Abstract
Research into treatments for diseases of the CNS has made impressive strides in the past few decades, but therapeutic options are limited for many patients with CNS disorders. Nanotechnology has emerged as an exciting and promising new means of treating neurological disease, with the potential to fundamentally change the way we approach CNS-targeted therapeutics. Molecules can be nanoengineered to cross the blood–brain barrier, target specific cell or signalling systems, respond to endogenous stimuli, or act as vehicles for gene delivery, or as a matrix to promote axon elongation and support cell survival. The wide variety of available nanotechnologies allows the selection of a nanoscale material with the characteristics best suited to the therapeutic challenges posed by an individual CNS disorder. In this Review, we describe recent advances in the development of nanotechnology for the treatment of neurological disorders—in particular, neurodegenerative disease and malignant brain tumours—and for the promotion of neuroregeneration.
Introduction
A number of obstacles present substantial challenges when attempting to treat CNS disorders. For example, systemically delivered products must pass through the blood–brain barrier (BBB), and substances delivered intracranially must withstand the substantial dynamic force of cerebrospinal fluid (CSF) flow in the brain interstitium.1 In addition, the complex cellular organization of the brain and spinal cord complicates the targeted treatment of specific cell populations. Nanotechnology presents a potential solution to these problems. The term ‘nanotechnology’ refers to the engineering of materials on the nanoscale, with functional organization of less than 100 nm in at least one dimension. The scale of nanoengineered materials enables the structures to interact with biological substrates at a molecular level, providing these materials with the potential to effect change in biological systems in unprecedented ways. As such, nanotechnologies can be broadly applied in the diagnosis, imaging and treatment of neurological disorders.
Nanoengineered materials are relevant, and indeed advantageous, for the treatment of CNS disease for a number of reasons. First and foremost, the materials can permeate the BBB—a common obstacle for CNS-targeted therapies. Nanomaterials can also be engineered to interact with defined cellular subsets or molecules, thereby affording specificity of treatment. Furthermore, inclusion of enzyme cleavage sequences in the nanomaterial enables modification of activity in response to biological stimuli, such as pH-sensitive modification or cation-triggered self-assembly. Nanofibres and nanoscaffolds can provide structural as well as trophic support for either endogenous or transplanted cells. Importantly, nanoengineered materials are multifaceted; multiple features can be incorporated into the structures to provide simultaneous targeting, bioactivity, gene delivery and imaging capabilities in a single material.
In this Review, we provide an overview of nanotechnologies that have been investigated in the context of neurological disease, discuss the evidence for efficacy and toxicity of nanomaterials in specific disorders of the CNS, and highlight the potential for translation of nanotechnology to the clinic.
Examples of nanotechnologies
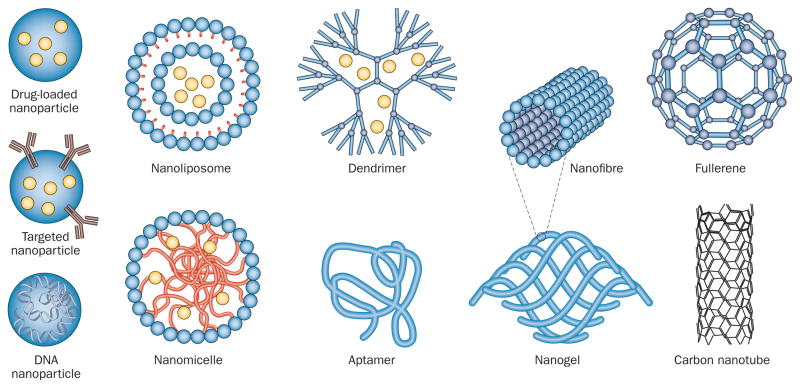
Many different forms of nanotechnology exist (Figure 1), each of which provides unique properties that can be utilized for CNS therapeutics. Nanoparticles are highly stable 3D polymeric encapsulation systems that can be loaded with drugs and functionalized with targeting ligands or antibodies, and can be used as nanocarriers to deliver drugs to the CNS. Nanomicelles and nanoliposomes are also used for CNS-targeted drug delivery; nanomicelles comprise a hydrophilic phospholipid monolayer (or polymer) with a hydrophobic core, whereas nanoliposomes have a lipid bilayer structure similar to that of a vesicle.
Figure 1.
Schematic representation of nanotechnologies that have been applied as therapies for CNS disease.
Dendrimers are highly organized structures with repeatedly branched polymers that arise from a central core that can also be loaded with drugs. Aptamers are single-stranded DNA or RNA molecules that are folded into specific 3D structures that can bind to targets with high affinity. Nanofibres are long, linear arrangements of nanomaterials that can self-assemble and provide structural support and guidance to neighbouring cells. This form of nanomaterial can be organized into nanogels that serve as 3D scaffolds to organize transplanted cells as well as promote cell adhesion and growth. Nanoscale materials can also be engineered from carbon; for example, fullerenes and their derivatives are spherical assemblies of 60 carbon atoms arranged in the same pattern as a soccer ball. A subset of fullerenes includes carbon nanotubes—hexagonal arrays of carbon that are similar in structure to graphite. This wide variety of available technologies enables the selection of a nanoscale material with the characteristics best suited to the therapeutic challenges posed by an individual CNS disorder, and is one of the main advantages provided by nanotechnology.
Neurodegenerative diseases
Neurodegenerative diseases are among the most debilitating of CNS disorders. Over 6 million Americans are affected by the two most common neurodegenerative diseases—Alzheimer disease (AD) and Parkinson disease (PD)—and this number can only rise as the population continues to age.2,3 Although advances have been made with regard to understanding the aetiology of these diseases, currently available treatments for CNS disorders are only able to temporarily alleviate symptoms, and delivery of therapeutics to the brain remains a considerable challenge. As such, novel approaches to the treatment of AD and PD have become a focus of nanotechnology research (Table 1).
Table 1.
Evidence regarding in vivo applications of nanotechnology in neurodegenerative disease
| Approach | Drug | Model | Outcome | Reference |
|---|---|---|---|---|
| Alzheimer disease | ||||
| Fullerenes | Not applicable | Rats with intraventricular injection of amyloid-β | Inhibited amyloid-β fibrillization and improved cognition | Podolski et al. (2007)26 |
| Carbon nanotubes | Acetylcholine | Kainic acid-induced Alzheimer disease mouse model | Restored learning and memory function | Yang et al. (2010)28 |
| PLGA or PBCA nanoparticles | Cholinesterase inhibitor (rivastigmine) | Scopolamine-treated mice | Improved Morris water maze performance | Joshi et al. (2010)32 |
| Parkinson disease | ||||
| PEGylated immunoliposomes (targeting transferrin) | Tyrosine hydroxylase gene delivery | 6-OHDA-lesioned rats | Restored thyrosine hydroxylase enzyme activity and motor function | Zhang et al. (2003)39 |
| DNA nanoparticles | GDNF gene delivery | 6-OHDA-lesioned rats | Enhanced survival of engrafted tyrosine hydroxylase-positive neurons with behavioural improvement | Yurek et al. (2009)40 |
| Fullerenes | Not applicable | Iron-infusion Parkinson disease rat model | Prevented striatal tyrosine hydroxylase-positive neuronal degeneration | Lin et al. (1999)42 |
Abbreviations: 6-OHDA, 6-hydroxydopamine; GDNF, glial cell line-derived neurotrophic factor; PBCA, Poly-n-butylcyanoacrylate; PEG, polyethylene glycol; PLGA, poly(lactic-coglycolic-acid).
Alzheimer disease
The pathological hallmarks of AD—the leading cause of dementia worldwide—include plaques of amyloid-β (Aβ) and neurofibrillary tangles of hyperphosphorylated tau. The peptides and aggregates of Aβ are neurotoxic and have been proposed as the inciting insult in AD,4 although tau aggregates are also neurotoxic and can impair cognition.5 The pathological features of AD are accompanied by increased oxidative stress,6 elevated levels of metal ions,7 and the eventual death of many neuronal subsets such as basal forebrain cholinergic neurons, which are among the earliest neurons affected by AD pathology.8
Nanoscale inhibition of Aβ aggregation
Inhibition of Aβ plaque formation is the most extensively investigated nanotechnology-based approach to AD treatment.9 Conditions that mimic the cell membrane environment are known to promote α-helical conformations of Aβ, which mitigates the capacity of Aβ to aggregate into plaques.10 Accordingly, nanomicelles composed of phospholipids (a major component of the cell membrane) stabilized by the addition of polyethylene glycol (PEG) inhibited β-pleated sheet formation and aggregation of Aβ, and attenuated Aβ-induced neurotoxicity in the SHSY-5Y human neuroblastoma cell line in vitro.11 The phytochemical curcumin was also found to alleviate Aβ oligomerization and cytotoxicity in vitro,12 but the compound exhibited poor bioavailability when injected into mice. Incorporation of curcumin into nanoliposomes improved its bioavailability while maintaining its capacity to potently inhibit Aβ aggregation.13,14
Chelating agents provide another tactic in AD therapeutics, owing to the fact that increased levels of metal ions, such as copper, precipitate the formation of Aβ plaques.15 For example, the copper–zinc chelator clioquinol reversed Aβ plaque formation and improved cognition in AD-like transgenic mice,16 and demonstrated modest efficacy in a pilot phase II clinical trial in patients with AD.17 Although clioquinol is hydrophobic and thus able to cross the BBB, the compound has been associated with adverse neurological effects, prompting investigations into alternative chelators for AD therapy. Microemulsion nanoparticles conjugated to the known copper chelator D-penicillamine were found to dissolve pre-existing Aβ aggregates in vitro.18 The combination of microemulsion nanoparticles with D-penicillamine should allow this intrinsically hydrophilic drug to cross the BBB, so this nanocarrier system could be a viable alternative to traditional chelating agents in the treatment of AD.
In addition to nanoparticles, self-assembling cholesterol-bearing pullulan nanogels have been investigated in the context of AD owing to their function as artificial molecular chaperones for misfolded proteins.19 These nanogels inhibited Aβ aggregation20 and reduced neurotoxicity of primary cortical neuron cultures in vitro.21
Although the various nanotechnology methodologies described above have not been investigated in vivo, the cellular findings that we have outlined suggest that further translational study of nanoscale inhibitors of Aβ aggregation as potential AD therapeutics is warranted.
Fullerenes as neuroprotectants
Oxidative damage is an early outcome of AD pathology,22 and has prompted interest in antioxidants as potential treatments for this disorder. On the nanoscale, derivatives of fullerenes are well-characterized as potent free-radical scavengers.23 These compounds are neuroprotective against the excitotoxicity induced by glutamate receptor agonists, including kainic acid, in mouse cortical neuron cultures.24 The neuroprotective efficacy of fullerene derivatives has been demonstrated in an in vivo proof-of-principle study using a genetic mouse model of amyotrophic lateral sclerosis (ALS).25 Fullerene-mediated neuroprotection against Aβ toxicity in vitro or in genetic mouse models of AD in vivo has not yet been demonstrated. However, the findings that fullerene derivatives inhibit fibrillization of Aβ peptide, and that intraventricular administration of hydrated fullerene in rats prevented Aβ-induced cognitive impairments,26 suggest that fullerenes have multiple synergistic mechanisms that could be utilized for the treatment of AD.
Cholinergic nanocarriers
Given the marked cholinergic deficit observed in patients with AD, current treatments approaches, including those based on nanotechnology, aim to enhance cholinergic activity through the use of cholinesterase inhibitors.27 Acetylcholine has a short half-life and does not readily cross the BBB, so nanocarriers for acetylcholine, such as single-walled carbon nanotubes that can cross the BBB and are taken up by cells, have been explored. When nanotubes were loaded with acetylcholine and administered systemically in a kainic-acid-induced mouse model of AD the acetylcholine-loaded particles restored cognitive function to pre-AD levels, as assessed on behavioural tests of learning and memory, whereas administration of free acetylcholine did not elicit any effect.28 As the biocompatibility of carbon nanotubes has been a concern (as discussed below),29 researchers also sought to investigate the basis of cellular toxicity by the nanomaterial. They identified a cytotoxicity-free dose range that was associated with carbon nanotube delivery specifically to lysosomes (the pharmacological target organelle), and not to mitochondria.28 Although the timeframe of disease in this study did not reflect the chronic course of AD, the promising results suggest that carbon nanotubes are viable nanocarriers that could be used in the treatment of neurodegenerative disorders.
Nanoparticles have also been used to encapsulate cholinesterase inhibitors, such as rivastigmine, which have been used clinically to treat patients with dementia. Poly(n-butylcyanoacrylate), also known as PBCA, adsorbs various apolipoproteins from the blood to enable binding of the particle to the LDL receptor on BBB endothelial cells, which facilitates transcytosis of the nanoparticle across the cell layer, thus delivering the encapsulated drug into the CNS.30 BBB-crossing is further enhanced when the PBCA nanoparticles are coated with polysorbate-80; compared with uncoated particles, polysorbate-80-coated PBCA particles significantly increased delivery of rivastigmine to the brains of rats when injected intravenously.31 Treatment with polysorbate-80-coated PBCA particles also improved spatial learning and memory in scopolamine-lesioned mice.32 These results suggest that nanocarriers are an effective means of enhancing the effects of cholinesterase inhibitors for the treatment of AD.
Parkinson disease
Selective loss of dopaminergic neurons in the substantia nigra pars compacta, and brainstem accumulation of α-synuclein aggregates (Lewy bodies), are the most prominent pathological features of PD—the second most common neurodegenerative disorder worldwide.33 Given the specific depletion of dopaminergic activity in PD, current treatments largely focus on enhancing levels of dopamine in the brain.34,35 Although these treatments have produced marked improvement of symptoms in patients with PD, they do not alter progression of the underlying disease process. Alternative approaches, including the use of nanotechnology, either alone or in combination with therapies such as gene therapy, targeted cell transplantation or deep brain stimulation, are currently being explored.
Nanoscale gene delivery
Gene delivery has been extensively evaluated in the context of PD, with efforts aimed at increasing striatal dopamine, inhibiting the subthalamic nucleus, or promoting survival of nigrostriatal dopaminergic neurons.36 Traditional gene therapy studies used viral vectors for DNA delivery, but concerns regarding the toxicity and immunogenicity of these materials have been raised. Nanotechnology offers attractive alternatives to virus-based systems.37 Complexing of nanogels of PEG and polyethylenimine with antisense oligonucleotides enabled efficient transport of the material across an in vitro BBB model.38 When injected intravenously, the nanogels also delivered the oligonucleotides to the brain, and the efficacy of this process was particularly enhanced when the gels were functionalized with insulin or transferrin molecules. Analogous to the pharmacological inhibition of dopamine degradation that is already in use clinically, this gene delivery system could be combined with antisense oligonucleotides that block monoamine oxidase B function to augment dopaminergic activity in patients with PD.
The capability of transferrin to facilitate BBB crossing via receptor-mediated transcytosis has also been exploited using nanoliposome-based gene therapy. A single intravenous administration of PEGylated liposomes encapsulating tyrosine hydroxylase-encoding plasmids conjugated to a transferrin receptor antibody successfully restored striatal tyrosine hydroxylase enzyme activity and reversed motor impairment in a 6-hydroxydopamine (6-OHDA) model of PD in rats.39
Single molecules of DNA can be compacted into nanoparticles using polycations, such as lysine oligomers, for direct delivery to the brain. Injection of DNA nanoparticles encoding glial cell line-derived neurotrophic factor (GDNF)—an established neurotrophic factor for mesencephalic dopamine neurons—into rat striatum lesioned with 6-OHDA strikingly increased the survival of subsequently grafted embryonic dopaminergic neurons.40 Importantly, pretreatment with GDNF-DNA nanoparticles also enhanced the behavioural improvement observed with engrafted tyrosine hydroxylase-positive neurons alone, suggesting that this method of gene delivery may be particularly effective when combined with therapeutic cell transplantation.
Fullerenes as neuroprotectants
As oxidative damage is also likely to be involved in the pathogenesis of PD,41 the neuroprotective effects of fullerenes have been explored in the context of this disease. Fullerene treatment resulted in potent neuroprotection of mesencephalic dopaminergic neuron cultures that had been treated with either 6-OHDA or 1-methyl-4-phenylpyridinium (MPP+), a metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine.42 Furthermore, in both the 6-OHDA and MPP+ models, fullerene treatment proved more effective than GDNF. Fullerenes have also been studied in an in vivo intranigral iron-infusion model of PD. Concomitant infusion of antioxidant carboxyfullerene prevented the degeneration of tyrosine hydroxylase-positive striatal neurons.43 The above findings suggest that fullerenes may improve the symptoms of PD, consistent with the demonstrated efficacy of this nanotechnology in models of AD and ALS.
Carbon nanotubes
Multiwalled carbon nanotubes loaded with nerve growth factor (NGF) have been shown to promote neurite outgrowth in both dorsal root ganglion neurons and PC12 cells in vitro.44 Another study found that the conductive properties of carbon nanotubes supported electrical activity of neurons.45 The findings from these two studies suggest that carbon nanotubes could enhance integration of cells transplanted into the striatum, particularly if the nanotubes are loaded with GDNF. Electrical stimulation of carbon nanotubes was found to directly stimulate neuronal activity,46 which raises the intriguing possibility of a carbon-nanotube-based deep brain stimulation method for the treatment of PD.
The translation of carbon nanotube technology to the treatment of human neurological disorders is not without obstacles, with biocompatibility of these materials as the primary concern.47 Carbon nanotubes were found to induce both apoptosis and necrosis in a variety of cell lines.48 Notably, single-walled nanotubes demonstrated more in vitro cytotoxicity than their multiwalled counterparts. No cytotoxicity was observed when cells cultured under identical conditions were treated with fullerene, indicating that the nanoarchitecture of the carbon nanotubes is the factor that causes the adverse effects.
Carbon nanotubes also increased oxidative stress in keratinocytes;49 given the importance of antioxidant neuroprotection outlined above, the cytotoxic response to this nanotechnology is particularly disappointing with regard to its potential application in neurodegenerative disease. Recent research that utilized improved purification methods in carbon nanotube synthesis and carbon nanotubes with additional side groups has achieved much lower levels of toxicity,50,51 suggesting that the relative biological incompatibility of carbon nanotubes could be overcome after sufficient optimization.
Neuroregeneration
Regeneration in the CNS constitutes a persistent clinical obstacle. As damaged or transected axons in the mammalian brain or spinal cord are generally unable to repair themselves, permanent neurological deficits commonly occur. Barriers that prevent regeneration of the CNS include the presence of extrinsic inhibitory molecules, intrinsic declines in regenerative capacity with age, the need to repair a heterogeneous population of cells, and the fact that repair requires careful guidance during axon regeneration for the successful rewiring of the intricate neuronal network.52,53 Current research is focused primarily on inhibition of glial scar formation after injury, promoting remyelination of axons that are attempting regeneration, and transplantation of stem cells to foster a permissive environment for CNS repair (Table 2).54 Nanotechnology is particularly well-suited for such multimodal therapy; for example, biodegradable porous poly-L-lactic acid nanoscaffolds can support neurite outgrowth from cultured neural stem cells,55 and when these scaffolds are assembled by electrospinning to create aligned channels they can direct neurite outgrowth parallel to the nanoscaffold longitudinal axis.56 These principles of generic neural tissue engineering have been applied in the areas of optic nerve regeneration and spinal cord injury (SCI).
Table 2.
Evidence regarding in vivo applications of nanotechnology in neuroregeneration
| Approach | Model | Drug | Outcome | Reference |
|---|---|---|---|---|
| Optic nerve regeneration | ||||
| RADA nanofibre scaffold | Hamster optic nerve acute transection model | NA | Promoted axon regeneration and partial visual restoration | Ellis-Behnke et al. (2006)61 |
| RADA nanofibre scaffold | Hamster optic nerve acute transection model | NA | Promoted axon regeneration | Liang et al. (2011)62 |
| PLGA nanospheres | Rat optic nerve crush injury model | EGFR kinase inhibitor (AG1478) | Promoted axon regeneration | Robinson et al. (2011)68 |
| Spinal cord injury | ||||
| PHPMA–RGD hydrogel | Rat transection model | NA | Promoted axon regeneration with partial recovery of hindlimb function | Woerly et al. (2001)76 |
| PLGA–PEG hydrogel | Rat transection model | Neurotrophin-3 | Promoted axon regeneration with functional recovery (BBB score) | Piantino et al. (2006)78 |
| Multilayer PLGA scaffold | Rat hemisection model | Neural stem cells | Enhanced axon regeneration and reduced glial scar with functional recovery (BBB score) | Teng et al. (2002)79 |
| Dextran sulphate-gelatin nanoscaffold | Rat transection model | Embryonic spinal cord cells | Promoted function recovery with evidence of reinnervation | Rochkind et al. (2006)80 |
| IKVAV peptide amphiphile nanofibre | Mouse contusion model | NA | Enhanced axon regeneration and reduced glial scar with functional recovery (BBB score) | Tysseling-Mattiace et al. (2008)88 |
Abbreviations: BBB, blood–brain barrier; EGFR, epidermal growth factor receptor; NA, not applicable; PEG, polyethylene glycol; PHPMA, poly(N-[2-hydroxypropyl]methacrylamide); PLGA, poly(lactic-co-glycolic-acid); RADA, Arg–Ala–Asp–Ala.
Optic nerve regeneration
Restoration of the retinal ganglion cell axons that comprise the optic nerve is relevant not only to the treatment of traumatic injury, but also for the treatment of numerous optic neuropathies such as optic neuritis, mitochondrial disorders (Leber hereditary optic neuropathy) and glaucoma.57 Current approaches to these disorders include immunomodulation via the targeting of resident macrophages, delivery of neurotrophic factors to the injury site, and inhibition of glial scar components. Notably, combined approaches that enable simultaneous targeting of numerous pathways yield the most favourable results.58 The scope for combining therapies is one of the main advantages of nanotechnology.
Nanofibre scaffolds
Self-assembling peptide nanofibre scaffolds have been utilized to enhance optic nerve regeneration. In the presence of physiological salt concentrations, these nanofibre scaffolds form spontaneously from individual peptides and, as they consist solely of L-amino acids, are entirely biocompatible.59 One such nanofibre scaffold, composed of Arg–Ala–Asp–Ala (RADA) peptides, can support neurite outgrowth by PC12 cells as well as synapse formation by hippocampal neurons in vitro.60 Furthermore, in a hamster model of optic tract transection, the RADA-based scaffold was able to support axon regeneration through the lesion site, which was accompanied by partial restoration of visual function.61
Although impressive, these findings are difficult to relate to the clinical setting, as the self-assembling peptide solution was applied at the time of injury, which would not be realistic in human patients. To address this issue, the same group established a model of chronic optic tract injury. The researchers also used Mn2+-enhanced MRI (MEMRI) as a noninvasive means to follow optic tract regeneration in real time. In this system, the nanofibre scaffold was applied 105 days after optic tract transection, with MEMRI and behavioural assessments being performed in the 45 days following treatment. Sparse regeneration of optic tract fibres was visible in scaffold-treated animals on both MEMRI and histological imaging,62 suggesting that the optic nerve maintains regenerative potential long after injury occurs. Notably, no functional recovery was observed following the nanoscaffold treatment. The authors suggest that this outcome might be explained by insufficient numbers of spared ganglion cell axons in the transection model, or by neuronal toxicity of the Mn2+ that was used as a contrast agent. Nevertheless, optimization of this nanofibre technology, perhaps in combination with other techniques that aim to provide a biological signal to regenerating axons, holds great promise for promoting CNS repair after injury.
Nanoscale drug delivery
Nanotechnology has also been used to enhance delivery of trophic factors and glial scar inhibitors to promote optic nerve repair. PEG hydrogels encapsulating ciliary neurotrophic factor (CNTF)—a molecule known to promote survival and axon regeneration of retinal ganglion cells63—provide a method for the controlled release of CNTF over weeks to months, with release kinetics that can be modified by the density of the hydrogel.64 In cultured retinal explants, CNTF-loaded hydrogels promoted neurite outgrowth that was indistinguishable from the outgrowth produced by media supplementation with CNTF alone.64 Although the treatment was not tested in an animal model, it seems likely that the slow, controlled release of CNTF provided by the PEG hydrogel would have a prolonged neuroregenerative effect in vivo.
Epidermal growth factor receptor (EGFR) inhibitors have also been investigated in neuroregeneration, as activation of this receptor following CNS injury promotes the deposition of growth-inhibitory molecules such as chondroitin sulphate proteoglycans.65,66 Indeed, local application of small-molecule EGFR kinase inhibitors promoted in vivo axon regeneration in an optic nerve crush injury model.67 However, the treatment required daily application of the EGFR inhibitor directly to the injured optic nerve, which would not be feasible in a clinical setting. To achieve similar local delivery in a minimally invasive manner, nanospheres composed of poly(lactic-co-glycolic acid)—also known as PLGA—were loaded with the EGFR kinase inhibitor AG1478 and delivered via the eye to rats after an optic nerve crush injury.68 AG1478-containing nanospheres potentiated axon regeneration beyond the site of injury at 4 weeks. Importantly, this result was not achieved when the AG1478 was encapsulated in PLGA microspheres, suggesting that nanoscale encapsulation of the drug directly contributed to its efficacy, perhaps owing to the constant rate of release of the drug from nanospheres, as opposed to an initial burst then decreased release as observed with microspheres.68
Spinal cord injury
Injuries to the spinal cord affect millions of people worldwide, costing the US health-care system alone over 40 billion dollars annually.69,70 Consequently, strategies to promote functional repair of the spinal cord after injury have been a major focus of translational neuroscience research. Techniques that encourage regrowth of severed nerve tracts, inhibit glial scar formation, enhance remyelination and augment plasticity of spared fibres have all shown some efficacy in animal models.71 In recent years, stem cell-based approaches have received much attention.72 Some of these methods have been translated to clinical trials and have shown good safety profiles, but few, if any, have demonstrated efficacy with regard to patient recovery.73 Nanotechnology seems likely to provide tools that are superior to our current inadequate arsenal of therapies for SCI.
Hydrogels
Owing to the porous hydrophilic nature, polymer hydrogels are particularly well suited to neuroregenerative applications. Implantation of hydrogels composed of poly(N-[2-hydroxypropyl] methacrylamide)—abbreviated to PHPMA—into spinal cord transection cavities can bridge the tissue defect and competently support angiogenesis and axonal ingrowth.74 Functionalization of the hydrogel with an Arg–Gly–Asp (RGD) peptide facilitates cell adhesion via fibronectin receptors75 and enables infiltration of the gel into surrounding cells, thus creating a beneficial environment for spinal cord repair. Indeed, in a rat model of SCI, implantation of PHPMA–RGD hydrogels led to axon regeneration through the lesion site (as observed using anterograde axonal labelling) and recovery of some hindlimb motor function.76 Although these findings are encouraging, the implantation of a preassembled hydrogel scaffold to fill the defect requires invasive surgery. To minimize this requirement, hydrogels that assemble in situ when exposed to light have been developed. Degradable poly(lactic acid)–PEG (PLA–PEG) hydrogels loaded with neurotrophin-3 (NT-3)—a molecule that has been extensively studied in the context of spinal cord repair77—have demonstrated efficacy in in vivo transection models of SCI. Treatment with NT3-loaded PLA–PEG hydrogels led to controlled neurotrophin release at the site of injury that resulted in reinnervation of both the corticospinal tract and the raphe spinal tract, as well as functional recovery.78 Nanotechnology provides a method to combine porous scaffolds and growth-promoting factors and so holds great potential to aid recovery in patients with SCI.
Nanoscaffolds for stem cell delivery
Stem cells have been subjected to intensive investigation as potential therapeutics for SCI. To augment the potential efficacy of such treatments, nanoscale scaffolds aimed at nurturing transplanted stem cells have been explored. Multilayer PLGA scaffolds designed with a porous inner layer for neural stem cell (NSC) seeding and an outer layer of aligned channels to facilitate axon guidance and regrowth have yielded promising results in SCI.79 Implantation of NSC-seeded PLGA scaffolds led to durable functional recovery in animals with a spinal cord transection, including substantial improvement in locomotion, as measured on the Basso, Beattie and Bresnahan scale, that was maintained at 1 year following injury. These improvements seemed to be mediated by enhanced axon regeneration as well as reduction of the glial scar.79
Nanoscaffolds with longitudinal organization to assist axon regeneration have been examined. A nanofibre scaffold of cross-linked dextran sulphate and gelatin seeded with human embryonic spinal cord cells promoted functional recovery in a rat transection model of SCI, with visible evidence of reinnervation in the scaffold.80 Self-assembling nanofibre scaffolds have also been investigated in the context of SCI. The same RGD-containing nanofibre scaffold described for optic nerve regeneration were seeded with neural cells and implanted into transected spinal cords.81 NSC-seeded scaffolds displayed increased axonal innervation compared with non-seeded scaffolds, although animals transplanted with Schwann cell-seeded scaffolds showed even greater innervation than animals given NSC-seeded scaffolds. The behavioural outcome of scaffold-induced neuronal regeneration was not explored. Notably, as preassembled scaffolds were used in this study, the utility of the self-assembling nature of this nanomaterial was not investigated.
Self-assembling bioactive peptide amphiphiles
Peptide amphiphile molecules consist of a hydrophobic aliphatic tail region, a linker region of amino acids that form β-pleated sheets, and a hydrophilic head group that can be functionalized with a bioactive peptide sequence of choice.82 In the presence of physiological cation concentrations, peptide amphiphile molecules undergo hydrophobic collapse and self-assemble into nanofibres. This material can, therefore, be injected into neural tissues as a liquid and will then self-assemble into nanofibres in the extracellular spaces. These nanofibres display the peptide head group on the surface at an extremely high density and, when in an aqueous solution, further organize into a 3D gel that is capable of acting as a surrogate extracellular matrix.83,84
A peptide amphiphile displaying the laminin-derived epitope IKVAV85,86 was found to potently promote the differentiation of NSCs into neurons while inhibiting their differentiation into astrocytes in vitro.87 In the context of SCI, injection of the IKVAV-containing peptide amphiphiles around the site of injury led to a dramatic recovery of motor function.88 Histologically, mice treated with the amphiphiles had few glial scars and showed evidence of regeneration of both ascending and descending nerve fibres. In addition, the remaining serotonergic fibres in mice that received the amphiphile injection showed enhanced plasticity compared with the fibres in sham-injected mice.89 The positive effects of this amphiphile treatment are entirely dependent on the bioactivity of the IKVAV epitope; studies that used non-bioactive peptide amphiphiles did not recapitulate the findings. Importantly, the above studies were performed in a contusion model of SCI that is much more akin to human injuries than the traditional transection model, implying that peptide amphiphiles could readily translate to clinical studies.
Brain tumours
Primary brain and CNS tumours constitute a substantial and formidable clinical entity, with over 22,000 new cases and 13,000 brain tumour-associated deaths estimated in the USA in 2011 alone.90 Patients diagnosed with the most common and aggressive form of these tumours, termed glioblastoma multiforme (GBM), have a median survival of less than 2 years.91 This bleak prognosis has remained relatively constant for decades, despite continued advances in surgical practices, the advent of new radiotherapy techniques,92 and the undertaking of clinical trials to optimize chemotherapy protocols.91,93,94 Consequently, treatment modalities for GBM remain palliative at best. The need for systemically delivered drugs to pass across the BBB has restricted chemotherapeutic options. In addition, attempts at GBM-tailored therapy using specific molecular candidates have yielded disappointing results in clinical trials.95 Thus, there is considerable room for improvement with respect to the ease of drug administration and molecular targeting to minimize side effects and optimize efficacy of therapies for brain tumours.
Drug delivery
Generic nanocarriers
As access to the brain is a considerable impediment to the application of standard chemotherapeutics for CNS neoplasms, nanocarriers that usher systemic drugs across the BBB have been extensively investigated. Examples of such nanocarriers include PBCA nanoparticles, which have been used to optimize the delivery of methotrexate96 and temozolomide97 to the brain, resulting in significantly increased intracerebral drug concentrations compared with treatment with the drugs alone.
Solid lipid nanoparticles (SLNs) have also been applied for delivery of chemotherapeutic agents, such as temozolomide,98 etoposide99 and paclitaxel,100 to the brain. The SLN formulation of etoposide inhibited proliferation of glioma cell lines more effectively than etoposide alone, with a concomitant decrease in cytotoxicity in normal astrocytes,99 thereby yielding a wider therapeutic window. Similarly, treatment with SLN-entrapped paclitaxel inhibited glioma cell growth in vitro by several orders of magnitude more than did the non-entrapped therapy.100 This finding translated in vivo to a significant reduction of tumour mass when animals were given the PCBA-mediated delivery of paclitaxel compared with standard drug administration. Interestingly, in the paclitaxel SLN study, inhibition of multidrug resistance pumps was observed in the cancerous cells following treatment with the solid lipid nanoparticle formulation. This finding has important implications for the retention of chemotherapeutics in specialized glioma cells that become drug-resistant owing to the development of drug efflux capabilities.101
Dendrimers have also been used to deliver antineoplastic treatments to the brain. Conjugation to polyether–copolyester (PEPE) dendrimers enhanced passage of D-glucosamine across an in vitro BBB model via glutamine transporter type 1, and increased the cytotoxicity of methotrexate against U87 and U343 cancer cell lines in culture.102 Notably, dendrimer encapsulation of methotrexate was able to overcome acquired resistance to the drug, suggesting that this mode of nanoscale delivery may be able to combat treatment resistance in gliomas. PEGylated polyamidoamine dendrimers loaded with doxorubicin also demonstrated an expanded therapeutic window by inhibiting C6 glioma spheroid proliferation while exhibiting little cytotoxicity against brain microvascular endothelial cells in vitro.103 In summary, generic nanocarriers show great promise for enhanced delivery of chemotherapeutics across the BBB, with curtailed effects on non-neoplastic cells.
Targeted nanocarriers
Nanocarriers can also be functionalized with molecules to enable more-precise targeting to the BBB and efficient drug transport into the brain (Table 3). PEG–PLGA nanoparticles incorporating a DNA aptamer to target nucleolin, a molecule that is highly expressed in the plasma membrane of both cancer cells and tumour endothelium,104,105 enhanced the antiproliferative effects of paclitaxel against C6 glioma cells in vitro.106 These aptamer nanoparticles strikingly reduced C6 glioma xenograft volumes in nude mice, and prolonged survival of animals with C6 intracranial gliomas compared with treatment with either paclitaxel alone or paclitaxel loaded into undecorated nanoparticles. Importantly, the aptamer-decorated nanoparticles showed greater efficacy than undecorated particles,106 highlighting the value of targeted delivery.
Table 3.
Evidence regarding in vivo applications of nanotechnology in malignant brain tumours
| Approach | Model | Drug | Outcome | Reference |
|---|---|---|---|---|
| PEG–PLGA nanoparticles with DNA aptamer targeting nucleolin | Intracranial C6 xenograft in nude mice | Paclitaxel | Reduced glioma volumes and prolonged survival | Guo et al. (2011)106 |
| PEG–PCL nanoparticles with LRP ligand | Intracranial U87 xenografts in nude mice | Paclitaxel | Increased drug accumulation in glioma cells | Xin et al. (2011)108 |
| Immunoliposomes targeting EGFR | U87 & U87–EGFRvIII xenografts in nude mice | Doxorubicin, epirubicin, vinorelbine | Enhanced cytotoxic effects against glioma cells | Mamot et al. (2005)110 |
| Iron oxide nanoparticles with EGFR antibody | U87–EGFRvIII xenograft mice | Not applicable | Prolonged survival and enhanced contrast on T2-weighted MRI | Hadjipanayis et al. (2010)111 |
| Cationic albumin-conjugated PEGylated nanoparticles | C6 intracranial xenografts in nude mice | TRAIL gene delivery | Enhanced glioma cell apoptosis and prolonged survival | Lu et al. (2006)112 |
| Immunoliposomes targeting insulin receptor and transferrin | Intracranial U87 xenograft mice | EGFR antisense RNA | Prolonged survival | Zhang et al. (2002)114 |
| PMLA nanoparticles | Intracranial U87 xenograft in nude mice | Laminin-411 antisense RNA (pH-dependent release) | Prolonged survival and inhibited glioma vascularization | Ding et al. (2010)115 |
| Iron oxide ‘nanoworms’ with CGKRK vascular-targeting peptide | Lentiviral mouse model of glioma | D-[KLAKLAK]2 proapoptotic peptide | Substantially reduced tumorigenicity | Agemy et al. (2011)116 |
Abbreviations: EGFR, epidermal growth factor receptor; LRP, LDL receptor-related protein; PCL, polycaprolactone; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic-acid); PMLA, poly(β-L-malic acid); TRAIL, tumour necrosis factor-related apoptosis-inducing ligand.
A similar dual-targeting approach has taken advantage of LDL receptor-related protein (LRP), a molecule that is expressed by the BBB as well as glioma cells. Compared with a non-targeted nanoparticle approach, conjugation of PEG-co-poly(ɛ-caprolactone) nanoparticles to angiopep—a ligand of LRP that mediates transcytosis across the BBB107—enhanced the inhibitory effects of paclitaxel against glioma cells in vitro.108 These angiopep-conjugated nanoparticles enabled increased accumulation of paclitaxel in intracranial U87 xenografts, but no in vivo efficacy data were presented.
Many GBM tumours exhibit amplification of the EGFR gene, and nanocarriers engineered to exploit this fact have been explored in the context of brain tumours. Immunoliposomes that targeted EGFR (via conjugation of a fragment of cetuximab to the liposome) significantly enhanced the cytotoxic effects of multiple different chemotherapeutics both in vitro109 and in mice with U87 xenografts, as well as in xenografts of U87 cells containing a constitutively active deletion mutant form of EGFR known as EGFRvIII.110 The multimodal capacity of nanotechnology has also been harnessed using EGFRvIII-targeted iron oxide nanoparticles (IONPs). Conjugation of IONPs to an EGFRvIII antibody provided a therapy that augmented apoptosis when administered to cultures of either U87 glioma cells (either with or without expression of EGFRvIII) or glioma stem cell neurospheres.111 For mice with xenografts of EGFRvIII-expressing U87 cells, convection-enhanced delivery of the EGFRvIII-targeted IONPs prolonged survival while simultaneously enhancing the visibility of tumours on T2-weighted MRI.
Targeted therapy
The studies described above focused on optimizing the packaging and delivery of drugs for the treatment of CNS neoplasms. However, nanotechnology has also been used to develop more-tailored therapies (Table 3). For instance, cationic albumin-conjugated PEGylated (CBSA) nanoparticles can serve as a nonviral vector for gene delivery of tumour necrosis factor–related apoptosis-inducing ligand (TRAIL) plasmid to glioma cells.112 Administration of CBSA–TRAIL nanoparticles enhanced apoptosis of intracranial C6 xenografts, and extended survival of the animals compared with treatment using non-CBSA nanoparticles. PEG immunoliposomes can also function as nonviral gene delivery systems.
Immunoliposomes loaded with antibodies against the human insulin receptor and antisense mRNA that targeted EGFR transcripts demonstrated cytotoxicity against U87 glioma cells in vitro.113 When coupled with a transferrin receptor antibody to enable endothelial transcytosis of the particle across the BBB, these immunoliposomes substantially improved survival of mice bearing intracranial U87 xenografts.114 Laminin-411, an extracellular matrix protein that is overexpressed by glioma cells and surrounding associated vasculature, has also been used as a target for nanoscale delivery of antisense mRNA. A poly(β-l-malic acid) nanoconjugate designed to release laminin-411 antisense mRNAs via a pH-dependent endosomal escape mechanism induced glioma cell apoptosis in vitro and suppressed xenograft growth and vascularization in vivo.115
The multimodal aptitude of nanotechnologies has been emphasized in an exciting new study of targeted therapy in a lentivirus-mediated genetic mouse model of GBM. Treatment with a nanosystem that combined tumour vasculature targeting (via addition of a CGKRK peptide), proapoptotic activity using a D-(KLAKLAK)2 peptide, and IONP ‘nanoworms’ to enhance tumour detection on MRI, caused a substantial reduction in tumorigenicity.116 90% of mice treated with the nanosystem survived for almost 1 year, whereas untreated animals or mice treated with a nanosystem that lacked the CGKRK targeting peptide all succumbed to disease within 3 months. The striking antineoplastic effect of this nanotreatment holds great promise for the development of novel GBM therapeutics that can improve patient survival.
Obstacles to clinical translation
Despite the abundance of data that suggests an unparalleled potential of nanomaterials to aid the treatment of neurological disease, this potential has not yet been realized. To date, only one phase I clinical trial involving nanotechnology in CNS therapeutics has been initiated; this trial, to investigate the use of nanoliposomal irinotecan for the treatment of recurrent glioma, is still recruiting patients.117 Notably, the trial began 5 years after publication of persuasive evidence on the in vivo efficacy of this nanomaterial,118 highlighting one of the main reasons why nanotechnology has failed to move into the clinical arena: insufficient evidence from physiological in vivo studies. Many promising in vitro results are not followed by in vivo correlations. Whether this lack of correlation is a consequence of unfamiliarity regarding the in vivo milieu on the part of many materials scientists (thus necessitating collaborations with biologists), or whether it is due to the inability of many researchers to replicate in vitro studies in the in vivo setting, is unclear.
Lack of standardization of nanomaterials presents another barrier to the undertaking of nanotechnology-based clinical trials. Many different physical and chemical properties of nanomaterials, such as size, charge and surface modifications, affect both their efficacy and toxicity.119 For instance, hydrophobic nanomaterials are quickly cleared by the reticuloendothelial system and so primarily accumulate in the liver and spleen before being eliminated. Surface modifications often render nanoparticles more hydrophilic, thereby allowing the particles to remain in the circulation for longer periods of time.120 In addition, nanomaterial size potently affects clearance: particles of 10–20 nm are efficiently excreted by the kidneys, but renal clearance is not possible for larger nanomaterials.121 These myriad properties present a challenge both to scientists when attempting to fully characterize nanoengineered materials and render them reproducible, and to regulatory agencies that must continually adapt current drug guidelines to facilitate the use of nanotechnology-based therapeutics.122
Toxicity of nanomaterials is of paramount concern, as underscored by the discussion of carbon nanotubes above. However, most studies of nanoscale therapeutics have failed to address the potential associated toxicities. The few dedicated studies of nanotoxicity highlight oxidative stress induction, genotoxicity, and immune modulation as three key areas of potential injury by nanoengineered therapies.123 Internalized nanoparticles cause mitochondrial damage, which leads to generation of reactive oxygen species, activation of proinflammatory signalling pathways and, ultimately, cell death.124 Oxidative stress is also implicated in the DNA damage associated with nanomaterials, raising concerns of tumorigenicity and infertility.125 Finally, nanoscale therapeutics interact with the immune system on multiple levels. The particles are ingested by macrophages and can induce complement activation and inflammatory reactions.126 This outcome is particularly important with regard to the use of nanoparticles in the treatment of malignant brain tumours, as the majority of in vivo preclinical studies in this field have utilized immunocompromised mice, which do not allow investigation of potential immunogenic properties of the nanotechnology.
Conclusions and future directions
The treatment of CNS disorders is stymied by a number of factors, such as the BBB, the complexity of the cellular environment, and the involvement of multiple signalling modalities in any given disease process. Nanotechnology is uniquely poised to address these issues. In models of neurodegenerative disorders, nanoscale approaches have been used to inhibit Aβ oligomerization, reduce reactive oxygen species, and enhance functional neuronal networks through use of carbon nanotubes. With regard to neuroregeneration, nanoscale scaffolds have successfully guided the appropriate rewiring of the optic nerve and spinal cord, with associated functional recovery in animal models. Nanocarriers have enabled targeted delivery of chemotherapeutics as well as antisense gene therapy, resulting in impressive inhibition of disease progression in both in vitro and in vivo models of malignant brain tumours.
Unfortunately, few—if any—of these promising preclinical studies have been successfully translated to the clinic to influence patient care. Barriers to this successful translation include insufficient studies of nanomaterial toxicity, immunological compatibility, and a relative paucity of in vivo studies. Moving forward, nanomaterials could be used as a surrogate niche for transplanted stem cells, providing not only structural support but also sustained release of signalling factors. In addition, the nanomaterials themselves can be functionalized to interact with stem cells, both by incorporating bioactive signals and by modifying the mechanical properties of the nanomaterial. Interdisciplinary collaboration will be critical to the successful clinical translation of nanotechnology.
Although nanotechnology has tremendous potential to affect treatment options in clinical neuroscience, it is unlikely that use of nanotechnology alone will accomplish the complicated task of repairing the CNS. The most efficacious applications of nanomaterials in the treatment of CNS disease have combined the power of nanoscale interventions with growth factors or cells that enhance the overall effect of the nanoscale treatment, highlighting the importance of a combined approach to nanotherapeutics. Furthermore, the utility of nanotechnology applications in CNS disease can only be augmented by advances in our biological understanding of the processes involved in these disorders. The synergy between new understanding of the molecular basis of neurological diseases and the multifunctional capabilities of nanotechnology is the factor that stands to fundamentally change the practice of neurology.
Key points.
To be effective, therapies for CNS disorders must overcome hurdles including the blood–brain barrier, the complex cellular architecture of the CNS, and the multifactorial nature of CNS disease
Nanotechnology—engineering of materials that measure less than 100 nm in at least one dimension—can combat these challenges, and enable multimodal therapeutic targeting at the molecular level
The efficacy of nanoscale treatments has been demonstrated in models of neurodegenerative disease, neuroregeneration and brain tumours, but few of these treatments have been successfully translated to the clinic
The future of nanotechnology in clinical neuroscience will rely on our ability to interface this technology with our burgeoning understanding of the molecular underpinnings of CNS disease
Review criteria.
We searched PubMed for articles published up to 4th March 2012, including electronic early release publications, using terms including “nanotechnology”, “nanoparticles”, “neuroscience”, “central nervous system”, and “neurology”. Searches for “neurodegenerative diseases”, “optic nerve or spinal cord regeneration”, “brain tumours”, and “toxicity” were also performed. Full-text versions of review and primary research articles were obtained, and references were checked for additional materials when appropriate. The ClinicalTrials.gov database was searched for registered trials of nanotechnology as therapeutics for neurological disorders, using the terms “nanotechnology”, “nanoparticles”, and “nanoliposomes”. Trial descriptions were read to determine whether patients with CNS disorders were included.
Footnotes
Competing interests The authors declare no competing interests.
Author contributions M. Srikanth researched data for the article, contributed to discussion of the content, wrote the article, and contributed to the review and editing of the manuscript before submission. J. A. Kessler provided substantial contribution to discussion of the content and to the review and editing of the manuscript before submission.
References
- 1.Abbott NJ. Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int. 2004;45:545–552. doi: 10.1016/j.neuint.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 2.Nutt JG, Wooten GF. Clinical practice. Diagnosis and initial management of Parkinson's disease. N Engl J Med. 2005;353:1021–1027. doi: 10.1056/NEJMcp043908. [DOI] [PubMed] [Google Scholar]
- 3.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 4.Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Santacruz K, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith MA, et al. Oxidative damage in Alzheimer's. Nature. 1996;382:120–121. doi: 10.1038/382120b0. [DOI] [PubMed] [Google Scholar]
- 7.Ehmann WD, Markesbery WR, Alauddin M, Hossain TI, Brubaker EH. Brain trace elements in Alzheimer's disease. Neurotoxicology. 1986;7:195–206. [PubMed] [Google Scholar]
- 8.Mesulam M, Shaw P, Mash D, Weintraub S. Cholinergic nucleus basalis tauopathy emerges early in the aging–MCI–AD continuum. Ann Neurol. 2004;55:815–828. doi: 10.1002/ana.20100. [DOI] [PubMed] [Google Scholar]
- 9.Brambilla D, et al. Nanotechnologies for Alzheimer's disease: diagnosis, therapy, and safety issues. Nanomedicine. 2011;7:521–540. doi: 10.1016/j.nano.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Kohno T, Kobayashi K, Maeda T, Sato K, Takashima A. Three-dimensional structures of the amyloid β peptide (25–35) in membrane-mimicking environment. Biochemistry. 1996;35:16094–16104. doi: 10.1021/bi961598j. [DOI] [PubMed] [Google Scholar]
- 11.Pai AS, Rubinstein I, Onyuksel H. PEGylated phospholipid nanomicelles interact with β-amyloid(1–42) and mitigate its β-sheet formation, aggregation and neurotoxicity in vitro. Peptides. 2006;27:2858–2866. doi: 10.1016/j.peptides.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 12.Yang F, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892–5901. doi: 10.1074/jbc.M404751200. [DOI] [PubMed] [Google Scholar]
- 13.Taylor M, et al. Effect of curcumin-associated and lipid ligand-functionalized nanoliposomes on aggregation of the Alzheimer's Aβ peptide. Nanomedicine. 2011;7:541–550. doi: 10.1016/j.nano.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Mourtas S, et al. Curcumin-decorated nanoliposomes with very high affinity for amyloid-β1-42 peptide. Biomaterials. 2011;32:1635–1645. doi: 10.1016/j.biomaterials.2010.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Lovell MA, Robertson JD, Teesdale WJ, Campbell JL, Markesbery WR. Copper, iron and zinc in Alzheimer's disease senile plaques. J Neurol Sci. 1998;158:47–52. doi: 10.1016/s0022-510x(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 16.Cherny RA, et al. Treatment with a copper–zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer's disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 17.Ritchie CW, et al. Metal-protein attenuation with iodochlorhydroxyquin (clioquinol) targeting Aβ amyloid deposition and toxicity in Alzheimer disease: a pilot phase 2 clinical trial. Arch Neurol. 2003;60:1685–1691. doi: 10.1001/archneur.60.12.1685. [DOI] [PubMed] [Google Scholar]
- 18.Cui Z, et al. Novel D-penicillamine carrying nanoparticles for metal chelation therapy in Alzheimer's and other CNS diseases. Eur J Pharm Biopharm. 2005;59:263–272. doi: 10.1016/j.ejpb.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Nomura Y, Ikeda M, Yamaguchi N, Aoyama Y, Akiyoshi K. Protein refolding assisted by self-assembled nanogels as novel artificial molecular chaperone. FEBS Lett. 2003;553:271–276. doi: 10.1016/s0014-5793(03)01028-7. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda K, Okada T, Sawada S, Akiyoshi K, Matsuzaki K. Inhibition of the formation of amyloid β-protein fibrils using biocompatible nanogels as artificial chaperones. FEBS Lett. 2006;580:6587–6595. doi: 10.1016/j.febslet.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 21.Boridy S, Takahashi H, Akiyoshi K, Maysinger D. The binding of pullulan modified cholesteryl nanogels to Aβ oligomers and their suppression of cytotoxicity. Biomaterials. 2009;30:5583–5591. doi: 10.1016/j.biomaterials.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Nunomura A, et al. Oxidative damage is the earliest event in Alzheimer disease. J Neuropathol Exp Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- 23.Krusic PJ, Wasserman E, Keizer PN, Morton JR, Preston KF. Radical reactions of c60. Science. 1991;254:1183–1185. doi: 10.1126/science.254.5035.1183. [DOI] [PubMed] [Google Scholar]
- 24.Dugan LL, Gabrielsen JK, Yu SP, Lin TS, Choi DW. Buckminsterfullerenol free radical scavengers reduce excitotoxic and apoptotic death of cultured cortical neurons. Neurobiol Dis. 1996;3:129–135. doi: 10.1006/nbdi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 25.Dugan LL, et al. Carboxyfullerenes as neuroprotective agents. Proc Natl Acad Sci USA. 1997;94:9434–9439. doi: 10.1073/pnas.94.17.9434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podolski IY, et al. Effects of hydrated forms of C60 fullerene on amyloid 1-peptide fibrillization in vitro and performance of the cognitive task. J Nanosci Nanotechnol. 2007;7:1479–1485. doi: 10.1166/jnn.2007.330. [DOI] [PubMed] [Google Scholar]
- 27.Trinh NH, Hoblyn J, Mohanty S, Yaffe K. Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA. 2003;289:210–216. doi: 10.1001/jama.289.2.210. [DOI] [PubMed] [Google Scholar]
- 28.Yang Z, et al. Pharmacological and toxicological target organelles and safe use of single-walled carbon nanotubes as drug carriers in treating Alzheimer disease. Nanomedicine. 2010;6:427–441. doi: 10.1016/j.nano.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 29.Smart SK, Cassady AI, Lu GQ, Martin DJ. The biocompatibility of carbon nanotubes. Carbon. 2006;44:1034–1047. [Google Scholar]
- 30.Kim HR, et al. Translocation of poly(ethylene glycol-co-hexadecyl)cyanoacrylate nanoparticles into rat brain endothelial cells: role of apolipoproteins in receptor-mediated endocytosis. Biomacromolecules. 2007;8:793–799. doi: 10.1021/bm060711a. [DOI] [PubMed] [Google Scholar]
- 31.Wilson B, et al. Poly(n-butylcyanoacrylate) nanoparticles coated with polysorbate 80 for the targeted delivery of rivastigmine into the brain to treat Alzheimer's disease. Brain Res. 2008;1200:159–168. doi: 10.1016/j.brainres.2008.01.039. [DOI] [PubMed] [Google Scholar]
- 32.Joshi SA, Chavhan SS, Sawant KK. Rivastigmine-loaded PLGA and PBCA nanoparticles: preparation, optimization, characterization, in vitro and pharmacodynamic studies. Eur J Pharm Biopharm. 2010;76:189–199. doi: 10.1016/j.ejpb.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 33.Lees AJ, Hardy J, Revesz T. Parkinson's disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 34.Fahn S, et al. Levodopa and the progression of Parkinson's disease. N Engl J Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- 35.Group PS. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol. 2002;59:1937–1943. doi: 10.1001/archneur.59.12.1937. [DOI] [PubMed] [Google Scholar]
- 36.Witt J, Marks WJ., Jr An update on gene therapy in Parkinson's disease. Curr Neurol Neurosci Rep. 2011;11:362–370. doi: 10.1007/s11910-011-0197-8. [DOI] [PubMed] [Google Scholar]
- 37.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 38.Vinogradov SV, Batrakova EV, Kabanov AV. Nanogels for oligonucleotide delivery to the brain. Bioconjug Chem. 2004;15:50–60. doi: 10.1021/bc034164r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y, Calon F, Zhu C, Boado RJ, Pardridge WM. Intravenous nonviral gene therapy causes normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism. Hum Gene Ther. 2003;14:1–12. doi: 10.1089/10430340360464660. [DOI] [PubMed] [Google Scholar]
- 40.Yurek DM, Flectcher AM, Kowalczyk TH, Padegimas L, Cooper MJ. Compacted DNA nanoparticle gene transfer of GDNF to the rat striatum enhances the survival of grafted fetal dopamine neurons. Cell Transplant. 2009;18:1183–1196. doi: 10.3727/096368909X12483162196881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jenner P. Oxidative mechanisms in nigral cell death in Parkinson's disease. Mov Disord. 1998;13(Suppl 1):24–34. [PubMed] [Google Scholar]
- 42.Lotharius J, Dugan LL, O'Malley KL. Distinct mechanisms underlie neurotoxin-mediated cell death in cultured dopaminergic neurons. J Neurosci. 1999;19:1284–1293. doi: 10.1523/JNEUROSCI.19-04-01284.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin AM, et al. Carboxyfullerene prevents iron-induced oxidative stress in rat brain. J Neurochem. 1999;72:1634–1640. doi: 10.1046/j.1471-4159.1999.721634.x. [DOI] [PubMed] [Google Scholar]
- 44.Matsumoto K, Sato C, Naka Y, Whitby R, Shimizu N. Stimulation of neuronal neurite outgrowth using functionalized carbon nanotubes. Nanotechnology. 2010;21:115101. doi: 10.1088/0957-4484/21/11/115101. [DOI] [PubMed] [Google Scholar]
- 45.Cellot G, et al. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat Nanotechnol. 2009;4:126–133. doi: 10.1038/nnano.2008.374. [DOI] [PubMed] [Google Scholar]
- 46.Mazzatenta A, et al. Interfacing neurons with carbon nanotubes: electrical signal transfer and synaptic stimulation in cultured brain circuits. J Neurosci. 2007;27:6931–6936. doi: 10.1523/JNEUROSCI.1051-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Foldvari M, Bagonluri M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomedicine. 2008;4:183–200. doi: 10.1016/j.nano.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 48.Jia G, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 49.Manna SK, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-κB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yehia HN, et al. Single-walled carbon nanotube interactions with HeLa cells. J Nanobiotechnology. 2007;5:8. doi: 10.1186/1477-3155-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davoren M, et al. In vitro toxicity evaluation of single walled carbon nanotubes on human A549 lung cells. Toxicol In Vitro. 2007;21:438–448. doi: 10.1016/j.tiv.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 52.Liu K, Tedeschi A, Park KK, He Z. Neuronal intrinsic mechanisms of axon regeneration. Ann Rev Neurosci. 2011;34:131–152. doi: 10.1146/annurev-neuro-061010-113723. [DOI] [PubMed] [Google Scholar]
- 53.Nash M, Pribiag H, Fournier AE, Jacobson C. Central nervous system regeneration inhibitors and their intracellular substrates. Mol Neurobiol. 2009;40:224–235. doi: 10.1007/s12035-009-8083-y. [DOI] [PubMed] [Google Scholar]
- 54.Hannila SS, Siddiq MM, Filbin MT. Therapeutic approaches to promoting axonal regeneration in the adult mammalian spinal cord. Int Rev Neurobiol. 2007;77:57–105. doi: 10.1016/S0074-7742(06)77003-9. [DOI] [PubMed] [Google Scholar]
- 55.Yang F, et al. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials. 2004;25:1891–1900. doi: 10.1016/j.biomaterials.2003.08.062. [DOI] [PubMed] [Google Scholar]
- 56.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26:2603–2610. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 57.Moore DL, Goldberg JL. Four steps to optic nerve regeneration. J Neuroophthalmol. 2010;30:347–360. doi: 10.1097/WNO.0b013e3181e755af. [DOI] [PubMed] [Google Scholar]
- 58.Benowitz LI, Yin Y. Optic nerve regeneration. Arch Ophthalmol. 2011;128:1059–1064. doi: 10.1001/archophthalmol.2010.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993;90:3334–3338. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holmes TC, et al. Extensive neurite outgrowth and active synapse formation on self-assembling peptide scaffolds. Proc Natl Acad Sci USA. 2000;97:6728–6733. doi: 10.1073/pnas.97.12.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ellis-Behnke RG, et al. Nano neuro knitting: peptide nanofiber scaffold for brain repair and axon regeneration with functional return of vision. Proc Natl Acad Sci USA. 2006;103:5054–5059. doi: 10.1073/pnas.0600559103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang YX, et al. CNS regeneration after chronic injury using a self-assembled nanomaterial and MEMRI for real-time in vivo monitoring. Nanomedicine. 2011;7:351–359. doi: 10.1016/j.nano.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 64.Burdick JA, Ward M, Liang E, Young MJ, Langer R. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459. doi: 10.1016/j.biomaterials.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 65.Smith GM, Strunz C. Growth factor and cytokine regulation of chondroitin sulfate proteoglycans by astrocytes. Glia. 2005;52:209–218. doi: 10.1002/glia.20236. [DOI] [PubMed] [Google Scholar]
- 66.Liu B, Chen H, Johns TG, Neufeld AH. Epidermal growth factor receptor activation: an upstream signal for transition of quiescent astrocytes into reactive astrocytes after neural injury. J Neurosci. 2006;26:7532–7540. doi: 10.1523/JNEUROSCI.1004-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koprivica V, et al. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- 68.Robinson R, et al. Nanospheres delivering the EGFR TKI AG1478 promote optic nerve regeneration: the role of size for intraocular drug delivery. ACS Nano. 2011;5:4392–4400. doi: 10.1021/nn103146p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Paralysis facts & figures. Christopher & Dana Reeve Foundation Paralysis Resource Center; 2010. [Google Scholar]
- 70.Ackery A, Tator C, Krassioukov A. A global perspective on spinal cord injury epidemiology. J Neurotrauma. 2004;21:1355–1370. doi: 10.1089/neu.2004.21.1355. [DOI] [PubMed] [Google Scholar]
- 71.Schwab ME. Repairing the injured spinal cord. Science. 2002;295:1029–1031. doi: 10.1126/science.1067840. [DOI] [PubMed] [Google Scholar]
- 72.Sahni V, Kessler JA. Stem cell therapies for spinal cord injury. Nat Rev Neurol. 2010;6:363–372. doi: 10.1038/nrneurol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- 74.Woerly S, et al. Neural tissue formation within porous hydrogels implanted in brain and spinal cord lesions: ultrastructural, immunohistochemical, and diffusion studies. Tissue Eng. 1999;5:467–488. doi: 10.1089/ten.1999.5.467. [DOI] [PubMed] [Google Scholar]
- 75.Tuckwell DS, Weston SA, Humphries MJ. Integrins: a review of their structure and mechanisms of ligand binding. Symp Soc Exp Biol. 1993;47:107–136. [PubMed] [Google Scholar]
- 76.Woerly S, Pinet E, de Robertis L, Van Diep D, Bousmina M. Spinal cord repair with PHPMA hydrogel containing RGD peptides (NeuroGel) Biomaterials. 2001;22:1095–1111. doi: 10.1016/s0142-9612(00)00354-9. [DOI] [PubMed] [Google Scholar]
- 77.Coumans JV, et al. Axonal regeneration and functional recovery after complete spinal cord transection in rats by delayed treatment with transplants and neurotrophins. J Neurosci. 2001;21:9334–9344. doi: 10.1523/JNEUROSCI.21-23-09334.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Piantino J, Burdick JA, Goldberg D, Langer R, Benowitz LI. An injectable, biodegradable hydrogel for trophic factor delivery enhances axonal rewiring and improves performance after spinal cord injury. Exp Neurol. 2006;201:359–367. doi: 10.1016/j.expneurol.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 79.Teng YD, et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc Natl Acad Sci USA. 2002;99:3024–3029. doi: 10.1073/pnas.052678899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rochkind S, et al. Development of a tissue-engineered composite implant for treating traumatic paraplegia in rats. Eur Spine J. 2006;15:234–245. doi: 10.1007/s00586-005-0981-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Guo J, et al. Reknitting the injured spinal cord by self-assembling peptide nanofiber scaffold. Nanomedicine. 2007;3:311–321. doi: 10.1016/j.nano.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 82.Silva GA. Nanotechnology approaches for the regeneration and neuroprotection of the central nervous system. Surg Neurol. 2005;63:301–306. doi: 10.1016/j.surneu.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 83.Hartgerink JD, Beniash E, Stupp SI. Self-assembly and mineralization of peptide-amphiphile nanofibers. Science. 2001;294:1684–1688. doi: 10.1126/science.1063187. [DOI] [PubMed] [Google Scholar]
- 84.Hartgerink JD, Beniash E, Stupp SI. Peptide-amphiphile nanofibers: a versatile scaffold for the preparation of self-assembling materials. Proc Natl Acad Sci USA. 2002;99:5133–5138. doi: 10.1073/pnas.072699999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tashiro K, et al. A synthetic peptide containing the IKVAV sequence from the A chain of laminin mediates cell attachment, migration, and neurite outgrowth. J Biol Chem. 1989;264:16174–16182. [PubMed] [Google Scholar]
- 86.Jucker M, Kleinman HK, Ingram DK. Fetal rat septal cells adhere to and extend processes on basement membrane, laminin, and a synthetic peptide from the laminin A chain sequence. J Neurosci Res. 1991;28:507–517. doi: 10.1002/jnr.490280407. [DOI] [PubMed] [Google Scholar]
- 87.Silva GA, et al. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science. 2004;303:1352–1355. doi: 10.1126/science.1093783. [DOI] [PubMed] [Google Scholar]
- 88.Tysseling-Mattiace VM, et al. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J Neurosci. 2008;28:3814–3823. doi: 10.1523/JNEUROSCI.0143-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tysseling VM, et al. Self-assembling peptide amphiphile promotes plasticity of serotonergic fibers following spinal cord injury. J Neurosci Res. 2010;88:3161–3170. doi: 10.1002/jnr.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.National Cancer Institute. 2012 [online], http://www.nci.nih.gov/cancertopics/types/brain/
- 91.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 92.Crowley RW, Pouratian N, Sheehan JP. Gamma knife surgery for glioblastoma multiforme. Neurosurg Focus. 2006;20:E17. doi: 10.3171/foc.2006.20.4.11. [DOI] [PubMed] [Google Scholar]
- 93.Dehdashti AR, Hegi ME, Regli L, Pica A, Stupp R. New trends in the medical management of glioblastoma multiforme: the role of temozolomide chemotherapy. Neurosurg Focus. 2006;20:E6. doi: 10.3171/foc.2006.20.4.3. [DOI] [PubMed] [Google Scholar]
- 94.Dixit S, Hingorani M, Achawal S, Scott I. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25:459–469. doi: 10.3109/02688697.2010.550342. [DOI] [PubMed] [Google Scholar]
- 95.Rich JN, et al. Phase II trial of gefitinib in recurrent glioblastoma. J Clin Oncol. 2004;22:133–142. doi: 10.1200/JCO.2004.08.110. [DOI] [PubMed] [Google Scholar]
- 96.Gao K, Jiang X. Influence of particle size on transport of methotrexate across blood brain barrier by polysorbate 80-coated polybutylcyanoacrylate nanoparticles. Int J Pharm. 2006;310:213–219. doi: 10.1016/j.ijpharm.2005.11.040. [DOI] [PubMed] [Google Scholar]
- 97.Tian XH, et al. Enhanced brain targeting of temozolomide in polysorbate-80 coated polybutylcyanoacrylate nanoparticles. Int J Nanomedicine. 2011;6:445–452. doi: 10.2147/IJN.S16570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Huang G, Zhang N, Bi X, Dou M. Solid lipid nanoparticles of temozolomide: potential reduction of cardial and nephric toxicity. Int J Pharm. 2008;355:314–320. doi: 10.1016/j.ijpharm.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 99.Lamprecht A, Benoit JP. Etoposide nanocarriers suppress glioma cell growth by intracellular drug delivery and simultaneous P-glycoprotein inhibition. J Control Release. 2006;112:208–213. doi: 10.1016/j.jconrel.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 100.Garcion E, et al. A new generation of anticancer, drug-loaded, colloidal vectors reverses multidrug resistance in glioma and reduces tumor progression in rats. Mol Cancer Ther. 2006;5:1710–1722. doi: 10.1158/1535-7163.MCT-06-0289. [DOI] [PubMed] [Google Scholar]
- 101.Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer. 2005;5:275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 102.Dhanikula RS, Argaw A, Bouchard JF, Hildgen P. Methotrexate loaded polyether-copolyester dendrimers for the treatment of gliomas: enhanced efficacy and intratumoral transport capability. Mol Pharm. 2008;5:105–116. doi: 10.1021/mp700086j. [DOI] [PubMed] [Google Scholar]
- 103.He H, et al. PEGylated Poly(amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials. 2010;32:478–487. doi: 10.1016/j.biomaterials.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 104.Hovanessian AG, et al. Surface expressed nucleolin is constantly induced in tumor cells to mediate calcium-dependent ligand internalization. PLoS ONE. 2010;5:e15787. doi: 10.1371/journal.pone.0015787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fogal V, Sugahara KN, Ruoslahti E, Christian S. Cell surface nucleolin antagonist causes endothelial cell apoptosis and normalization of tumor vasculature. Angiogenesis. 2009;12:91–100. doi: 10.1007/s10456-009-9137-5. [DOI] [PubMed] [Google Scholar]
- 106.Guo J, et al. Aptamer-functionalized PEG–PLGA nanoparticles for enhanced anti-glioma drug delivery. Biomaterials. 2011;32:8010–8020. doi: 10.1016/j.biomaterials.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 107.Demeule M, et al. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J Neurochem. 2008;106:1534–1544. doi: 10.1111/j.1471-4159.2008.05492.x. [DOI] [PubMed] [Google Scholar]
- 108.Xin H, et al. Angiopep-conjugated poly(ethylene glycol)-co-poly(ɛ-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials. 2011;32:4293–4305. doi: 10.1016/j.biomaterials.2011.02.044. [DOI] [PubMed] [Google Scholar]
- 109.Mamot C, et al. Epidermal growth factor receptor (EGFR)-targeted immunoliposomes mediate specific and efficient drug delivery to EGFR- and EGFRvIII-overexpressing tumor cells. Cancer Res. 2003;63:3154–3161. [PubMed] [Google Scholar]
- 110.Mamot C, et al. Epidermal growth factor receptor-targeted immunoliposomes significantly enhance the efficacy of multiple anticancer drugs in vivo. Cancer Res. 2005;65:11631–11638. doi: 10.1158/0008-5472.CAN-05-1093. [DOI] [PubMed] [Google Scholar]
- 111.Hadjipanayis CG, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70:6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lu W, Sun Q, Wan J, She Z, Jiang XG. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer Res. 2006;66:11878–11887. doi: 10.1158/0008-5472.CAN-06-2354. [DOI] [PubMed] [Google Scholar]
- 113.Zhang Y, Jeong Lee H, Boado RJ, Pardridge WM. Receptor-mediated delivery of an antisense gene to human brain cancer cells. J Gene Med. 2002;4:183–194. doi: 10.1002/jgm.255. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Y, Zhu C, Pardridge WM. Antisense gene therapy of brain cancer with an artificial virus gene delivery system. Mol Ther. 2002;6:67–72. doi: 10.1006/mthe.2002.0633. [DOI] [PubMed] [Google Scholar]
- 115.Ding H, et al. Inhibition of brain tumor growth by intravenous poly (beta-L-malic acid) nanobioconjugate with pH-dependent drug release [corrected] Proc Natl Acad Sci USA. 2010;107:18143–18148. doi: 10.1073/pnas.1003919107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Agemy L, et al. Targeted nanoparticle enhanced proapoptotic peptide as potential therapy for glioblastoma. Proc Natl Acad Sci USA. 2011;108:17450–17455. doi: 10.1073/pnas.1114518108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.US National Library of Medicine. A phase I trial of nanoliposomal CPT-11 (NL CPT-11) in patients with recurrent high-grade gliomas. ClinicalTrials.gov. 2011 [online], http://clinicaltrials.gov/ct2/show/NCT00734682?term=NCT00734682&rank=1.
- 118.Noble CO, et al. Novel nanoliposomal CPT-11 infused by convection-enhanced delivery in intracranial tumors: pharmacology and efficacy. Cancer Res. 2006;66:2801–2806. doi: 10.1158/0008-5472.CAN-05-3535. [DOI] [PubMed] [Google Scholar]
- 119.Kunzmann A, et al. Toxicology of engineered nanomaterials: focus on biocompatibility, biodistribution and biodegradation. Biochim Biophys Acta. 2010;1810:361–373. doi: 10.1016/j.bbagen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 120.Garnett MC, Kallinteri P. Nanomedicines and nanotoxicology: some physiological principles. Occup Med (Lond) 2006;56:307–311. doi: 10.1093/occmed/kql052. [DOI] [PubMed] [Google Scholar]
- 121.Zolnik BS, Sadrieh N. Regulatory perspective on the importance of ADME assessment of nanoscale material containing drugs. Adv Drug Deliv Rev. 2009;61:422–427. doi: 10.1016/j.addr.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 122.Nanotechnology Task Force Report 2007. US Food and Drug Administration. 2007 [online], http://www.fda.gov/ScienceResearch/SpecialTopics/Nanotechnology/NanotechnologyTaskForceReport2007/default.htm.
- 123.Vega-Villa KR, et al. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60:929–938. doi: 10.1016/j.addr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 124.Marano F, Hussain S, Rodrigues-Lima F, Baeza-Squiban A, Boland S. Nanoparticles: molecular targets and cell signalling. Arch Toxicol. 2011;85:733–741. doi: 10.1007/s00204-010-0546-4. [DOI] [PubMed] [Google Scholar]
- 125.Singh N, et al. NanoGenotoxicology: the DNA damaging potential of engineered nanomaterials. Biomaterials. 2009;30:3891–3914. doi: 10.1016/j.biomaterials.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 126.Dobrovolskaia MA, McNeil SE. Immunological properties of engineered nanomaterials. Nat Nanotechnol. 2007;2:469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]