet ER. Corticosterone and serotonergic neurotransmission in the hippocampus: Functional implications of central corticosteroid receptor diversity. Critical Reviews in Neurobiology. 1998;12:1–20. [PubMed] [Google Scholar]
- Meyer JH, McMain S, Kennedy SH, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. American Journal of Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB. Neurobiological consequences of childhood trauma. Journal of Clinical Psychiatry. 2004;65(Suppl 1):18–28. [PubMed] [Google Scholar]
- Nolan KA, Volavka J, Czobor P, et al. Suicidal behavior in patients with schizophrenia is related to COMT polymorphism. Psychiatric Genetics. 2000;10:117–124. doi: 10.1097/00041444-200010030-00003. [DOI] [PubMed] [Google Scholar]
- Ono H, Shirakawa O, Nushida H, et al. Association between catechol-O-methyltransferase functional polymorphism and male suicide completers. Neuropsychopharmacology. 2004;29:1374–1377. doi: 10.1038/sj.npp.1300470. 2004. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Galfalvy H, Russo S, et al. Prospective study of clinical predictors of suicidal acts after a major depressive episode in patients with major depressive disorder or bipolar disorder. American Journal of Psychiatry. 2004;161:1433–1441. doi: 10.1176/appi.ajp.161.8.1433. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Malone KM, Mann JJ. Suicide: Risk factors and prevention in refractory major depression. Depression and Anxiety. 1997;5:202–211. [PubMed] [Google Scholar]
- Oquendo MA, Russo SA, Underwood MD, et al. Higher postmortem prefrontal 5-HT(2A) receptor binding correlates with lifetime aggression in suicide. Biological Psychiatry. 2006;59:235–243. doi: 10.1016/j.biopsych.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Ordway GA, Widdowson PS, Smith KS, et al. Agonist binding to alpha 2-adrenoceptors is elevated in the locus coeruleus from victims of suicide. Journal of neurochemistry. 1994;63:617–624. doi: 10.1046/j.1471-4159.1994.63020617.x. [DOI] [PubMed] [Google Scholar]
- Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacological Reviews. 1991;43:425–473. [PubMed] [Google Scholar]
- Pandey GN, Dwivedi Y, Rizavi HS, et al. Higher expression of serotonin 5-HT(2A) receptors in the postmortem brains of teenage suicide victims. American Journal of Psychiatry. 2002;159:419–429. doi: 10.1176/appi.ajp.159.3.419. [DOI] [PubMed] [Google Scholar]
- Papadimitriou GN, Linkowski P, Delarbre C, et al. Suicide on the paternal and maternal sides of depressed patients with a lifetime history of attempted suicide. Acta Psychiatrica Scandinavica. 1991;83:417–419. doi: 10.1111/j.1600-0447.1991.tb05567.x. [DOI] [PubMed] [Google Scholar]
- Persson ML, Geijer T, Wasserman D, et al. Lack of association between suicide attempt and a polymorphism at the dopamine receptor D4 locus. Psychiatric Genetics. 1999;9:97–100. doi: 10.1097/00041444-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, Ansseau M. Role of dopamine in non-depressed patients with a history of suicide attempts. European Psychiatry: The Journal of the Association of European Psychiatrists. 2001;16:424–427. doi: 10.1016/s0924-9338(01)00601-0. [DOI] [PubMed] [Google Scholar]
- Pitchot W, Hansenne M, Moreno AG, et al. Suicidal behavior and growth hormone response to apomorphine test. Biological Psychiatry. 1992;31:1213–1219. doi: 10.1016/0006-3223(92)90340-6. [DOI] [PubMed] [Google Scholar]
- Pollock LR, Williams JM. Problem-solving in suicide attempters. Psychological Medicine. 2004;34:163–167. doi: 10.1017/s0033291703008092. [DOI] [PubMed] [Google Scholar]
- Rifai AH, George CJ, Stack JA, et al. Hopelessness in suicide attempters after acute treatment of major depression in late life. American Journal of Psychiatry. 1994;151:1687–1690. doi: 10.1176/ajp.151.11.1687. [DOI] [PubMed] [Google Scholar]
- Roy A. Family history of suicide and neuroticism: a preliminary study. Psychiatry Research. 2002;110:87–90. doi: 10.1016/s0165-1781(02)00011-2. [DOI] [PubMed] [Google Scholar]
- Rujescu D, Giegling I, Gietl A, et al. A functional single nucleotide polymorphism (V158M) in the COMT gene is associated with aggressive personality traits. Biological Psychiatry. 2003;54:34–39. doi: 10.1016/s0006-3223(02)01831-0. [DOI] [PubMed] [Google Scholar]
- Russ MJ, Lachman HM, Kashdan T, et al. Analysis of catechol-O-methyltransferase and 5-hydroxytryptamine transporter polymorphisms in patients at risk for suicide. Psychiatry Research. 2000;93:73–78. doi: 10.1016/s0165-1781(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Ryding E, Ahnlide JA, Lindstrom M, et al. Regional brain serotonin and dopamine transporter binding capacity in suicide attempters relate to impulsiveness and mental energy. Psychiatry Research. 2006;148:195–203. doi: 10.1016/j.pscychresns.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Fisher P, et al. Psychiatric diagnosis in child and adolescent suicide. Archives of General Psychiatry. 1996;53:339–348. doi: 10.1001/archpsyc.1996.01830040075012. [DOI] [PubMed] [Google Scholar]
- Smith GS, Lotrich FE, Malhotra AK, et al. Effects of serotonin transporter promoter polymorphisms on serotonin function. Neuropsychopharmacology. 2004;29:2226–2234. doi: 10.1038/sj.npp.1300552. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Lynch KG, Kelly TM. Childhood abuse as a risk factor for suicidal behavior in borderline personality disorder. Journal of Personality Disorders. 2002;16:201–214. doi: 10.1521/pedi.16.3.201.22542. [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Ebner K, Day TA. Differential involvement of rat medial prefrontal cortex dopamine receptors in modulation of hypothalamic-pituitary-adrenal axis responses to different stressors. The European Journal of Neuroscience. 2004;20:1008–1016. doi: 10.1111/j.1460-9568.2004.03569.x. [DOI] [PubMed] [Google Scholar]
- Stanley M, Mann JJ. Increased serotonin-2 binding sites in frontal cortex of suicide victims. Lancet. 1983;1:214–216. doi: 10.1016/s0140-6736(83)92590-4. [DOI] [PubMed] [Google Scholar]
- Stanley M, Traskman-Bendz L, Dorovini-Zis K. Correlations between aminergic metabolites simultaneously obtained from human CSF and brain. Life Science. 1985;37:1279–1286. doi: 10.1016/0024-3205(85)90242-5. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Dilley GE, Shapiro LA, et al. Serotonin receptors in suicide victims with major depression. Neuropsychopharmacology. 1997;16:162–173. doi: 10.1016/S0893-133X(96)00170-4. [DOI] [PubMed] [Google Scholar]
- Stoff DM, Mann JJ. Suicide research. Overview and introduction. Annals of the New York Academy of Sciences. 1997;29:1–11. doi: 10.1111/j.1749-6632.1997.tb52352.x. [DOI] [PubMed] [Google Scholar]
- Terwilliger JD, Goring HH. Gene mapping in the 20th and 21st centuries: Statistical methods, data analysis, and experimental design. Human Biology. 2000;72:63–132. [PubMed] [Google Scholar]
- Tork I. Anatomy of the serotonergic system. Annals of the New York Academy of Sciences. 1990;600:9–34. doi: 10.1111/j.1749-6632.1990.tb16870.x. [DOI] [PubMed] [Google Scholar]
- Traskman L, Asberg M, Bertilsson L, et al. Monoamine metabolites in CSF and suicidal behavior. Archives of General Psychiatry. 1981;38:631–636. doi: 10.1001/archpsyc.1981.01780310031002. [DOI] [PubMed] [Google Scholar]
- Turecki G, Briere R, Dewar K, et al. Prediction of level of serotonin 2A receptor binding by serotonin receptor 2A genetic variation in postmortem brain samples from subjects who did or did not commit suicide. American Journal of Psychiatry. 1999;156:1456–1458. doi: 10.1176/ajp.156.9.1456. [DOI] [PubMed] [Google Scholar]
- Van Heeringen C, Audenaert K, van Laere K, et al. Prefrontal 5-HT2a receptor binding index, hopelessness and personality characteristics in attempted suicide. Journal of Affective Disorders. 2003;74:149–158. doi: 10.1016/s0165-0327(01)00482-7. [DOI] [PubMed] [Google Scholar]
- Van Heeringen C, Marusic A. Understanding the suicidal brain. British Journal of Psychiatry. 2003;3:282–284. doi: 10.1192/bjp.183.4.282. [DOI] [PubMed] [Google Scholar]
- Van Heeringen K. The neurobiology of suicide and suicidality. Canadian Journal of Psychiatry. 2003;48:292–300. doi: 10.1177/070674370304800504. [DOI] [PubMed] [Google Scholar]
- Van Heeringen K, Audenaert K, van de Wiele L, et al. Cortisol in violent suicidal behaviour: association with personality and monoaminergic activity. Journal of Affective Disorders. 2000;60:181–189. doi: 10.1016/s0165-0327(99)00180-9. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress. I. Preclinical studies. Depression and Anxiety. 2002;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, et al. Depression and anxiety: Role of the locus coeruleus and corticotropin-releasing factor. Brain Research Bulletin. 1994;35:561–275. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Pollock L. Psychological aspects of the suicidal process. In: van Heeringen C, editor. Understanding suicidal behaviour: The suicidal process approach to research, treatment and prevention. JohnWiley; Chichester: 2001. pp. 76–94. [Google Scholar]
- Ystgaard M, Hestetun I, Loeb M, et al. Is there a specific relationship between childhood sexual and physical abuse and repeated suicidal behavior? Child Abuse & Neglect. 2004;28:863–875. doi: 10.1016/j.chiabu.2004.01.009. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Frisch A, Bromberg M, et al. Family-based association study of serotonin transporter promoter in suicidal adolescents: No association with suicidality but possible role in violence traits. American Journal of Medical Genetics. 2001;105:239–245. doi: 10.1002/ajmg.1261. [DOI] [PubMed] [Google Scholar]
- Zalsman G, Frisch A, Lewis R, et al. DRD4 receptor gene exon III polymorphism in inpatient suicidal adolescents. Journal of Neural Transmission. 2004;111:1593–1603. doi: 10.1007/s00702-004-0182-3. [DOI] [PubMed] [Google Scholar]
- Zhang HY, Ishigaki T, Tani K, et al. Serotonin2A receptor gene polymorphism in mood disorders. Biological Psychiatry. 1997;41:768–773. doi: 10.1016/S0006-3223(96)00160-6. [DOI] [PubMed] [Google Scholar]
- Ziegler DR, Cass WA, Herman JP. Excitatory influence of the locus coeruleus in hypothalamic-pituitary-adrenocortical axis responses to stress. Journal of Neuroendocrinology. 1999;11:361–369. doi: 10.1046/j.1365-2826.1999.00337.x. [DOI] [PubMed] [Google Scholar]
- Zill P, Buttner A, Eisenmenger W, et al. Single nucleotide polymorphism and haplotype analysis of a novel tryptophan hydroxylase isoform (TPH2) gene in suicide victims. Biological Psychiatry. 2004;56:581–586. doi: 10.1016/j.biopsych.2004.07.015. [DOI] [PubMed] [Google Scholar]


