Abstract
The bone morphogenetic proteins (BMPs) are a group of powerful morphogens that are critical for development of the nervous system. The effects of BMP signaling on neural stem cells are myriad and dynamic, changing with each stage of development. During early development inhibition of BMP signaling differentiates neuroectoderm from ectoderm, and BMP signaling helps to specify neural crest. Thus modulation of BMP signaling underlies formation of both the central and peripheral nervous systems. BMPs secreted from dorsal structures then form a gradient which helps pattern the dorsal–ventral axis of the developing spinal cord and brain. During forebrain development BMPs sequentially induce neurogenesis and then astrogliogenesis and participate in neurite outgrowth from immature neurons. BMP signaling also plays a critical role in maintaining adult neural stem cell niches in the subventricular zone (SVZ) and subgranular zone (SGZ). BMPs are able to exert such diverse effects through closely regulated temporospatial expression and interaction with other signaling pathways.
Keywords: BMP, neural stem cells, patterning, neurogenesis, gliogenesis
Introduction
Neural stem cells are multipotent progenitors that can differentiate into neurons, astrocytes, and oligodendrocytes in the central nervous system (CNS). Neural crest progenitor cells generate neurons or Schwann cells in the peripheral nervous system (PNS). Bone morphogenetic proteins (BMPs) are a class of morphogens that are critical for establishing tissue structure throughout the body. Though they were first discovered for their osteoinductive properties, it is now evident that BMP signaling is a pivotal regulator of stem cells in both the CNS and PNS. BMP signaling exhibits a dynamic range of functions and plays a role in neural stem cell fate and maturation at virtually every stage of neural development. BMP signaling is tightly regulated both temporally and spatially, and the effects of BMP signaling are modulated by other spatially and temporally specific signals. This four dimensional specificity enables BMPs to have wide ranging and often opposing effects during distinct stages of development. Here, we elaborate the changing role of BMP signaling in neural stem cell fate and maturation.
Mechanisms of BMP Signaling
BMP Ligands and Their Receptors
BMPs are members of the transforming growth factor beta (TGFβ) superfamily of signaling ligands. Over 30 different BMPs have been identified across various species, and they can be divided into numerous subgroups based on structural similarities (Table 1). One subgroup composed of BMP2 and BMP4 in vertebrates and decapentaplegic (Dpp) in drosophila plays a particularly major role throughout CNS development (Mehler et al, 1997). Other members, such as BMP6, BMP7, and BMP9, are also critical during certain developmental events. BMPs are initially synthesized as large precursor proteins which dimerize and are then proteolytically cleaved at a consensus Arg-X-X-Arg site (Wozney et al., 1988; Rosen et al., 1989; Israel et al., 1992, 1996). The resulting carboxy-terminal mature dimers, which are generally held together by an intermolecular disulfide bond, are then secreted from the cell (Daopin et al., 1992; Schlunegger and Grutter, 1992; Griffith et al., 1996).
Table 1.
BMP Ligands Implicated in CNS Development and Maintenance
| BMP Ligand | Selected References |
|---|---|
| BMP2 | Mujtaba et al., 1998; Mabie et al., 1999; Sailer et al., 2005 |
| BMP3b (GDF10) | Takao et al., 1996; Soderstrom and Ebendal, 1999 |
| BMP4 | Gross et al., 1996; Rajan and McKay, 1998; Pierani et al., 1999; Lillien and Raphael, 2000 |
| BMP5 | Vogel-Hopker and Rohrer, 2002; Holzschuh et al., 2005; Tilleman et al., 2010 |
| BMP6 | Schluesener and Meyermann, 1994; Gratacos et al., 2002; Crews et al., 2010 |
| BMP7 (OP1) | Perides et al., 1992; Zhu et al., 1999; de Rivero Vaccari et al., 2009 |
| BMP9 (GDF9) | Lopez-Coviella et al., 2005, 2006; Bissonnette et al., 2011 |
| BMP11 (GDF11) | Wu et al., 2003; Kim et al., 2005; Shi and Liu, 2011 |
| BMP12 (GDF7) | Lee et al,. 1998; Alder et al., 1999; Currle et al., 2005 |
| BMP13 (GDF6) | Chang and Hemmati-Brivanlou, 1999; Hanel and Hensey, 2006; den Hollander et al., 2010 |
| BMP14 (GDF5) | Krieglstein et al., 1995; O'Keeffe et al., 2004; Gajavelli et al., 2004 |
Mature BMP dimers exert their effects by binding to BMP receptors (BMPRs) on neighboring cells (paracrine) or on the cell that secreted the signal (autocrine). BMPRs are tetrameric complexes composed of pairs of Type I and Type II serine/threonine kinase receptor subunits (Fig. 1) (Wrana et al., 1992; Massague, 1998). BMP ligands bind with high affinity when both receptor subunits are preassembled in the membrane, but they may also bind one subunit and then recruit the other subunit to the complex. Each BMP family member has a unique affinity for different combinations of receptor subunits. For example, BMP2 and BMP4 preferentially bind to two kinds of Type I receptors (BMPRIa/Alk3 and BMPRIb/Alk6) and one kind of Type II receptor (BMPRII). Type II receptors are constitutively active kinases which, upon ligand binding, transphosphorylate and activate Type I receptors. Type I receptors in turn phosphorylate intracellular targets that mediate their downstream effects (Fig. 1).
Figure 1.
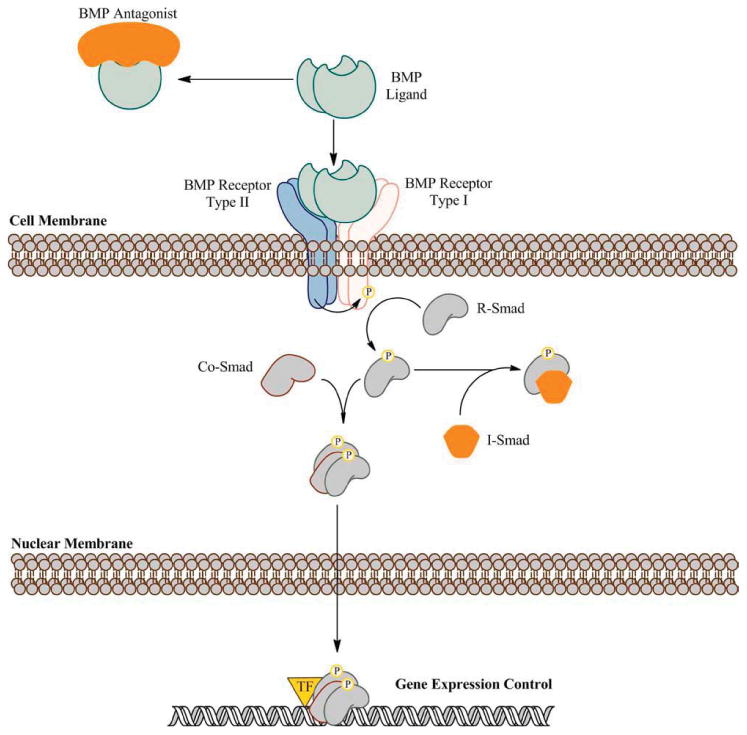
Canonical BMP signaling. BMP signaling is transduced by a series of phosphorylation events. When BMP ligands bind their receptors, BMP Type II receptors transphosphorylate Type I receptors, which in turn phosphorylate R-Smads. Activated R-Smads bind with Co-Smads and translocate into the nucleus. In the nucleus, the R-Smad-Co-Smad complexes interact with transcription factors (TF) and other coregulatory proteins to control gene expression. BMP signaling can be inhibited extracellularly by antagonists, such as Noggin, which competitively bind BMP ligands. Intracellulary, inhibitory Smads (I-Smads) bind to activated R-Smads and prevent their binding with Co-Smads. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Expression patterns of the three BMPRs (BMPRIa, BMPRIb, and BMPRII) do not always overlap and fluctuate with development. Though structurally similar, BMPRIa and BMPRIb have different expression patterns in the mammalian nervous system. After gastrulation BMPRIa is expressed in neural tube proliferative zones. In contrast, BMPRIb expression is not detectable until embryonic day 9 and is restricted to the dorsal aspect of the neural tube (Dewulf et al., 1995). BMPRII expression in the developing embryo is primarily restricted to proliferative regions in the CNS (Zhang et al., 1998). All three receptors are also expressed in the adult, with BMPRIa more abundant and broadly distributed than BMPRIb and BMPRII. BMPRIa is expressed heterogeneously in gray matter structures, while strong BMPRIb expression is localized to the anterior olfactory nucleus. BMPRII is found in all regions of the hippocampus as well as in the cerebellar Purkinje layer (Zhang et al., 1998).
Differences in BMPR expression patterns reflect their individual functions. BMPRIa signaling both maintains precursor cell proliferation and induces BMPRIb expression. Predominant BMPRIb signaling induces mitotic arrest through either differentiation or apoptosis (Panchision et al., 2001). Functional differences between the two Type I receptors have also been demonstrated in mice with conditional Olig1-lineage BMPRIa ablation. In these mice, disproportionate numbers of cortical oligodendrocytes and interneurons are formed, indicating that BMPRIb signaling cannot compensate for loss of BMPRIa signaling (Brederlau et al., 2004; Samanta et al., 2007). However, in other aspects of development, such as limb patterning and cerebellar formation, the roles of BMPRIa and BMPRIb are not as distinct, indicating that BMPR subunit function is flexible and cell-type specific (Yi et al., 2000; Ahn et al., 2001; Qin et al., 2006).
BMP Signaling Pathways
In C. elegans the downstream signaling molecule Sma is phosphoyrlated by BMP-orthologs, and in Drosophila Mothers against dpp (Mad) is similarly activated (Sekelsky et al., 1995; Savage et al., 1996). Vertebrate members of this protein family, named Smad (fusion of Sma and Mad), constitute the canonical intracellular pathway of BMP signaling (Fig. 1). Receptor-regulated Smads (R-Smads) −1/5/8 are directly phosphorylated and activated by BMPRIa and BMPRIb at their C-terminus (Wrana, 2000). These in turn bind with co-Smad4, and the resulting heteromeric complex translocates into the nucleus (Hoodless et al., 1996; Heldin et al., 1997; Nishimura et al., 1998; Kawai et al., 2000). Once in the nucleus, the complex interacts with transcription factors as well as co-activators/repressors to modulate gene expression.
There are also a number of non-canonical pathways for BMP signaling, adding complexity to its downstream mechanisms. X-chromosome-linked inhibitor of apoptosis (XIAP) mediates signaling between BMP receptors and TAB1, an activator of TAK1, which is a member of the MAP kinase kinase kinase family (Shibuya et al., 1996, 1998; Shirakabe et al., 1997; Yamaguchi et al., 1999; Takaesu et al., 2000; Nohe et al., 2003; Matluk et al., 2010). During early development the XIAP-TAB1-TAK1 pathway is proposed to mediate BMP signaling's switch from a proliferation to an apoptosis signal through neurotrophin receptor interacting MAGE (NRAGE)-mediated activation of p38 (Kendall et al., 2005). Another non-canonical mediator of BMP signaling is the LIM-domain-containing protein kinase 1 (LIMK1). LIMK1 associates with the carboxy tail of BMPRII and is important for actin stabilization and neurite outgrowth (Foletta et al., 2003; Lee-Hoeflich et al., 2004; Eaton and Davis, 2005; Matsuura et al., 2007; Hocking et al., 2009).
Inhibition of BMP Signaling
While BMP signaling is crucial for many events in development, its inhibition is equally necessary for others. As a result, there are a number of extracellular and intracellular mechanisms that block the influence of BMP signaling. Extracellular signaling is inhibited by competitive binding of BMPR ligands with more than 20 different antagonists, including noggin, chordin, dan, cerebrus, twisted gastrulation, follistatin, and others (Fig. 1) (Holley et al., 1996; Piccolo et al., 1996; Zimmerman et al., 1996; Fainsod et al., 1997); for a review of BMP inhibitors see (Rider and Mulloy, 2010). These proteins seem to have evolved independently of one another as there is little primary sequence similarity amongst them. Moreover, these inhibitors have different binding affinities for various BMP ligands, and some inhibit other signaling pathways as well. For example, follistatin binds a number of BMP family members, but binds Activan A with the highest affinity. Cerebrus binds BMP2, BMP4, and BMP7, but it also binds members of the Wnt family. Similarly, twisted gastrulation acts as a competitive antagonist of BMP signaling but also may act as an agonist by enhancing cleavage of BMP-chordin complexes. At each stage of development a unique cocktail of inhibitors works to specifically target the impact of BMPs.
BMP signaling can also be inhibited intracellularly by blocking downstream signaling pathways. Inhibitory Smads (I-Smads) −6 and −7 directly compete with R-Smads for binding Type I receptors, preventing R-Smad phosphorylation (Fig. 1). I-Smads can also bind with Co-Smads to prevent nuclear translocation of phosphorylated R-Smads (Itoh et al., 2001). Some BMPs, such as BMP7, can induce expression of Smad-6 and −7, suggesting that BMPs can self-regulate through a negative feedback loop. Smad ubiquitin regulatory factors (Smurfs) target R-Smads for ubiquitin mediated degradation (Lo and Massague, 1999; Zhu et al., 1999). BAMBI (BMP and Activin membrane-bound inhibitor) is a Xenopus pseudoreceptor (mammalian homologue, Nma) that contains an extracellular domain similar to the Type I receptors, but lacks the catalytic intracellular domain (Onichtchouk et al., 1999; Grotewold et al., 2001). This allows single BAMBI subunits to bind Type I receptor subunits, creating an unstable dimer that prevents formation of active BMP receptor complexes.
BMPs in Early CNS Development and Patterning
BMP signaling is a potent regulator of CNS development and patterning. Initially BMP signaling promotes the formation of non-neural ectoderm and inhibition of BMP signaling is required to establish primitive neural tissue (neuroectoderm). Thereafter BMP gradients are essential for patterning the dorsal–ventral axis. Thus, BMP signaling in early embryonic stem cells plays a major role in sculpting the CNS.
BMP Inhibition Induces Neuroectoderm
The level of BMP signaling determines the fate of embryonic ectodermal stem cells as either epidermal or neuroectodermal [Fig. 2(A,B)]. In Xenopus embryos, neural tissue formation is normally restricted to the dorsal side of the embryo. However, when dorsal tissue was transplanted on the ventral side of the embryo, neural tissue formed at the expense of epidermal tissue (Spemann and Mangold, 1924). This dorsal tissue contains a collection of cells called the Spemann organizer which express BMP antagonists noggin, chordin, and follistatin (Smith and Harland, 1992; Smith et al., 1993). Ectopic expression of noggin on the ventral side of Xenopus embryos demonstrates that inhibition of BMPs induces neural fate (Lamb et al., 1993). In addition, blocking BMP signaling through overexpression of dominant-negative Type I receptors or dominant-negative forms of BMP4 and BMP7 promotes neurulation (Hemmati-Brivanlou and Melton, 1994; Hawley et al., 1995; Wilson and Hemmati-Brivanlou, 1995). Alternatively, depletion of BMP antagonists inhibits neuroectoderm formation (Oelgeschlager et al., 2003; Kuroda et al., 2004; Khokha et al., 2005). The role of BMP signaling in neurulation is highly conserved among species including both vertebrates and non-vertebrates; for example, the drosophila BMP antagonist Short gastrulation (SOG) mimics the effects of chordin in vertebrates and vice versa (Holley et al., 1995; Schmidt et al., 1995). These findings demonstrate that neuroectoderm is the default fate of naïve ectoderm and that active BMP signaling is required to form non-neural tissue.
Figure 2.
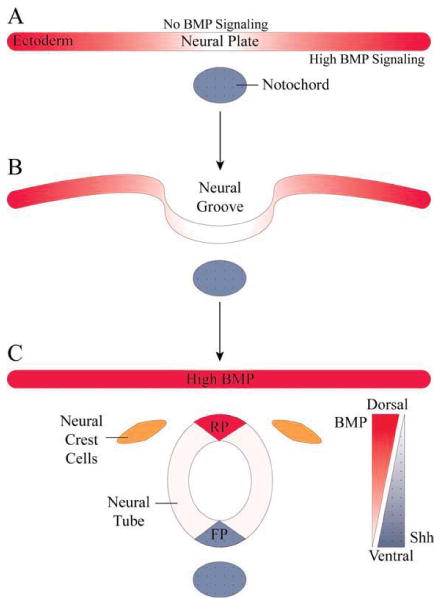
BMP signaling during neurulation. A: A gradient of BMP signaling is established in ectoderm by BMP inhibitors. Neural plate formation is induced where BMP signaling is absent. B: The neural plate folds to create the neural groove and finally pinches off from the overlying ectoderm, forming the neural tube. C: The ectoderm and neural tube roof plate (RP) continue to secrete BMP ligands, which directly oppose ventrally secreted Shh from the notochord and floor plate (FP). The dual morphogen gradient of BMP and Shh establishes the dorsal–ventral axis and specifies neuron populations along this axis. Intermediate levels of BMPs induce neural crest cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Neural induction in the embryo cannot progress simply in the presence of BMP antagonists, but instead requires coordination with multiple other signals including fibroblast growth factor (FGF) and insulinlike growth factor (IGF). FGF signaling downregulates BMP4 and BMP7 expression and is required for neural fate acquisition (Ishimura et al., 2000; Wilson et al., 2000). In addition, FGF and IGF signaling pathways negatively regulate Smad phosphorylation to inhibit downstream BMP signaling (Pera et al., 2001, 2003). Normally, BMPRI phosphorylates Smad on its C-terminus leading to nuclear localization. However, in the presence of FGF or IGF, MAPK phosphorylates Smad on its linker region, preventing translocation to the nucleus. These molecular insights explain why BMP signaling seems to oppose the effects of FGF, IGF, and other growth factors that activate tyrosine kinase receptors (Liu and Niswander, 2005).
Neural Crest Formation
After closure of the neural tube, a column of multipotent stem/progenitor cells—the neural crest—forms along the dorsal aspect of the neural tube at the border of ectoderm and neuroectoderm. These cells give rise to the sympathetic, peripheral sensory, and enteric nervous systems, as well as a variety of other cells such as melanocytes and facial osteocytes. When explant cultures from early neural tissue are exposed to BMP4 or BMP7 they give rise to neural crest, suggesting that BMP signaling plays a role in neural crest induction (Liem et al., 1995). Given the location of the neural crest between high levels of BMP in the ectoderm and low levels of BMP signaling in neuroectoderm, a hypothesis has emerged that an intermediate level of BMP signaling is necessary for proper induction of neural crest [Fig. 2(C)] (Huang and Saint-Jeannet, 2004). Evidence supporting this idea comes from Xenopus studies where exacerbated BMP4 expression leads to reduction of neural crest markers, whereas overexpression of BMP antagonists expands neural crest (Mayor et al., 1995; Marchant et al., 1998). Similarly, zebrafish mutations in BMP signaling members, such as snailhouse (BMP7), swirl (BMPRIIb), and somitobun (Smad5), lead to reduced neural crest formation (Kishimoto et al., 1997; Nguyen et al., 1998, 2000; Hild et al., 1999; Dick et al., 2000; Schmid et al., 2000). However, the level of BMP signaling alone cannot account for neural crest induction, and other signaling pathways including WNT, FGF, and Notch are also crucial for neural crest formation (Coffman et al., 1993; Kengaku and Okamoto, 1993; Saint-Jeannet et al., 1997; LaBonne and Bronner-Fraser, 1998); for review see Sauka-Spengler and Bronner-Fraser (2008). This suggests a multistep process in which BMP signaling sensitizes cells to assume a neural crest identity, while other signaling pathways maintain this identity (Liu and Niswander, 2005).
A Gradient of BMPs Establishes Spinal Cord Patterning
Gradients of BMP signaling also play a central role in development of the spinal cord, which arises from the neural tube. BMPs are secreted from the ectoderm and the dorsal most aspect of the neural tube known as the roof plate. BMP2, BMP4, BMP7 as well as activin and GDF7 are expressed by the ectoderm and neural tube roof plate and form a dorsal–ventral concentration gradient of BMP signaling [Fig. 2(C)] (Basler et al., 1993; Liem et al., 1995; Lee et al., 1998). When this gradient of BMPs is perturbed, the balance of dorsal to ventral neurons becomes skewed. For example, loss of BMP secreting structures results in loss of the dorsal most population of sensory interneurons (marked by LIM homeodomain transcription factors 2a and 2b), which arise from Math1+ precursor cells (Lee et al., 2000; Millonig et al., 2000). Similarly, deletion of both BMPRIa and Ib from the dorsal spinal cord leads to a complete loss of the dorsal most dI1 interneurons with a concurrent dorsal expansion of more ventral dI3 and dI4 interneurons (Wine-Lee et al., 2004). Inhibition of BMP signaling through mis-expression of noggin or knockdown of Smad4 also causes loss of dI1 interneurons and expansion of dI2-4 interneurons (Chesnutt et al., 2004). In contrast, overexpression of BMPRIa and Ib in chick or mouse embryos leads to an expansion of dorsal and intermediate interneurons at the expense of ventral interneurons (Panchision et al., 2001; Timmer et al., 2002). It is increasingly evident that differentiation of some specific spinal cord interneuron subtypes is directly dependent on the BMP signaling gradient. Lim1+ intermediate interneurons are increased in various zebrafish BMP mutants such as swr, sbn, and snh. Strikingly, a further decrease of BMP signaling through overexpression of chordin in the swr mutants results in a decrease of Lim1+ cells, suggesting that cell type specification is dependent upon specific ranges of BMP signaling (Nguyen et al., 2000). Moreover, NGN1+ interneurons are lost in embryos that have either high levels or no expression of BMPs, while a ventral expansion of NGN1+ cells is seen with low levels of BMPs (Lee et al., 2000; Timmer et al., 2002).
Sonic Hedgehog (Shh) secreted from the floor plate and notochord directly opposes the dorsalizing effects of BMP signaling and promotes ventral patterning of the spinal cord [Fig. 2(C)] (Chiang et al., 1996). However, active inhibition of BMP signaling is also required for proper ventralization. BMP inhibitors, such as noggin and follastin, are expressed in the notochord, and knockout of noggin results in loss of ventral interneurons and progenitors, such as those expressing Nkx2.2 (McMahon et al., 1998; Liem et al., 2000). In addition, expression of Smad7 in intermediate spinal cord confines BMP signaling to dorsal spinal cord (Hazen et al., 2011). Interestingly, BMP signaling rapidly induces noggin expression, establishing an effective feedback loop that limits the effects of BMP signaling (Akizu et al., 2010). As a whole these studies suggest that discrete levels of BMP signaling are required for spinal cord dorsalization and for proper patterning.
Once spinal cord neurons have differentiated, BMP signaling influences their axonal projections. Commissural neuron axons grow ventrally, away from the roof plate, due to the repulsive effects of BMP7 and GDF7 (Augsburger et al., 1999; Butler and Dodd, 2003). Roof plate explants affect axon orientation of cultured neurons through the actions of BMPs secreted from the roof plate. Furthermore, BMPRIb, but not BMPRIa, mediates the effects of BMPs on axonal projections in the spinal cord (Yamauchi et al., 2008).
BMPs also regulate spinal cord proliferation. When noggin is over-expressed in the neural tube, proliferation is decreased, implying that BMP signaling promotes proliferation (Chesnutt et al., 2004). BMP-induced proliferation is mediated, in part, by Wnt signaling, another group of morphogens important for embryonic patterning (Zechner et al., 2003). Deletion of BMPRIa and Ib in double-knockout mice reduces Wnt1 and Wnt3a expression, suggesting that BMPs induce Wnts (Wine-Lee et al., 2004). Moreover, Wnt signaling has been shown to induce BMP signaling by increasing the levels of phosphorylated Smad1/5/8 (Ille et al., 2007). These studies propose that Wnt signaling mediates BMP-induced proliferation through a positive feedback loop and illustrate how the effects of BMPs can change through interaction with other signaling pathways.
BMP Signaling Patterns the Forebrain and Cerebellum
As in the spinal cord, BMPs secreted from the dorsal midline pattern brain structures such as forebrain and cerebellum. BMPs are secreted from two signaling centers in the dorsal midline of the telencephalon, the roofplate and cortical hem. These BMPs promote dor-somedial identity and local apoptosis (Furuta et al., 1997). Forebrain explants exposed to BMP4 down-regulate anterior forebrain markers, whereas deletion of BMP antagonists causes loss of anterior brain structures (Anderson et al., 2002). BMPRIa knockout in telencephalon results only in loss of the most dor-salmedial derivative, choroid plexus, whose progenitors remained proliferative and cannot differentiate (Hebert et al., 2002). In contrast, double-knockout of BMPRIa and Ib in dorsal telencephalon causes holoprosencephaly and complete loss of all dorsal midline cell types (Fernandes et al., 2007). However, when BMP signaling is conditionally knocked out only after neural tube closure, holoprosencephaly does not occur, but there are serious defects in dentate gyrus formation (Caronia et al., 2010).
Cerebellar granule neurons originate in the rhombic lip, part of the rhombencephalon, located adjacent to the dorsal midline. BMPs secreted from the dorsal midline initiate the differentiation of granule neurons by inducing expression of granule neuron markers, such as the basic helix-loop-helix (bHLH) transcription factor Math1 (Alder et al., 1999). Multiple BMPs work in concert to promote granule neuron differentiation through distinct mechanisms. BMP2 suppresses the proliferative effect of Shh signaling through phosphorylation of Smad5 and initiates differentiation by causing exit from cell cycle (Rios et al., 2004). BMP4 stimulates granule neuron differentiation by increasing Smad1 expression and translocating it to the nucleus (Angley et al., 2003). BMP6 promotes granule neuron survival through non-canonical activation of the MEK/ERK/CREB pathway (Barneda-Zahonero et al., 2009). Furthermore, double-knockout of BMPRIa and Ib results in severe cerebellum patterning defects and almost complete loss of granule neurons, despite correct specification of Purkinje cells (Qin et al., 2006). Single knockout of BMPRIa or Ib displays no defects, demonstrating the functional redundancy of Type I BMPRs in cerebellar development.
BMPs in Forebrain Cell Fate Specification
Neural fate specification involves both acquisition and maintenance of a particular phenotype and suppression of all other phenotypes. This manifests as a cell type-specific transcriptional profile in which some gene expression is stimulated, while other gene expression is repressed. For example, to commit to the neuronal lineage progenitors must express pro-neuronal genes and inhibit expression of astrocyte-and oligodendrocyte-specific genes. Remarkably, BMP signaling is critical for progenitor fate specification in both neurogenesis and later in astrogliogenesis through dynamic transcriptional regulation.
Neurogenesis
During neural fate specification, BMP signaling initially promotes neurogenesis by pushing progenitors toward a neuronal fate and suppressing the oligodendroglial fate (Li et al., 1998; Mabie et al., 1999; Mehler et al., 2000; Moon et al., 2009). BMP signaling induces expression of the neuron-specific class II beta-tubulin (Tuj1) through activation of the extracellular-signal-related kinase (ERK) pathway (Moon et al., 2009). This mechanism of neuronal induction is similar to that of other pro-neurogenic growth factors, such as platelet-derived growth factor (PDGF) (Menard et al., 2002; Moon et al., 2009). BMP signaling's role in neuronal fate specification can be dissociated from its suppression of oligodendroglial differentiation, suggesting separate mechanisms which will be discussed later (Mabie et al., 1999). Notably, BMP signaling switches from a pro-neuronal to pro-astroglial cue towards the end of neurogenesis, before the transition to gliogenesis (Mabie et al., 1999; Mehler et al., 2000). Levels of expression of the pro-neuronal bHLH transcription factor neurogenin (Ngn1) help to regulate the changing role of BMP signaling in cell fate determination. Ngn1 expression is tightly linked to the time course of neurogenesis; it is highly expressed during the peak of neurogenesis and gradually decreases with the transition to gliogenesis (Ma et al., 1996). During the peak of neurogenesis high levels of Ngn1 expression are thought to contribute to the pro-neuronal effects of BMPs. As neurogenesis proceeds, Ngn1 then works to suppress BMP-mediated astrocyte differentiation by sequestering downstream transcriptional regulators away from glial gene promoters, such as the glial fibrillary acid protein (GFAP) promoter (Sun et al., 2001). As Ngn1 expression diminishes during with the transition to gliogenesis, the pro-astroglial effects of BMP signaling are unmasked. Together these data suggest that the temporally restricted expression of Ngn1 regulates, at least in part, the transition of BMP signaling from being pro-neuronal to being pro-astroglial.
Neuronal Subtype Specification
Different BMP family members are highly expressed in embryonic signaling centers and are critical for the specification of certain CNS cell types. For example, BMP9 is transiently expressed in the embryonic septum during a critical period for basal forebrain cholinergic neuron (BFCN) development. Treatment of septal cultures with BMP9 induces the BFCN phenotype and transcriptome, suggesting that BMP9 may be sufficient for the generation of BFCNs (Lopez-Coviella et al., 2000, 2005). Recent work done with human embryonic stem cells confirms that BMP9 induces expression of two transcription factors, Lhx6 and Gbx1, which are necessary and sufficient to generate basal forebrain cholinergic neurons (Bissonnette et al., 2011). BMP signaling in the cortex also determines the fate of GABAergic interneuron precursors, promoting differentiation of parvalbumin-positive interneurons and blocking differentiation of calbindin- and somatostatin-positive interneurons (Samanta et al., 2007; Mukhopadhyay et al., 2009).
Neurite Outgrowth
After neurogenesis has been completed, BMP signaling regulates neurite outgrowth and dendritogenesis in post-mitotic neurons throughout the CNS, including cortical neurons (Li et al., 1998; Le Roux et al., 1999; Lee-Hoeflich et al., 2004; Podkowa et al., 2010), hippocampal neurons (Withers et al., 2000), and cerebellar neurons (Matsuura et al., 2007). BMP-mediated dendritogenesis is transduced non-canonically through BMPRII and requires local cytoskeleton rearrangement by LIMK1. Activated LIMK1 regulates actin dynamics by phosphorylating and inactivating actin depolymerizing proteins like actin-depolymerizing factor (ADF) and cofilin (Foletta et al., 2003). Interestingly, ADF/cofilin-mediated actin dynamics regulate AMPAR trafficking during synaptic plasticity in cultured hippocampal neurons (Gu et al., 2010). The Drosophila homologue to BMPRII, wishful thinking, also regulates actin dynamics and synaptic stability through LIMK, suggesting that this mechanism of dendritogenesis is evolutionarily conserved (Eaton and Davis, 2005). In addition to regulating actin dynamics, signaling through BMPRII stabilizes dendritic microtubules through another kinase, c-Jun N-terminal kinase (JNK) (Podkowa et al., 2010). Together, these studies demonstrate that non-canonical signaling through BMPRII regulates cytoskeletal rearrangements and ultimately dendritogenesis in post-mitotic neurons.
Astrogliogenesis
During the late embryonic and postnatal periods BMP signaling promotes astroglial differentiation (Gross et al., 1996; Mehler et al., 2000). Similarly, members of the ciliary neurotrophic factor-leukemia inhibitory factor (CNTF-LIF) cytokine family are also potent astrocytic differentiation factors that operate through activation of the Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway (Bonni et al., 1997). LIF-STAT3 and BMP-Smad signaling cascades synergize to promote astrocytic differentiation by activating the GFAP promoter through a STAT3-p300/CBP-Smad1 complex (Nakashima et al., 1999). p300 and CBP (CREB-binding protein) are co-activating proteins that interact with multiple transcription factors to enhance expression of their target genes. However, BMP-mediated astroglial fate commitment may also occur by non-canonical mechanisms. Under high density conditions BMP4 treatment has been reported to activate STAT proteins through the serine-threonine kinase FKBP12/rapamycin-associated protein (FRAP), mammalian target of rapamycin (mTOR) (Rajan et al., 2003). FKBP12 is sequestered to the BMPRIa receptor and, upon BMP4 binding, is released to activate FRAP. FRAP in turn catalyzes phosphorylation of STAT, which ultimately promotes astrocytic gene expression. This suggests that BMP-and LIF-mediated astroglial fate commitment can occur through distinct mechanisms and may explain in part why LIF and BMP signaling generate different types of astrocytes. LIF signaling promotes the generation of GFAP+ progenitor cells, while BMP signaling promotes the generation of mature GFAP+ astrocytes which lack stem/progenitor cell properties (Bonaguidi et al., 2005).
In addition to promoting an astrocytic identity, BMP signaling also activates downstream signals that inhibit oligodendroglial and neuronal lineage commitment (Gross et al., 1996, Mehler et al., 2000). BMPs induce expression of the Hes (homologue of hairy and enhancer of split) and Id (inhibitor of differentiation) gene families, which negatively regulate bHLH transcription factors. Oligodendrogenesis is promoted by the bHLH transcription factors Olig1 and Olig2 (Ross et al., 2003). BMP-induced expression of Id2 and Id4 in neural progenitors prevents oligodendrocyte lineage commitment by binding Olig1 and Olig2 and sequestering them away from their targets (Samanta and Kessler, 2004). bHLH transcription factors such as Mash1 (mammalian achaete-scute homologue), neurogenin, and NeuroD promote neurogenesis and are negatively regulated by Hes-1, Hes-5 and the Id family of proteins (Bertrand et al., 2002; Ross et al., 2003). Exposure to BMP2 upregulates the expression of Id1, Id3, and Hes-5 in neural progenitors, which inhibits neuronal differentiation and promotes astroglial commitment (Nakashima et al., 2001). As mentioned earlier, fate specification requires both acquisition and maintenance of a particular phenotype. BMP-induced expression of the Ids and Hes-5 is transient and likely contributes to the acquisition but not the maintenance of the astrocytic fate (Nakashima et al., 2001; Kohyama et al., 2010). Thus, BMPs rely on the transcriptional repressor RE1 silencer of transcription/neuron-restrictive silencer factor (REST/NRSF) for continual suppression of neuronal gene expression. Canonical BMP signaling upregulates and sustains expression of REST/NRSF, which blocks neuronal gene expression throughout the life of the astrocyte (Kohyama et al., 2010).
BMP Signaling in Adult Neurogenesis
Though the mammalian brain continues to develop postnatally, by adulthood most regions of the brain no longer maintain a dividing progenitor population. The subventricular zone (SVZ) of the forebrain and the subgranular zone (SGZ) of the hippocampus are the only two regions that contain stem cell niches and continue to generate new neurons in adulthood. Both regions contain stem cells that self-renew and can give rise to all neural lineages (Altman and Das, 1965; Morshead et al., 1994; Palmer et al., 1997; Bonaguidi et al., 2008; Walker et al., 2008). As might be expected, similar signaling systems are important during developmental and adult neurogenesis, including BMP signaling.
Subventricular Zone Neurogenesis
The SVZ is located along the lateral ventricles and is made up of various cell types. Ependymal cells line the ventricle to provide a barrier between ventricular cerebrospinal fluid and the niche, and there are suggestions that ependymal cells themselves have stem cell properties (Johansson et al., 1999). Adjacent to the ependymal cells is the subependymal layer which consists of radial glial-like stem cells (Type B), transient amplifying progenitors (TAPs) (Type C), and neuroblasts (Type A) (Ihrie and Alvarez-Buylla, 2011). Neurogenesis starts when radial glial-like stem cells divide to give rise to TAPs which rapidly divide and become neuroblasts [Fig. 3(A)]. BMP2, 4, and 7 are expressed in radial glial-like stem cells and TAPs, but not in ependymal cells or neuroblasts (Lim et al., 2000; Peretto et al., 2002); noggin is expressed in ependymal cells (Lim et al., 2000; Peretto et al., 2002). BMP signaling in the SVZ promotes astroglial lineage commitment and inhibits the differentiation of neurons and oligodendrocytes (Lim et al., 2000; Colak et al., 2008). In contrast, noggin suppresses astroglial lineage commitment (Chmielnicki et al., 2004). Interestingly, BMP signaling also seems to promote the survival of dividing neuroblasts which have already committed to the neuronal lineage (Lim et al., 2000; Colak et al., 2008). Brain-derived neurotrophic factor (BDNF) is also an important regulator of adult neurogenesis in the SVZ (Ahmed et al., 1995; Kirschenbaum and Goldman, 1995), and overexpression of BDNF in SVZ induces addition of neurons to the olfactory bulb and striatum (Benraiss et al., 2001). Notably, co-overexpression of noggin along with BDNF results in a marked and sustained increase in recruitment of new medium spiny neurons to the striatum, which is usually non-neurogenic (Chmielnicki et al., 2004; Benraiss et al., 2011). Long-term overexpression of BDNF/noggin in SVZ is sufficient to maintain ongoing addition of medium spiny neurons to striatum and delays motor impairment and increases survival of a mouse model of Huntington's disease (Cho et al., 2007; Benraiss et al., 2011). These behavioral effects require the synergy of BDNF and noggin and their positive effects on neurogenesis. As during development, it is likely that BMP signaling in adult SVZ has differential effects at various stages of the lineage and interacts with other signaling pathways.
Figure 3.
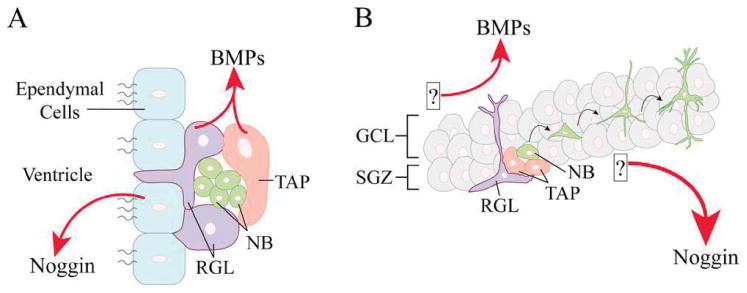
BMP signaling in adult neurogenic niches. Adult brains contain two neurogenic niches, the SVZ and the SGZ. A: In the SVZ, radial glial-like (RGL) stem cells divide to give rise to transient amplifying cells (TAP) which rapidly divide to become neuroblasts (NB). These immature neurons travel through the rostral migratory stream to the olfactory bulb where they become interneurons. RGL stem cells and TAPs secrete BMP ligands, whereas ependymal cells secrete Noggin. BMP signaling in the SVZ promotes astroglial lineage commitment and promotes survival of neuronally committed neuroblasts. B: In the hippocampal SGZ of the dentate gyrus, RGL stem cells divide to give rise to TAPs, which in turn produce NBs, similar to the SVZ. NBs migrate radially into the granule cell layer (GCL) where they become mature dentate granule cells. Unlike in the SVZ, the sources of BMP ligands and Noggin have not yet been clearly identified in the SGZ. While noggin is required to maintain the proliferating, self-renewing population of stem cells, BMP signaling seems to block neurogenesis, indicating that a balance of BMP signaling regulates neurogenesis in the SGZ niche. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Subgranular Zone Neurogenesis
In the hippocampus, the SGZ is the innermost layer of the dentate gyrus, adjacent to the hilus. The SGZ contains no ependymal cells, but still contains radial glial-like stem cells which give rise to TAPs that divide to form neuroblasts [Fig. 3(B)]. Neuroblasts migrate radially into the granule cell layer and mature into dentate granule cells. Postnatally BMPs, their receptors, and noggin are expressed in the dentate gyrus (Charytoniuk et al., 2000; Fan et al., 2003a; Mikawa et al., 2006; Sato et al., 2010; Mikawa and Sato, 2011), however, it is not exactly known which cell types express the receptors and secreted factors.
The existence of bona fide stem cells in the SGZ has been a contentious topic in the field of neurogenesis (Bonaguidi et al., 2011; Encinas et al., 2011; Kempermann, 2011). Cultured SGZ stem cells require the addition of either noggin or depolarizing levels of KCl to propagate in vitro, however, these treatments have no effect on SVZ stem cells (Bonaguidi et al., 2008, Walker et al., 2008). The fact that noggin specifically unveils the self-renewal capacity of SGZ neural stem cells suggests that BMP signaling is a critical factor in regulating adult hippocampal neurogenesis. In vivo noggin treatment increases neurogenesis and neuronal commitment (Gobeske et al., 2009, Mira et al., 2010), while exacerbated BMP signaling in the dentate gyrus decreases neurogenesis (Fan et al., 2003b) and promotes astroglial lineage commitment (Brederlau et al., 2004; Bonaguidi et al., 2005). One study proposes that long-term noggin treatment depletes the stem cell pool, suggesting that stem cells in the SGZ have a limited capacity to self-renew (Mira et al., 2010). However, other studies find that the proneurogenic effects of noggin are enduring and renewable (Bonaguidi et al., 2008, Gobeske et al., 2009; Guo et al., 2011). Though there is dissonance regarding the exact role of BMP signaling in the SGZ, it is clear that the antagonistic balance between noggin and BMPs is critical to the regulation of adult hippocampal neurogenesis.
Adult SGZ neurogenesis is influenced by various factors such as exercise, environmental enrichment, and aging. Not surprisingly, BMP signaling may be involved in modulating neurogenesis under these conditions. Neurogenesis is required for the formation of hippocampus-dependent memory (Saxe et al., 2006; Deng et al., 2009; Marin-Burgin and Schinder, 2011). Because BMP signaling limits neurogenesis, it could be hypothesized that BMP signaling also limits the formation of new memories. In fact, increased BMP signaling results in cognitive impairments on hippocampus-dependent tasks whereas decreased BMP signaling improves cognition (Fan et al., 2003b; Gobeske et al., 2009). Running is known to enhance SGZ neurogenesis and proliferation (van Praag et al., 1999) and has been associated with increased levels of noggin coincident with decreased levels of BMP4 protein in the hippocampus (Gobeske et al., 2009). Additionally, overexpression of BMP4 seems to block the neurogenic effect of running (Gobeske et al., 2009). SGZ neurogenesis declines with age (Kuhn et al., 1996; Knoth et al., 2010) and in mouse models of Alzheimer's disease (AD) (Rodriguez and Verkhratsky, 2011). Both decreased neurogenesis and an increase in BMP4 or BMP6 levels have been associated with mouse models of AD (Li et al., 2008; Crews et al., 2010). Additionally, infusion of noggin rescues the decrease in neurogenesis associated with models of AD (Tang et al., 2009). Together these studies suggest that changes in adult neurogenesis caused by environmental factors are modulated by BMP signaling.
Conclusion
BMP signaling affects neural stem cell fate and maturation at almost every stage of CNS development, from neurulation through adult life (Fig. 4). Spatiotemporal signals mediate its effects, enabling BMP signaling to exert a wide range of functions. In addition to its role in development, recent studies implicate BMP signaling in the cognitive decline associated with normal aging and neurodegenerative diseases. Continued study of the role of BMP signaling in neural stem cells will have important clinical implications and could lead to novel therapeutic targets.
Figure 4.
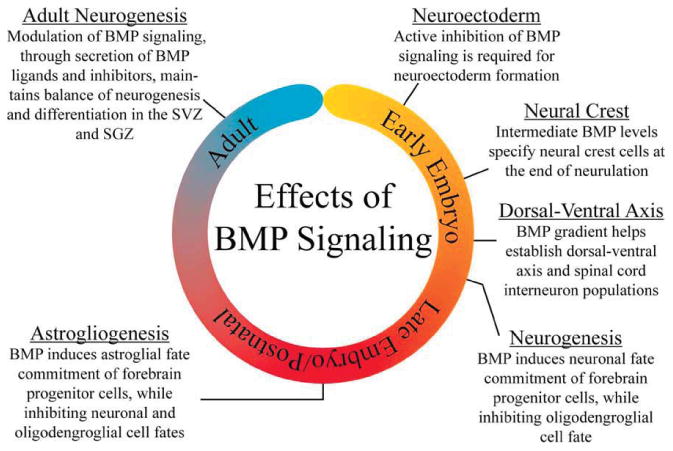
The changing roles of BMP signaling. BMP signaling is involved in numerous aspects of neural stem cell development. Initially, inhibition of BMP signaling is required for neuroectoderm induction. A gradient of BMP signaling establishes the dorsal–ventral axis and specifies neural crest cells and distinct interneuron subgroups within the spinal cord. During forebrain development, BMP signaling promotes neuronal lineage commitment while inhibiting oligodendrocyte formation. In late embryogenesis and postnatally, BMP signaling changes to promote astroglial commitment while repressing commitment to the other two neural lineages. In adulthood, a balance of BMP signaling is critical for maintenance and differentiation of neural stem cell populations in the SVZ and SGZ. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
References
- Ahmed S, Reynolds BA, Weiss S. BDNF enhances the differentiation but not the survival of CNS stem cell-derived neuronal precursors. J Neurosci. 1995;15:5765–5778. doi: 10.1523/JNEUROSCI.15-08-05765.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB., III BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development. 2001;128:4449–4461. doi: 10.1242/dev.128.22.4449. [DOI] [PubMed] [Google Scholar]
- Akizu N, Estaras C, Guerrero L, Marti E, Martinez-Balbas MA. H3K27me3 regulates BMP activity in developing spinal cord. Development. 2010;137:2915–2925. doi: 10.1242/dev.049395. [DOI] [PubMed] [Google Scholar]
- Alder J, Lee KJ, Jessell TM, Hatten ME. Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci. 1999;2:535–540. doi: 10.1038/9189. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Lawrence AR, Stottmann RW, Bachiller D, Klingensmith J. Chordin and noggin promote organizing centers of forebrain development in the mouse. Development. 2002;129:4975–4987. doi: 10.1242/dev.129.21.4975. [DOI] [PubMed] [Google Scholar]
- Angley C, Kumar M, Dinsio KJ, Hall AK, Siegel RE. Signaling by bone morphogenetic proteins and Smad1 modulates the postnatal differentiation of cerebellar cells. J Neurosci. 2003;23:260–268. doi: 10.1523/JNEUROSCI.23-01-00260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- Barneda-Zahonero B, Minano-Molina A, Badiola N, Fado R, Xifro X, Saura CA, Rodriguez-Alvarez J. Bone morphogenetic protein-6 promotes cerebellar granule neurons survival by activation of the MEK/ERK/CREB pathway. Mol Biol Cell. 2009;20:5051–5063. doi: 10.1091/mbc.E09-05-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler K, Edlund T, Jessell TM, Yamada T. Control of cell pattern in the neural tube: Regulation of cell differentiation by dorsalin-1, a novel TGF beta family member. Cell. 1993;73:687–702. doi: 10.1016/0092-8674(93)90249-p. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Bruel-Jungerman E, Lu G, Economides AN, Davidson B, Goldman SA. Sustained induction of neuronal addition to the adult rat neostriatum by AAV4-delivered noggin and BDNF. Gene Ther. 2011 doi: 10.1038/gt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. J Neurosci. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Castro DS, Guillemot F. Proneural genes and the specification of neural cell types. Nat Rev Neurosci. 2002;3:517–530. doi: 10.1038/nrn874. [DOI] [PubMed] [Google Scholar]
- Bissonnette CJ, Lyass L, Bhattacharyya BJ, Belmadani A, Miller RJ, Kessler JA. The controlled generation of functional basal forebrain cholinergic neurons from human embryonic stem cells. Stem Cells. 2011;29:802–811. doi: 10.1002/stem.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, McGuire T, Hu M, Kan L, Samanta J, Kessler JA. LIF and BMP signaling generate separate and discrete types of GFAP-expressing cells. Development. 2005;132:5503–5514. doi: 10.1242/dev.02166. [DOI] [PubMed] [Google Scholar]
- Bonaguidi MA, Peng CY, McGuire T, Falciglia G, Gobeske KT, Czeisler C, Kessler JA. Noggin expands neural stem cells in the adult hippocampus. J Neurosci. 2008;28:9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi MA, Wheeler MA, Shapiro JS, Stadel RP, Sun GJ, Ming GL, Song H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell. 2011;145:1142–1155. doi: 10.1016/j.cell.2011.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, et al. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. doi: 10.1126/science.278.5337.477. [DOI] [PubMed] [Google Scholar]
- Brederlau A, Faigle R, Elmi M, Zarebski A, Sjoberg S, Fujii M, Miyazono K, et al. The bone morphogenetic protein type Ib receptor is a major mediator of glial differentiation and cell survival in adult hippocampal progenitor cell culture. Mol Biol Cell. 2004;15:3863–3875. doi: 10.1091/mbc.E03-08-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- Caronia G, Wilcoxon J, Feldman P, Grove EA. Bone morphogenetic protein signaling in the developing telencephalon controls formation of the hippocampal dentate gyrus and modifies fear-related behavior. J Neurosci. 2010;30:6291–6301. doi: 10.1523/JNEUROSCI.0550-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Hemmati-Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development. 1999;126:3347–3357. doi: 10.1242/dev.126.15.3347. [DOI] [PubMed] [Google Scholar]
- Charytoniuk DA, Traiffort E, Pinard E, Issertial O, Seylaz J, Ruat M. Distribution of bone morphogenetic protein and bone morphogenetic protein receptor transcripts in the rodent nervous system and up-regulation of bone morphogenetic protein receptor type II in hippocampal dentate gyrus in a rat model of global cerebral ischemia. Neuroscience. 2000;100:33–43. doi: 10.1016/s0306-4522(00)00246-3. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, Beachy PA. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. J Neurosci. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. J Clin Invest. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman CR, Skoglund P, Harris WA, Kintner CR. Expression of an extracellular deletion of Xotch diverts cell fate in Xenopus embryos. Cell. 1993;73:659–671. doi: 10.1016/0092-8674(93)90247-n. [DOI] [PubMed] [Google Scholar]
- Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, Monteiro R, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews L, Adame A, Patrick C, Delaney A, Pham E, Rock-enstein E, Hansen L, et al. Increased BMP6 levels in the brains of Alzheimer's disease patients and APP transgenic mice are accompanied by impaired neurogenesis. J Neurosci. 2010;30:12252–12262. doi: 10.1523/JNEUROSCI.1305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Currle DS, Cheng X, Hsu CM, Monuki ES. Direct and indirect roles of CNS dorsal midline cells in choroid plexus epithelia formation. Development. 2005;132:3549–3559. doi: 10.1242/dev.01915. [DOI] [PubMed] [Google Scholar]
- Daopin S, Piez KA, Ogawa Y, Davies DR. Crystal structure of transforming growth factor-beta 2: An unusual fold for the superfamily. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Marcillo A, Nonner D, Dietrich WD, Keane RW. Neuroprotective effects of bone morphogenetic protein 7 (BMP7) treatment after spinal cord injury. Neurosci Lett. 2009;465:226–229. doi: 10.1016/j.neulet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- den Hollander AI, Biyanwila J, Kovach P, Bardakjian T, Traboulsi EI, Ragge NK, Schneider A, et al. Genetic defects of GDF6 in the zebrafish out of sight mutant and in human eye developmental anomalies. BMC Genet. 2010;11:102. doi: 10.1186/1471-2156-11-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Saxe MD, Gallina IS, Gage FH. Adult-born hippocampal dentate granule cells undergoing maturation modulate learning and memory in the brain. J Neurosci. 2009;29:13532–13542. doi: 10.1523/JNEUROSCI.3362-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, et al. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, et al. Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development. 2000;127:343–354. doi: 10.1242/dev.127.2.343. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. LIM Kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron. 2005;47:695–708. doi: 10.1016/j.neuron.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Encinas JM, Michurina TV, Peunova N, Park JH, Tordo J, Peterson DA, Fishell G, et al. Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell. 2011;8:566–579. doi: 10.1016/j.stem.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fainsod A, Deissler K, Yelin R, Marom K, Epstein M, Pillemer G, Steinbeisser H, et al. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech Dev. 1997;63:39–50. doi: 10.1016/s0925-4773(97)00673-4. [DOI] [PubMed] [Google Scholar]
- Fan X, Xu H, Cai W, Yang Z, Zhang J. Spatial and temporal patterns of expression of Noggin and BMP4 in embryonic and postnatal rat hippocampus. Brain Res Dev Brain Res. 2003a;146:51–58. doi: 10.1016/j.devbrainres.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Fan XT, Cai WQ, Yang Z, Xu HW, Zhang JH. Effect of antisense oligonucleotide of noggin on spatial learning and memory of rats. Acta Pharmacol Sin. 2003b;24:394–397. [PubMed] [Google Scholar]
- Fernandes M, Gutin G, Alcorn H, McConnell SK, Hebert JM. Mutations in the BMP pathway in mice support the existence of two molecular classes of holoprosencephaly. Development. 2007;134:3789–3794. doi: 10.1242/dev.004325. [DOI] [PubMed] [Google Scholar]
- Foletta VC, Lim MA, Soosairajah J, Kelly AP, Stanley EG, Shannon M, He W, et al. Direct signaling by the BMP type II receptor via the cytoskeletal regulator LIMK1. J Cell Biol. 2003;162:1089–1098. doi: 10.1083/jcb.200212060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Gajavelli S, Wood PM, Pennica D, Whittemore SR, Tsoulfas P. BMP signaling initiates a neural crest differentiation program in embryonic rat CNS stem cells. Exp Neurol. 2004;188:205–223. doi: 10.1016/j.expneurol.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Gobeske KT, Das S, Bonaguidi MA, Weiss C, Radulovic J, Disterhoft JF, Kessler JA. BMP signaling mediates effects of exercise on hippocampal neurogenesis and cognition in mice. PLoS One. 2009;4:e7506. doi: 10.1371/journal.pone.0007506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratacos E, Gavalda N, Alberch J. Bone morphogenetic protein-6 is a neurotrophic factor for calbindin-positive striatal neurons. J Neurosci Res. 2002;70:638–644. doi: 10.1002/jnr.10438. [DOI] [PubMed] [Google Scholar]
- Griffith DL, Keck PC, Sampath TK, Rueger DC, Carlson WD. Three-dimensional structure of recombinant human osteogenic protein 1: Structural paradigm for the transforming growth factor beta superfamily. Proc Natl Acad Sci USA. 1996;93:878–883. doi: 10.1073/pnas.93.2.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross RE, Mehler MF, Mabie PC, Zang Z, Santschi L, Kessler JA. Bone morphogenetic proteins promote astroglial lineage commitment by mammalian subventricular zone progenitor cells. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- Grotewold L, Plum M, Dildrop R, Peters T, Ruther U. Bambi is coexpressed with Bmp-4 during mouse embryogenesis. Mech Dev. 2001;100:327–330. doi: 10.1016/s0925-4773(00)00524-4. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komlos D, Tang X, Sun C, Yu K, et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Zhang L, Christopher DM, Teng ZQ, Fausett SR, Liu C, George OL, Klingensmith J, Jin P, Zhao X. RNA-binding protein FXR2 regulates adult hippocampal neurogenesis by reducing Noggin expression. Neuron. 2011;70:924–938. doi: 10.1016/j.neuron.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanel ML, Hensey C. Eye and neural defects associated with loss of GDF6. BMC Dev Biol. 2006;6:43. doi: 10.1186/1471-213X-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley SH, Wunnenberg-Stapleton K, Hashimoto C, Laurent MN, Watabe T, Blumberg BW, Cho KW. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995;9:2923–2935. doi: 10.1101/gad.9.23.2923. [DOI] [PubMed] [Google Scholar]
- Hazen VM, Phan KD, Hudiburgh S, Butler SJ. Inhibitory Smads differentially regulate cell fate specification and axon dynamics in the dorsal spinal cord. Dev Biol. 2011;356:566–575. doi: 10.1016/j.ydbio.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert JM, Mishina Y, McConnell SK. BMP signaling is required locally to pattern the dorsal telencephalic midline. Neuron. 2002;35:1029–1041. doi: 10.1016/s0896-6273(02)00900-5. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton DA. Inhibition of activin receptor signaling promotes neuralization in Xenopus. Cell. 1994;77:273–281. doi: 10.1016/0092-8674(94)90319-0. [DOI] [PubMed] [Google Scholar]
- Hild M, Dick A, Rauch GJ, Meier A, Bouwmeester T, Haffter P, Hammerschmidt M. The smad5 mutation somitabun blocks Bmp2b signaling during early dorsoventral patterning of the zebrafish embryo. Development. 1999;126:2149–2159. doi: 10.1242/dev.126.10.2149. [DOI] [PubMed] [Google Scholar]
- Hocking JC, Hehr CL, Bertolesi G, Funakoshi H, Nakamura T, McFarlane S. LIMK1 acts downstream of BMP signaling in developing retinal ganglion cell axons but not dendrites. Dev Biol. 2009;330:273–285. doi: 10.1016/j.ydbio.2009.03.027. [DOI] [PubMed] [Google Scholar]
- Holley SA, Jackson PD, Sasai Y, Lu B, De Robertis EM, Hoffmann FM, Ferguson EL. A conserved system for dorsal-ventral patterning in insects and vertebrates involving sog and chordin. Nature. 1995;376:249–253. doi: 10.1038/376249a0. [DOI] [PubMed] [Google Scholar]
- Holley SA, Neul JL, Attisano L, Wrana JL, Sasai Y, O'Connor MB, De Robertis EM, et al. The Xenopus dorsalizing factor noggin ventralizes Drosophila embryos by preventing DPP from activating its receptor. Cell. 1996;86:607–617. doi: 10.1016/s0092-8674(00)80134-8. [DOI] [PubMed] [Google Scholar]
- Holzschuh J, Wada N, Wada C, Schaffer A, Javidan Y, Tallafuss A, Bally-Cuif L, et al. Requirements for endoderm and BMP signaling in sensory neurogenesis in zebrafish. Development. 2005;132:3731–3742. doi: 10.1242/dev.01936. [DOI] [PubMed] [Google Scholar]
- Hoodless PA, Haerry T, Abdollah S, Stapleton M, O'Connor MB, Attisano L, Wrana JL. MADR1, a MAD-related protein that functions in BMP2 signaling pathways. Cell. 1996;85:489–500. doi: 10.1016/s0092-8674(00)81250-7. [DOI] [PubMed] [Google Scholar]
- Huang X, Saint-Jeannet JP. Induction of the neural crest and the opportunities of life on the edge. Dev Biol. 2004;275:1–11. doi: 10.1016/j.ydbio.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Ihrie RA, Alvarez-Buylla A. Lake-front property: A unique germinal niche by the lateral ventricles of the adult brain. Neuron. 2011;70:674–686. doi: 10.1016/j.neuron.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ille F, Atanasoski S, Falk S, Ittner LM, Marki D, Buchmann-Moller S, Wurdak H, et al. Wnt/BMP signal integration regulates the balance between proliferation and differentiation of neuroepithelial cells in the dorsal spinal cord. Dev Biol. 2007;304:394–408. doi: 10.1016/j.ydbio.2006.12.045. [DOI] [PubMed] [Google Scholar]
- Ishimura A, Maeda R, Takeda M, Kikkawa M, Daar IO, Maeno M. Involvement of BMP-4/msx-1 and FGF pathways in neural induction in the Xenopus embryo. Dev Growth Differ. 2000;42:307–316. doi: 10.1046/j.1440-169x.2000.00514.x. [DOI] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Kaufman RJ, Rosen V, Cox KA, Wozney JM. Heterodimeric bone morphogenetic proteins show enhanced activity in vitro and in vivo. Growth Factors. 1996;13:291–300. doi: 10.3109/08977199609003229. [DOI] [PubMed] [Google Scholar]
- Israel DI, Nove J, Kerns KM, Moutsatsos IK, Kaufman RJ. Expression and characterization of bone morphogenetic protein-2 in Chinese hamster ovary cells. Growth Factors. 1992;7:139–150. doi: 10.3109/08977199209046403. [DOI] [PubMed] [Google Scholar]
- Itoh F, Asao H, Sugamura K, Heldin CH, ten Dijke P, Itoh S. Promoting bone morphogenetic protein signaling through negative regulation of inhibitory Smads. EMBO J. 2001;20:4132–4142. doi: 10.1093/emboj/20.15.4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- Kawai S, Faucheu C, Gallea S, Spinella-Jaegle S, Atfi A, Baron R, Roman SR. Mouse smad8 phosphorylation downstream of BMP receptors ALK-2, ALK-3, and ALK-6 induces its association with Smad4 and transcriptional activity. Biochem Biophys Res Commun. 2000;271:682–687. doi: 10.1006/bbrc.2000.2704. [DOI] [PubMed] [Google Scholar]
- Kempermann G. The pessimist's and optimist's views of adult neurogenesis. Cell. 2011;145:1009–1011. doi: 10.1016/j.cell.2011.06.011. [DOI] [PubMed] [Google Scholar]
- Kendall SE, Battelli C, Irwin S, Mitchell JG, Glackin CA, Verdi JM. NRAGE mediates p38 activation and neural progenitor apoptosis via the bone morphogenetic protein signaling cascade. Mol Cell Biol. 2005;25:7711–7724. doi: 10.1128/MCB.25.17.7711-7724.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kengaku M, Okamoto H. Basic fibroblast growth factor induces differentiation of neural tube and neural crest lineages of cultured ectoderm cells from Xenopus gastrula. Development. 1993;119:1067–1078. doi: 10.1242/dev.119.4.1067. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann's organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kim J, Wu HH, Lander AD, Lyons KM, Matzuk MM, Calof AL. GDF11 controls the timing of progenitor cell competence in developing retina. Science. 2005;308:1927–1930. doi: 10.1126/science.1110175. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proc Natl Acad Sci USA. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Knoth R, Singec I, Ditter M, Pantazis G, Capetian P, Meyer RP, Horvat V, et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS One. 2010;5:e8809. doi: 10.1371/journal.pone.0008809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohyama J, Sanosaka T, Tokunaga A, Takatsuka E, Tsujimura K, Okano H, Nakashima K. BMP-induced REST regulates the establishment and maintenance of astrocytic identity. J Cell Biol. 2010;189:159–170. doi: 10.1083/jcb.200908048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieglstein K, Suter-Crazzolara C, Hotten G, Pohl J, Unsicker K. Trophic and protective effects of growth/differentiation factor 5, a member of the transforming growth factor-beta superfamily, on midbrain dopaminergic neurons. J Neurosci Res. 1995;42:724–732. doi: 10.1002/jnr.490420516. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda H, Wessely O, De Robertis EM. Neural induction in Xenopus: Requirement for ectodermal and endomesodermal signals via chordin, noggin, beta-catenin, and cerberus. PLoS Biol. 2004;2:E92. doi: 10.1371/journal.pbio.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBonne C, Bronner-Fraser M. Neural crest induction in Xenopus: Evidence for a two-signal model. Development. 1998;125:2403–2414. doi: 10.1242/dev.125.13.2403. [DOI] [PubMed] [Google Scholar]
- Lamb TM, Knecht AK, Smith WC, Stachel SE, Economides AN, Stahl N, Yancopolous GD, et al. Neural induction by the secreted polypeptide noggin. Science. 1993;262:713–718. doi: 10.1126/science.8235591. [DOI] [PubMed] [Google Scholar]
- Le Roux P, Behar S, Higgins D, Charette M. OP-1 enhances dendritic growth from cerebral cortical neurons in vitro. Exp Neurol. 1999;160:151–163. doi: 10.1006/exnr.1999.7194. [DOI] [PubMed] [Google Scholar]
- Lee-Hoeflich ST, Causing CG, Podkowa M, Zhao X, Wrana JL, Attisano L. Activation of LIMK1 by binding to the BMP receptor, BMPRII, regulates BMP-dependent dendritogenesis. EMBO J. 2004;23:4792–4801. doi: 10.1038/sj.emboj.7600418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: A requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Tang J, Xu H, Fan X, Bai Y, Yang L. Decreased hippocampal cell proliferation correlates with increased expression of BMP4 in the APPswe/PS1DeltaE9 mouse model of Alzheimer's disease. Hippocampus. 2008;18:692–698. doi: 10.1002/hipo.20428. [DOI] [PubMed] [Google Scholar]
- Li W, Cogswell CA, LoTurco JJ. Neuronal differentiation of precursors in the neocortical ventricular zone is triggered by BMP. J Neurosci. 1998;18:8853–8862. doi: 10.1523/JNEUROSCI.18-21-08853.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liem KF, Jr, Jessell TM, Briscoe J. Regulation of the neural patterning activity of sonic hedgehog by secreted BMP inhibitors expressed by notochord and somites. Development. 2000;127:4855–4866. doi: 10.1242/dev.127.22.4855. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Roelink H, Jessell TM. Dorsal differentiation of neural plate cells induced by BMP-mediated signals from epidermal ectoderm. Cell. 1995;82:969–979. doi: 10.1016/0092-8674(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Lillien L, Raphael H. BMP and FGF regulate the development of EGF-responsive neural progenitor cells. Development. 2000;127:4993–5005. doi: 10.1242/dev.127.22.4993. [DOI] [PubMed] [Google Scholar]
- Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–726. doi: 10.1016/s0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- Liu A, Niswander LA. Bone morphogenetic protein signalling and vertebrate nervous system development. Nat Rev Neurosci. 2005;6:945–954. doi: 10.1038/nrn1805. [DOI] [PubMed] [Google Scholar]
- Lo RS, Massague J. Ubiquitin-dependent degradation of TGF-beta-activated smad2. Nat Cell Biol. 1999;1:472–478. doi: 10.1038/70258. [DOI] [PubMed] [Google Scholar]
- Lopez-Coviella I, Berse B, Krauss R, Thies RS, Blusztajn JK. Induction and maintenance of the neuronal cholinergic phenotype in the central nervous system by BMP-9. Science. 2000;289:313–316. doi: 10.1126/science.289.5477.313. [DOI] [PubMed] [Google Scholar]
- Lopez-Coviella I, Follettie MT, Mellott TJ, Kovacheva VP, Slack BE, Diesl V, Berse B, et al. Bone morphogenetic protein 9 induces the transcriptome of basal forebrain cholinergic neurons. Proc Natl Acad Sci USA. 2005;102:6984–6989. doi: 10.1073/pnas.0502097102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Coviella I, Mellott TM, Kovacheva VP, Berse B, Slack BE, Zemelko V, Schnitzler A, et al. Developmental pattern of expression of BMP receptors and Smads and activation of Smad1 and Smad5 by BMP9 in mouse basal forebrain. Brain Res. 2006;1088:49–56. doi: 10.1016/j.brainres.2006.02.073. [DOI] [PubMed] [Google Scholar]
- Ma Q, Kintner C, Anderson DJ. Identification of neurogenin, a vertebrate neuronal determination gene. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- Mabie PC, Mehler MF, Kessler JA. Multiple roles of bone morphogenetic protein signaling in the regulation of cortical cell number and phenotype. J Neurosci. 1999;19:7077–7088. doi: 10.1523/JNEUROSCI.19-16-07077.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant L, Linker C, Ruiz P, Guerrero N, Mayor R. The inductive properties of mesoderm suggest that the neural crest cells are specified by a BMP gradient. Dev Biol. 1998;198:319–329. [PubMed] [Google Scholar]
- Marin-Burgin A, Schinder AF. Requirement of adult-born neurons for hippocampus-dependent learning. Behav Brain Res. 2012;227:391–399. doi: 10.1016/j.bbr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Matluk N, Rochira JA, Karaczyn A, Adams T, Verdi JM. A role for NRAGE in NF-kappaB activation through the non-canonical BMP pathway. BMC Biol. 2010;8:7. doi: 10.1186/1741-7007-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura I, Endo M, Hata K, Kubo T, Yamaguchi A, Saeki N, Yamashita T. BMP inhibits neurite growth by a mechanism dependent on LIM-kinase. Biochem Biophys Res Commun. 2007;360:868–873. doi: 10.1016/j.bbrc.2007.06.157. [DOI] [PubMed] [Google Scholar]
- Mayor R, Morgan R, Sargent MG. Induction of the prospective neural crest of Xenopus. Development. 1995;121:767–777. doi: 10.1242/dev.121.3.767. [DOI] [PubMed] [Google Scholar]
- McMahon JA, Takada S, Zimmerman LB, Fan CM, Harland RM, McMahon AP. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhang D, Kessler JA. Bone morphogenetic proteins in the nervous system. Trends Neurosci. 1997;20:309–317. doi: 10.1016/s0166-2236(96)01046-6. [DOI] [PubMed] [Google Scholar]
- Mehler MF, Mabie PC, Zhu G, Gokhan S, Kessler JA. Developmental changes in progenitor cell responsiveness to bone morphogenetic proteins differentially modulate progressive CNS lineage fate. Dev Neurosci. 2000;22:74–85. doi: 10.1159/000017429. [DOI] [PubMed] [Google Scholar]
- Menard C, Hein P, Paquin A, Savelson A, Yang XM, Lederfein D, Barnabe-Heider F, et al. An essential role for a MEK-C/EBP pathway during growth factor-regulated cortical neurogenesis. Neuron. 2002;36:597–610. doi: 10.1016/s0896-6273(02)01026-7. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Sato K. Noggin expression in the adult rat brain. Neuroscience. 2011;184:38–53. doi: 10.1016/j.neuroscience.2011.03.036. [DOI] [PubMed] [Google Scholar]
- Mikawa S, Wang C, Sato K. Bone morphogenetic protein-4 expression in the adult rat brain. J Comp Neurol. 2006;499:613–625. doi: 10.1002/cne.21125. [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Mira H, Andreu Z, Suh H, Lie DC, Jessberger S, Consiglio A, San Emeterio J, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Moon BS, Yoon JY, Kim MY, Lee SH, Choi T, Choi KY. Bone morphogenetic protein 4 stimulates neuronal differentiation of neuronal stem cells through the ERK pathway. Exp Mol Med. 2009;41:116–125. doi: 10.3858/emm.2009.41.2.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, et al. Neural stem cells in the adult mammalian forebrain: A relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS and PNS. Dev Biol. 1998;200:1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay A, McGuire T, Peng CY, Kessler JA. Differential effects of BMP signaling on parvalbumin and somatostatin interneuron differentiation. Development. 2009;136:2633–2642. doi: 10.1242/dev.034439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima K, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Kawabata M, Miyazono K, Taga T. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Takizawa T, Ochiai W, Yanagisawa M, Hisatsune T, Nakafuku M, Miyazono K, et al. BMP2-mediated alteration in the developmental pathway of fetal mouse brain cells from neurogenesis to astrocytogenesis. Proc Natl Acad Sci USA. 2001;98:5868–5873. doi: 10.1073/pnas.101109698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Nishimura R, Kato Y, Chen D, Harris SE, Mundy GR, Yoneda T. Smad5 and DPC4 are key molecules in mediating BMP-2-induced osteoblastic differentiation of the pluripotent mesenchymal precursor cell line C2C12. J Biol Chem. 1998;273:1872–1879. doi: 10.1074/jbc.273.4.1872. [DOI] [PubMed] [Google Scholar]
- Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Effect of the distribution and clustering of the type I A BMP receptor (ALK3) with the type II BMP receptor on the activation of signalling pathways. J Cell Sci. 2003;116:3277–3284. doi: 10.1242/jcs.00519. [DOI] [PubMed] [Google Scholar]
- O'Keeffe GW, Hanke M, Pohl J, Sullivan AM. Expression of growth differentiation factor-5 in the developing and adult rat brain. Brain Res Dev Brain Res. 2004;151:199–202. doi: 10.1016/j.devbrainres.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Oelgeschlager M, Kuroda H, Reversade B, De Robertis EM. Chordin is required for the Spemann organizer transplantation phenomenon in Xenopus embryos. Dev Cell. 2003;4:219–230. doi: 10.1016/s1534-5807(02)00404-5. [DOI] [PubMed] [Google Scholar]
- Onichtchouk D, Chen YG, Dosch R, Gawantka V, Delius H, Massague J, Niehrs C. Silencing of TGF-beta signalling by the pseudoreceptor BAMBI. Nature. 1999;401:480–485. doi: 10.1038/46794. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Takahashi J, Gage FH. The adult rat hippocampus contains primordial neural stem cells. Mol Cell Neurosci. 1997;8:389–404. doi: 10.1006/mcne.1996.0595. [DOI] [PubMed] [Google Scholar]
- Panchision DM, Pickel JM, Studer L, Lee SH, Turner PA, Hazel TG, McKay RD. Sequential actions of BMP receptors control neural precursor cell production and fate. Genes Dev. 2001;15:2094–2110. doi: 10.1101/gad.894701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev. 2003;17:3023–3028. doi: 10.1101/gad.1153603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera EM, Wessely O, Li SY, De Robertis EM. Neural and head induction by insulin-like growth factor signals. Dev Cell. 2001;1:655–665. doi: 10.1016/s1534-5807(01)00069-7. [DOI] [PubMed] [Google Scholar]
- Peretto P, Cummings D, Modena C, Behrens M, Venkatraman G, Fasolo A, Margolis FL. BMP mRNA and protein expression in the developing mouse olfactory system. J Comp Neurol. 2002;451:267–278. doi: 10.1002/cne.10343. [DOI] [PubMed] [Google Scholar]
- Perides G, Safran RM, Rueger DC, Charness ME. Induction of the neural cell adhesion molecule and neuronal aggregation by osteogenic protein 1. Proc Natl Acad Sci USA. 1992;89:10326–10330. doi: 10.1073/pnas.89.21.10326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo S, Sasai Y, Lu B, De Robertis EM. Dorsoventral patterning in Xenopus: Inhibition of ventral signals by direct binding of chordin to BMP-4. Cell. 1996;86:589–598. doi: 10.1016/s0092-8674(00)80132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Brenner-Morton S, Chiang C, Jessell TM. A sonic hedgehog-independent, retinoid-activated pathway of neurogenesis in the ventral spinal cord. Cell. 1999;97:903–915. doi: 10.1016/s0092-8674(00)80802-8. [DOI] [PubMed] [Google Scholar]
- Podkowa M, Zhao X, Chow CW, Coffey ET, Davis RJ, Attisano L. Microtubule stabilization by bone morphogenetic protein receptor-mediated scaffolding of c-Jun N-terminal kinase promotes dendrite formation. Mol Cell Biol. 2010;30:2241–2250. doi: 10.1128/MCB.01166-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Wine-Lee L, Ahn KJ, Crenshaw EB., III Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development. J Neurosci. 2006;26:1896–1905. doi: 10.1523/JNEUROSCI.3202-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P, McKay RD. Multiple routes to astrocytic differentiation in the CNS. J Neurosci. 1998;18:3620–3629. doi: 10.1523/JNEUROSCI.18-10-03620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajan P, Panchision DM, Newell LF, McKay RD. BMPs signal alternately through a SMAD or FRAP-STAT pathway to regulate fate choice in CNS stem cells. J Cell Biol. 2003;161:911–921. doi: 10.1083/jcb.200211021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider CC, Mulloy B. Bone morphogenetic protein and growth differentiation factor cytokine families and their protein antagonists. Biochem J. 2010;429:1–12. doi: 10.1042/BJ20100305. [DOI] [PubMed] [Google Scholar]
- Rios I, Alvarez-Rodriguez R, Marti E, Pons S. Bmp2 antagonizes sonic hedgehog-mediated proliferation of cerebellar granule neurones through Smad5 signalling. Development. 2004;131:3159–3168. doi: 10.1242/dev.01188. [DOI] [PubMed] [Google Scholar]
- Rodriguez JJ, Verkhratsky A. Neurogenesis in Alzheimer's disease. J Anat. 2011;219:78–89. doi: 10.1111/j.1469-7580.2011.01343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen V, Wozney JM, Wang EA, Cordes P, Celeste A, McQuaid D, Kurtzberg L. Purification and molecular cloning of a novel group of BMPs and localization of BMP mRNA in developing bone. Connect Tissue Res. 1989;20:313–319. doi: 10.3109/03008208909023902. [DOI] [PubMed] [Google Scholar]
- Ross SE, Greenberg ME, Stiles CD. Basic helix-loop-helix factors in cortical development. Neuron. 2003;39:13–25. doi: 10.1016/s0896-6273(03)00365-9. [DOI] [PubMed] [Google Scholar]
- Sailer MH, Hazel TG, Panchision DM, Hoeppner DJ, Schwab ME, McKay RD. BMP2 and FGF2 cooperate to induce neural-crest-like fates from fetal and adult CNS stem cells. J Cell Sci. 2005;118:5849–5860. doi: 10.1242/jcs.02708. [DOI] [PubMed] [Google Scholar]
- Saint-Jeannet JP, He X, Varmus HE, Dawid IB. Regulation of dorsal fate in the neuraxis by Wnt-1 and Wnt-3a. Proc Natl Acad Sci USA. 1997;94:13713–13718. doi: 10.1073/pnas.94.25.13713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Burke GM, McGuire T, Pisarek AJ, Mukhopadhyay A, Mishina Y, Kessler JA. BMPR1a signaling determines numbers of oligodendrocytes and calbindin-expressing interneurons in the cortex. J Neurosci. 2007;27:7397–7407. doi: 10.1523/JNEUROSCI.1434-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samanta J, Kessler JA. Interactions between ID and OLIG proteins mediate the inhibitory effects of BMP4 on oligodendroglial differentiation. Development. 2004;131:4131–4142. doi: 10.1242/dev.01273. [DOI] [PubMed] [Google Scholar]
- Sato T, Mikawa S, Sato K. BMP2 expression in the adult rat brain. J Comp Neurol. 2010;518:4513–4530. doi: 10.1002/cne.22469. [DOI] [PubMed] [Google Scholar]
- Sauka-Spengler T, Bronner-Fraser M. Evolution of the neural crest viewed from a gene regulatory perspective. Genesis. 2008;46:673–682. doi: 10.1002/dvg.20436. [DOI] [PubMed] [Google Scholar]
- Savage C, Das P, Finelli AL, Townsend SR, Sun CY, Baird SE, Padgett RW. Caenorhabditis elegans genes sma-2, sma-3, and sma-4 define a conserved family of transforming growth factor beta pathway components. Proc Natl Acad Sci USA. 1996;93:790–794. doi: 10.1073/pnas.93.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, et al. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci USA. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluesener HJ, Meyermann R. Expression of BMP-6, a TGF-beta related morphogenetic cytokine, in rat radial glial cells. Glia. 1994;12:161–164. doi: 10.1002/glia.440120210. [DOI] [PubMed] [Google Scholar]
- Schlunegger MP, Grutter MG. An unusual feature revealed by the crystal structure at 2.2 A resolution of human transforming growth factor-beta 2. Nature. 1992;358:430–434. doi: 10.1038/358430a0. [DOI] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Connors SA, Trout J, Thisse B, Thisse C, Mullins MC. Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development. 2000;127:957–967. doi: 10.1242/dev.127.5.957. [DOI] [PubMed] [Google Scholar]
- Schmidt J, Francois V, Bier E, Kimelman D. Drosophila short gastrulation induces an ectopic axis in Xenopus: Evidence for conserved mechanisms of dorsal-ventral patterning. Development. 1995;121:4319–4328. doi: 10.1242/dev.121.12.4319. [DOI] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Liu JP. Gdf11 facilitates temporal progression of neurogenesis in the developing spinal cord. J Neurosci. 2011;31:883–893. doi: 10.1523/JNEUROSCI.2394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Iwata H, Masuyama N, Gotoh Y, Yamaguchi K, Irie K, Matsumoto K, et al. Role of TAK1 and TAB1 in BMP signaling in early Xenopus development. EMBO J. 1998;17:1019–1028. doi: 10.1093/emboj/17.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya H, Yamaguchi K, Shirakabe K, Tonegawa A, Gotoh Y, Ueno N, Irie K, et al. TAB1: An activator of the TAK1 MAPKKK in TGF-beta signal transduction. Science. 1996;272:1179–1182. doi: 10.1126/science.272.5265.1179. [DOI] [PubMed] [Google Scholar]
- Shirakabe K, Yamaguchi K, Shibuya H, Irie K, Matsuda S, Moriguchi T, Gotoh Y, et al. TAK1 mediates the ceramide signaling to stress-activated protein kinase/c-Jun N-terminal kinase. J Biol Chem. 1997;272:8141–8144. doi: 10.1074/jbc.272.13.8141. [DOI] [PubMed] [Google Scholar]
- Smith WC, Harland RM. Expression cloning of noggin, a new dorsalizing factor localized to the Spemann organizer in Xenopus embryos. Cell. 1992;70:829–840. doi: 10.1016/0092-8674(92)90316-5. [DOI] [PubMed] [Google Scholar]
- Smith WC, Knecht AK, Wu M, Harland RM. Secreted noggin protein mimics the Spemann organizer in dorsalizing Xenopus mesoderm. Nature. 1993;361:547–549. doi: 10.1038/361547a0. [DOI] [PubMed] [Google Scholar]
- Soderstrom S, Ebendal T. Localized expression of BMP and GDF mRNA in the rodent brain. J Neurosci Res. 1999;56:482–492. doi: 10.1002/(SICI)1097-4547(19990601)56:5<482::AID-JNR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Spemann H, Mangold H. Uber induktion von embryonanlagen durch implantation artfremder organisatoren. Arch Mikrosk Anat. 1924;100:599–638. [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, Fan G, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- Takaesu G, Kishida S, Hiyama A, Yamaguchi K, Shibuya H, Irie K, Ninomiya-Tsuji J, et al. TAB2, a novel adaptor protein, mediates activation of TAK1 MAPKKK by linking TAK1 to TRAF6 in the IL-1 signal transduction pathway. Mol Cell. 2000;5:649–658. doi: 10.1016/s1097-2765(00)80244-0. [DOI] [PubMed] [Google Scholar]
- Takao M, Hino J, Takeshita N, Konno Y, Nishizawa T, Matsuo H, Kangawa K. Identification of rat bone morphogenetic protein-3b (BMP-3b), a new member of BMP-3. Biochem Biophys Res Commun. 1996;219:656–662. doi: 10.1006/bbrc.1996.0289. [DOI] [PubMed] [Google Scholar]
- Tang J, Song M, Wang Y, Fan X, Xu H, Bai Y. Noggin and BMP4 co-modulate adult hippocampal neurogenesis in the APP(swe)/PS1(DeltaE9) transgenic mouse model of Alzheimer's disease. Biochem Biophys Res Commun. 2009;385:341–345. doi: 10.1016/j.bbrc.2009.05.067. [DOI] [PubMed] [Google Scholar]
- Tilleman H, Hakim V, Novikov O, Liser K, Nashelsky L, Di Salvio M, Krauthammer M, et al. Bmp5/7 in concert with the mid-hindbrain organizer control development of noradrenergic locus coeruleus neurons. Mol Cell Neurosci. 2010;45:1–11. doi: 10.1016/j.mcn.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix-loop-helix transcription factors. Development. 2002;129:2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2:266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- Vogel-Hopker A, Rohrer H. The specification of noradrenergic locus coeruleus (LC) neurones depends on bone morphogenetic proteins (BMPs) Development. 2002;129:983–991. doi: 10.1242/dev.129.4.983. [DOI] [PubMed] [Google Scholar]
- Walker TL, White A, Black DM, Wallace RH, Sah P, Bartlett PF. Latent stem and progenitor cells in the hippocampus are activated by neural excitation. J Neurosci. 2008;28:5240–5247. doi: 10.1523/JNEUROSCI.0344-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Induction of epidermis and inhibition of neural fate by Bmp-4. Nature. 1995;376:331–333. doi: 10.1038/376331a0. [DOI] [PubMed] [Google Scholar]
- Wilson SI, Graziano E, Harland R, Jessell TM, Edlund T. An early requirement for FGF signalling in the acquisition of neural cell fate in the chick embryo. Curr Biol. 2000;10:421–429. doi: 10.1016/s0960-9822(00)00431-0. [DOI] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB., III Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–5403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- Withers GS, Higgins D, Charette M, Banker G. Bone morphogenetic protein-7 enhances dendritic growth and receptivity to innervation in cultured hippocampal neurons. Eur J Neurosci. 2000;12:106–116. doi: 10.1046/j.1460-9568.2000.00889.x. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, et al. Novel regulators of bone formation: Molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Wrana JL. Regulation of Smad activity. Cell. 2000;100:189–192. doi: 10.1016/s0092-8674(00)81556-1. [DOI] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Carcamo J, Zentella A, Doody J, Laiho M, Wang XF, et al. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992;71:1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]
- Wu HH, Ivkovic S, Murray RC, Jaramillo S, Lyons KM, Johnson JE, Calof AL. Autoregulation of neurogenesis by GDF11. Neuron. 2003;37:197–207. doi: 10.1016/s0896-6273(02)01172-8. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Nagai S, Ninomiya-Tsuji J, Nishita M, Tamai K, Irie K, Ueno N, et al. XIAP, a cellular member of the inhibitor of apoptosis protein family, links the receptors to TAB1-TAK1 in the BMP signaling pathway. EMBO J. 1999;18:179–187. doi: 10.1093/emboj/18.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135:1119–1128. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chon-drogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- Zechner D, Fujita Y, Hulsken J, Muller T, Walther I, Taketo MM, Crenshaw EB, III, et al. Beta-catenin signals regulate cell growth and the balance between progenitor cell expansion and differentiation in the nervous system. Dev Biol. 2003;258:406–418. doi: 10.1016/s0012-1606(03)00123-4. [DOI] [PubMed] [Google Scholar]
- Zhang D, Mehler MF, Song Q, Kessler JA. Development of bone morphogenetic protein receptors in the nervous system and possible roles in regulating trkC expression. J Neurosci. 1998;18:3314–3326. doi: 10.1523/JNEUROSCI.18-09-03314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Kavsak P, Abdollah S, Wrana JL, Thomsen GH. A SMAD ubiquitin ligase targets the BMP pathway and affects embryonic pattern formation. Nature. 1999;400:687–693. doi: 10.1038/23293. [DOI] [PubMed] [Google Scholar]
- Zimmerman LB, De Jesus-Escobar JM, Harland RM. The Spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell. 1996;86:599–606. doi: 10.1016/s0092-8674(00)80133-6. [DOI] [PubMed] [Google Scholar]


