Summary
Perilipin is the most abundant adipocyte-specific protein that coats lipid droplets, and it is required for optimal lipid incorporation and release from the droplet. We identified two heterozygous frameshift mutations in the perilipin gene (PLIN1) in three families with partial lipodystrophy, severe dyslipidemia, and insulin-resistant diabetes. Subcutaneous fat from the patients was characterized by smaller-than-normal adipocytes, macrophage infiltration, and fibrosis. In contrast to wild-type perilipin, mutant forms of the protein failed to increase triglyceride accumulation when expressed heterologously in preadipocytes. These findings define a novel dominant form of inherited lipodystrophy and highlight the serious metabolic consequences of a primary defect in the formation of lipid droplets in adipose tissue.
Although the adverse consequences of an excessive fat mass (obesity) are widely appreciated, adipose tissue also has key metabolic functions, including storage of surplus energy and buffering of the postprandial influx of free fatty acids and their release in the fasting state or during exercise for oxidation by other tissues — all of which are essential for health.1 Small intracytoplasmic lipid droplets are found in most cell types, whereas white fat cells (adipocytes) store triglycerides within a large, single lipid droplet, which occupies up to 90% of the cell volume. The unilocular droplet of white adipocytes allows optimal energy storage and, by virtue of its reduced surface-to-volume ratio, as compared with that of smaller droplets in other cell types, facilitates precise lipolytic regulation.
Several proteins involved in the structural organization of the adipocyte lipid droplet (i.e., seipin, CIDEC, caveolin-1, and cavin-1) have been implicated in the pathophysiology of lipodystrophic syndromes.2-8 In these diseases, the inability to store triglycerides safely in adipocytes results in ectopic deposition of lipids in tissues such as skeletal muscle and liver, leading to insulin resistance and hypertriglyceridemia.9 Perilipin is the most abundant protein coating lipid droplets in adipocytes, where it is required for droplet formation and maturation, optimal triglyceride storage, and the release of free fatty acids from the droplet.10 In addition, although perilipin-null mice have decreased fat mass and increased insulin resistance, they do not exhibit a complete lipodystrophic phenotype.11-13 Therefore, we sought to determine whether mutations in PLIN1 could be responsible for lipodystrophic syndromes in humans.
Here we describe the clinical and metabolic findings in three families, all of self-reported white, French ancestry, carrying two different heterozygous loss-of-function mutations in PLIN1.
CASE REPORTS
PATIENT 1
Patient 1, a 54-year-old woman of self-reported white, French ancestry, was referred because of partial lipodystrophy and insulin-resistant diabetes (Table 1). Although her body-mass index (BMI, the weight in kilograms divided by the square of the height in meters) was never above 24, she was noted to have severe hypertriglyceridemia (triglyceride level, 2600 mg per deciliter [29.4 mmol per liter]) and hypertension at the age of 23 years, insulin resistance at the age of 33 years (with a fasting insulin level of 29.9 μU per milliliter [208 pmol per liter] and a fasting glucose level of 110 mg per deciliter [6.1 mmol per liter]), and diabetes and liver steatosis at the age of 45 years. Lipoatrophy was most striking in the lower limbs and femorogluteal depot, faciocervical fat was normal, and muscular hypertrophy was prominent in the calves (Fig. 1A, and Fig. 1 in the Supplementary Appendix, available with the full text of this article at NEJM.org); this habitus was reportedly apparent since childhood. Acanthosis nigricans was present in the axillary and nuchal folds. Menarche occurred at the age of 10 years and preceded chronic oligomenorrhea. She had 10 miscarriages of unknown cause and two full-term pregnancies complicated by hypertension. Both her daughters had a generally muscular appearance and peripheral lipoatrophy (also reported as having been present since childhood), with unaltered faciocervical fat, and axillary acanthosis nigricans (Fig. 1A and 1B, and Fig. 1 in the Supplementary Appendix). They had hypertriglyceridemia (triglyceride level, 250 to 450 mg per deciliter [2.8 to 5.1 mmol per liter]) from late puberty (Table 1), and liver steatosis was noted on ultrasonographic imaging. Peripheral lipoatrophy was confirmed by skinfold-thickness measurements (Fig. 2 in the Supplementary Appendix), and a reduced fat mass with an increased lean mass was documented on dual-energy x-ray absorptiometry (DEXA) in all three patients (Table 1).14
Table 1.
Metabolic Characteristics, Adipose-Tissue Distribution, and Liver Steatosis in Patients with Mutations in PLIN1.*
| Variable | Patient 1 | Daughter 1 of Patient 1 |
Daughter 2 of Patient 1 |
Patient 2 | Patient 3 | Reference Range |
|---|---|---|---|---|---|---|
| PLIN1 mutation | p.Leu404fs | p.Leu404fs | p.Leu404fs | p.Val398fs | p.Val398fs | |
| Age (yr) | 54 | 31 | 25 | 50 | 32 | |
| Sex | Female | Female | Female | Female | Female | |
| Body-mass index† | 23.0 | 24.7 | 27.5 | 24.7 | 26.9 | 18–25 |
| Glucose (mg/dl) | 138 | 87 | 94 | 200 | 288 | 80–101 |
| Insulin (pmol/liter) | 88 | 52 | 263 | Insulin-treated | 195 | 13.9–62.5 |
| Triglycerides (mg/dl) | 464 | 265 | 250 | 796 | 2655 | 35–135 |
| Cholesterol (mg/dl) | ||||||
| Total | 219 | 211 | 149 | 292 | 480 | 160–240 |
| HDL | 29 | 50 | 24 | 26 | 25 | 50–80 |
| Nonesterified fatty acids (μmol/liter) |
340 | 307 | 860 | 100–600 | ||
| Leptin (μg/liter)‡ | 2.5 | 5.9 | 3.5 | 4.7 | <0.1 | |
| Adiponectin (mg/liter) | 0.4 | 1.8 | 0.6 | 1.0 | 1.4 | 3.9–12.9 |
| DEXA results (%) | ||||||
| Total fat | 19.4 | 21.4 | 18.6 | 17.7 | 27.2–33.3 | |
| Upper-limb fat | 21.0 | 21.6 | 17.1 | 18.8 | 26.5–33.9 | |
| Trunk fat | 18.5 | 22.0 | 18.2 | 19.0 | 25.7–32.3 | |
| Lower-limb fat | 20.5 | 21.3 | 19.7 | 14.0 | 30.1–36.1 | |
| Total lean mass | 76.2 | 74.4 | 77.6 | 78.8 | 60.5–70.7 | |
| Fatty liver§ | Yes | Yes | Yes | Yes | Yes |
To convert the values for glucose into millimoles per liter, multiply by 0.05551. To convert the values for insulin to microunits per milliliter, divide by 6.945. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129. To convert the values for cholesterol and high-density lipoprotein (HDL) cholesterol to millimoles per liter, multiply by 0.02586. DEXA denotes dual-energy x-ray absorptiometry.
The body-mass index (BMI) is the weight in kilograms divided by the square of the height in meters.
Leptin reference ranges for women have been adjusted for BMI: in women with a BMI of less than 25, leptin levels range from 2.4 to 24.4 μg per liter; in women with a BMI of 25 to 29, leptin levels range from 8.6 to 38.9 μg per liter.
Liver steatosis was assessed by means of ultrasonography.
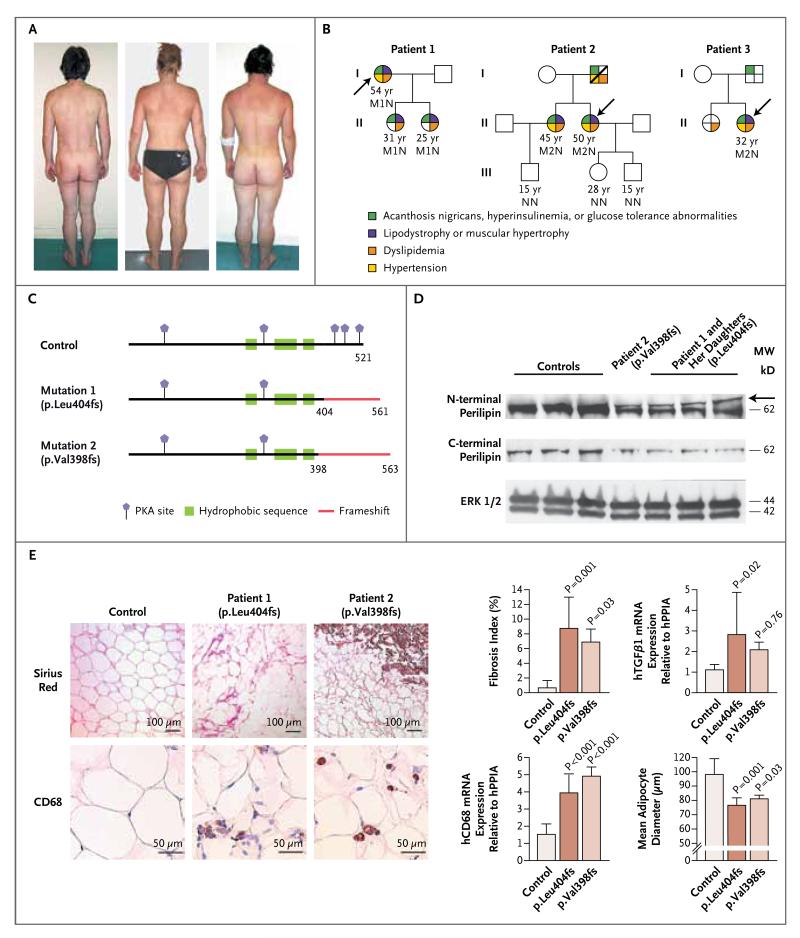
Figure 1. Cosegregation of Heterozygous Frameshift PLIN1 Mutations with Insulin-Resistant Diabetes, Dyslipidemia, and Partial Lipodystrophy.
In Panel A, photographs of Patient 1 (left) and her affected daughters (center and right) reveal the paucity of subcutaneous fat and the muscular appearance. Panel B shows the family pedigrees of Patients 1, 2, and 3 (the patients are designated by arrows), with the family members’ ages and genotypes. N denotes wild type, M1 mutation 1 (PLIN1 p.Leu404AlafsX158), and M2 mutation 2 (PLIN1 p.Val398GlyfsX166). There is complete concordance between features of severe insulin resistance and the presence of the PLIN1 mutations. Heterozygous family members were variably affected by additional features of the metabolic syndrome. Dyslipidemia was defined by triglyceride levels that were higher than 177 mg per deciliter (2 mmol per liter) and high-density-lipoprotein (HDL) cholesterol levels that were less than 39 mg per deciliter (1 mmol per liter). Panel C shows the effects of the PLIN1 p.Leu404AlafsX158 and p.Val398GlyfsX166 frameshift variants on the perilipin protein, indicating the predicted protein kinase A (PKA) sites in human perilipin. In Panel D, immunoblots show perilipin expression in samples of white adipose tissue from Patient 2, for the p.Val398GlyfsX166 mutation, and from Patient 1 and her two daughters, for the p.Leu404AlafsX158 mutation. Extracellular-signal-regulated kinase (ERK) 1/2 was used as a gel-loading control. Samples of abdominal subcutaneous adipose tissue from three healthy, lean, nondiabetic women were used as controls. Immunoblotting was done with N-terminal and C-terminal perilipin antibodies. The mutant isoform was recognized by the N-terminal antibody as a frameshifted band (arrow) just above the 62-kD molecular-weight (MW) marker. This extra band was not detected by the C-terminal antibody. Panel E (left) shows light micrographs of biopsy specimens of subcutaneous abdominal adipose tissue from a lean, healthy control subject and from Patients 1 and 2. Sirius red staining highlights the marked increase in fibrosis and CD68 immunostaining shows the increase in macrophage infiltration of the adipose tissue in the patients. The graphs at the right show the results of analyses that are in keeping with these findings. In the patients, as compared with the control, the fibrosis index (calculated as described in the Supplementary Appendix), transforming growth factor β1 (TGF-β1) messenger RNA (mRNA) levels, and hCD68 mRNA expression, a marker of proinflammatory macrophages, were significantly increased and the average adipocyte diameter (see the Supplementary Appendix) was significantly reduced. Results are given as means ±SD. hPPIA denotes peptidyl-prolyl isomerase A (cyclophilin A), used as an internal control for gene expression.
PATIENT 2
Patient 2, a 50-year-old woman of self-reported white, French ancestry, was first evaluated when she was 25 years of age because of her cushingoid appearance, reported as having been present since childhood. Cushing’s syndrome was ruled out, but severe hypertension, acanthosis nigricans, hypertriglyceridemia, insulin resistance, and ovarian hyperandrogenemia were documented. Diabetes was diagnosed at the age of 26 years. She had two strokes in her 40s. At the age of 50 years, her BMI was 24.7, and clinical examination revealed acromegaloid features of her face, hands, and feet with no excess fat accumulation; lipoatrophy and muscular hypertrophy of her lower limbs; and axillary acanthosis nigricans. Acromegaly was ruled out biochemically. Despite treatment with very high doses of insulin (about 280 U per day), glycemic and dyslipidemic control remained suboptimal (glycated hemoglobin level, 7.9%; triglyceride level, 796 mg per deciliter [9.0 mmol per liter]) (Table 1). Severe insulin resistance was confirmed by a hyperinsulinemic euglycemic clamp (see the table in the Supplementary Appendix), reduced fat mass on DEXA, and liver steatosis on ultrasonographic imaging (Table 1). She has two phenotypically normal children, but her 45-year-old sister (BMI, 21.3) has diabetes, dyslipidemia, hypertension, and limb lipoatrophy with calf hypertrophy (Fig. 1B).
PATIENT 3
Patient 3, who was 32 years of age and of self-reported white, French ancestry, had had insulin-resistant diabetes with extensive acanthosis nigricans, severe hypertriglyceridemia (triglyceride level, 2655 mg per deciliter [30.0 mmol per liter]), and steatohepatitis since the age of 21 years (Table 1). She had a cushingoid appearance and was slightly overweight (BMI, 26.9). Severe insulin resistance was confirmed by a hyperinsulinemic euglycemic clamp (see the table in the Supplementary Appendix). She was lost to follow-up after her evaluation.
METHODS
GENETIC ANALYSES
We screened DNA from all three unrelated probands for mutations in genes previously implicated in lipodystrophies (LMNA, PPARG, AKT2, CIDEC, BSCL2, AGPAT2, and CAV1) and, detecting no mutations, we then sequenced all the exons and intron-extron junctions of the PLIN1 gene. The study was conducted in accordance with the Declaration of Helsinki and approved by the national research ethics committee. Written informed consent was obtained from all participants.
Genomic DNA was extracted from peripheral-blood leukocytes, and the entire coding region and splice junctions of PLIN1 (GenBank accession number, NM_002666.4) were amplified and sequenced (the primer sequences are described in the Supplementary Appendix).
PHENOTYPING STUDIES
Body composition and fat distribution were assessed on the basis of skinfold thickness and the findings on DEXA and on magnetic resonance imaging or computed tomographic scanning at the level of the L4 vertebrae. Biopsy specimens of subcutaneous abdominal adipose tissue were obtained from Patient 1 and her two daughters and from Patient 2. Tissue samples were studied with the use of conventional light microscopy, immunohistochemistry, and reverse-transcriptase–polymerase-chain-reaction assays (see the Supplementary Appendix).
IN VITRO STUDIES OF MUTANT PLIN1
We characterized the functional consequences of the mutations in stably transfected 3T3L1 preadipocytes (for details, see the Supplementary Appendix).
RESULTS
IDENTIFICATION OF MUTATIONS IN PLIN1 AND COSEGREGATION ANALYSIS
Sequencing of all the exons and splicing regions of PLIN1 revealed a heterozygous transversion of guanine to thymine affecting the intron 8 splice-acceptor site (c.1210-1G→T) in Patient 1 and a heterozygous deletion of adenine and guanine in exon 8 (c.1191_1192delAG) in Patients 2 and 3. Direct sequencing of complementary DNA (cDNA) derived from reverse transcription of RNA isolated from an adipose tissue sample from Patient 1 showed that the PLIN1 c.1210-1G→T mutation results in alternative splicing at position +9 of exon 9, with a frameshift in translation leading to the incorporation of 158 aberrant amino acid residues (p.Leu404AlafsX158); the c.1191_1192delAG mutation predicts a frameshift translation leading to the synthesis of 166 aberrant amino acids (p.Val398GlyfsX166) (Fig. 1C, and Fig. 3 in the Supplementary Appendix). Coincidentally, both mutations induce the synthesis of a longer mutated protein with the same 158 aberrant C-terminal amino acids. Both mutations cosegregated with insulin resistance, dyslipidemia, and partial lipodystrophy in affected relatives (Fig. 1B) and were absent in 203 unrelated white control subjects, including at least 66 of French origin. Haplotype analysis revealed that Patients 2 and 3 had different disease-associated alleles, suggesting the recurrence of independent mutational events.
EFFECTS OF THE PLIN1 VARIANTS ON ADIPOSE TISSUE
Samples of subcutaneous abdominal adipose tissue were obtained from four affected patients. Perilipin immunoblots with an antibody targeting an N-terminal epitope revealed a reduction in full-length perilipin and an extra band that ran marginally above wild-type perilipin, in keeping with the predicted elongated C-terminal tail of the mutant proteins (Fig. 1D). Immunoblots with an antibody targeting the C-terminal region of perilipin, which does not bind the perilipin mutants, confirmed the expected reduction in wild-type perilipin (Fig. 1D). The most striking histologic abnormalities included a significant reduction in adipocyte size, as compared with that in controls, increased macrophage infiltration, and a greater degree of adipose-tissue fibrosis (Fig. 1E, and Fig. 4 in the Supplementary Appendix).
FUNCTIONAL CHARACTERIZATION OF THE PLIN1 FRAMESHIFT MUTATIONS
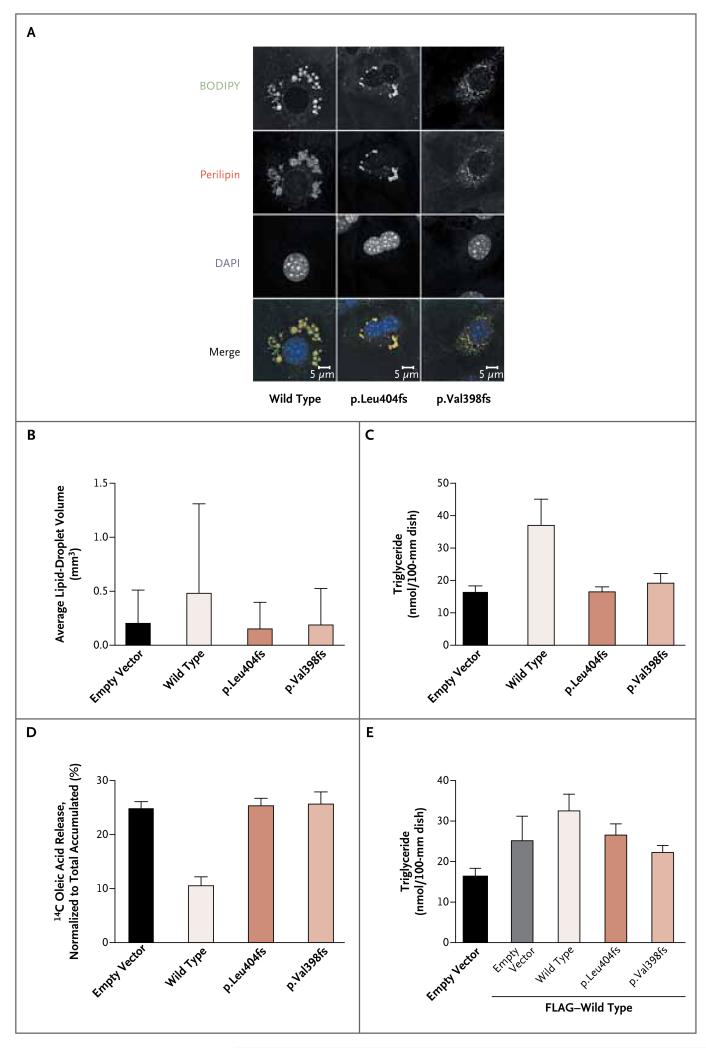
To understand the consequences of the C-terminal frameshift mutations in PLIN1, wild-type and mutant perilipin were stably expressed in 3T3L1 preadipocytes, which do not otherwise express perilipin before differentiation. Both mutants and wild-type perilipin were correctly targeted to the lipid-droplet surface in these cells, but droplets were smaller in cells expressing perilipin mutants (Fig. 2A and 2B). Biochemical assessment confirmed that expression of wild-type perilipin resulted in triglyceride levels that were increased by a factor of 2, whereas expression of mutant perilipin failed to alter intracellular triglyceride levels in preadipocytes, as compared with cells transfected with the control vector (Fig. 2C) (P<0.01). Mutant and wild-type PLIN1 messenger RNA (mRNA) were expressed at similar levels, whereas mutant protein levels were lower than wild-type perilipin levels (Fig. 5 in the Supplementary Appendix).
Figure 2. Functional Properties of the PLIN1 Variants.
Preadipocytes (3T3L1) were retrovirally transfected with an empty vector (pBABE-puro or pLXSN [EV]) or with pBABE–wild-type PLIN1, pBABE–PLIN1 p.Leu404AlafsX158, or pBABE–PLIN1 p.Val398GlyfsX166, either alone or in combination with pLXSN–wild-type PLIN1. PLIN1 was N-terminally myc-tagged when expressed in pBABE and FLAG-tagged when expressed in pLXSN. Panel A shows intracellular localization of wild-type and mutant perilipin. Boron-dipyrromethene (BODIPY) was used to stain lipid droplets, and 4′,6′-diamidine-2-phenylindole dihydrochloride (DAPI) is a nuclear stain. Perilipin was stained with an N-terminal perilipin antibody that recognizes both wild-type and mutant perilipin. BODIPY staining suggested that the lipid droplets were smaller in cells that expressed mutant perilipin. In Panel B, automated assessment of lipid-droplet volume from five representative fields confirms the reduced size of lipid droplets in cells expressing the perilipin mutants, with an average of 355 lipid droplets per field. Panel C shows the biochemical measurement of triglyceride levels in preadipocytes expressing wild-type or mutant perilipin in response to treatment with oleic acid. Triglyceride levels were determined in duplicate in lysates from 12 to 18 dishes per cell line. An average of the duplicate measurements was used for each 100-mm dish. Data are mean values for the 12 to 18 dishes. Panel D shows the radiometric assessment of basal lipolysis in preadipocytes treated with 14C-labeled oleic acid. Data are mean values for nine independent measurements per cell line. In Panels B, C, and D, P<0.001 for the comparisons between empty vector and wild-type cells and between wild-type and mutant cells. Panel E shows triglyceride levels in preadipocytes coexpressing FLAG-tagged wild-type perilipin with myc-tagged wild-type or mutant perilipin. Triglyceride levels were determined in duplicate in lysates from 12 to 18 dishes (100 mm) per cell line. An average of the duplicate measurements was used for each 100-mm dish. Data are mean values for the 12 to 18 dishes. In Panel E, P<0.001 for the comparison between empty vector and FLAG-tagged wild-type cells, P<0.01 for the comparisons between FLAG-tagged and myc-tagged wild-type coexpressing cells and between FLAG-tagged wild-type and myc-tagged p.Leu404fs co-expressing cells, and P<0.001 for the comparisons between FLAG-tagged and myc-tagged wild-type coexpressing cells and between FLAG-tagged wild-type and myc-tagged p.Val398fs coexpressing cells. FLAG-tagged wild-type expressing cells were not statistically different from FLAG-tagged wild-type and myc-tagged mutant coexpressing cells. T bars in Panels B through E indicate standard deviations.
Measurements of 14C-labeled oleic acid released into the culture medium showed that expression of wild-type perilipin significantly inhibited basal lipolysis in preadipocytes (by more than 50%), as compared with cells transfected with the empty vector (P<0.001), whereas basal lipolysis was unaltered in cells expressing the perilipin mutants (Fig. 2D). Coexpression of wild-type perilipin partially restored the effects of the perilipin mutants on triglyceride accumulation (i.e., to the same extent as in cells cotransfected with empty vector and wild-type PLIN1). However, triglyceride accumulation remained significantly lower than in doubly transfected wild-type PLIN1 cells (Fig. 2E), suggesting that the dominantly inherited phenotype is a consequence of perilipin haploinsufficiency. Coexpression of wild-type and mutant perilipin had a similar effect on basal lipolysis in cotransfected cells (data not shown).
DISCUSSION
The adipocyte lipid droplet is now recognized as a dynamic cell organelle, and studies suggest that perilipin functions as a scaffold protein that organizes triglyceride storage and orchestrates lipolytic effector proteins.15,16 Lipolytic stimuli act by inducing protein kinase A–mediated phosphorylation of perilipin at five sites, three of which are located in the C-terminal region of perilipin.15 In addition, the C-terminal tail of perilipin has been shown to be involved in the inhibition of basal lipolysis independently of hormone-sensitive lipase.17
In this study, we found that two different heterozygous PLIN1 mutations, which substantially alter the C-terminal of perilipin (p.Leu404AlafsX158 and p.Val398GlyfsX166), cosegregated in three families with partial lipodystrophy, insulin resistance, hepatic steatosis, and dyslipidemia, defining a novel subtype of familial partial lipodystrophy. Morphometric analyses of adipose tissue specimens from the patients showed adipocytes that were smaller than those in unaffected persons, suggesting impaired triglyceride storage in the lipid droplets. Accordingly, when the mutant proteins were expressed in preadipocytes, they were associated with smaller lipid droplets, reduced triglyceride accumulation, and increased basal lipolysis. This third finding may indicate that these patients might benefit from treatment with lipolytic inhibitors such as acipimox.
Although the levels of mRNA expression of wild-type and mutant perilipin were similar, mutant protein levels were reduced, in keeping with research suggesting that lipid accumulation stabilizes perilipin protein levels.18 Artificial murine perilipin mutants (e.g., constructs encoding amino acids 1–364, 1–405, or 1–429) lacking the C-terminal tail of perilipin have properties similar to the mutant protein in our patients, in terms of the reduction in triglyceride accumulation in transfected preadipocytes, enhancement of basal lipolysis, and the associated reduction in perilipin protein levels.17,18 The fact that the centrally located hydrophobic regions are conserved in the protein in patients with the mutations is consistent with their correct targeting to lipid droplets.10,19 When coexpressed with wild-type perilipin in vitro, the truncated mutants do not appear to impair the effect of the wild-type protein on triglyceride accumulation or basal lipolysis, but they do lead to significantly reduced total triglyceride accumulation and accelerated basal lipolysis, as compared with the effect of coexpression of two copies of wild-type perilipin. These data suggest that haploinsufficiency accounts for the autosomal dominant phenotype (Fig. 2E).
Fat mass was reduced in carriers of both mutations, an observation that is consonant with the findings in perilipin-null mice and mice with haploinsufficiency.11,12 The pattern of lipodystrophy associated with PLIN1 variants differs from the previously described forms of familial partial lipodystrophy, with a more uniform reduction in all fat depots.7,20 Leptin levels in our patients broadly reflected the extent of adiposetissue loss, as previously described in human lipodystrophies,21,22 with the exception of one patient in whom serum leptin was undetectable. The associated metabolic disturbances were similar to those seen in all other forms of familial partial lipodystrophy.23 The identification and evaluation of a greater number of affected patients will be required to confirm these findings, and the possibility of leptin-substitution therapy in this disorder warrants exploration.
In contrast to lamin A/C and, to a lesser extent, peroxisome proliferator–activated receptor γ, perilipin is almost exclusively expressed in adipocytes. Thus, the findings in our patients with perilipin mutations demonstrate the metabolic consequences of a primary defect in adipocytes. Although adipocyte size (which is typically increased in obesity) was reduced in the affected patients, the increases in macrophage infiltration and fibrosis were similar to those seen in adipose-tissue samples from obese patients with insulin resistance.24 This concordant histologic appearance suggests that lipolytic dysregulation may be involved in triggering the fibrogenic response. Halberg et al. have recently suggested that hypoxia triggers an increase in hypoxia-inducible factor 1α (HIF1α) expression and the fibrotic response of adipose tissue in the obese state.25 However, HIF1α mRNA expression was not increased in our patients (data not shown), suggesting that alternative pathways may also be involved.
We describe a unique subtype of familial partial lipodystrophy and provide strong human genetic evidence that perilipin is required for optimal triglyceride storage within adipocyte lipid droplets. Our findings suggest that primary perturbations in fat metabolism can lead to severe insulin resistance and impaired glucose regulation.
Supplementary Material
Acknowledgments
Supported by grants from the Wellcome Trust (077016/Z/05/Z, to Drs. Gandotra, Bottomley, Barroso, Semple, O’Rahilly, and Savage), GlaxoSmithKline (to Dr. Savage), the U.K. National Institute for Health Research Cambridge Biomedical Research Centre, the Medical Research Council Center for Obesity and Related Metabolic Disease, Société Francophone du Diabète (Association de Langue Française pour l’Etude du Diabète et des Maladies Métaboliques) (to Dr. Magré), INSERM (to Drs. Cervera, Lascols, Capeau, Magré, and Vigouroux), and the French Ministère de l’Enseignement Supérieur et de la Recherche (to Dr. Le Dour).
We thank the patients who participated in these studies, Muriel Meier for help with molecular analyses, Drs. Camille Vatier and Véronique Béréziat and Ms. Sylvie Dumont for help in histologic analyses of adipose tissue, Drs. Soraya Fellahi and Jean-Philippe Bastard for adipokine measurements, Dr. Sylvia Franc for providing clinical and biologic data, Gregory Strachan for training and support for confocal microscopy, Koini Lim for technical support, and Yves Chrétien for help with statistical analyses.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Frayn KN. Adipose tissue as a buffer for daily lipid flux. Diabetologia. 2002;45:1201–10. doi: 10.1007/s00125-002-0873-y. [DOI] [PubMed] [Google Scholar]
- 2.Boutet E, El Mourabit H, Prot M, et al. Seipin deficiency alters fatty acid Delta9 desaturation and lipid droplet formation in Berardinelli-Seip congenital lipodystrophy. Biochimie. 2009;91:796–803. doi: 10.1016/j.biochi.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Fei W, Shui G, Gaeta B, et al. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J Cell Biol. 2008;180:473–82. doi: 10.1083/jcb.200711136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayashi YK, Matsuda C, Ogawa M, et al. Human PTRF mutations cause secondary deficiency of caveolins resulting in muscular dystrophy with generalized lipodystrophy. J Clin Invest. 2009;119:2623–33. doi: 10.1172/JCI38660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim CA, Delépine M, Boutet E, et al. Association of a homozygous nonsense caveolin-1 mutation with Berardinelli-Seip congenital lipodystrophy. J Clin Endocrinol Metab. 2008;93:1129–34. doi: 10.1210/jc.2007-1328. [DOI] [PubMed] [Google Scholar]
- 6.Rajab A, Straub V, McCann LJ, et al. Fatal cardiac arrhythmia and long-QT syndrome in a new form of congenital generalized lipodystrophy with muscle rippling (CGL4) due to PTRF-CAVIN mutations. PLoS Genet. 2010;6(3):e1000874. doi: 10.1371/journal.pgen.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio-Cabezas O, Puri V, Murano I, et al. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol Med. 2009;1:280–7. doi: 10.1002/emmm.200900037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szymanski KM, Binns D, Bartz R, et al. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A. 2007;104:20890–5. doi: 10.1073/pnas.0704154104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savage DB, Petersen KF, Shulman GI. Disordered lipid metabolism and the pathogenesis of insulin resistance. Physiol Rev. 2007;87:507–20. doi: 10.1152/physrev.00024.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Botas J, Anderson JB, Tessier D, et al. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet. 2000;26:474–9. doi: 10.1038/82630. [DOI] [PubMed] [Google Scholar]
- 12.Tansey JT, Sztalryd C, Gruia-Gray J, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci U S A. 2001;98:6494–9. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saha PK, Kojima H, Martinez-Botas J, Sunehag AL, Chan L. Metabolic adaptations in the absence of perilipin: increased beta-oxidation and decreased hepatic glucose production associated with peripheral insulin resistance but normal glucose tolerance in perilipin-null mice. J Biol Chem. 2004;279:35150–8. doi: 10.1074/jbc.M405499200. [DOI] [PubMed] [Google Scholar]
- 14.Mazess RB, Barden HS, Bisek JP, Hanson J. Dual-energy x-ray absorptiometry for total-body and regional bone-mineral and soft-tissue composition. Am J Clin Nutr. 1990;51:1106–12. doi: 10.1093/ajcn/51.6.1106. [DOI] [PubMed] [Google Scholar]
- 15.Brasaemle DL. Thematic review series: adipocyte biology — the perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–59. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 16.Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl) J Biol Chem. 2009;284:34538–44. doi: 10.1074/jbc.M109.068478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HH, Souza SC, Muliro KV, Kraemer FB, Obin MS, Greenberg AS. Lipase-selective functional domains of perilipin A differentially regulate constitutive and protein kinase A-stimulated lipolysis. J Biol Chem. 2003;278:51535–42. doi: 10.1074/jbc.M309591200. [DOI] [PubMed] [Google Scholar]
- 18.Garcia A, Subramanian V, Sekowski A, Bhattacharyya S, Love MW, Brasaemle DL. The amino and carboxyl termini of perilipin A facilitate the storage of triacylglycerols. J Biol Chem. 2004;279:8409–16. doi: 10.1074/jbc.M311198200. [DOI] [PubMed] [Google Scholar]
- 19.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–91. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 20.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–34. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 21.Haque WA, Shimomura I, Matsuzawa Y, Garg A. Serum adiponectin and leptin levels in patients with lipodystrophies. J Clin Endocrinol Metab. 2002;87:2395. doi: 10.1210/jcem.87.5.8624. [DOI] [PubMed] [Google Scholar]
- 22.Antuna-Puente B, Boutet E, Vigouroux C, et al. Higher adiponectin levels in patients with Berardinelli-Seip congenital lipodystrophy due to seipin as compared with 1-acylglycerol-3-phosphate-O-acyltransferase-2 deficiency. J Clin Endocrinol Metab. 2010;95:1463–8. doi: 10.1210/jc.2009-1824. [DOI] [PubMed] [Google Scholar]
- 23.Decaudain A, Vantyghem MC, Guerci B, et al. New metabolic phenotypes in laminopathies: LMNA mutations in patients with severe metabolic syndrome. J Clin Endocrinol Metab. 2007;92:4835–44. doi: 10.1210/jc.2007-0654. [DOI] [PubMed] [Google Scholar]
- 24.Henegar C, Tordjman J, Achard V, et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008;9(1):R14. doi: 10.1186/gb-2008-9-1-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Halberg N, Khan T, Trujillo ME, et al. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol Cell Biol. 2009;29:4467–83. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.