Abstract
In patients with chronic-phase traumatic brain injury (TBI), structural MRI is readily attainable and provides rich anatomical information, yet the relationship between whole-brain structural MRI measures and neurocognitive outcome is relatively unexplored and can be complicated by the presence of combined focal and diffuse injury. In this study, sixty-three patients spanning the full range of TBI severity received high-resolution structural MRI concurrent with neuropsychological testing. Multivariate statistical analysis assessed covariance patterns between volumes of grey matter, white matter, and sulcal/subdural and ventricular CSF across 38 brain regions and neuropsychological test performance. Patients with diffuse and diffuse + focal injury were analyzed both separately and together. Tests of speeded attention, working memory, and verbal learning and memory robustly covaried with a distributed pattern of volume loss over temporal, ventromedial prefrontal, right parietal regions, and cingulate regions. This pattern was modulated by the presence of large focal lesions, but held even when analyses were restricted to those with diffuse injury. Effects were most consistently observed within grey matter. Relative to regional brain volumetric data, clinically defined injury severity (depth of coma at time of injury) showed only weak relation to neuropsychological outcome. The results showed that neuropsychological test performance in patients with TBI is related to a distributed pattern of volume loss in regions mediating mnemonic and attentional processing. This relationship holds for patients with and without focal lesions, indicating that diffuse injury alone is sufficient to cause significant neuropsychological disability in relation to regional volume loss. Quantified structural brain imaging data provides a highly sensitive index of brain integrity that is related to cognitive functioning in chronic phase TBI.
Keywords: Structural MRI, Neuropsychological assessment, Executive Function, Attention, Memory, Traumatic Brain Injury
Highlights
-
•
Cognitive deficits in TBI associated with distributed volume loss in grey matter.
-
•
Brain-behavior relationship modulated by focal lesions, but holds in diffuse injury.
-
•
Volumetrics more sensitive to cognitive deficits than clinical classification.
1. Introduction
Disability due to traumatic brain injury (TBI) is estimated to affect 2% of U.S. population (Thurman et al., 1999). While the neurocognitive effects of TBI on speeded attention, memory, and executive functioning are well known (Levin et al., 1982), the nature of the relationship between TBI neuropathology and neuropsychological outcome remains unclear. Certain brain–behavior relationships have been described for focal lesions in ventral frontal and anterior temporal regions (Mattson and Levin, 1990; Stuss and Gow, 1992), yet the primary neuropathology of TBI is diffuse axonal injury (DAI) (Gennarelli et al., 1982; Povlishock and Katz, 2005). Relative to focal lesion effects, less is known about the effects of DAI on behavior, and it is not known how these effects interact with focal lesions.
Quantified structural MRI can reveal volume loss not appreciated in clinical interpretation. Yet the volume of specific structures visualized on MRI (e.g., the corpus callosum, fornix, and hippocampus) is only inconsistently correlated with neuropsychological outcome (Gale et al., 1993; Serra-Grabulosa et al., 2005; Tomaiuolo et al., 2004; Yount et al., 2002), possibly because additional damage outside these regions of interest is unaccounted for. Whole brain analysis with voxel-based morphometry has been applied in TBI, with variable relationships to behavior (Bendlin et al., 2008; Gale et al., 2005; Tomaiuolo et al., 2005; Warner et al., 2010a; Wilde et al., 2011) or with comparison to global outcome rather than to cognitive test performance (Sidaros et al., 2009; Warner et al., 2010b).
Many of the foregoing studies are complicated by small sample sizes, variance in imaging methods, and confounding the effects of focal and diffuse injury (Levine et al., 2006). Automated voxel-based methods pose significant problems with registration (Bookstein, 2001), especially given the distorted brain morphology in TBI (Kim et al., 2008). Using non-linear deformation field matching to preserve patients' anatomy, robust tissue compartment segmentation, regional parcellation of the cerebrum, and multivariate analysis, we previously found systematic relationships between quantified volume loss and TBI severity (Levine et al., 2008). Here we investigate the relationship of this volume loss to behavior as measured by concurrent neuropsychological testing.
2. Methods
2.1. Participants
Sixty-three TBI patients were recruited from consecutive admissions to a level I trauma center at approximately one year post-injury as part of the Toronto TBI Study (Levine et al., 2008). Injury severity was determined by the GCS at the time of discharge from the Trauma Unit (corresponding to the 6-hour GCS score; Teasdale and Jennett, 1974). Severity classification (mild/moderate/severe) was upgraded in eight cases where extended loss of consciousness (> 2 h), post-traumatic amnesia (> 48 h) or focal lesions suggested more severe injury than indicated by the GCS.
To minimize confounds from psychosocial effects specific to the TBI cohort, 27 comparison participants were recruited from friends and family members of the TBI patients. Exclusion criteria for all subjects included significant psychiatric history, substance abuse, learning disability, multiple traumatic brain injuries, severe injury to organs other than the brain, prior history of neurological disease, major medical problems or medications impacting cognition, and lack of English proficiency. As the patients were recruited from consecutive hospital admissions, compensation-related symptom exaggeration was minimized; all but one patient with large bifrontal lesions performed normally on a test of symptom validity (Green et al., 1996).
As seen in Table 1, participants in different groups were well matched for sex, age, education, vocabulary knowledge (Zachary, 1986), and time since injury. All participants were informed of the experimental aim of the study and gave their consent to participate.
Table 1.
Characteristics of TBI patients and controls.
| Group | Na | Age |
Education (yrs) |
Vocabularyb |
GCSc |
LOC (h)d |
PTA (d)e |
TSI (yr)f |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Median | IQR | Median | IQR | Mean | SD | ||
| Mild | 13 (8) | 33.7 | 13.1 | 13.2 | 2.0 | 28.8 | 3.6 | 14.6 | 0.7 | 0.00 | 0–0.13 | 0.0 | 0–0.30 | 1.19 | 0.42 |
| Moderate | 29 (15) | 32.2 | 11.2 | 15.0 | 2.3 | 29.6 | 6.2 | 11.0 | 2.1 | 26.0 | 2–144 | 10.0 | 4.5–21 | 1.12 | 0.38 |
| Severe | 21 (14) | 28.5 | 8.4 | 14.6 | 2.7 | 27.8 | 5.2 | 6.3 | 2.5 | 122 | 63–336 | 36 | 18–60 | 1.03 | 0.2 |
| Controls | 27 (11) | 27.7 | 7.6 | 15.1 | 1.8 | 30.4 | 4.0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Number of males in parentheses.
Raw score on the vocabulary subtest of the Shipley Institute of Living Scale (Zachary, 1986).
Glasgow Coma Scale score (Teasdale and Jennett, 1974).
Duration of loss of consciousness.
Duration of post-traumatic amnesia.
Time since injury.
2.2. Neuropsychological tests
All participants received a battery of standardized neuropsychological tests sensitive to information processing deficits in attention, memory, and executive functioning caused by TBI. These included the Symbol-Digit Modalities Test (oral and written versions, SDMTo and SDMTw) (Smith, 1978), assessing clerical speed and accuracy; the Trail-Making Test, parts A and B (TRa, TRb) (Army Individual Test Battery, 1944), a paper-and-pencil test of speeded information processing; the Self-Ordered Pointing Test (six, eight, ten, and twelve item forms, SOP6, SOP8, SOP10, and SOP12) (Petrides and Milner, 1982), a measure of working memory; the Hopkins Verbal Learning Test—Revised (Benedict et al., 1998), involving verbal learning acquisition (HVLTln) and delayed recall (HVLTdl), Phonemic Word List Generation (FAS) (Spreen and Strauss, 1998) requiring speeded word retrieval to phonemic cues; and the Wisconsin Card Sorting Test (categories, criterion perseverations, response perseverations, and set loss errors, WCSTc, WCSTpc, WCSTpr, and WCSTs) (Stuss et al., 2000), a test of executive and frontal lobe functioning. Neuropsychological test data were scaled so that high scores indicate intact performance.
2.3. MRI acquisition and image processing
TBI patients were scanned with a 1.5 T-MR system (Signa, CV/i hardware, LX software, General Electric Healthcare, Waukesha, WI) at the time of testing. A sagittal T1-weighted 3D volume technique produced 124 1.3 mm slices (TR/TE of 35/5 ms, flip angle of 35°, 1.0 NEX, and FOV of 22 cm, acquisition matrix: 256 × 256 X 124). Proton density and T2-weighted images with a slice thickness of 3 mm were obtained using an interleaved sequence (TR/TE of 3000/30, 80 ms, 0.5 NEX, and FOV of 22 cm, acquisition matrix: 256 × 256). For TBI patients, gradient echo T2 sequences with a slice thickness of 6 mm were obtained to emphasize hemosiderin deposits (TR/TE of 750/35 ms, flip angle of 20°, 2.0 NEX, and FOV of 22 cm, acquisition matrix: 256 × 256). For technical reasons, gradient echo images were unavailable for 21 patients.
The image-processing pipeline is described in our earlier publication (Levine et al., 2008) (see also Fujiwara et al., 2008; McKinnon et al., 2008; Rosenbaum et al., 2008). In brief, each patient's T1-weighted image was registered to a template brain (Woods et al., 1998a, 1998b), preserving the original size of the brain while standardizing the position and orientation. Template matching was accomplished via non-linear registration of T1-weighted images to the template image (Collins and Evans, 1997). Removal of non-brain tissue from the image incorporated thresholding information derived from the PD- and T2-weighted images, facilitating the distinction between dura mater and gray matter (Kovacevic et al., 2002), optimizing estimation of subdural CSF.
Focal cortical contusions, appearing on at least two slices with a minimal diameter of 3 mm were manually defined in the axial plane in 20 of the 63 patients following a ventral frontal/anterior temporal distribution typical for TBI (Courville, 1937; Gentry et al., 1988) (see Fig. 1). Scans were also clinically interpreted by a board-certified neuroradiologist specializing in TBI. In addition to the large focal lesions, evidence of DAI (e.g., hemosiderin deposits) was present for 91% of the moderate and severe TBI patients and 15% of the mild TBI patients. These clinically evident lesions represent only the regions where the confluence of damage is sufficient to be detected by the naked eye (Gentry, 1990) and are provided for reference as volumetric measures are considered to be more sensitive markers of diffuse injury (Levine et al., 2008).
Fig. 1.
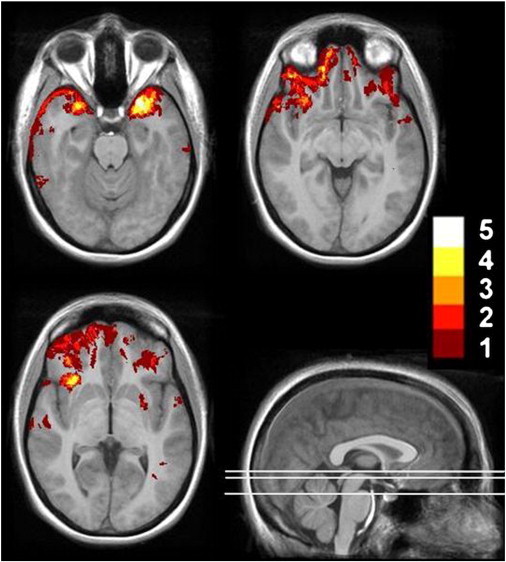
Distribution of focal lesions (contusions) in 20 TBI patients. Lesion tracings are projected on selected axial slices of a template brain derived from 12 healthy control subjects. The color scale indicates degree of lesion overlap across patients (max = 5).
The voxels on the T1-image were then classified as representing gray matter, white matter, or CSF using a robust automated tissue classification method (Kovacevic et al., 2002). A trained operator reclassified CSF-segmented voxels inside the ventricles, allowing for the separate assessment of ventricular and sulcal/subdural CSF (vCSF and sCSF, respectively). A modified Semi-Automated Brain Region Extraction (SABRE) (Dade et al., 2004) method was then used to create 38 ROIs on the template brain (19 per hemisphere; see Fig. 2). Non-linear deformation field matching of the template to individual images was used to customize these regions to fit each patient's brain anatomy, again preserving inter-individual topographical variability. Regional volumes were adjusted for total intracranial capacity using a regression-based method (Arndt et al., 1991).
Fig. 2.
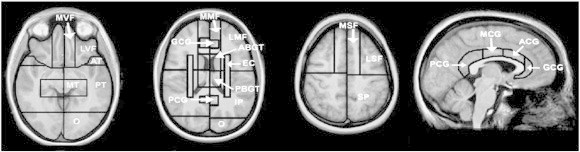
SABRE regional cortical divisions in axial and sagittal views. Abbreviations: LSF: lateral superior frontal, MSF: medial superior frontal, LMF: lateral middle frontal, MMF: medial middle frontal, LVF: lateral ventral frontal, MVF: medial ventral frontal, GCG: genual cingulate gyrus, ACG: anterior cingulate gyrus, MCG: middle cingulate gyrus, PCG: posterior cingulate gyrus, AT: anterior temporal, MT: medial temporal, PT: posterior temporal, O: occipital, ABGT: anterior basal ganglia/thalamus, PBGT: posterior basal ganglia/thalamus, EC: external capsule/corona radiata, IP: inferior parietal, SP: superior parietal.
2.4. Image analysis
Partial Least Squares (PLS), a flexible, widely used multivariate neuroimage analysis technique (McIntosh and Lobaugh, 2004; McIntosh et al., 1996) was used to identify patterns of regional volume loss related to performance on neuropsychological tests. PLS uses singular value decomposition (SVD) applied to the brain–behavior correlation matrix to identify latent variables [LVs] that indicate optimal relations between patterns of regional brain volume loss and the profile of test scores (McIntosh and Lobaugh, 2004; McIntosh et al., 1996). The statistical significance of each LV is assessed by 1500 permutation tests (Edgington, 1980). The stability of each brain region's contribution to the LV is determined through bootstrap resampling (subjects were resampled 500 times) (Wasserman and Bockenholt, 1989). Brain regions were considered reliable if they had a ratio of salience to tandard error (hereafter referred to as the bootstrap ratio, interpreted similar to a Z-score McIntosh and Lobaugh, 2004) greater than 3, corresponding to 99% confidence limits.
Two PLS analyses were conducted. The first set incorporated the full sample of 63 TBI patients, providing a global picture of brain–behavior relationships in this sample. The second set was restricted to 43 patients without traceable focal lesions, hereafter referred to as the “diffuse injury” group. Each analysis included CSF, grey matter, and white matter volumes. Additional analyses were conducted on separate vCSF and sCSF volumes. We also used PLS to assess the relationship of TBI presence and severity classification and test performance (i.e., without considering brain imaging data). This provided a comparison of injury severity measures to brain imaging data in their relationship to cognitive functioning.
3. Results
3.1. Relationship of regional brain volumes to test performance
The PLS analysis for the full sample indicated one significant latent variable for each of the full and diffuse injury samples, ps = .0007 and .0133, respectively, by permutation test. These latent variables accounted for 70% and 58% of the covariance between test scores and regional volumes, respectively.
3.1.1. Contribution of test scores to patterns of brain volume changes
Figs. 3 and 4 display the latent variables for the full sample and diffuse injury PLS analyses. The contribution of each test to the patterns of volume changes described below can be determined by examining the correlations and their 99% confidence intervals as plotted in the upper panels of Figs. 3 and 4. For the full sample, correlations for all test scores except those associated with the WCST were significantly different from 0, with the highest correlations noted for tests of speeded information processing (SDMTo, SDMTw, TRa, TRb), although tests of mnemonic function were also significant. This pattern held for the diffuse injury sample, except that SOP10 was no longer significant, and WCSTpc and WCSTpr were marginally significant.
Fig. 3.
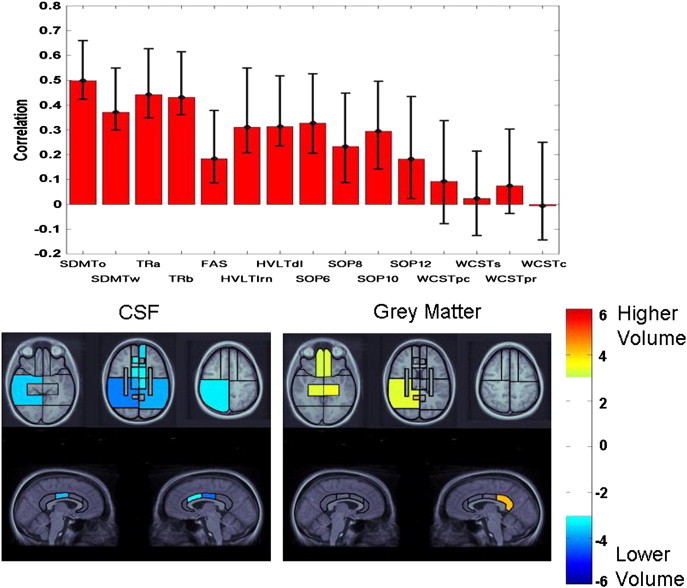
Latent variable from PLS analysis indicating the association of test performance in the full sample with patterns of volume changes across CSF, grey matter, and white matter. Top panel: pattern of test performance associated with the latent variable, expressed as correlations between test scores with the pattern of volume changes. Error bars represent 99% confidence intervals. Tests with error bars crossing the horizontal axis did not significantly contribute to the latent variable. For ease of interpretation, all tests were scaled so that higher scores represent better performance. Remaining panels: Regional plots of bootstrap ratios indicating pattern of CSF and grey matter volume changes associated with the pattern of test scores depicted in the top panel. The color bar indicates the coding scheme according to the level of the bootstrap ratio, interpreted similar to a Z-score. CSF values are negative, indicating volume increases associated with worse test performance, whereas grey matter values are positive, indicating volume loss associated with worse test performance. Images were thresholded at a bootstrap ratio of 3.0, corresponding approximately to p < .001. Axial images are displayed in radiological convention (right hemisphere displayed on left side of image). The right and left cingulate volumes are displayed on the right and left side of the images, respectively.
Fig. 4.
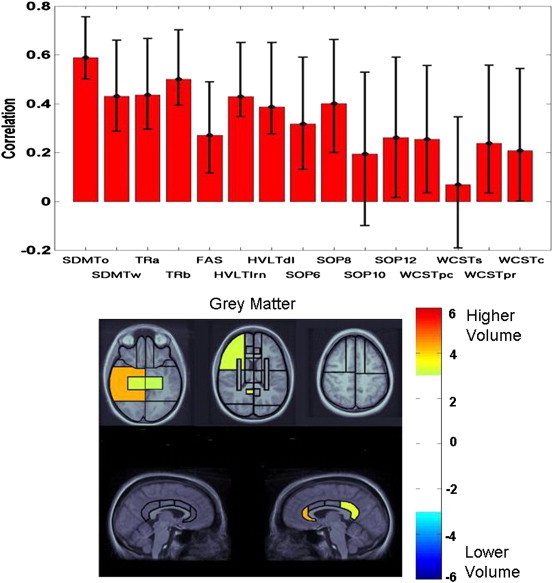
Latent variable from PLS analysis indicating the association of test performance in the diffuse injury group with the patterns of grey matter volume changes. See Fig. 3 caption for interpretation of plots.
3.1.2. Patterns of volumetric changes
The color-coded plots of bootstrap ratios in Figs. 3 and 4 indicate the patterns of CSF, white, and grey matter volume changes associated with the above-described pattern of test scores. For the full sample, significant bootstrap ratios for CSF volumes were noted in a right-lateralized pattern, including the right inferior parietal and posterior temporal regions. The left inferior parietal lobe was also significant. The middle cingulate region was significant, right greater than left, with additional involvement of the right anterior cingulate region. All basal ganglia/thalamic regions were significant. In the frontal lobes, the medial sector of the middle frontal region was significant. Within grey matter, the highest bootstrap ratio was observed in the right posterior cingulate region. The medial temporal lobes were significant bilaterally, as was the right posterior temporal lobe. The ventromedial frontal regions were significant bilaterally.
The regional pattern for the diffuse injury sample was limited to grey matter, where the highest bootstrap ratio was observed for the right posterior temporal lobe and the right anterior cingulate gyrus, with additional involvement of the right posterior cingulate gyrus. Other posterior regions included the medial temporal lobes bilaterally and the right inferior parietal lobe. Within frontal lobes, significant bootstrap ratios were observed for the lateral sectors of the middle frontal regions and the ventromedial sectors bilaterally.
3.1.2.1. Ventricular vs. subdural/sulcal CSF
As above, PLS analyses for the full sample and diffuse injury group yielded a single latent variable for each compartment. For the full sample, p values for permutation tests for sCSF and vCSF were 0.009 and 0, respectively, accounting for 73 and 96% of the covariance between imaging and neuropsychological data, respectively. For the diffuse injury group, these values were .03 and .003, respectively, with 61 and 94% of the covariance accounted for, respectively. Overall, vCSF was more closely related to behavior than was sCSF. For the full sample, all tests but the WCST measures, certain SOP measures, and FAS (vCSF only) contributed to the pattern of volume loss. All regions of vCSF contributed to the pattern, with peaks in the right medial and left posterior temporal lobe, but for sCSF only the right superior parietal and left inferior parietal regions were significant. For the diffuse injury group, all tests but the HVLTd, SOP, and WCST contributed to a generalized pattern of subtle sCSF volume changes, with no specific region of sCSF emerging as significant in this analysis. All tests but the HVLTln, certain SOP measures, and the WCST covaried with vCSF loss in a single region: the left posterior temporal lobe.
3.2. Relationship of TBI severity classification to test performance
PLS analysis assessing the relationship between TBI presence/severity and test performance for the full sample and non-injured comparison subjects indicated one significant latent variable. The permutation test yielded a p value of 0, indicating that none of the 1500 permuted orderings of observations produced a latent variable exceeding the value of the observed one. A single latent variable also emerged from the analysis of the diffuse injury sample, p = .0007 by permutation test. The latent variables accounted for 82% and 78% of the covariance between test scores and severity for the full sample and diffuse injury samples, respectively. For both samples, the non-injured and severe TBI groups contributed to the identified pattern of test scores; the mild and moderate TBI groups did not significantly contribute to the test score pattern. In other words, with respect to their test performance, patients with mild or moderate TBI could not be statistically distinguished from non-injured or severe TBI patients. By comparison, in this same sample, mild and moderate TBI were associated with significant volume loss relative to non-injured comparison subjects (Levine et al., 2008).
Relative to the brain imaging data, injury severity classification was related to a comparatively constrained profile of test performance. Bootstrap ratios for SDMTo, SDMTw, TMTb, HVLTln and HVLTdl were statistically significant for the full sample. The diffuse injury group showed a similar pattern, with SDMTo, HVLTln, HVLTdl, and SOP10 identified as significant.
4. Discussion
Understanding the nature of brain–behavior relationships in TBI has implications for both management of patients with TBI and theory. Clinical interpretation of brain MRI findings has been inconsistently related to behavior as measured by test performance (Levin et al., 1992; Scheid et al., 2006). While quantified MRI has yielded correlations between structural brain volumes and neuropsychological test performance (Bendlin et al., 2008; Gale et al., 1993; Tomaiuolo et al., 2004; Warner et al., 2010a; Wilde et al., 2011), these correlations can be elusive or lacking in neuroanatomical specificity (Serra-Grabulosa et al., 2005; Yount et al., 2002), possibly due to heterogeneity in patient ascertainment, sample size, test selection, image acquisition and analysis methods, and confounding focal and diffuse injury effects.
In this study, we investigated the relationship between structural brain integrity and cognition in a large sample of chronic-phase TBI patients drawn from consecutive admissions to a major trauma center. We found systematic relationships between quantified measures of regional brain volume and neuropsychological test performance, even when patients with focal cortical contusions were excluded. The relationships to brain volume were spatially distributed, indicating that interpretation of brain–behavior relationships in TBI requires whole-brain assessment. Regional brain volume measures were more closely related to behavior than indicators of acute consciousness disruption routinely used to classify TBI severity.
Whereas our previous study in this cohort related regional brain volumes to TBI severity (Levine et al., 2008), in this study we considered regional brain volumes expressing focal or diffuse lesion load as more precise measures of TBI severity effects at the chronic stage. Accordingly, these regional volumetric measures demonstrated a finer-grained relationship to concurrent neuropsychological test performance than did acute TBI severity measures, where patients with mild and moderate TBI did not significantly contribute to the pattern of test scores even though these groups were differentiated in terms of patterns of regional brain volume changes (Levine et al., 2008).
The measures most robustly associated with TBI severity and to regional brain volumes involved speeded attention, working memory, and verbal learning and memory, which covaried with frontal, temporal, and parietal volumes in patients with and without focal lesions. Grey matter volumes in the medial and posterior temporal lobe, the ventromedial prefrontal cortex, and the right inferior parietal lobe covaried with test performance. These regions are classically associated with learning, memory, attention, and self-regulation as well as complaints of working memory deficits. Cingulate regions also figured prominently in the pattern, particularly in the posterior region that is vulnerable in TBI (Gentry et al., 1988; Kim et al., 2008; Levine et al., 2008). This region, closely interconnected to the medial temporal lobes, frontal lobes, and anterior cingulate gyrus (Morris et al., 1999) is considered a connector hub region crucial to integration of information across the cortex (Hagmann et al., 2008; Raja Beharelle et al., 2012).
The oral version of the SDMT, which requires rapid shifting of visual attention and maintenance and manipulation of information on-line, is an excellent measure of such integrated brain function that is highly sensitive to brain disease in other patient populations (Christodoulou et al., 2003; Parmenter et al., 2007; Turken et al., 2008). This test showed the strongest relation to volume loss in both samples. On the other hand, a canonical measure of executive/frontal function, the WCST, did not significantly covary with regional volumetric measures in the full sample. This may reflect the fact that lesions in this group, concentrated in ventral frontal regions, are not in the critical dorsal regions sensitive to WCST (Stuss et al., 2000). In the diffuse injury group, WCST performance subtly contributed to a pattern of test scores that covaried with lateral frontal grey matter (among other regions). Tasks sensitive to ventral frontal damage may show greater specificity in TBI. Indeed, in this same sample, we found a relationship between the tests of ventral frontal function, particularly the Smell Identification Test (Doty et al., 1984) and ventral frontal integrity (Fujiwara et al., 2008). Our data suggest that executive control deficits classically associated with TBI may be secondary to slowed information processing and impaired working memory, which were more robustly related to structural changes following TBI.
In relation to TBI severity, volume loss was demonstrated in grey matter, white matter, and CSF compartments (Levine et al., 2008). In relation to behavior, however, only CSF and grey matter contributed. Thus white matter volume loss is sensitive to TBI, yet the functional consequences as measured by the tests in this study are related to grey matter loss, which is assumed to reflect cell death due to delayed axotomy (Povlishock and Christman, 1995). Indeed, the contribution of white matter volume loss to increased CSF may have attenuated relationships between CSF and behavior. The lack of covariance between test behavior and white matter volumes may relate to limitations of functional localization within white matter as assessed by the T1-weighted image. This should not imply that white matter damage does not contribute to cognitive disability in TBI. On the contrary, axonal damage due to DAI leads to neuronal death and deafferentation of connected fields, affecting neuronal processing in select cortical regions (Maxwell et al., 2010; Povlishock and Christman, 1995). Accordingly, specific effects have been noted using diffusion tensor imaging (DTI) (Bendlin et al., 2008; Kinnunen et al., 2011; Wilde et al., 2006), which can show axonal damage effects in normally appearing white matter on the T1-weighted image. Nonetheless, DTI and the associated processing pipelines have yet to achieve the accessibility of standard T1-weighted imaging.
The relationship between vCSF and behavior in this study replicates prior work (Blatter et al., 1997). This finding, however, may appear difficult to reconcile with our finding that sCSF is more strongly related to injury severity and to whole brain grey matter volume (Levine et al., 2008) (see also Kim et al., 2008). This discrepancy may relate to the regional pattern of brain–behavior relationships that included midline regions with greater vCSF volume, particularly in the temporal lobes, which has been related to mnemonic deficits in patients with TBI (Gale et al., 1994).
4.1. Conclusions
TBI causes widely distributed brain damage with associated impairments on measures of integrated brain function mediating attentional, executive, and mnemonic capacities. In a large sample of patients with chronic-phase TBI drawn from consecutive admissions, variance in temporal, frontal, parietal, and cingulate regional volume covaried with performance on concurrently administered neuropsychological tests of speeded attention, working memory, and verbal learning and memory. Although both white and grey matter are affected in TBI, grey matter volumes more strongly covaried with behavior. The brain–behavior covariance patterns were modified by presence of focal lesions, yet regionally-specific effects held even when patients with focal lesions were excluded, illustrating that diffuse injury alone can cause significant neuropsychological disability. Quantified structural MRI provides a highly sensitive index of brain damage in TBI that is more closely related to behavior than are indicators of acute consciousness disruption routinely used to classify TBI patients into severity groups.
Acknowledgments
Ann Campbell, Catherine Hynes, Sabitha Kanagasabai, Colleen O'Toole, Marina Mandic, Karen Philp, Adriana Restagno, Joel Ramirez, Jovanka Skocic, and Gary Turner are thanked for technical assistance. We gratefully thank the TBI patients and non-injured volunteers for participating in this research. This research was supported by grants from the Canadian Institutes of Health Research (Grant #s MT-14744, MOP-37535, and MOP-108540), and the NIH-NICHD (Grant # HD42385-01) to B.L. B.L. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data and analysis.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- Army Individual Test Battery . War Department, Adjutant General's Office; Washington, D.C.: 1944. Manual of Directions and Scoring. [Google Scholar]
- Arndt S., Cohen G., Alliger R.J., Swayze V.W., II, Andreasen N.C. Problems with ratio and proportion measures of imaged cerebral structures. Psychiatry Research: Neuroimaging. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Bendlin B.B., Ries M.L., Lazar M. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. NeuroImage. 2008;42(2):503–514. doi: 10.1016/j.neuroimage.2008.04.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict R.-H.B., Schretlen D., Groninger L., Brandt J. Hopkins verbal learning test—revised: normative data and analysis of inter-form and test–retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. [Google Scholar]
- Blatter D.D., Bigler E.D., Gale S.D. MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. AJNR. American Journal of Neuroradiology. 1997;18(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Bookstein F.L. “Voxel-based morphometry” should not be used with imperfectly registered images. NeuroImage. 2001;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. (Dec) [DOI] [PubMed] [Google Scholar]
- Christodoulou C., Krupp L.B., Liang Z. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60(11):1793–1798. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- Collins D.L., Evans A.C. ANIMAL: validation and applications of non-linear registration based segmentation. Int J Pattern Recogn. 1997;11:1271–1294. [Google Scholar]
- Courville C.B. Pacific Press Publishing; Mountain View, CA: 1937. Pathology of the central nervous system, part 4. [Google Scholar]
- Dade L.A., Gao F.Q., Kovacevic N. Semiautomatic brain region extraction: a method of parcellating brain regions from structural magnetic resonance images. NeuroImage. 2004;22(4):1492–1502. doi: 10.1016/j.neuroimage.2004.03.023. (Aug) [DOI] [PubMed] [Google Scholar]
- Doty R.L., Shaman P., Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiology and Behavior. 1984:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Edgington E.S. Marcel Dekker; New York: 1980. Randomization Tests. [Google Scholar]
- Fujiwara E., Schwartz M.L., Gao F., Black S.E., Levine B. Ventral frontal cortex functions and quantified MRI in traumatic brain injury. Neuropsychologia. 2008;46(2):461–474. doi: 10.1016/j.neuropsychologia.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale S.D., Burr R.B., Bigler E.D., Blatter D. Fornix degeneration and memory in traumatic brain injury. Brain Research Bulletin. 1993;32(4):345–349. doi: 10.1016/0361-9230(93)90198-k. [DOI] [PubMed] [Google Scholar]
- Gale S.D., Johnson S.C., Bigler E.D., Blatter D.D. Traumatic brain injury and temporal horn enlargement: correlates with tests of intelligence and memory. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1994;7(3):60–165. (Jul 1994) [Google Scholar]
- Gale S.D., Baxter L., Roundy N., Johnson S.C. Traumatic brain injury and grey matter concentration: a preliminary voxel based morphometry study. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(7):984–988. doi: 10.1136/jnnp.2004.036210. (Jul) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennarelli T.A., Thibault L.E., Adams J.H., Graham D.I., Thompson C.J., Marcincin R.P. Diffuse axonal injury and traumatic coma in the primate. Annals of Neurology. 1982;1982:564–572. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- Gentry L.R. Head Trauma. In: Atlas S.W., editor. Magnetic Resonance Imaging of the Brain and Spine. Raven; New York, NY: 1990. pp. 439–466. [Google Scholar]
- Gentry L.R., Godersky J.C., Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. American Journal of Neuroradiology. 1988;9:101–110. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- Green P., Allen L.M.I., Astner K. CogniSyst, Inc.; Durham, NC: 1996. The Word Memory Test. [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X. Mapping the structural core of human cerebral cortex. PLoS Biology. 2008;6(7):e159. doi: 10.1371/journal.pbio.0060159. (Jul 1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J., Avants B., Patel S. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. NeuroImage. 2008;39(3):1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen K.M., Greenwood R., Powell J.H. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134(Pt. 2):449–463. doi: 10.1093/brain/awq347. (Feb) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic N., Lobaugh N.J., Bronskill M.J., Levine B., Feinstein A., Black S.E. A robust method for extraction and automatic segmentation of brain images. NeuroImage. 2002;17(3):1087–1100. doi: 10.1006/nimg.2002.1221. (Nov) [DOI] [PubMed] [Google Scholar]
- Levin H.S., Benton A.L., Grossman R.G. Oxford University Press; New York: 1982. Neurobehavioral Consequences of Closed Head Injury. [Google Scholar]
- Levin H.S., Williams D.H., Eisenberg H.M., High W.M., Jr., Guinto F.C., Jr. Serial MRI and neurobehavioural findings after mild to moderate closed head injury. Journal of Neurology, Neurosurgery, and Psychiatry. 1992;55(4):255–262. doi: 10.1136/jnnp.55.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Fujiwara E., O'Connor C. In vivo characterization of traumatic brain injury neuropathology with structural and functional neuroimaging. Journal of Neurotrauma. 2006;23(10):1396–1411. doi: 10.1089/neu.2006.23.1396. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B., Kovacevic N., Nica E.I. The Toronto traumatic brain injury study: injury severity and quantified MRI. Neurology. 2008;70(10):771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Mattson A.J., Levin H.S. Frontal lobe dysfunction following closed head injury. The Journal of Nervous and Mental Disease. 1990;178(5):282–291. doi: 10.1097/00005053-199005000-00002. [DOI] [PubMed] [Google Scholar]
- Maxwell W.L., MacKinnon M.A., Stewart J.E., Graham D.I. Stereology of cerebral cortex after traumatic brain injury matched to the Glasgow outcome score. Brain. 2010;133(Pt 1):139–160. doi: 10.1093/brain/awp264. (Jan) [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Lobaugh N.J. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23(Suppl. 1):S250–S263. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- McIntosh A.R., Bookstein F.L., Haxby J.V., Grady C.L. Spatial pattern analysis of functional brain images using partial least squares. NeuroImage. 1996;3:143–157. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- McKinnon M.C., Nica E.I., Sengdy P. Autobiographical memory and patterns of brain atrophy in fronto-temporal lobar degeneration. Journal of Cognitive Neuroscience. 2008;20(10):1839–1853. doi: 10.1162/jocn.2008.20126. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris R., Petrides M., Pandya D.N. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) European Journal of Neuroscience. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. (Jul) [DOI] [PubMed] [Google Scholar]
- Parmenter B.A., Weinstock-Guttman B., Garg N., Munschauer F., Benedict R.H. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Multiple Sclerosis. 2007;13(1):52–57. doi: 10.1177/1352458506070750. (Jan) [DOI] [PubMed] [Google Scholar]
- Petrides M., Milner B. Deficits on subject-ordered tasks after frontal- and temporal-lobe lesions in man. Neuropsychologia. 1982;20:249–262. doi: 10.1016/0028-3932(82)90100-2. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T., Christman C.W. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. Journal of Neurotrauma. 1995;12(4):555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Povlishock J.T., Katz D.I. Update of neuropathology and neurological recovery after traumatic brain injury. The Journal of Head Trauma Rehabilitation. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. (Jan-Feb) [DOI] [PubMed] [Google Scholar]
- Raja Beharelle A., Kovacevic N., McIntosh A.R., Levine B. Brain signal variability relates to stability of behavior after recovery from diffuse brain injury. NeuroImage. 2012;60(2):1528–1537. doi: 10.1016/j.neuroimage.2012.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum R.S., Moscovitch M., Foster J.K. Patterns of autobiographical memory loss in medial-temporal lobe amnesic patients. Journal of Cognitive Neuroscience. 2008;20(8):1490–1506. doi: 10.1162/jocn.2008.20105. (Aug) [DOI] [PubMed] [Google Scholar]
- Scheid R., Walther K., Guthke T., Preul C., von Cramon D.Y. Cognitive sequelae of diffuse axonal injury. Archives of Neurology. 2006;63(3):418–424. doi: 10.1001/archneur.63.3.418. (Mar) [DOI] [PubMed] [Google Scholar]
- Serra-Grabulosa J.M., Junque C., Verger K., Salgado-Pineda P., Maneru C., Mercader J.M. Cerebral correlates of declarative memory dysfunctions in early traumatic brain injury. Journal of Neurology, Neurosurgery, and Psychiatry. 2005;76(1):129–131. doi: 10.1136/jnnp.2004.027631. (Jan) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A., Skimminge A., Liptrot M.G. Long-term global and regional brain volume changes following severe traumatic brain injury: a longitudinal study with clinical correlates. NeuroImage. 2009;44(1):1–8. doi: 10.1016/j.neuroimage.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Smith A. Western Psychological Services; Los Angeles: 1978. Symbol Digit Modalities Test. [Google Scholar]
- Spreen O., Strauss E. Oxford University Press; New York: 1998. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. [Google Scholar]
- Stuss D.T., Gow C.A. “Frontal dysfunction” after traumatic brain injury. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1992;5(4):272–282. [Google Scholar]
- Stuss D.T., Levine B., Alexander M.P. Wisconsin Card Sorting Test performance in patients with focal frontal and posterior brain damage: effects of lesion location and test structure on separable cognitive processes. Neuropsychologia. 2000;38(4):388–402. doi: 10.1016/s0028-3932(99)00093-7. [DOI] [PubMed] [Google Scholar]
- Teasdale G., Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thurman D.J., Alverson C., Dunn K.A., Guerrero J., Sniezek J.E. Traumatic brain injury in the United States: a public health perspective. The Journal of head trauma rehabilitation. 1999;14(6):602–615. doi: 10.1097/00001199-199912000-00009. (Dec) [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F., Carlesimo G.A., Di Paola M. Gross morphology and morphometric sequelae in the hippocampus, fornix, and corpus callosum of patients with severe non-missile traumatic brain injury without macroscopically detectable lesions: a T1 weighted MRI study. Journal of Neurology, Neurosurgery, and Psychiatry. 2004;75(9):1314–1322. doi: 10.1136/jnnp.2003.017046. (Sep) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaiuolo F., Worsley K.J., Lerch J. Changes in white matter in long-term survivors of severe non-missile traumatic brain injury: a computational analysis of magnetic resonance images. Journal of Neurotrauma. 2005;22(1):76–82. doi: 10.1089/neu.2005.22.76. (Jan) [DOI] [PubMed] [Google Scholar]
- Turken A., Whitfield-Gabrieli S., Bammer R., Baldo J.V., Dronkers N.F., Gabrieli J.D. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. NeuroImage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A., Marquez de la Plata C., Spence J. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. Journal of Neurotrauma. 2010;27(12):2121–2130. doi: 10.1089/neu.2010.1429. (Dec) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner M.A., Youn T.S., Davis T. Regionally selective atrophy after traumatic axonal injury. Archives of Neurology. 2010;67(11):1336–1344. doi: 10.1001/archneurol.2010.149. (Nov) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman S., Bockenholt U. Bootstrapping: applications to psychophysiology. Psychophysiology. 1989;26(2):208–221. doi: 10.1111/j.1469-8986.1989.tb03159.x. (Mar) [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Chu Z., Bigler E.D. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde E.A., Newsome M.R., Bigler E.D. Brain imaging correlates of verbal working memory in children following traumatic brain injury. International Journal of Psychophysiology. 2011;82(1):86–96. doi: 10.1016/j.ijpsycho.2011.04.006. (Oct) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods R.P., Grafton S.T., Holmes C.J., Cherry S.R., Mazziotta J.C. Automated image registration: I. General methods and intrasubject, intramodality validation. Journal of Computer Assisted Tomography. 1998;22(1):139–152. doi: 10.1097/00004728-199801000-00027. (Jan-Feb) [DOI] [PubMed] [Google Scholar]
- Woods R.P., Grafton S.T., Watson J.D., Sicotte N.L., Mazziotta J.C. Automated image registration: II. Intersubject validation of linear and nonlinear models. Journal of Computer Assisted Tomography. 1998;22(1):153–165. doi: 10.1097/00004728-199801000-00028. (Jan-Feb) [DOI] [PubMed] [Google Scholar]
- Yount R., Raschke K.A., Biru M. Traumatic brain injury and atrophy of the cingulate gyrus. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14(4):416–423. doi: 10.1176/jnp.14.4.416. (Fall) [DOI] [PubMed] [Google Scholar]
- Zachary R.A. Western Psychological Services; Los Angeles: 1986. Shipley Institute of Living Scale. Revised Manual. [Google Scholar]


