Abstract
Migraine without aura is the most common form of migraine, characterized by recurrent disabling headache and associated autonomic symptoms. To identify common genetic variants for this migraine type, we analyzed genome-wide association data of 2,326 clinic-based German and Dutch patients and 4,580 population-matched controls. We selected SNPs from 12 loci with two or more SNPs with P-values < 1 × 10−5 for follow-up in 2,508 patients and 2,652 controls. Two loci, i.e. 1q22 (MEF2D) and 3p24 (near TGFBR2) replicated convincingly (P = 4.9 × 10−4, P = 1.0 × 10−4, respectively). Meta-analysis of the discovery and replication data yielded two additional genome-wide significant (P < 5 × 10−8) loci in PHACTR1 and ASTN2. In addition, SNPs in two previously reported migraine loci in or near TRPM8 and LRP1 significantly replicated. This study reveals the first susceptibility loci for migraine without aura, thereby expanding our knowledge of this debilitating neurological disorder.
Main text
Migraine is a disabling episodic neurovascular brain disorder affecting 12% of the general population1-4. Migraine attacks are typically characterized by severe throbbing unilateral headache and nausea, vomiting and photo- and phonophobia (migraine without aura; MO). In up to one third of patients attacks may be associated with neurological aura symptoms (migraine with aura; MA). Previous genome-wide association studies (GWAS) identified a migraine susceptibility locus on chromosome 8q22, close to MTDH, in the clinic-based International Headache Genetics Consortium (IHGC) MA study5 and three other loci in or near PRDM16, LRP1, and TRPM8 in the population-based migraine Women’s Genome Health Study (WGHS)6. For TRPM8 there was suggestive association (P < 1 × 10−5) also in the clinic-based IHGC MA GWAS5. Here we report the first GWAS in MO, the most common form of migraine. We analyzed two large samples from headache centres in Germany and the Netherlands including 2,326 MO patients and 4,580 population-matched controls (Supplementary Note and Supplementary Fig. 1). A quantile-quantile plot of the joint analysis (Supplementary Fig. 2) and an overall inflation factor (λ1000) of 1.03 were used as final quality control measures. The discovery dataset identified one genome-wide significant (P < 5 × 10−8) locus on chromosome 1q22 as well as eleven additional loci containing multiple SNPs with suggestive association (P < 1 × 10−5) (Supplementary Table 1). Eighteen SNPs from these 12 loci were taken forward to the replication stage in four independent clinic-based European MO samples (2,508 cases and 2,652 controls) (Supplementary Fig. 1 and Supplementary Table 1). Eight SNPs in six loci showed P-values < 0.05 in the replication study, and five of these SNPs also showed P-values < 5 × 10−8 in the meta-analysis combining the discovery and replication cohorts (Table 1, Fig. 1 and Supplementary Fig. 3). Four loci (1q22, 3p24, 6p24, 9q33) replicated, although replication was less convincing for loci on 6p24 and 9q33 with replication P-values of 0.012 and 0.018, respectively, although P-values were < 5 × 10−8 in the overall meta-analysis. In addition, we tested top SNPs of the four previously identified migraine loci (1p36, 2q37, 8q22, 12q13)5,6 in the replication stage (Fig. 2 and Supplementary Table 1), of which 2q37 and 12q13 convincingly replicated. Because migraine is more prevalent in women, we performed a gender interaction analysis for the reported SNPs (Supplementary Table 2). No significant interactions were observed and all SNPs had relatively similar odds ratios in both genders. To further extend our analyses, we searched for expression quantitative trait loci (eQTLs) in available data sets of tissues and cell lines7,8, but did not observe consistently significant eQTLs for any of the SNPs of Table 1.
Table 1. SNPs of the six migraine without aura loci.
SNPs showing significant association either in the discovery stage (i.e. locus 1) or after meta-analysis of the discovery and replication samples (i.e. loci 2-6).
| General SNP information | Discovery samples | All replications | Overall meta-analysis | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SNP | Chr | Positiona | Location | Geneb | Minor allele |
minor allele frequency |
Gen./Imp.c | P-Value | OR (95% CI) | I2 | P-value | OR (95% CI) | I2 | P-value | OR (95% CI) | I2 | r2 with top SNP |
| Locus 1: | |||||||||||||||||
| rs1050316 | 1 | 154.701.327 | Intragenic | MEF2D | G | 0.34 | Imputed | 2.59×10−8 | 1.24 [1.15-1.33] | 0.00 | 1.15×10−3 | 1.14 [1.06-1.24] | 0.00 | 3.21×10−10 | 1.19 [1.13-1.26] | 0.00 | 0.987 |
| rs2274316 | 1 | 154.712.866 | Intragenic | MEF2D | C | 0.35 | Genotyped | 3.60×10−8 | 1.23 [1.14-1.33] | 0.00 | - | - | - | - | - | - | 0.986 |
| rs1925950 | 1 | 154.717.364 | Intragenic | MEF2D | G | 0.35 | Imputed | 2.97×10−8 | 1.24 [1.15-1.33] | 0.00 | - | - | - | - | - | - | 0.988 |
| rs3790455 | 1 | 154.722.925 | Intragenic | MEF2D | C | 0.34 | Genotyped | 1.71×10−8 | 1.24 [1.15-1.34] | 0.00 | 4.85×10−4 | 1.16 [1.07-1.26] | 0.00 | 7.06×10−11 | 1.20 [1.14-1.27] | 0.00 | - |
| rs3790459 | 1 | 154.728.331 | Intragenic | MEF2D | A | 0.35 | Imputed | 2.85×10−8 | 1.24 [1.15-1.33] | 0.00 | - | - | - | - | - | - | 0.989 |
| rs12136856 | 1 | 154.739.738 | Intergenic | MEF2D | C | 0.34 | Imputed | 3.90×10−8 | 1.23 [1.15-1.33] | 0.00 | - | - | - | - | - | - | 0.985 |
| Locus 2: | |||||||||||||||||
| rs7640543 | 3 | 30.437.407 | Intergenic | TGFBR2 | A | 0.32 | Genotyped | 2.72×10−6 | 1.20 [1.11-1.30] | 0.00 | 1.02×10−4 | 1.18 [1.09-1.29] | 0.51 | 1.17×10−9 | 1.19 [1.13-1.26] | 0.19 | - |
| Locus 3: | |||||||||||||||||
| rs9349379 | 6 | 13.011.943 | Intragenic | PHACTR1 | G | 0.38 | Genotyped | 2.06×10−7 | 0.82 [0.77-0.89] | 0.00 | 0.01 | 0.90 [0.83-0.98] | 0.00 | 3.20×10−8 | 0.86 [0.81-0.91] | 0.10 | - |
| Locus 4: | |||||||||||||||||
| rs6478241 | 9 | 118.292.450 | Intragenic | ASTN2 | A | 0.38 | Genotyped | 1.14×10−7 | 1.22 [1.13-1.31] | 0.00 | 0.02 | 1.10 [1.02-1.19] | 0.57 | 3.86×10−8 | 1.16 [1.10-1.23] | 0.56 | - |
| Locus 5: | |||||||||||||||||
| rs10166942 | 2 | 234.489.832 | Intergenic | TRPM8 | C | 0.18 | Genotyped | 1.32×10−5 | 0.82 [0.74-0.89] | 0.00 | 5.62×10−9 | 0.74 [0.67-0.82] | 0.00 | 9.83×10−13 | 0.78 [0.73-0.84] | 0.00 | - |
| rs17862920 | 2 | 234.492.734 | Intragenic | TRPM8 | T | 0.10 | Genotyped | 2.19×10−5 | 0.78 [0.69-0.87] | 0.00 | 6.44×10−5 | 0.75 [0.66-0.87] | 0.00 | 5.97×10−9 | 0.77 [0.70-0.84] | 0.00 | 0.520 |
| Locus 6: | |||||||||||||||||
| rs11172113 | 12 | 55.813.550 | Intragenic | LRP1 | C | 0.40 | Genotyped | 3.38×10−5 | 0.86 [0.80-0.92] | 0.00 | 2.33×10−4 | 0.86 [0.79-0.93] | 0.67 | 2.97×10−8 | 0.86 [0.81-0.91] | 0.45 | - |
Genome-wide significant P-values and successful replications are shown in boldface. ORs are reported for the minor allele.
Chromosomal positions are based on NCBI build 36.
For intragenic SNPs the gene is listed in which the SNP is located, whereas for intergenic SNPs the nearest gene is listed.
Gen./Imp. (Genotyping/imputation) indicates whether a SNP is genotyped or imputed. I2 = heterogeneity index. r2 indicates the LD between the SNP and the top SNP in the respective locus.
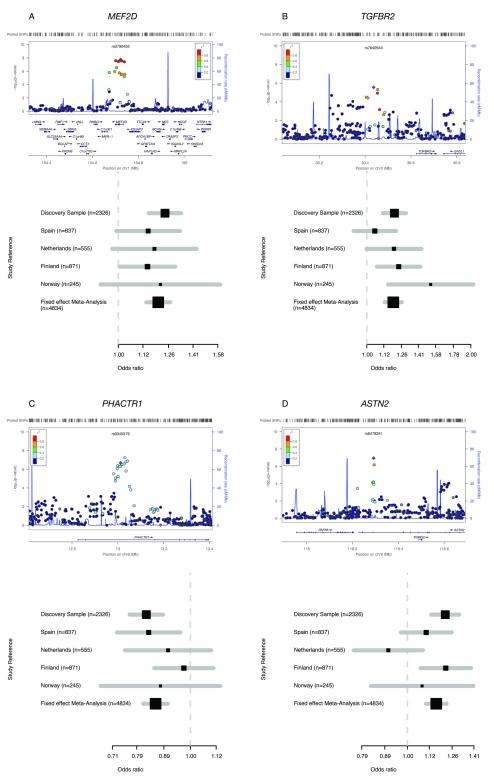
Figure 1. Regional and forest plots for the novel migraine loci.
Regional association plots (generated using LocusZoom (see Web resources)) are shown for the four novel migraine loci together with a forest plot for the SNP with highest association for the respective locus (with the number of cases for each sample indicated) (a-d). Each regional plot shows the chromosomal position (NCBI build 36) of SNPs in the specific region against the –log10 P-values. The SNP with the highest association signal in each locus is represented as a purple diamond; the additional SNPs are color-coded according to the extent of LD with that SNP. Estimated recombination rates (cM/Mb) from HapMap CEU release 22 are shown as light blue lines. Forest plots are shown for: rs3790455 (MEF2D) (a), rs7640543 (TGFBR2) (b), rs9349379 (PHACTR1) (c), and rs6478241 (ASTN2) (d). Black squares represent the odds ratios for the individual cohorts; the horizontal lines represent the 95% confidence intervals. Numbers in parentheses indicate the number of cases in each cohort.
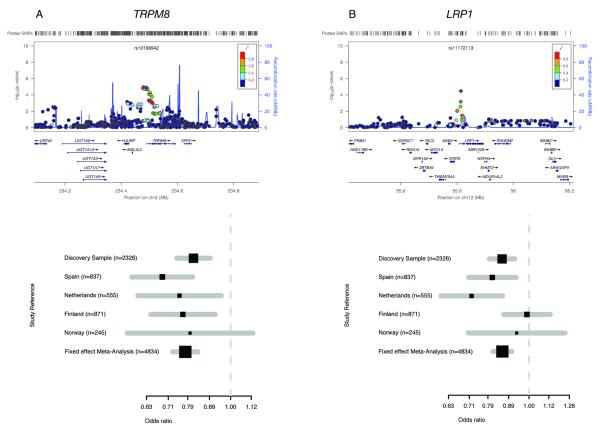
Figure 2. Regional and forest plots for the previously reported migraine loci that replicate in the current MO study.
Regional association plots (generated using LocusZoom (see Web resources)) are shown for the two previously reported migraine loci6 that significantly replicated in the current study as well as forest plots for the SNP with highest association in these loci (with the number of cases for each sample indicated) (a and b).
Each regional plot shows the chromosomal position (NCBI build 36) of SNPs in the specific region against the –log10 P-values. SNP with the highest association signal in each locus is represented as a purple diamond; the additional SNPs are color-coded according to the extent of LD with this SNP. Estimated recombination rates (cM/Mb) from HapMap CEU release 22 are shown as light blue lines. Forest plots are shown for: rs10166942 (TRPM8) (a) and rs11127113 (LRP1) (b). Black squares represent the odds ratio for the individual cohorts; the horizontal lines represent the 95% confidence intervals. Numbers in parentheses indicate the number of cases in each cohort.
The 1q22 locus contained six SNPs with genome-wide significant association (P < 5 × 10−8) already in the discovery stage of the analysis and that were all in close LD (r2 > 0.98). SNPs rs1050316 and rs3790455 were taken forward to the replication stage and successfully replicated (overall meta-analysis P-values: 3.21 × 10−10 (OR = 1.19) and 7.06 × 10−11 (OR = 1.20), respectively) (Table 1, Fig. 1 and Supplementary Fig. 3). All associated SNPs are located within the MEF2D (myocyte enhancer factor 2D) gene (i.e. intronic and 3′-UTR) that encodes a transcription factor highly expressed in brain. MEF2D regulates neuronal differentiation by supporting survival of newly formed neurons9. Perhaps even more relevant to migraine, neuronal activity-dependent activation of MEF2D restricts the number of excitatory synapses10. As the migraine brain is hyperexcitable11, it is tempting to speculate that MEF2D dysregulation may affect neuronal excitatory neurotransmission in MO patients. There is some evidence for increased glutamate (the main brain excitatory neurotransmitter) levels in migraine patients12,13 and increased glutamatergic neurotransmission was reported in a transgenic knock-in mouse model with a pathogenic human hemiplegic migraine gene mutation14. A role for MEF2D dysregulation in migraine is also plausible given that several MEF2 target genes have been associated with other neurological disorders, such as epilepsy15-17. Notably, pituitary adenylate cyclase-activating polypeptide-38 (PACAP-38), which is encoded by one of the activity-regulated MEF2D target genes and modulates excitatory synaptic transmission, may trigger migraine-like attacks in patients with MO18. In mice, PACAP-38 is involved in nitroglycerol-induced trigeminovascular activation19, the presumed origin of the migraine headache20.
The 3p24 locus contained top SNP rs7640543 (initial P = 2.72 × 10−6, OR = 1.20). This SNP showed strong replication (P = 1.02 × 10−4) and reached genome-wide significance in the meta-analysis of the discovery and replication samples (P = 1.17 × 10−9, OR = 1.19) (Table 1, Fig. 1 and Supplementary Fig. 3). Rs7640543 is located ~200 kb upstream of TGFBR2 (transforming growth factor beta receptor 2), which encodes a serine-threonine kinase involved in the regulation of cell proliferation and differentiation as well as extracellular matrix production21. TGFBR2 is an attractive candidate gene for migraine as the p.Arg460His missense mutation in TGFBR2 not only causes seemingly monogenic, familial aortic dissection, but also migrainous headaches in 11 of 14 mutation carriers in a large multigenerational family22. This may fit with the observation that migraineurs seemed to have a two-fold increased risk for cervical artery dissection23.
For the 6p24 locus, SNP rs9349379 reached genome-wide significance when combining the data of the discovery and replication samples (P = 3.20 × 10−8, OR = 0.86) (Table 1, Fig. 1 and Supplementary Fig. 3). Five SNPs (P < 10−5) were taken forward to the replication stage (see Supplementary Table 1). All five SNPs are located in PHACTR1 (phosphastase and actin regulator 1), a member of the PHACTR/scapinin family, which controls synaptic activity and synapse morphology through regulation of protein phosphatase 1 and actin binding24,25. PHACTR1 has further been implicated in endothelial cell functioning26 and susceptibility to early-onset myocardial infarction27. Thus, the link between PHACTR1 and migraine could be neuronal through aberrant synaptic transmission or vascular since endothelial dysfunction, cardiovascular disease and myocardial infarction all seemed to be linked with migraine28. Like TGFBR2, PHACTR1 may also be involved in systemic vascular disease through a TGFβ signalling pathway.
The 9p33 locus with top SNP rs6478241 reached genome-wide significance in the meta-analysis of the discovery and replication samples, although there is heterogeneity (I2 = 0.57) in the replication samples (P = 3.86 × 10−8, OR = 1.16) (Table 1, Fig. 1 and Supplementary Fig. 3). In one of the four replication cohorts the effect direction was opposite to the discovery sample and the other replication samples. This locus should be considered tentative and further studies are needed to confirm its relevance to migraine. Rs6478241 is located in ASTN2, a member of the astrotactin gene family, which plays a role in glial-guided migration that seems important for development of the laminar architecture of cortical regions in the brain29. Although structural abnormalities in migraineurs have been reported in the somatosensory cortex30 and the cerebellum31, they more likely reflect degenerative processes related to severe migraine attacks than developmental problems. Therefore, it remains at present unclear how ASTN2 could play a role in migraine pathophysiology.
In addition, two of the four previously reported migraine loci5,6 showed significant association in the current clinic-based MO GWAS. Two top SNPs (r2 = 0.52) in the 2q37 migraine locus reached genome-wide significance in the overall MO meta-analysis (rs10166942, P = 9.83 × 10−13, OR = 0.78; rs17862920, P = 5.97 × 10−9, OR = 0.77) (Table 1, Fig. 2 and Supplementary Fig. 3). SNP rs10166942 is located about 1 kb upstream of the predicted transcription start of TRPM8 (transient receptor potential melastatin 8), whereas rs17862920 is located in the first intron of TRPM8. TRPM8 encodes a cold- and menthol-activated ion channel that is expressed in sensory neurons. The gene was identified as a migraine susceptibility gene both in the population-based WGHS migraine GWAS6 and the clinic-based IHGC MA GWAS5. The effect direction of these SNPs is the same in all three studies, and the effect size estimates are similar (OR = 0.78 in the clinic-based IHGC MA study for rs17862920 versus 0.77 in the present study, and OR = 0.85 in the population-based WGHS migraine study for rs10166942 versus 0.78 in the present study). TRPM8 could be involved in cutaneous allodynia32-34, which is defined as pain due to thermal or mechanical stimuli that normally do not provoke pain that is present in the majority of migraine patients.
The top SNP rs11172113 of the previously reported6 12q13 migraine locus reached genome-wide significance in the overall MO meta-analysis (rs11172113, overall P = 2.97 × 10−8, OR = 0.86) (Table 1, Fig. 2 and Supplementary Fig. 3). SNP rs11172113 is located within the first intron of LRP1 that codes for the low density lipoprotein receptor-related protein 1 which is expressed in multiple tissues including neurons and the vasculature. LRP1 is a cell surface receptor that acts as a sensor of the extracellular environment: it is involved in the proliferation of vascular smooth muscle cells, and modulates synaptic transmission35,36. A possible role for LRP1 in migraine can be envisaged because of its neuronal and/or vascular function.
Addressing the question whether MA and MO represent different disease entities37,38, we tested the top SNPs from the six loci from the current MO study in silico in our previous IHGC MA GWAS data set5 (Supplementary Table 3). Except for the 3p24 locus, all loci showed P-values below 0.05 in the MA data set and ORs going in the same direction in both studies. The TRPM8 locus showed the most significant P-value (rs1786920: 2.19 × 10−5, OR = 0.78 and rs10166942: 1.32 × 10−5, OR = 0.82) in the MA data set. Interestingly, rs10166942 also showed association in the WGHS migraine study (P = 2.30 × 10−7; OR = 0.86 in the initial scan)6. This suggests that TRPM8 may play a role in various forms of migraine. In contrast, the SNP rs1835740 in the 8q22 locus in the IHGC MA GWAS5 pointing at MTDH as the putative migraine susceptibility gene5, did not show association in the present GWAS (P = 0.70) nor the population-based WGHS migraine GWAS6 (P = 0.22). This may suggest that MTDH confers more susceptibility to aura than to headache.
In conclusion, we present the first GWAS in MO, the most common migraine type, and identified several loci. Two loci (MEF2D and TGFBR2) showed convincing replication signals, whereas replication for the PHACTR1 and ASTN2 loci was weaker. Future studies will need to confirm their role as migraine susceptibility loci. In addition, two of the four previously identified migraine loci (i.e. TRPM8 and LRP1) replicated in this clinic-based MO study. Functional studies are necessary to dissect the exact underlying molecular pathways to identify putative treatment targets for this common debilitating brain disorder.
ONLINE METHODS
Overall study design
The discovery stage of the study was based on an analysis of de novo genotyping in two large MO sample sets from headache clinics in Germany (Munich/Kiel) and the Netherlands (Leiden) (Supplementary Fig. 1). Population-matched controls were recruited from studies with existing genotyping data (for details on study cohorts and controls cf. Supplementary Note). For both sample sets, raw data were imputed to approximately 1.4M SNPs using HapMap3 release 239 as reference panel. As an initial step, genome-wide logistic regression analysis was performed independently in both samples, followed by meta-analysis of the two datasets. Subsequently, the top SNPs of the meta-analysis were tested for replication in four smaller clinic-based MO samples from Finland (Helsinki), Spain (Barcelona), Norway (Trondheim) and the Netherlands (Leiden) (Fig. 1 and Supplementary Fig. 1).
Ethical aspects
Written informed consent was obtained from all participants, and the study was approved by the respective local research ethics committees of the Klinikum Großhadern, Ludwig-Maximilians-University in Munich (Germany), the University of Leiden Medical Centre (The Netherlands), the Helsinki University Central Hospital (Finland), the Vall d’Hebron Research Institute in Barcelona (Spain), and the Regional Committee for Medical and Health Research Ethics in Trondheim (Norway).
Discovery stage genotyping
Genomic DNA was extracted from peripheral blood samples according to standard protocols. Genotyping of the German GWAS sample was performed at Genome Analysis Center, Helmholtz Zentrum München, Germany using the Illumina Human 610-Quad v1 (n = 838) or Illumina Human 660W-Quad v1 SNP microarrays according to the Infinium II protocol from the manufacturer (Illumina Inc., San Diego, CA, USA) (n = 391). Genotype calling was performed using the Illumina Gencall data analyses software. Genotyping of the complete Dutch GWAS sample was performed at the Wellcome Trust Sanger Institute using the Illumina 660W technology. Genotype calling was performed using the Illuminus software.
Replication stage genotyping
For the replication study, all cases and controls were genotyped at the Wellcome Trust Sanger Institute using matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry (MS), using the Sequenom MassARRAY™ methodology (Sequenom Inc, San Diego, CA, USA). Amplification reactions and parameters were based on the manufacturer’s instructions. Each 384-wells plate contained positive (CEPH DNA) and negative controls, to check for assay performance and contaminations, respectively. Spectrocaller software supplied by the manufacturer was used to automatically call the genotypes. Clusters were checked manually and all doubtful calls were evaluated.
In the replication stage, we selected all loci with at least two SNPs with P < 1 × 10−5 for follow-up in the discovery stage (see Supplementary Table 1). Two SNPs each were selected from loci deemed to be most convincing (MEF2D, PHACTR1, near TGFBR2, FHL5), and one SNP each from the remaining loci (see Supplementary Table 1). At the PHACTR1 locus, as we observed both effect directions for minor alleles in the discovery stage, we chose three additional SNPs (for a total of five) for follow-up at this locus to robustly cover possible heterogeneity at this locus. In addition, the top reported SNPs from the four previously reported migraine loci (see Supplementary Table 1) were included in the replication stage.
Quality control
To ensure high data quality, the datasets from the primary study were subjected to per-SNP and per-sample quality control (QC) before and after imputation. In both cases, cut-offs of 1% for minor allele frequency and 1 × 10−6 for HWE were used for cases and controls independently (as the cases and controls were genotyped independently in both populations), and the latter again for the combined set of cases and controls. Further, SNPs with call rates < 97% were excluded. Subjects with a genotyping rate <97% and subjects which were closely related to each other (π_hat > 0.15) were removed, as were those with cryptic relatedness and those deemed to be heterozygosity outliers. In addition, population outliers were excluded using a manual selection from a multidimensional scaling plot of the genome-wide IBS pair-wise distance matrix in PLINK.
Before imputation, 452,154 SNPs for 3,772 individuals (1,208 cases and 2,564 controls) were in the German dataset, and 494,760 SNPs for 3,134 individuals (1,118 cases and 2,016 controls) were in the Dutch dataset.
Imputation
Imputation of the German and Dutch discovery samples was performed using IMPUTE 2 (v2.1.0 for the German samples, v2.1.2 for the Dutch samples)40. For the phased haploid reference panel, we used HapMap3 release 2 data for the 120 unrelated CEU trios41. We used the recommended parameters for the imputation, with the exception of a different number of copying states (κ = 60 for Germans, κ = 80 for Dutch) and a larger buffer size for the Dutch (500 kb instead of 250 kb). After imputation, we used individual call posterior probability > 0.9 and info measure I(A) > 0.6 as cut-offs to ensure high imputation data quality. From 1,411,821 SNPs after imputation, 165,433 SNPs were dropped due to QC reasons.
Statistical analysis
For the GWA data from the two initial study samples, we analyzed the imputed allele dosage data with the SNPTEST software (version 2.2.0; see Web Resources) to generate population-specific summary statistics. We used the presence of migraine as a binary phenotype, and assumed an additive model. Consistent with our previous clinic-based MA GWA study5 the association analysis was not corrected for age and gender. The primary reason for not using age as a covariate was that we lack age information for some of the control cohorts. However the majority of the individuals in the cohorts were of working age, similar to our case samples. The missing data score likelihood option was used for handling missing data. For the replication studies, genotyped markers were analyzed using the same model as the discovery samples for the population-specific results.
A fixed-effect meta-analysis of the summary statistics was conducted using GWAMA42 (see Web Resources) first on the two discovery samples for the primary results. In the discovery sample meta-analysis, only SNPs that were present in both datasets were retained and filtered for the heterogeneity measure I2 < 0.5. This moderately high threshold for I2 was chosen to reflect the expectation of some differences in association signals of common markers due to varying LD structure. After replication, all six study sets (two discovery and four replication samples) were included in the overall meta-analysis. Reasonable genomic inflation was observed (λ = 1.095, λ1000 = 1.031). Consistency of allelic effects across studies was examined utilising the Cochran’s Q43 and I2 metrics44. Between-study (effect) heterogeneity was indicated by Q-statistic p-values (P ≤ 0.1) and moderate (25-50%) or larger I2 values45. Meta-analysis of SNPs associated with P ≤ 1 × 10−5 and showing evidence of effect heterogeneity were also analysed using a random-effects model46. Manhattan and quantile-quantile plots were generated from the resulting data of 1,246,388 SNPs (Supplementary Fig. 2).
In the gender effects analysis, we analysed the effect of including gender information in the association analysis for the directly genotyped SNPs at each of the newly identified loci. We analysed the SNPs in PLINK47 using a logistic regression model assuming additive effects and covariate adjustment for population identity, and compared the output with results from a males- and females-only analyses. In addition, we compared the results to those of a regression analysis where an additional interaction component between gender and genotype was included in the model.
eQTL analysis
In the expression QTL (eQTL) analysis, we assessed publicly available data from two published eQTL studies7,8. In these datasets, as described in the original publications, association between the genotypes of the most interesting SNPs and gene expression were analysed using Spearman rank correlation for all genes within a 2-Mb window surrounding the SNP of interest. Significance was assessed by comparing the observed P-value at a 0.001 threshold with the minimum P-values from each of the 10,000 permutations of the expression values relative to genotypes7,8,48. As an additional approach to eQTL, we explored the NIH Genotype-Tissue Expression (GTEx) database. The GTEx data did not provide any association (data not shown).
Supplementary Material
Acknowledgements
We wish to thank all individuals in the respective cohorts for their generous participation. This work was supported by the German Federal Ministry of Education and Research (BMBF) (grant 01GS08121 to M.D. along with support to H.E.W. in the context of the German National Genome Research Network, (NGFN-2 and NGFN-plus) for the Heinz Nixdorf Recall Study); the Spanish Ministry of Science and Innovation, grant SAF2009-13182-C03 (to A.M. and B.C.); the Agència de Gestió d’Ajuts Universitaris i de Recerca (AGAUR), grants 2009SGR78 and 2009SGR971 (to A.M and B.C, respectively); an unrestricted grant of the Vascular Dementia Research Foundation (to M.D.) and the Netherlands Organization for the Health Research and Development (ZonMw) no.90700217 and VIDI (ZonMw) no.91711319 (to G.M.T.); the Netherlands Organisation for Scientific Research (NWO) VICI (918.56.602) and Spinoza (2009) grants (to M.D.F.); and the Center for Medical Systems Biology (CMSB) established in the Netherlands Genomics Initiative/Netherlands Organisation for Scientific Research (NGI/NWO), project nr. 050-060-409 (to C.M.v.D., R.R.F., M.D.F. and A.M.J.M.v.d.M.) and 050-060-810 (to C.M.v.D) and the generation and management of GWAS genotype data for the Rotterdam Study (175.010.2005.011, 911-03-012) (that is funded by the Erasmus Medical Center & Erasmus University Rotterdam, & the Ministries of Education, Culture & Science, Health, Welfare & Sports) as well as the NGI-sponsored Netherlands Consortium for Healthy Aging (NCHA) and the Research Institute for Diseases in the Elderly (014-93-015; RIDE2); the German Federal Ministry of Education and Research (BMBF) within the framework of the National Genome Research Network (NGFN-Plus; grants 01GS08120 and 01GS1103 to C.K; the German Federal Ministry of Education and Research and by the State of Bavaria and supported within the Munich Center of Health Sciences (MC Health) as part of LMUinnovativ) for the KORA research platform, which was initiated by the Helmholtz Center Munich, German Research Center for Environmental Health; the NHMRC Research Fellowship (613674) and the ARC Future Fellowship (FT0991022) schemes (to D.R.N.); the Wellcome Trust (grant number 098051 to AP); the Academy of Finland (grant number 251704 to AP, and 139795 to MW); the Academy of Finland, Center of Excellence in Complex Disease Genetics, (grant numbers 213506 and 129680 to AP and JK); the South-Eastern Norway Regional Health Authority (2010075 and 2011083 to BW and JAZ), Unger-Vetlesen Medical Fund (to BW), and the Ullevaal fund (to BW); the European Community’s Seventh Framework Programme (FP7/2007-2013), ENGAGE Consortium, (grant agreement HEALTH-F4-2007-201413); EU/SYNSYS-Synaptic Systems (grant number 242167 to AP); the Sigrid Juselius Foundation, Finland (to AP); the Folkhälsan Research Foundation, Helsinki (to MW); and the Helsinki University Central Hospital (to MK, VAr, MF).
Footnotes
Competing financial interests
The authors declare no competing financial interests.
Web resources
GWAMA - www.well.ox.ac.uk/gwama
NCBI36 - www.ncbi.nlm.nih.gov
Hapmap3 data - www.hapmap.org
Impute 2 - http://mathgen.stats.ox.ac.uk/impute/impute_v2.html
SNPTEST - http://www.stats.ox.ac.uk/~marchini/software/gwas/snptest.html
Locuszoom - http://csg.sph.umich.edu/locuszoom/
References
- 1.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a population-based cohort: the GEM study. Neurology. 1999;53:537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 2.Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J. Epidemiology of headache in Europe. Eur. J. Neurol. 2006;13:333–345. doi: 10.1111/j.1468-1331.2006.01184.x. [DOI] [PubMed] [Google Scholar]
- 3.Stovner L, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27:193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- 4.Olesen J, Lekander I, Andlin-Sobocki P, Jönsson B. Funding of headache research in Europe. Cephalalgia. 2007;27:995–999. doi: 10.1111/j.1468-2982.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 5.Anttila V, et al. Genome-wide association study of migraine implicates a common susceptibility variant on 8q22.1. Nat. Genet. 2010;42:869–873. doi: 10.1038/ng.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chasman DI, et al. Genome-wide association study reveals three susceptibility loci for common migraine in the general population. Nat. Genet. 2011;43:695–698. doi: 10.1038/ng.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dimas AS, et al. Common regulatory variation impacts gene expression in a cell type-dependent manner. Science. 2009;325:1246–1250. doi: 10.1126/science.1174148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nica AC, et al. The architecture of gene regulatory variation across multiple human tissues: the MuTHER study. PLoS Genet. 2011;7:e1002003. doi: 10.1371/journal.pgen.1002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin X, Shah S, Bulleit RF. The expression of MEF2 genes is implicated in CNS neuronal differentiation. Brain Res. Mol. Brain Res. 1996;42:307–316. doi: 10.1016/s0169-328x(96)00135-0. [DOI] [PubMed] [Google Scholar]
- 10.Flavell SW, et al. Activity-dependent regulation of MEF2 transcription factors suppresses excitatory synapse number. Science. 2006;311:1008–1012. doi: 10.1126/science.1122511. [DOI] [PubMed] [Google Scholar]
- 11.Aurora SK, Wilkinson F. The brain is hyperexcitable in migraine. Cephalalgia. 2007;27:1442–1453. doi: 10.1111/j.1468-2982.2007.01502.x. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari MD, Odink J, Bos KD, Malessy MJ, Bruyn GW. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology. 1990;40:1582–1586. doi: 10.1212/wnl.40.10.1582. [DOI] [PubMed] [Google Scholar]
- 13.Martínez F, Castillo J, Rodríguez JR, Leira R, Noya M. Neuroexcitatory amino acid levels in plasma and cerebrospinal fluid during migraine attacks. Cephalalgia. 1993;13:89–93. doi: 10.1046/j.1468-2982.1993.1302089.x. [DOI] [PubMed] [Google Scholar]
- 14.Tottene A, et al. Enhanced excitatory transmission at cortical synapses as the basis for facilitated spreading depression in Ca(v)2.1 knockin migraine mice. Neuron. 2009;61:762–773. doi: 10.1016/j.neuron.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 15.Flavell SW, et al. Genome-wide analysis of MEF2 transcriptional program reveals synaptic target genes and neuronal activity-dependent polyadenylation site selection. Neuron. 2008;60:1022–1038. doi: 10.1016/j.neuron.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow EM, et al. Identifying Autism Loci and Genes by Tracing Recent Shared Ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pfeiffer BE, et al. Fragile X Mental Retardation Protein Is Required for Synapse Elimination by the Activity-Dependent Transcription Factor MEF2. Neuron. 2010;66:191–197. doi: 10.1016/j.neuron.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schytz HW, et al. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain. 2009;132:16–25. doi: 10.1093/brain/awn307. [DOI] [PubMed] [Google Scholar]
- 19.Markovics A, et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2011;45:633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Goadsby PJ, Lipton RB, Ferrari MD. Migraine - current understanding and treatment. N. Engl. J. Med. 2002;346:257–270. doi: 10.1056/NEJMra010917. [DOI] [PubMed] [Google Scholar]
- 21.Lin HY, Wang XF, Ng-Eaton E, Weinberg RA, Lodish HF. Expression cloning of the TGF-beta type 2 receptor, a functional transmembrane serine/threonine kinase. Cell. 1992;68:775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- 22.Law C, et al. Clinical features in a family with an R460H mutation in transforming growh factor β receptor 2 gene. J. Med. Genet. 2006;43:908–916. doi: 10.1136/jmg.2006.042176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rist PM, Diener HC, Kurth T, Schürks M. Migraine, migraine aura, and cervical artery dissection: a systematic review and meta-analysis. Cephalalgia. 2011;31:886–896. doi: 10.1177/0333102411401634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allen PB, Greenfield AT, Svenningsson P, Haspeslagh DC, Greengard P. Phactrs 1-4: a family of protein phosphatise 1 and actin regulatory proteins. Proc. Nat. Acad. Sci. 2004;101:7187–7192. doi: 10.1073/pnas.0401673101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greengard P, Allen PB, Nairn AC. Beyond the Dopamine Receptor: the DARPP-32/Protein Phosphatase-1 Cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 26.Jarray R, et al. Depletion of the novel protein PHACTR-1 from human endothelial cells abolishes tube formation and induces cell death receptor apoptosis. Biochemie. 2011;93:1668–1675. doi: 10.1016/j.biochi.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Myocardial Infarction Genetics Consortium Genome-wide association of early-onset myocardial infarction with single nucleotide polymorphisms and copy number variants. Nat. Genet. 2009;41:334–341. doi: 10.1038/ng.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tietjen GE. Migraine as a systemic vasculopathy. Cephalalgia. 2009;29:987–996. doi: 10.1111/j.1468-2982.2009.01937.x. [DOI] [PubMed] [Google Scholar]
- 29.Wilson PM, Fryer RH, Fang Y, Hatten ME. Astn2, a novel member of the astrotactin gene family, regulates the trafficking of ASTN1 during glial-guided neuronal migration. J. Neurosci. 2010;30:8529–8540. doi: 10.1523/JNEUROSCI.0032-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DaSilva AF, Granziera C, Snyder J, Hadjikhani N. Thickening in the somatosensory cortex of patients with migraine. Neurology. 2007;69:1990–1995. doi: 10.1212/01.wnl.0000291618.32247.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruit MC, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291:427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 32.Proudfoot CJ, et al. Anagesie mediated by the TRPM8 cold receptor in chronic neuropathic pain. Curr. Biol. 2006;16:1591–1605. doi: 10.1016/j.cub.2006.07.061. [DOI] [PubMed] [Google Scholar]
- 33.Caspani O, Zurborg S, Labuz D, Heppenstall PA. The contribution of TRPM8 and TRPA1 channels to cold allodynia and neuropathic pain. PLoS One. 2009;4:e7383. doi: 10.1371/journal.pone.0007383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Agostino VC, Francia E, Licursi V, Cerbo R. Clinical and personality features of allodynic migraine. Neurol. Sci. 2010;31(Suppl1):S159–161. doi: 10.1007/s10072-010-0316-3. [DOI] [PubMed] [Google Scholar]
- 35.Lillis AP, van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May P, et al. Neuronal LRP1 functionally associates with postsynaptic proteins and is required for normal motor function in mice. J. Mol Cell Biol. 2004;24:8872–8883. doi: 10.1128/MCB.24.20.8872-8883.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Russell MB, Rasmussen BK, Fenger K, Olesen J. Migraine without aura and migraine with aura are distinct clinical entities: a study of four hundred and eighty-four male and female migraineurs from the general population. Cephalalgia. 1996;16:239–245. doi: 10.1046/j.1468-2982.1996.1604239.x. [DOI] [PubMed] [Google Scholar]
- 38.Kallela M, Wessman M, Havanka H, Palotie A, Färkkilä M. Familial migraine with and without aura: clinical characteristics and co-occurrence. Eur. J. Neurol. 2001;8:441–449. doi: 10.1046/j.1468-1331.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 39.Frazer KA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howie BN, Donnelly P, Marchini J. A flexible and accurate genotype imputation method for the next generation of genome-wide association studies. PLoS Genet. 2009;5:e1000529. doi: 10.1371/journal.pgen.1000529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Altshuler DM, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC bioinformatics. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 44.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP, Patsopoulos NA, Evangelou E. Heterogeneity in meta-analyses of genome-wide association investigations. PLoS One. 2007;2:e841. doi: 10.1371/journal.pone.0000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 47.Purcell S, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stranger BE, et al. Population genomics of human gene expression. Nat. Genet. 2007;39:1217–1224. doi: 10.1038/ng2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.