Abstract
Background
The effect of supplementation with calcium alone on risk fractures in a healthy population is not clear.
Objective
The objective was to determine whether 4 y of calcium supplementation would reduce the fracture risk during treatment and subsequent follow-up in a randomized placebo-controlled trial.
Design
The participants were aged <80 y at study entry (mean age: 61 y), were generally healthy, and had a recent diagnosis of colorectal adenoma. A total of 930 participants (72% men; mean age: 61 y) were randomly assigned to receive 4 y of treatment with 3 g CaCO3 (1200 mg elemental Ca) daily or placebo and were followed for a mean of 10.8 y. The primary outcomes of this analysis were all fractures and minimal trauma fractures (caused by a fall from standing height or lower while sitting, standing, or walking).
Results
There were 46 fractures (15 from minimal trauma) in 464 participants in the calcium group and 54 (29 from minimal trauma) in 466 participants in the placebo group. The overall risk of fracture differed significantly between groups during the treatment phase [hazard ratio (HR): 0.28; 95% CI: 0.09, 0.85], but not during the subsequent posttreatment follow-up (HR: 1.10; 95% CI: 0.71, 1.69). Minimal trauma fractures were also less frequent in the calcium group during treatment (HR: 0; 95% CI: 0, 0.50).
Conclusion
Calcium supplementation reduced the risk of all fractures and of minimal trauma fractures among healthy individuals. The benefit appeared to dissipate after treatment was stopped.
INTRODUCTION
Several national organizations recommend a high calcium intake to achieve optimum bone health (1–3). Epidemiologic studies indicate that calcium supplementation (without vitamin D) may have a small beneficial effect on bone mass (4), but double-blind randomized controlled trials (5–9) and observational studies (10–16) provide only conflicting evidence regarding the effect of calcium intake on fracture risk. A recent meta-analysis summarized the efficacy of calcium alone for the prevention of nonvertebral and hip fractures (17). Based on 5 studies (5666 primarily postmenopausal women plus 1074 men) with 814 nonvertebral fractures, the pooled RR for nonvertebral fractures comparing calcium supplementation (800–1600 mg/d) with placebo was 0.92 (95% CI: 0.81, 1.05). Based on 4 studies with separate results for hip fracture (6504 individuals with 139 hip fractures), the pooled RR comparing calcium with placebo was 1.64 (95% CI: 1.02, 2.64) (17).
To help clarify whether calcium, given without vitamin D, affects fracture risk, we analyzed data collected during the treatment and observational follow-up phases of a randomized trial of calcium supplementation for the prevention of colorectal adenomas. We tested the hypothesis that 4 y of supplementation with 3 g CaCo3 (1200 mg elemental Ca) daily would protect healthy individuals against fractures, particularly against fractures caused by low trauma injuries.
SUBJECTS AND METHODS
Subjects
The Calcium Polyp Prevention Study was a randomized, 4-y (mean: 38 mo), double-blind, placebo-controlled trial of calcium carbonate for the prevention of colorectal adenomas. Recruitment took place at 6 sites in the United States between 1988 and 1992 (18). Eligible participants had at least one histologically confirmed large-bowel adenoma removed within the preceding 3 mo; the entire large bowel mucosa was judged to be free of polyps. At enrollment, participants were <80 y of age, in good health, and willing to forego calcium supplementation (including calcium-containing multivitamins or antacids). They had no history of familial polyposis, invasive large-bowel cancer, malabsorption syndromes, or any condition that might be worsened by calcium supplementation. All participants provided written informed consent, and the study was approved by the Human Subjects Committee at each of the involved institutions.
The participants completed a baseline interview regarding lifestyle habits and medical history, completed a validated food-frequency questionnaire (19), and provided a blood sample (stored at −70 °C) and had height and weight measured. We assayed 25-hydroxyvitamin D [25(OH)D] concentrations in the baseline blood specimens using competitive protein binding (Nichol’s Institute Diagnostics, Capistrano, CA). The sensitivity of the assay was 5 ng/mL. To monitor the precision of the measurements, serum samples from 182 subjects were split, and the paired aliquots were distributed among batches shipped for analysis. The interbatch Pearson correlation for the 25(OH) vitamin D measurements was 0.95.
Randomization and treatment
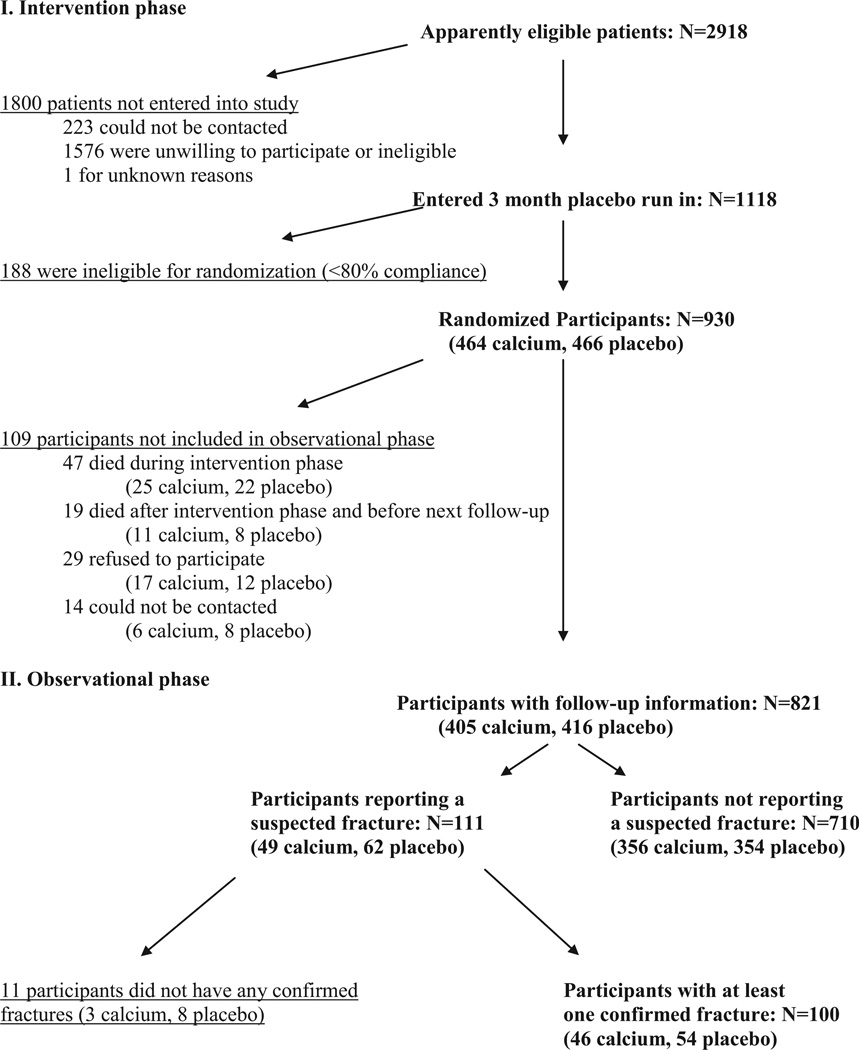
Of 2918 individuals potentially eligible for the study, 1118 (38%) provided consent and began a 3-mo single-blind placebo run-in period (Figure 1). At the end of the run-in period, 930 (83%) had taken ≥80% of their tablets and were deemed eligible for randomization. Participants were assigned to calcium or placebo using computer-generated random numbers, stratified by study center. The daily intervention consisted of 2 tablets, each containing either 1.5 g CaCO3 (600 mg elemental Ca) or cellulose-sucrose placebo. Pills were mailed directly to participants by an unblinded pharmacy technician; treatment allocation was concealed from participants and all study personnel but the study analyst. During the intervention phase, participants were asked to avoid taking calcium supplements other than those provided by the trial. Every 6 mo, they were telephoned to record major changes in health (including hospitalizations, physician visits, use of nutritional supplements, and compliance with the study intervention).
FIGURE 1.
Numbers of subjects studied and dropouts during the intervention and observational phases of the trial, by treatment allocation.
Posttreatment follow-up
The treatment phase of the trial ended in 1996, and the participants were informed of their treatment allocation in August 1997. In January 1999, 3–7 y after the subjects had completed their study treatment, the primary analyses relating to adenoma recurrence were published, which showed that calcium supplementation had conferred a moderate but significant reduction in risk (18). At that time, we informed participants of the study’s primary outcome by mail and sent a follow-up questionnaire addressing medical events and use of medications and nutritional supplements since the last study contact at the end of randomized treatment. In this questionnaire, information was specifically requested regarding fractures that had occurred since enrollment in the study. The questionnaire also asked about “regular” use of nutritional supplements (≥75 d during a year, ie, at least twice per week for a whole year or daily for 2.5 mo). Subsequent questionnaires, similar to the initial one, were sent annually, each of which covered the period since the preceding questionnaire. If participants did not return a mailed questionnaire within 21 d, the study coordinator attempted to obtain interviews by telephone. If unsuccessful, they contacted the friend or relative previously designated by each subject as a source of follow-up information, and the subject’s participation in the study ended after that interview. By the end of the study in September 2003, the participants had been followed from randomization for a mean (±SD) of 10.8 ± 3.2 y.
Fracture assessment
Medical records for all reported fractures were reviewed independently by 2 reviewers (JAB and HAB-F) who were blinded to the subjects’ treatment allocations. Fractures were considered to be “definite” if the medical records contained radiological evidence of a new fracture or (if radiological records were not available) evidence that a fracture was diagnosed by a physician. Fractures were defined as “probable” if the radiological report was equivocal or negative but the treating clinician had clearly considered a fracture to have taken place. When the clinical and radiological reports were consistent with no fracture having occurred, the fracture was classified as “ruled out.” For all confirmed (definite or probable) fractures, we used the available clinical information to grade the degree of trauma associated with the event. “Minimal trauma” was defined as a fall from standing position or lower while standing or walking, but not running. “Significant trauma” was defined as a fall on stairs or higher levels (eg, ladder) or higher impact trauma including running, sports injuries, and car accidents. The reviewers’ independent confirmations of fracture diagnosis were identical in all cases. In 6 cases, the independent assessments of trauma level differed; consensus was reached after discussion. For 10 fractures, the exact date of the event could not be ascertained from the medical records or subject recall; for these fractures we assigned the date to be the 15th of the month in which the fracture occurred. Fracture assessment was performed throughout the trial phase and in yearly questionnaires sent out during the follow-up period.
Statistical analysis
On the basis of the analysis plan set out before the blinded fracture review, we focused our analyses on 2 main outcomes: all fractures and minimal trauma fractures. Fracture rates were computed as the total number of confirmed fractures per 10 000 person-years of observation. Baseline characteristics in the 2 intervention groups were compared by using chi-square contingency table analyses and t tests. All treatment comparisons were performed according to the intention to treat principle with the use of Cox proportional hazard models to estimate the hazard ratio (HR) for time to first fracture and time to first low-trauma fracture. We generated models with and without adjustments for age, sex, 25(OH)D concentration, alcohol use, smoking, and body mass index [BMI (in kg/m2) <25, 25–30, and >30]. Significance of the difference in HRs between the treatment and post-treatment periods was tested by assessing the interaction of a marker variable for the 2 study phases with study treatment in the Cox models. For the analysis of minimal trauma fractures during the treatment period, small numbers of events hampered a time to event analysis; consequently an odds ratio was estimated and 95% CIs were computed using exact methods. Analyses were performed using Stata version 7.0 (Stata Corporation, College Station, TX) and R.
RESULTS
Baseline characteristics
Participants in the 2 intervention groups were generally similar with respect to their baseline characteristics (Table 1). The subjects had a mean age of 61 y at study entry (range: 27–80 y), and 72% were men. There was a slight excess of obese participants in the calcium group, although the mean BMI was similar in the 2 groups. A small proportion of the randomized participants used calcium supplements before enrollment (3% in the placebo group and 2% in the calcium group), but agreed to discontinue them during the intervention study. At baseline, the mean dietary calcium intake was 865 mg/d in the placebo group and 889 mg/d in the calcium group. Mean serum 25(OH)D was 72.8 nmol/L in the placebo group and 73.0 nmol/L in the calcium group.
TABLE 1.
Baseline characteristics of the study participants
| Placebo group (n = 466) |
Calcium group (n = 464) |
|
|---|---|---|
| Men [n (%)] | 327 (70) | 345 (74) |
| Women [n (%)] | 139 (30) | 119 (26) |
| Age (y) | 61.0 ± 9.11 | 61.0 ± 9.1 |
| BMI [n (%)]2 | 27.1 ± 4.3 | 27.6 ± 4.4 |
| <18.5 kg/m2 | 4/465 (0.9) | 2/463 (0.4) |
| 18.5–24.9 kg/m2 | 151/465 (32.5) | 134/463 (28.9) |
| 25–30 kg/m2 | 211/465 (45.4) | 200/463 (43.2) |
| >30 kg/m2 | 99/465 (21.3) | 127/463 (27.4) |
| Dietary calcium intake (mg/d) | 865 ± 4233 | 889 ± 4514 |
| Use of calcium supplements [n (%)] | 13/456 (3) | 11/451 (2) |
| Current smoker [n (%)] | 85 (18) | 94 (20) |
| Alcoholic drinks consumed (no./d) | 0.60 ± 1.085 | 0.60 ± 1.164 |
| Serum 25-hydroxyvitamin D (nmol/L) | 72.8 ± 26.7 | 73.0 ± 28.0 |
| ≥75 nmol/L [n (%)] | 184/403 (46) | 183/395 (46) |
x̄ ± SD (all such values).
Mean BMI did not differ significantly between groups. The number of obese subjects differed significantly between groups (χ2 = 4.72, P = 0.03). Because of the small numbers, individuals with a BMI < 18.5 were added to the next category (<24.9) in the regression analyses.
n = 456.
n = 451.
n = 455.
Follow-up and adherence
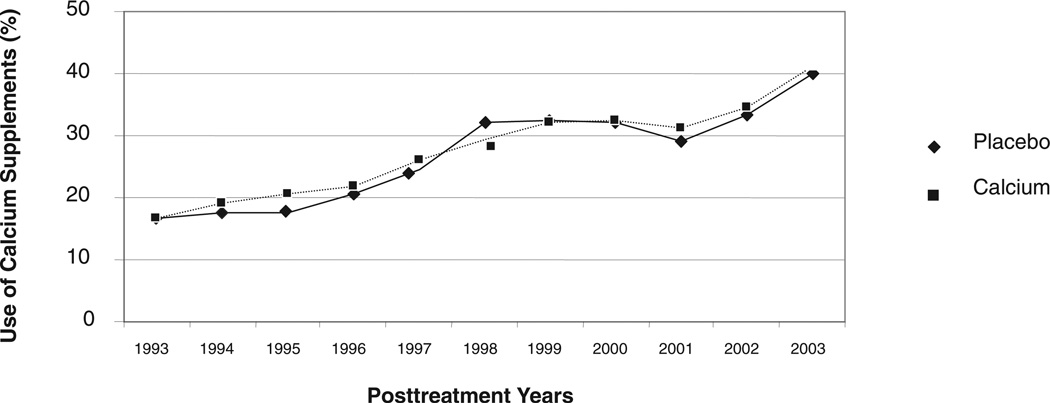
Self-reported adherence to study treatment was excellent during the trial; even during the fourth year of treatment, ≥90% adherence to the study pills was reported by 346 (75%) of 464 participants randomly assigned to receive calcium and by 358 (77%) of 466 participants randomly assigned to placebo. During the observational phase, data were collected for 821 of 930 (88%) participants: 760 completed questionnaires themselves and for 61 the information was provided by a surrogate respondent. Of the 821 respondents, 364 (48%) reported using calcium supplements regularly for one or more years during the observational phase, and 196 (26%) did so for at least half of the follow-up years for which questionnaire data were available. Personal use of calcium supplements during the observational phase did not differ materially between the randomized groups (P = 0.68); both showed an increase over time that accelerated after 2001 (Figure 2).
FIGURE 2.
Use of calcium supplements in the observational follow-up after the treatment phase. Of 821 individuals followed after the treatment phase, 48% reported using calcium supplements regularly for ≥1 y, and 26% did so for at least half of the follow-up years for which questionnaire data were available. Personal use of calcium supplements during the observational phase was similar between the randomized groups (Students t test: P = 0.68).
Confirmed fractures
During the combined intervention and observational phases of the study (an average of 38 mo of randomized treatment and 10.8 y of follow-up in total), 141 possible fractures were reported by 111 participants. Definite (n = 117) or probable (n = 10) fractures were confirmed by medical record review among 100 participants, including 5 participants with multiple (2, 3, or 4) fractures on the same day and 15 participants with multiple (2 or 3) fractures on different days. Eighteen subjects sustained their fractures during the treatment period. The reviewers ruled out a diagnosis of fracture for 11 participants (3 in the calcium group and 8 in the placebo group) (Table 2). The rate of all confirmed fractures in the placebo group during the combined intervention and observational phases was 98/10 000 person-years in men and 145 in women.
TABLE 2.
Individuals with confirmed fractures and type of fractures by treatment1
| Placebo group (n = 466) |
Calcium group (n = 464) |
Total (n = 930) |
|
|---|---|---|---|
| All confirmed fractures | |||
| Number of participants with fracture | 54 | 46 | 100 |
| Men | 33 | 31 | 64 |
| Women | 21 | 15 | 36 |
| Number of fractures | 66 | 61 | 127 |
| Upper limb | 22 | 28 | 50 |
| Lower limb (hip fractures) | 34 (4) | 27 (6) | 61 (10) |
| Axial | 10 | 6 | 16 |
| Minimal trauma fractures | |||
| Number of participants | 29 | 15 | 44 |
| Men | 17 | 10 | 27 |
| Women | 12 | 5 | 17 |
| Number of fractures | 30 | 15 | 45 |
| Upper limb (including clavicle) | 14 | 7 | 21 |
| Lower limb | 14 | 7 | 21 |
| Hip fractures | 3 | 2 | 5 |
| Axial | 2 | 1 | 3 |
| Fracture trauma | |||
| Fall from stairs or higher level | |||
| Number of participants | 3 | 6 | 9 |
| High impact trauma | |||
| Number of participants | 11 | 12 | 23 |
| Unknown trauma | |||
| Number of participants | 11 | 13 | 24 |
Upper limb includes clavicle; axial includes face, vertebrae, pelvis, and ribs.
Overall (during the combined treatment and observational phases of the study), one or more fractures occurred in 46 of 464 individuals in the calcium group and in 54 of 466 in the placebo group (unadjusted HR: 0.86; 95% CI: 0.58, 1.28; Table 3). Adjustment for sex, baseline age, BMI, cigarette smoking, alcohol consumption, and serum 25(OH)D concentrations changed the HR only minimally [adjusted HR: 0.91; 95% CI: 0.60, 1.36]. Fractures occurred in 31 of 345 men in the calcium group and in 33 of 327 in the placebo group (unadjusted HR: 0.89; 95% CI: 0.55, 1.45). For women, the proportions were 15 of 119 and 21 of 139, respectively (unadjusted HR: 0.84, 95% CI: 0.43, 1.63). Findings were essentially identical when the analysis was limited to definite fractures (data not shown).
TABLE 3.
Unadjusted hazard ratios for one or more fractures, by type of fracture and study period
| Entire follow-up | Treatment period | Posttreatment period | ||||
|---|---|---|---|---|---|---|
| Calcium/placebo | Hazard ratio (95% CI) |
Calcium/placebo | Hazard ratio (95% CI) |
Calcium/placebo | Hazard ratio (95% CI) |
|
| n | n | n | ||||
| All confirmed fractures | 46/54 | 0.86 (0.58, 1.28) | 4/14 | 0.28 (0.09, 0.85) | 42/40 | 1.10 (0.71, 1.69) |
| Minimal trauma fractures | 15/29 | 0.52 (0.28, 0.97) | 0/9 | 0.00 (0.00, 0.50)1 | 15/20 | 0.78 (0.40, 1.52) |
Computed by using exact methods.
During the 4-y intervention phase, there were 14 subjects with fractures in the placebo group and 4 in the calcium group, a pattern that indicated a marked reduction in risk (unadjusted HR: 0.28; 95% CI: 0.09, 0.85). After adjustment, the HR was 0.21 (95% CI: 0.6, 0.76). There were more fractures during the observational, posttreatment follow-up: 42 calcium subjects and 40 placebo subjects had one or more fractures, which yielded an unadjusted HR of 1.10 (95% CI: 0.71, 1.69). After multivariate adjustment, the HR was 1.17 (95% CI: 0.75, 1.82). The unadjusted HRs were significantly different after treatment than during treatment (P = 0.026).
Minimal trauma fractures
Of the 930 randomized subjects, 44 experienced at least one minimal trauma fracture, (15 of 464 in the calcium group and 29 of 466 in the placebo group), 9 of which occurred during the treatment period. About equal numbers of subjects experienced fractures of the upper and lower limbs (Table 2); axial fractures were uncommon, occurring in only 3 (7%) subjects (Table 2). Overall, the risk of one or more minimal trauma fractures was significantly lower in the calcium group (unadjusted HR: 0.52; 95% CI: 0.28, 0.97; P = 0.04) (Table 3). After multivariate adjustment, the HR was similar (0.56; 95% CI: 0.30, 1.05; P = 0.07) (Table 3).
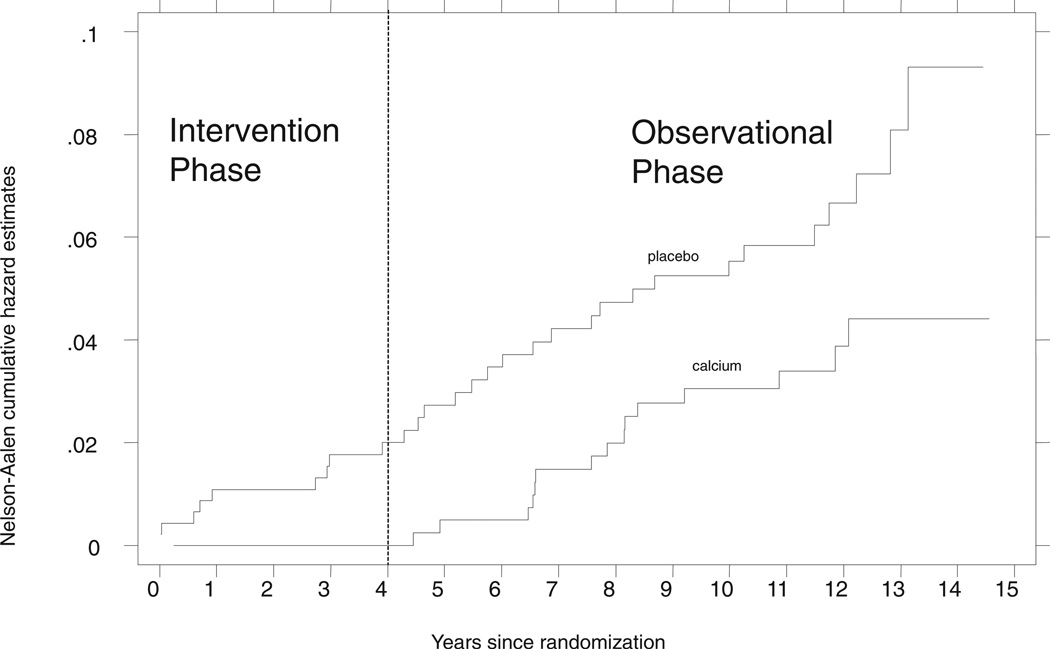
During the intervention phase of the study, there were no minimal trauma fractures in the calcium group; the odds ratio was 0.0 (95% CI: 0.0, 0.50) (Figure 3, Table 3). Fracture events occurred at roughly similar rates after the end of treatment, which left almost parallel event curves over the later time interval (Figure 3). During the posttreatment phase, the unadjusted HR was 0.78 (95% CI: 0.40, 1.52); with adjustment it was 0.85 (95% CI: 0.43, 1.68).
FIGURE 3.
Cumulative incidence of minimal trauma fractures, by treatment group. The unadjusted hazard ratio for the comparison of calcium with placebo was 0.52 (95% CI: 0.28, 0.97; P = 0.04) across the total follow-up, including both the intervention and the observational phases of the study. The odds ratio during the treatment phase was 0.00 (95% CI: 0.00, 0.50); during the posttreatment phase, the unadjusted hazard ratio was 0.78 (95% CI: 0.40, 1.52).
The HRs for minimal trauma fractures were similar in men (HR: 0.55; 95% CI: 0.25, 1.20) and women (HR: 0.50; 95% CI: 0.18, 1.42), and findings did not change materially when the analysis was limited to the 42 definite fractures (data not shown). When we included fractures caused by either minimal trauma or falls due to unknown trauma, the unadjusted HR during the combined treatment and posttreatment periods was 0.72 (95% CI: 0.43, 1.14; P = 0.21).
DISCUSSION
In this randomized intervention trial of calcium supplementation without vitamin D, there was a marked reduction in the risk of fractures—particularly minimal trauma fractures— during the 4-y treatment period. In the subsequent follow-up, this benefit was not apparent. The effects were similar in men and women.
Calcium is a structural component of bone, and calcium supplementation may improve bone density and reduce fractures by suppressing parathyroid hormone secretion and thus reducing bone resorption (20). However, the recommended intake of 1200 mg/d for postmenopausal women and adult men (21, 22) is typically not achieved through diet alone (15). Although this discordance between ideal and actual calcium intakes in the population appeared to provide a good opportunity for intervention, observational studies of the association between calcium supplementation and fracture risk have given inconsistent results (10–16). Randomized trials of calcium supplementation alone have also reported conflicting findings, summarized in a recent meta-analysis, calcium supplementation has a neutral effect on nonvertebral fractures among primarily female populations at risk of osteoporosis (pooled RR: 0.92; 95% CI: 0.81, 1.05) (17). Several studies included in this meta-analysis reported a reduction in risk that was not statistically significant (5, 7–9); another study found a risk reduction in the as-treated analysis that was not confirmed by the intention to treat analysis (6). The interpretation of all of these studies is limited by differences in the prescribed dose of calcium, small sample sizes (5, 7, 9), and short duration of follow-up (5).
Randomized trials of both calcium and vitamin D are also potentially relevant to the debate over the effectiveness of calcium supplementation, because the effect of the combination may give an indication of the maximum possible benefit from calcium alone. Of the trials that have reported the effects on fracture risk of combined calcium and vitamin D supplementation, 2 showed a significant benefit (23, 24), but 4 did not (25–28). The interpretation of these trials is hindered by the different doses of vitamin D used, low adherence (26–28), concurrent use of supplements outside the study protocol (27, 28), length of follow-up (25), and different risk profiles, including primary (23–25, 27, 28) and secondary (26) fracture prevention. Taken together, the calcium and calcium–vitamin D trials fail to clearly suggest a benefit of calcium supplementation alone in high-risk populations, but provide little information about its effect in healthier populations or among men.
A particular advantage of our analysis is its relevance to healthy adults at a relatively low risk of fractures. Fracture rates among participants in the placebo group (145 and 98 fractures per 10 000 person-years among women and men, respectively) were lower than the rates reported in several other trials and surveillance studies (28 –31). The effect of calcium in a healthy population is of interest because there is evidence that its benefit on bone density and fracture risk depends on factors associated with general health. For example, one meta-analysis found that calcium supplementation has a greater effect on bone density if combined with exercise (32). Higher serum 25(OH)D concentrations are associated with more efficient calcium absorption (33), which suggests that a lower calcium intake may be sufficient for optimal suppression of parathyroid hormone secretion among individuals with higher serum 25(OH)D concentrations (34). Our participants’ mean baseline serum 25(OH)D concentration of 70 nmol/L is substantially higher than concentrations reported among individuals at high risk of osteoporosis (23, 35), and this may have promoted the beneficial effect of calcium on fracture risk. In addition, the participants’ mean baseline calcium intake exceeded the estimated mean intake of individuals aged ≥50 y in the general population at the time of the study (763 mg/d in men and 558 mg/d in women) (15).
The apparent effect of calcium in our healthy population suggests that the potential benefits of calcium supplementation on fracture risk are not necessarily limited to individuals with the greatest calcium deficit and raises questions about the types of population in which the effects of calcium supplementation should be studied. It is possible that calcium supplementation will not reduce fracture risk in the long run, eg, if the physiologic changes that precede osteoporosis have progressed too far or if too many additional risk factors are present. If so, it may be more productive to target younger, healthier populations with higher physical activity and better 25(OH)D status than populations already at high risk of osteoporosis. Future studies could attempt to define the characteristics that predict the best clinical response to calcium supplementation and hence identify the age and circumstances in which calcium supplementation will provide the greatest long-term benefit.
Our study had the advantage of a having a randomized period of calcium supplementation or placebo. Randomization ensures that any differences between the treatment groups is due to chance, which minimizes the problem of confounding. As expected in a study of this size, there were no material imbalances in baseline characteristics between the calcium and placebo groups, and adjustment for baseline factors had only a small impact on the point estimate of effect. However, the numbers of endpoints observed in the study were modest, and we had limited ability to adjust for whatever differences might have emerged between the treatment groups.
Our study was also limited by the participants’ use of supplemental calcium during the observational phase. However, supplement use began only after the completion of the 4-y intervention phase and could not have distorted the relative risk estimates for events during the treatment period. Moreover, supplement use after the end of randomized treatment was similar in the intervention groups (Figure 2), so we would expect any bias in the HR estimates for the posttreatment period to be conservative. Another limitation of the study was the lack of trauma data for 24 of 100 (24%) first confirmed fractures; however, it is reassuring that these cases were distributed similarly in the 2 treatment groups and that findings were similar whether we included fractures caused by minimal trauma only or both minimal and unknown trauma.
Other possible limitations of the study result from its status as a secondary analysis within a trial focused on an unrelated primary endpoint (colorectal adenomas) (18). In particular we collected information about possible fractures during the intervention phase as part of our general medical follow-up, and specific fracture questions were added only during the observational posttreatment period. Also, we did not conduct prospective follow-up between the intervention and observational phases of the study. Instead, in 1999, we asked participants to recall health information from several years past at a time when they were aware of their previous assignment to calcium or placebo. It is possible that these factors affected fracture ascertainment and the participants’ self reports of personal calcium supplement use.
Finally, it is worth considering the basis of the quantitative difference between our results for minimal trauma fractures and those for all fractures. Many high-trauma fractures arguably are not preventable through metabolic interventions such as calcium supplementation, whereas minimal trauma fractures are potentially preventable. It is conceivable that the inclusion of high trauma fractures would tend to cause a conservative bias if the outcome of interest is preventable fractures. Thus, it is not unreasonable that our findings in relation to calcium and minimal trauma fractures were stronger than those for all fractures.
The benefits of calcium supplementation for all fractures and minimal trauma fractures in this trial were restricted to the intervention phase (mean of 38 mo out of 14 y), with no sustained residual benefit after treatment was stopped. In a 2004 meta-analysis of randomized controlled trials, calcium supplementation of 500 to 2000 mg/d in postmenopausal women provided a modest benefit on bone density: 2.05% difference in total body bone density, 1.66% for lumbar spine, and 1.64% for the hip (4, 36). However, the effects of calcium supplementation on bone mineral density appear to represent a one-time increment that does not continue to accrue with time (8), a finding that may explain why we did not see a sustained benefit after the end of treatment even for minimal trauma fractures.
In conclusion, we showed that 4 y of supplementation with 1200 mg elemental Ca is associated with a reduction in risk of all fractures and minimal trauma fractures among healthy community-dwelling older men and women. Our findings in the observational phase of the trial suggest that this benefit of calcium is lost after treatment is stopped. Thus, calcium supplementation may be beneficial in the prevention of fractures among relatively healthy individuals who have close to adequate 25(OH)D concentrations and who are willing to take the supplements on a continuous basis.
Footnotes
Supported by grants (CA37287 and CA23108) from the National Institutes of Health, a fellowship from the Swiss Foundation for Nutrition Research and the International Foundation for the Promotion of Nutrition Research and Nutrition Education, and a Swiss National Foundation Professorship grant. The calcium and placebo tablets were provided by Lederle (now Whitehall-Robins), Pearl River, NY.
The authors’ responsibilities were as follows—HAB-F: had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis; HAB-F, JRR, and JAB: study concept and design; JAB: administrative, technical, and material support; HAB-F and JAB: obtained funding; HAB-F, JRR, MVG, and JAB: acquisition of data; and HAB-F, JRR, and MVG: conducted the statistical analysis. All authors drafted the manuscript, critically revised the manuscript for important intellectual content, and analyzed and interpreted the data. No sponsors participated in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript. No conflicts of interest were declared.
REFERENCES
- 1.AACE. American Association of Clinical Endocrinologists medical guidelines for clinical practice for the prevention and treatment of post-menopausal osteoporosis: 2001 ed, with selected updates for 2003. [accessed 29 March 2004]; Internet: http://www.guideline.gov/summary/summary.aspx?doc_id=4157&nbr=003185&string=calcium#s23.
- 2.General S. Bone health and osteoporosis: a report of the Surgeon General. 2004 Internet: http://www.surgeongeneral.gov/library/bonehealth/content.html. [PubMed]
- 3.Health NIo. Osteoporosis prevention, diagnosis, and therapy. NIH Consensus Statement. 2000 Internet: http://consensus.nih.gov/2000/2000Osteoporosis111html.htm. [PubMed]
- 4.Shea B, Wells G, Cranney A, et al. Meta-analyses of therapies for postmenopausal osteoporosis. VII. Meta-analysis of calcium supplementation for the prevention of postmenopausal osteoporosis. Endocr Rev. 2002;23(4):552–559. doi: 10.1210/er.2001-7002. [DOI] [PubMed] [Google Scholar]
- 5.Chevalley T, Rizzoli R, Nydegger V, et al. Effects of calcium supplements on femoral bone mineral density and vertebral fracture rate in vitamin-D-replete elderly patients. Osteoporos Int. 1994;4(5):245–252. doi: 10.1007/BF01623348. [DOI] [PubMed] [Google Scholar]
- 6.Prince RL, Devine A, Dhaliwal SS, Dick IM. Effects of calcium supplementation on clinical fracture and bone structure: results of a 5-year, double-blind, placebo-controlled trial in elderly women. Arch Intern Med. 2006;166(8):869–875. doi: 10.1001/archinte.166.8.869. [DOI] [PubMed] [Google Scholar]
- 7.Reid IR, Ames RW, Evans MC, Gamble GD, Sharpe SJ. Long-term effects of calcium supplementation on bone loss and fractures in post-menopausal women: a randomized controlled trial. Am J Med. 1995;98(4):331–335. doi: 10.1016/S0002-9343(99)80310-6. [DOI] [PubMed] [Google Scholar]
- 8.Reid IR, Mason B, Horne A, et al. Randomized controlled trial of calcium in healthy older women. Am J Med. 2006;119(9):777–785. doi: 10.1016/j.amjmed.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ., III Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res. 1998;13(2):168–174. doi: 10.1359/jbmr.1998.13.2.168. [DOI] [PubMed] [Google Scholar]
- 10.Feskanich D, Willett WC, Colditz GA. Calcium, vitamin D, milk consumption, and hip fractures: a prospective study among postmenopausal women. Am J Clin Nutr. 2003;77(2):504–511. doi: 10.1093/ajcn/77.2.504. [DOI] [PubMed] [Google Scholar]
- 11.Holbrook TL, Barrett-Connor E, Wingard DL. Dietary calcium and risk of hip fracture: 14-year prospective population study. Lancet. 1988;2(8619):1046–1049. doi: 10.1016/s0140-6736(88)90065-7. [DOI] [PubMed] [Google Scholar]
- 12.Cumming RG, Cummings SR, Nevitt MC, et al. Calcium intake and fracture risk: results from the study of osteoporotic fractures. Am J Epidemiol. 1997;145(10):926–934. doi: 10.1093/oxfordjournals.aje.a009052. [DOI] [PubMed] [Google Scholar]
- 13.Owusu W, Willett WC, Feskanich D, Ascherio A, Spiegelman D, Colditz GA. Calcium intake and the incidence of forearm and hip fractures among men. J Nutr. 1997;127(9):1782–1787. doi: 10.1093/jn/127.9.1782. [DOI] [PubMed] [Google Scholar]
- 14.Paganini-Hill A, Chao A, Ross RK, Henderson BE. Exercise and other factors in the prevention of hip fracture: the Leisure World study. Epidemiology. 1991;2(1):16–25. doi: 10.1097/00001648-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Looker AC, Harris TB, Madans JH, Sempos CT. Dietary calcium and hip fracture risk: the NHANES I Epidemiologic Follow-Up Study. Osteoporos Int. 1993;3(4):177–184. doi: 10.1007/BF01623673. [DOI] [PubMed] [Google Scholar]
- 16.Michaelsson K, Melhus H, Bellocco R, Wolk A. Dietary calcium and vitamin D intake in relation to osteoporotic fracture risk. Bone. 2003;32(6):694–703. doi: 10.1016/s8756-3282(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 17.Bischoff-Ferrari HA, Dawson-Hughes B, Baron JA, et al. Calcium intake and hip fracture risk in men and women: a meta-analysis of prospective cohort studies and randomized controlled trials. Am J Clin Nutr. 2007;86:1579–1580. doi: 10.1093/ajcn/86.5.1780. [DOI] [PubMed] [Google Scholar]
- 18.Baron JA, Beach M, Mandel JS, et al. Calcium Polyp Prevention Study Group. Calcium supplements for the prevention of colorectal adenomas. N Engl J Med. 1999;340(2):101–107. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 19.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol. 1986;124(3):453–469. doi: 10.1093/oxfordjournals.aje.a114416. [DOI] [PubMed] [Google Scholar]
- 20.Blumsohn A, Herrington K, Hannon RA, Shao P, Eyre DR, Eastell R. The effect of calcium supplementation on the circadian rhythm of bone resorption. J Clin Endocrinol Metab. 1994;79(3):730–735. doi: 10.1210/jcem.79.3.8077353. [DOI] [PubMed] [Google Scholar]
- 21.National Academy Press. Dietary reference intakes: calcium, phosphorus, magnesium, vitamin D, and fluoride. Washington, DC: National Academy Press; 1997. [PubMed] [Google Scholar]
- 22.Spencer H, Kramer L, Lesniak M, De Bartolo M, Norris C, Osis D. Calcium requirements in humans. Report of original data and a review. Clin Orthop Relat Res. 1984;(184):270–280. [PubMed] [Google Scholar]
- 23.Chapuy MC, Arlot ME, Duboeuf F, et al. VitaminD3andcalcium to prevent hip fractures in the elderly women. N Engl J Med. 1992;327(23):1637–1642. doi: 10.1056/NEJM199212033272305. [DOI] [PubMed] [Google Scholar]
- 24.Dawson-Hughes B, Harris SS, Krall EA, Dallal GE. Effect of calcium and vitamin D supplementation on bone density in men and women 65 years of age or older. N Engl J Med. 1997;337(10):670–676. doi: 10.1056/NEJM199709043371003. [DOI] [PubMed] [Google Scholar]
- 25.Chapuy MC, Pamphile R, Paris E, et al. Combined calcium and vitamin D3 supplementation in elderly women: confirmation of reversal of secondary hyperparathyroidism and hip fracture risk: the Decalyos II study. Osteoporos Int. 2002;13(3):257–264. doi: 10.1007/s001980200023. [DOI] [PubMed] [Google Scholar]
- 26.Grant AM, Avenell A, Campbell MK, et al. Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (Randomised Evaluation of Calcium Or vitamin D, RECORD): a randomised placebo-controlled trial. Lancet. 2005;365(9471):1621–1628. doi: 10.1016/S0140-6736(05)63013-9. [DOI] [PubMed] [Google Scholar]
- 27.Porthouse J, Cockayne S, King C, et al. Randomised controlled trial of calcium and supplementation with cholecalciferol (vitamin D3) for prevention of fractures in primary care. BMJ. 2005;330(7498):1003. doi: 10.1136/bmj.330.7498.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jackson RD, LaCroix AZ, Gass M, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–683. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM, Nicholson GC, Ugoni AM, Seeman E, Pasco JA, Kotowicz MA. Fracture rates lower in rural than urban communities: the Geelong Osteoporosis Study. J Epidemiol Community Health. 2002;56(6):466–470. doi: 10.1136/jech.56.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffin MR, Ray WA, Fought RL, Melton LJ., III Black-white differences in fracture rates. Am J Epidemiol. 1992;136(11):1378–1385. doi: 10.1093/oxfordjournals.aje.a116450. [DOI] [PubMed] [Google Scholar]
- 31.Jones S, Johansen A, Brennan J, Butler J, Lyons RA. The effect of socioeconomic deprivation on fracture incidence in the United Kingdom. Osteoporos Int. 2004;15(7):520–524. doi: 10.1007/s00198-003-1564-3. [DOI] [PubMed] [Google Scholar]
- 32.Specker BL. Evidence for an interaction between calcium intake and physical activity on changes in bone mineral density. J Bone Miner Res. 1996;11(10):1539–1544. doi: 10.1002/jbmr.5650111022. [DOI] [PubMed] [Google Scholar]
- 33.Heaney RP, Dowell MS, Hale CA, Bendich A. Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr. 2003;22(2):142–146. doi: 10.1080/07315724.2003.10719287. [DOI] [PubMed] [Google Scholar]
- 34.Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G. Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA. 2005;294(18):2336–2341. doi: 10.1001/jama.294.18.2336. [DOI] [PubMed] [Google Scholar]
- 35.Lips P, Graafmans WC, Ooms ME, Bezemer PD, Bouter LM. Vitamin D supplementation and fracture incidence in elderly persons. A randomized, placebo-controlled clinical trial. Ann Intern Med. 1996;124(4):400–406. doi: 10.7326/0003-4819-124-4-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 36.Shea B, Wells G, Cranney A, et al. Calcium supplementation on bone loss in postmenopausal women. Cochrane Database Syst Rev. 2004;1(1):CD004526. doi: 10.1002/14651858.CD004526.pub2. [DOI] [PubMed] [Google Scholar]