Abstract
Objective
Tongue papillae are critical organs in mastication. There are four different types of tongue papillae; fungiform, circumvallate, foliate, and filiform papillae. Unlike the other three taste papillae, non-gustatory papillae, filiform papillae cover the entire dorsal surface of the tongue and are important structures for the mechanical stress of sucking. Filiform papillae are further classified into two subtypes with different morphologies, depending on their location on the dorsum of the tongue. The filiform papillae at the intermolar eminence have pointed tips, whereas filiform papillae with rounded tips are found in other regions (anterior tongue). It remains unknown how the shape of each type of filiform papillae are determined during their development. Bmp signalling pathway has been known to regulate mechanisms that determine the shapes of many ectodermal organs. The aim of this study was to investigate the role of Bmp signalling in filiform papillae development.
Design
Comparative in situ hybridization analysis of six Bmps (Bmp2–Bmp7) and two Bmpr genes (Bmpr1a and Bmpr1b) were carried out in filiform papillae development. We further examined tongue papillae in mice over-expressing Noggin under the keratin14 promoter (K14-Noggin).
Results
We identified a dynamic temporo-spatial expression of Bmps in filiform papillae development. The K14-Noggin mice showed pointed filiform papillae in regions of the tongue normally occupied by the rounded type.
Conclusions
Bmp signalling thus regulates the shape of filiform papillae.
Keywords: Tongue, Filiform papillae, Anterior tongue, Intermolar eminence
1. Introduction
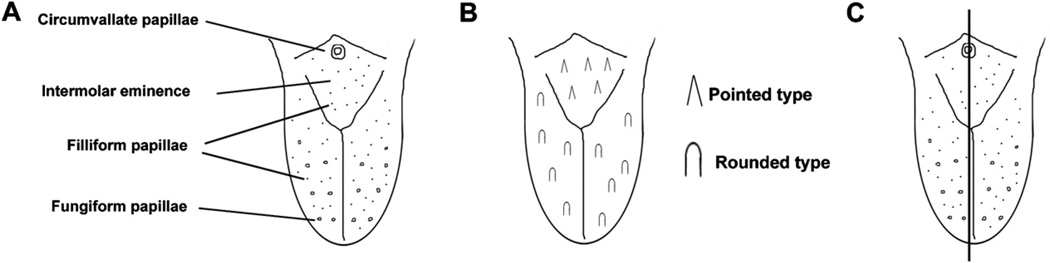
The dorsal surface of the tongue is covered by papillae that play critical roles in the mastication and gustatory system. Inrodents, there are four different types of tongue papillae; fungiform, circumvallate, foliate, and filiform. The fungiform, circumvallate and foliate papillae contain taste buds and are referred to as taste papillae, whereas filiform papillae contain no taste buds (non-gustatory papillae). These papillae are distributed in a specific pattern over the tongue. A single circumvallate papilla is located in the centre of the terminal sulcus, which divides the tongue from the pharynx. Fungiform papillae are present on the anterior tongue in a pattern of longitudinal rows, bracketing a median furrow. Unlike the taste papillae, non-gustatory filiform papillae cover the entire dorsal surface of the tongue (Fig. 1A).1 To date, the molecular mechanisms of filiform papillae development remain largely unknown, whereas it has been well studied in gustatory papillae development.2–9
Fig. 1.
Mouse tongue papillae. (A) Schematic diagram of mouse tongue papillae. (B) Schematic diagram of the two types of filiform tongue papillae; pointed type in the intermolar eminence and rounded type in the anterior region. (C) Schematic diagram of the direction of sections; sagittal sections including circumvallate papillae of the intermolar eminence.
The filiform papillae are important structures for the mechanical stress of sucking.10,11 In rodents, filiform papillae are further classified into two subtypes with different morphologies, depending on their location on the dorsum of the tongue. In the intermolar eminence, the filiform papillae have pointed tips in both the anterior–posterior and the left-right axis (pointed type; Fig. 1B).12 The pointed type filiform papillae are frequently bifurcated. Unlike the intermolar eminence, in other parts of the tongue surface (anterior tongue), the filiform papillae have rounded tips in the left-right axis, whereas their spines point posteriorly in the anterior–posterior axis (rounded type; Fig. 1B).12 It is unclear how the shape of each type of filiform papillae is determined during their development.
It is known that multiple signalling pathways such as Bmp play critical roles in regulating the shape of ectodermal organs, including taste tongue papillae and teeth.2,4,12–15 However, the role of Bmp signalling in filiform papillae development remains unknown.
We show here that Bmp signalling is activated in filiform papillae development. Bmp ligands and their receptors show a dynamic temporo-spatial expression pattern in filiform papillae development. Mice over-expressing Noggin under the keratin14 promoter (K14-Noggin) develop abnormal pointed filiform papillae in the anterior tongue where the rounded type are normally located. This result provides evidence of a role for Bmp signalling in regulating the morphogenesis of filiform papillae.
2. Materials and methods
2.1. Production and analysis of transgenic mice
K14-Noggin transgenic mice were produced as described by Guha et al.16 Five K14-Noggin mice (five month-old) and twenty mice (E14.5, E16.5 and newborn) of the CD-1 strain were used. Noon of the day on which the plugs were detected was considered as E0.5. To accurately assess the age of embryos, somite pairs were counted and the stage confirmed using morphological criteria, e.g., relative sizes of maxillary and mandibular primodia, extent of nasal placode invagination, and the size of limb buds.
2.2. In situ hybridization
Embryo heads were fixed in 4% buffered paraformaldehyde, wax embedded, and serially sectioned at 7 µm. Sections were split over three to ten slides and prepared for histology or radioactive in situ hybridization. In situ hybridization with [35S]UTP-labelled riboprobes was carried out as described previously.17
2.3. Scanning electron microscope (SEM) analysis
Tongues were fixed with 2% glutaraldehyde in 0.1 M Nacacodylate buffer and 0.1 M sucrose, followed by post-fixation with 1% osmium tetroxide in 0.1 M Na-cacodylate buffer. After critical point drying, the samples were coated with gold and photographed using scanning electron microscopy.
2.4. Immunohistochemistry
After deparaffinization, sections were treated by proteinase K and then incubated with antibody to phosphorylated Smad1, Smad5 and Smad8 (p-Smad1/5/8; 1:100; Cell signalling Technology; #9511). As a negative control, normal rabbit serum was used instead of primary antibody. Secondary antibodies conjugated with biotin (Abcam; ab6828) were used at dilution of 1:100. Tyramide signal amplification system was performed (Perkin Elmer Life Science; NEL701) for detecting the antibody. DAPI (4′,6-diamidino-2-phenylindole dihydrochloride; Sigma) was used for nuclear staining. Pictures were taken with same exposure between control wild-type and mutant mice.
3. Results
3.1. Expression of Bmps and Bmp receptors in filiform papillae development
At 11.5 days gestation (E11.5) the tongue emerges as a swelling on the floor of the developing mandible, with a spatulate tongue being observed at E12.5. At E13.5, the tongue has a distinctive topography. The well-formed taste bud papillae can be detected by E13.5. Unlike taste papillae development, filiform papillae start to develop later, at around E15.5.18 In common with other ectodermal appendages, such as hair and tooth, the first morphological sign of tongue papillae development is localized thickenings of the epithelium and condensation of the underlying mesenchymal cells. The subsequent formation of epithelial arch-like structures is proceeded by their vertical elongation into a cornified stratified squamous epithelium.19 Filiform papillae seem to develop rapidly during the few days before birth.19 At birth, filiform papillae show a similar distribution to those seen in the adult.1
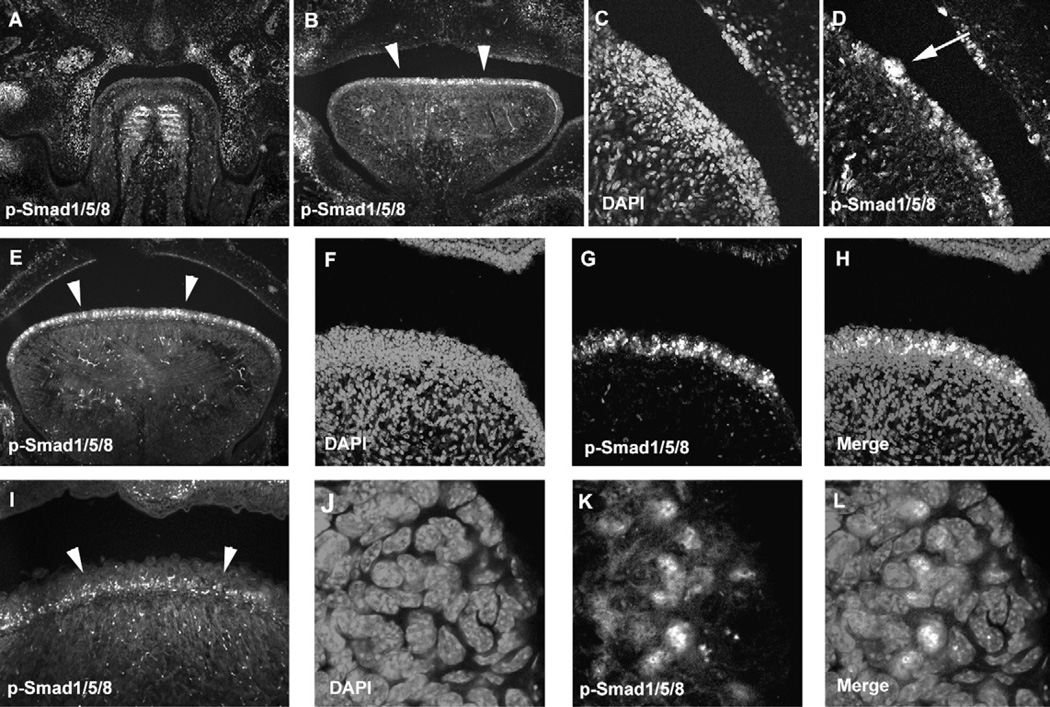
In order to investigate whether Bmp signalling is involved in filiform papillae development, we examined Bmp signalling in filiform papillae development by immuno his to chemistry using anti-phosphorylated Smad1/Smad5/Smad8 (p-Smad1/5/8) antibody. Smad1, Smad5 and Smad8 are phosphorylated by Bmp receptors following binding of ligands.20 P-Smad1/5/8 positive cells could not be detected in either tongue epithelium or underneath mesenchyme at E13.5, although they were observed in developing tongue muscle (Fig. 2A). P-Smad1/5/8 was localized in entire tongue epithelium at E14.5 and E16.5, whereas they were found in basal epithelial cells at birth (Fig. 2B–I). These suggested that Bmp signalling is activated in filiform papillae development from E14.5.In addition to filiform papillae, p-Smad1/5/8 positive cells were observed in fungiform papillae (Fig. 2D). Most of p-Smad1/5/8 was localized in nuclear of filiform papillae epithelial cells (Fig. 2J–L).
Fig. 2.
P-Smad1/5/8 in filiform papilla development. Immunohistochemistry of p-Smad1/5/8 in frontal head sections of the developing tongue at E13.5 (A), E14.5 (B–D), E16.5 (E–H, J–L) and birth (I). Arrowheads indicating p-Smad1/5/8 positive cells in tongue papillae (B, E, I). Arrow indicating p-Smad1/5/8 positive cells in fungiform papillae (D). DAPI (C, F, J), p-Smad1/5/8 (A, B, D, E, G, I, K), Merge images (H, L). (F–H, J–L) High magnification of E.
A comprehensive expression analysis of Bmps in filiform papillae development was therefore carried out between E14.5 and E18.5 using radioactive in situ hybridization to understand the significance of this gene family in filiform papillae organogenesis. In addition to the presence of Bmp signalling, this period also encompasses development preceding the morphological appearance of filiform papillae to maturation of the organs. In order to investigate Bmp expression in both pointed and rounded type filiform papillae development, we examined the expression at the intermolar eminence anterior to the circumvallate papillae for the pointed type, and in the anterior part of tongue (anterior tongue) for the rounded type (Fig. 1C).
3.2. E14.5
At E14.5, the presumptive filiform papilla epithelium is composed of a few layers of cuboidal cells.18,19
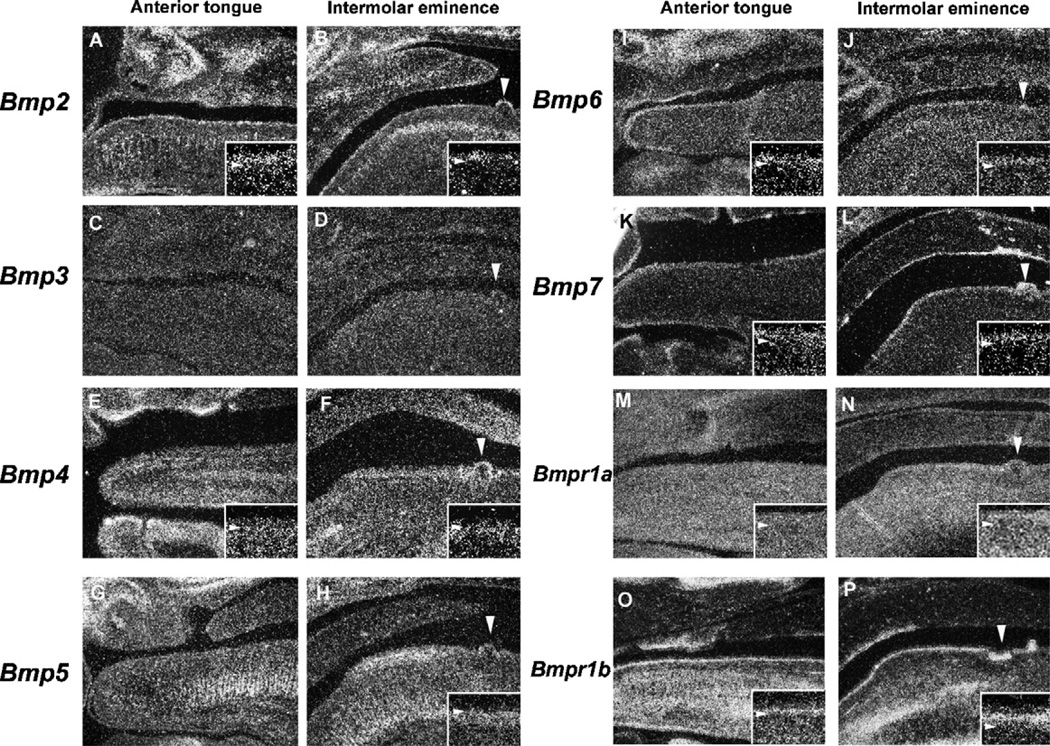
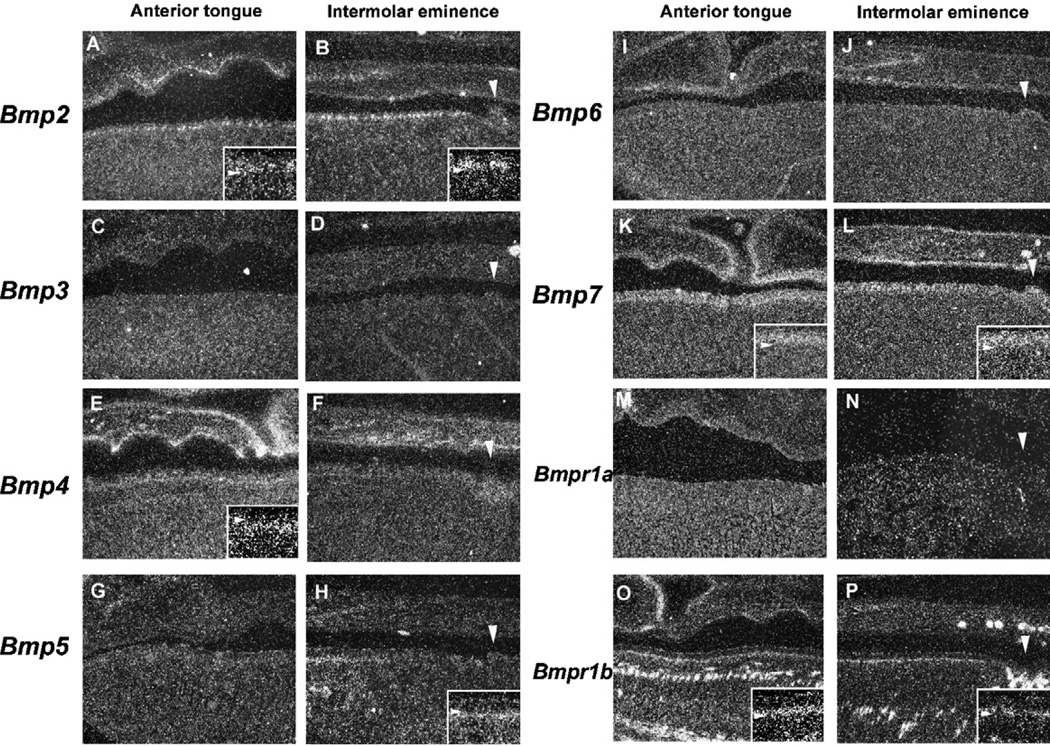
In both the anterior tongue and the intermolar eminence, Bmp2 was expressed in the filiform papillae epithelium (Fig. 3A and B). Bmp4 expression was observed in mesenchymal cells facing the epithelium in the intermolar eminence, whereas very little expression was seen in the equivalent mesenchymal cells in the anterior tongue (Fig. 3E and F). In the intermolar eminence, Bmp5 expression was found in the filiform papillae mesenchyme of the anterior portion but not seen in the posterior region (Fig. 3H). Bmp5 expression was not observed in the anterior tongue (Fig. 3G). Bmp6 and Bmp7 was weakly expressed in the filiform papillae epithelium of both the anterior tongue and the intermolar eminence, with no significant difference in their expression between the two regions (Fig. 3I–L). Bmp3 expression could not be detected in either the epithelium or mesenchyme of the filiform papillae at this stage (Fig. 3C and D). The biological activities of Bmps are mediated by heterodimeric receptor complexes consisting of type I Bmp receptors (Bmpr1a and Bmpr1b) and the type II receptor (Bmpr2). The formation of heterodimers of type I and type II receptors is essential for Bmp signal transduction.21–23 It is possible that filiform papillae type is regulated by differences in expression of these receptors. We further examined Bmpr1a and Bmpr1b expression in filiform papillae development. Bmpr1a expression was observed in both the epithelium and mesenchyme, with a slightly weaker expression seen in the latter (Fig. 3M and N). Bmpr1b expression was restricted to the basal epithelial cells in both the anterior tongue and the intermolar eminence (Fig. 3O and P). There was no significant difference in the both receptor expression between in the anterior tongue and the intermolar eminence.
Fig. 3.
Bmp expression in filiform papilla development at E14.5. In situ hybridization of Bmps (A–L) and Bmp receptors (M–P) showing expression in sagittal head sections of the anterior tongue (A, C, E, G, I, K, M, O) and the intermolar eminence (B, D, F, H, J, L, N, P) at E14.5 Bmp2 (A, B) Bmp3 (C, D) Bmp4 (E, F) Bmp5 (G, H) Bmp6 (I, J) Bmp7 (K, L) Bmpr1a (M, N) and Bmpr1a (O, P). Arrowheads indicating the circumvallate papillae (B, D, F, H, J, L, N, P). (A, B, E, F, H, I–P) Inset is high magnification view of the filiform papillae. Arrowhead indicating the boundary between epithelium and mesenchyme in inset.
3.3. E16.5
At E16.5, primitive arch-like epithelial structures are found in regions of the dorsal surface of tongue.18,19
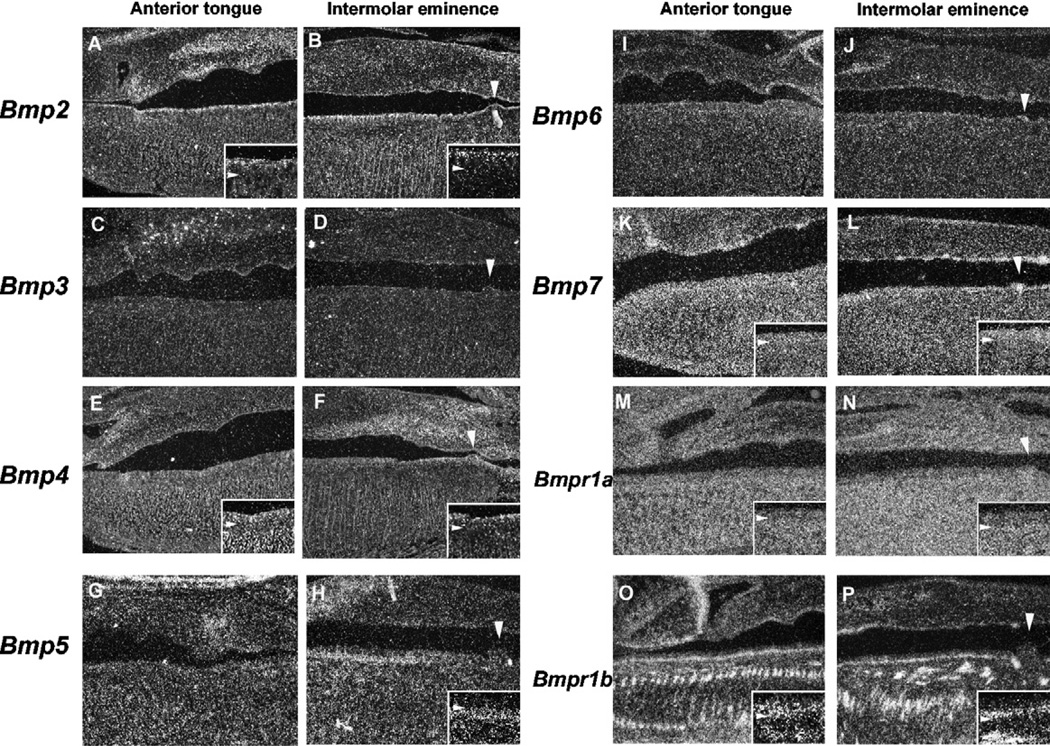
Bmp2 expression was only observed in the epithelial cells facing the oral cavity (Fig. 4A and B). There was no significant difference in expression between the anterior tongue and the intermolar eminence. Bmp4 was weakly expressed in epithelium of both the anterior tongue and the intermolar eminence (Fig.4E and F). In the intermolar eminence, Bmp5 expression was only found in the filiform papillae mesenchymal cells facing the epithelium at the anterior portion, but not seen in the posterior region (Fig. 4H). Bmp5 expression was not observed in the anterior tongue (Fig. 4G). Bmp7 was weakly expressed in the entire filiform papillae epithelium of both the anterior tongue and the intermolar eminence (Fig. 4K and L). Bmp3 and Bmp6 showed no expression in the filiform papillae epithelium or the mesenchyme at this stage (Fig.4C,D,I and J).Bmpr1a expression was observed in both the epithelium and the mesenchyme, with a slightly weaker expression in the epithelium (Fig.4M and N). Bmpr1b was expressed in the basal epithelial cells of both the anterior tongue and the intermolar eminence (Fig. 4O and P). There was no significant difference in expression between the anterior tongue and the intermolar eminence.
Fig. 4.
Bmp expression in filiform papilla development at E16.5. In situ hybridization of Bmps (A–L) and Bmp receptors (M–P) showing expression in sagittal head sections of the anterior tongue (A, C, E, G, I, K, M, O) and the intermolar eminence (B, D, F, H, J, L, N, P) at E16.5. Arrowheads indicate the circumvallate papillae Bmp2 (A, B) Bmp3 (C, D) Bmp4 (E, F) Bmp5 (G, H) Bmp6 (I, J) Bmp7 (K, L) Bmpr1a (M, N) and Bmpr1a (O, P) Arrowheads indicating the circumvallate papillae (B, D, F, H, J, L, N, P). (A, B, E, F, H, K–P) Insets are high magnification views of the filiform papillae. Arrowheads indicating the boundary between epithelium and mesenchyme in insets.
3.3.1. Newborn
At birth, the dorsal tongue epithelium is composed of a typical stratified squamous epithelium. Filiform papillae become more distinct and the connective tissue of the lamina propria penetrate the centre of each structure.18,19
Bmp2 was expressed in the basal epithelial cells of both the intermolar eminence and the anterior tongue (Fig. 5A and B). Bmp4 was weakly expressed in the filiform papillae epithelium and the mesenchymal cells facing the epithelium in the anterior tongue but not in the intermolar eminence (Fig. 5E and F). In the intermolar eminence, Bmp5 showed restricted expression only in the mesenchymal cells facing the epithelium to the anterior domain, with no expression seen in posterior region (Fig. 5H). Bmp5 expression could not be detected in the anterior tongue (Fig. 5G). Bmp7 was expressed in the epithelial cells in both the anterior tongue and the intermolar eminence (Fig. 5K and L). Bmp3 and Bmp6 expression could not be detected in the epithelium or the mesenchyme of the filiform papillae at this stage (Fig. 5C, D, I and J). No expression of Bmpr1a was observed in either the anterior tongue or the intermolar eminence (Fig. 5M and N). Bmpr1b expression was found in the basal epithelial cells in both the intermolar eminence and the anterior tongue, with no significant difference in expression between the two regions (Fig. 5O and P).
Fig. 5.
Bmp expression in filiform papilla development at birth. In situ hybridization of Bmps (A–L) and Bmp receptors (M–P) showing expression in sagittal head sections of the anterior tongue (A, C, E, G, I, K, M, O) and the intermolar eminence (B, D, F, H, J, L, N, P) at birth. Arrowheads indicate the circumvallate papillae Bmp2 (A, B) Bmp3 (C, D) Bmp4 (E, F) Bmp5 (G, H) Bmp6 (I, J) Bmp7 (K, L) Bmpr1a (M, N) and Bmpr1a (O, P). Arrowheads indicating the circumvallate papillae (B, D, F, H, J, L, N, P). (A, B, E, H, K, L, O, P) Insets are high magnification views of the filiform papillae. Arrowheads indicating the boundary between epithelium and mesenchyme in insets.
Bmp ligands and their receptors thus show a dynamic temporo-spatial expression pattern in filiform papillae development.
The expression of Bmps and Bmprs during filiform papillae development is summarized in Table 1.
Table 1.
Summary of the expression of Bmps and Bmprs.
| E14.5 |
E16.5 |
NB |
||||
|---|---|---|---|---|---|---|
| Anterior | Intermolar | Anterior | Intermolar | Anterior | Intermolar | |
| Bmp2 | ++ | ++ | ++ | ++ | ++ | ++ |
| Bmp3 | − | − | − | − | − | − |
| Bmp4 | + | ++ | + | + | + | − |
| Bmp5 | − | +++ | − | +++ | − | + |
| Bmp6 | + | + | − | − | − | − |
| Bmp7 | + | + | ++ | ++ | ++ | ++ |
| Bmpr1a | ++ | ++ | ++ | ++ | − | − |
| Bmpr1b | +++ | +++ | +++ | +++ | ++ | ++ |
(−) no, (+) low, (++) medium, and (+++) high level of expression, Anterior; Anterior tongue, Intermolar; Intermolar eminence.
3.4. Tongue papillae in K14-Noggin mice
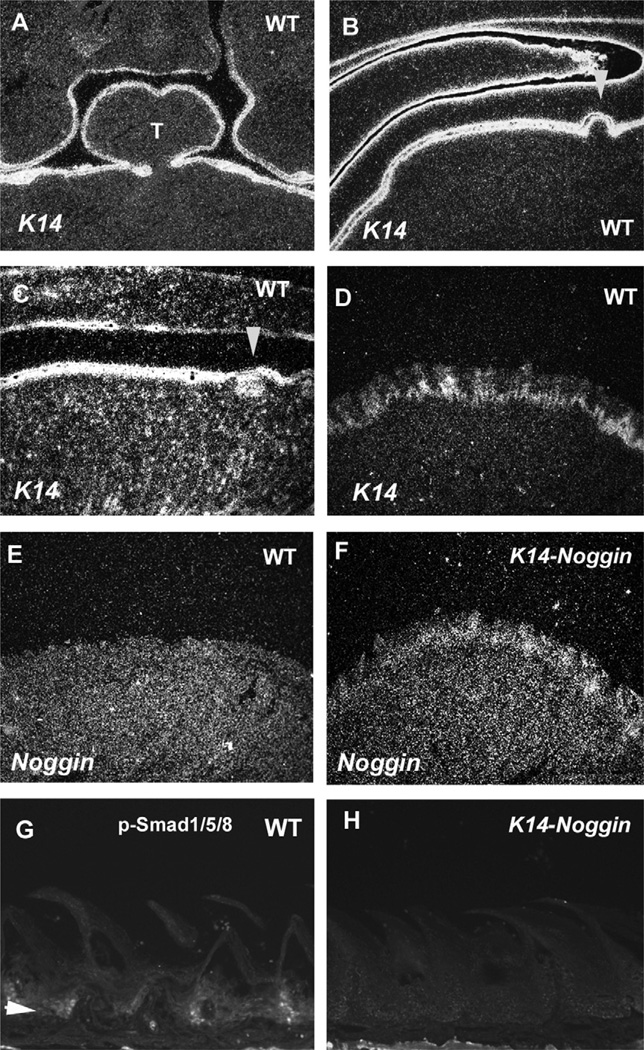
In order to investigate the role of Bmp signalling in filiform papilla development, we examined the tongue papillae of transgenic mice over-expressing the BMP antagonist, Noggin, under the control of the Keratin 14 (K14) promotor (K14-Noggin). K14 is expressed in tongue epithelium from E12.5 to adult in both the anterior tongue and the intermolar eminence (Fig. 6A–D). 19,24 In the wild-type tongue, significant Noggin expression could not be detected in the filiform papillae within the embryonic or adult stages, whereas expression was seen in the fungiform papillae at E14.5 (Fig. 6E; data not shown).4 Ectopic Noggin expression and reduced p-Smad1/5/8 positive cells were seen in the filiform papillae epithelium of K14-Noggin adult mice (Fig. 6F and H).
Fig. 6.
K14 and Noggin expression and p-Smad1/5/8 localization in tongue papilla of wild-type and K14-Noggin mice. In situ hybridization showing the expression of K14 (A–D) and Noggin (E, F) in frontal head sections (A, D–F) and in sagittal head sections (B, C) of wild-type (A–E) and K14-Noggin (.F) at E12.5 (A), E14.5 (B), E16.5 (C) and five months of age (D–H). Arrowheads indicating the circumvallate papillae (B, C). (G, H) Immunohistochemistry of p-Smad1/5/8 in sagittal head sections of wild-type (G) and K14-Noggin mice (H) at five months of age. Arrowhead indicating p-Smad1/5/8 positive cells (G). T = Tongue.
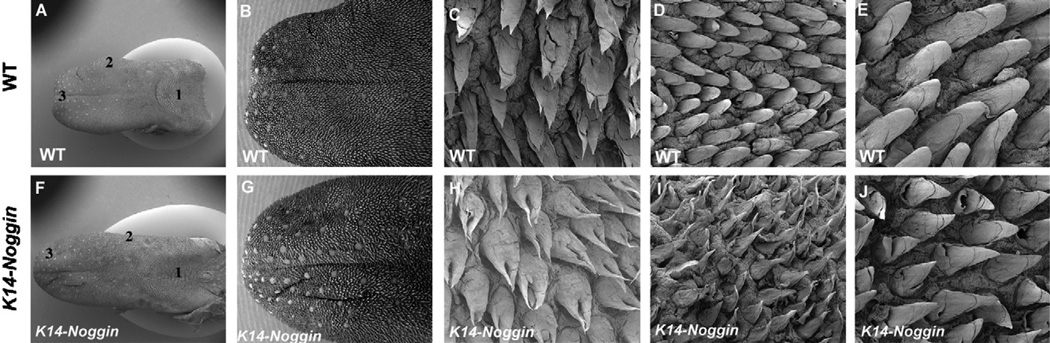
In the wild-type tongue, the pointed tips of the filiform papillae were observed in the intermolar eminence (Fig. 7C). In other regions, including the anterior part and edges of the tongue, filiform papillae have rounded tips (Fig. 7D and E). In K14-Noggin mice, the fungiform papillae were more prominent than those seen in wild-type mice (Fig. 7G). This supports previous reports that primitive fungiform papillae are enlarged by addition of exogenous Noggin in vitro.4 In K14-Noggin mice, the anterior part and edge of the tongue showed abnormally pointed tips of the filiform papillae, where rounded types are usually presented (Fig. 7I and J). Significant differences could not be found in the filiform papillae in the intermolar eminence in the K14-Noggin mice (Fig. 7H). Unlike the left-right axis, the filiform papillae were normal in the anterior–posterior axis in K14-Noggin mice (data not shown).
Fig. 7.
SEMs of Filiform papillae in K14-Noggin (A, F) Oral view of the entire tongue. (B, G) Oral view of the anterior part of the tongue. (C, H) High magnification of the intermolar eminence (1 in A, F). (D, I) High magnification of the side edge of the tongue (2 in A, F). (E, J) High magnification of the anterior part of the tongue (3 in A, F). Wild-type (A–E) and K14-Noggin mice (F–J) at five months of age.
4. Discussion
Bmps show a dynamic temporo-spatial expression pattern in filiform papillae development of both anterior tongue and intermolar eminence. Reduced Bmp signalling induces pointed type papillae formation in the anterior tongue that usually have rounded type papillae. This suggests that Bmp signalling is required for regulating the shape of filiform papillae in anterior tongue. Unlike anterior tongue, the filiform papillae in the intermolar eminence retained their shape as pointed type in K14-Noggin mice. Mice with mutation of follistatin, an antagonist of a subset of TGFβ superfamily molecules, however show a lack of the filiform papillae only in the intermolar eminence which is believed to be caused by upregulation of Bmp7.2 This suggests that Bmp signalling is involved in the formation of both types of filiform papillae, but that there are differences in sensitivity to the Bmp signalling between filiform papillae in the anterior tongue and the intermolar eminence.
It is also conceivable that filiform papillae development is regulated by the expression of different ligands between the anterior tongue and the intermolar eminence. In fact, the Bmp ligands and Bmp receptors examined in this study show slightly difference in expression between the anterior tongue and the intermolar eminence. Bmp5 expression was found in the intermolar eminence but not in the anterior tongue at E14.5 and E16.5. Bmp4 also showed slightly restricted expression pattern. In addition to these ligands, it is feasible that differences in the expression patterns of other Bmp signalling related ligands, such as Gdf5 and Gdf11, could contribute to the different shapes of filiform papillae between the tongue regions.
Mutation of Hoxc13 is known to lead to weaken filiform papillae, which results in break of tip of filiform papillae. Hoxc13 mutant mice therefore show abnormal rounded filiform papillae.25 However, any sign of broken-off filiform papillae pieces could not be detected in K14-Noggin mice. Unlike the left-right axis, the filiform papillae were normal shape in the anterior–posterior axis in K14-Noggin mice, ruling out possibility that abnormal shape of filiform papillae in K14-Noggin mice is caused by wearing papillae during usage. In the anterior tongue, Bmps thus only regulate the shape of filiform papillae in the left-right axis. In Pax9 mutant mice, filiform papillae lack anterior–posterior polarity and a cornified layer is absent.26 This suggests that the pathways regulated by Pax9 are related to shape in the anterior–posterior polarity, whereas Bmps regulate the left-right axis morphology.
K14-Noggin mice also show significant increase in the number of trigeminal neurons in skin.27 Innervation is known to play a critical role in morphology of tongue papillae.28–30 It is possible that alteration of innervation lead to abnormal shape of filiform papillae formation in K14-Noggin mice.
Epithelium of oral mucosa including tongue papillae provides permeability barrier function. Follistatin mutants show defects in barrier formation in intermolar eminence, suggesting that Bmp signalling play a critical role in barrier formation.2 In K14-Noggin mice, any significant signs of disruption of barrier formation such as oedema, erythema, blister and infusion of inflammatory cells could not be detected in anterior tongue where shapes of filiform papillae are altered. In common with filiform papillae shape, barrier formation is probably regulated by different molecular mechanisms between the anterior tongue and the intermolar eminence.
Similar transgenic mouse model (overexpression of Noggin in epithelium) has shown that strength of phenotypes correlates with transgene copy number in the mouse genome and Noggin expression level.31 Filiform papillae phenotypes could be variable by intensity of Bmp signalling.
Acknowledgements
We would like to thank Heiko Peters for helpful comments, David Rice for the Bmp6 plasmid, Masato Ota for the Bmp3 plasmid and Tony Brain for the SEM analysis. This work was supported by the MRC. T.P. is supported by the W J B Houston Research Scholarship (European Orthodontic Society). S.G. is supported by the Higher Education of Pakistan under the Overseas Scholarship Phase II (Batch 2) scheme. Y.O.-T. was supported by Nihon University. K.K. is supported by JSPS International Program for Young Researcher Overseas Visits.
Funding This work was supported by the MRC.
Footnotes
Competing interests
None declared.
Ethical approval
Not required.
REFERENCES
- 1.Iwasaki S, Okumura Y, Kumakura M. Ultrastructural study of the relationship between the morphogenesis of filiform papillae and the keratinization of the lingual epithelium in the mouse. Cells Tissues Organs. 1999;165(2):91–103. doi: 10.1159/000016679. [DOI] [PubMed] [Google Scholar]
- 2.Beites CL, Hollenbeck PL, Kim J, Lovell-Badge R, Lander AD, Calof AL. Follistatin modulates a BMP autoregulatory loop to control the size and patterning of sensory domains in the developing tongue. Development. 2009;136(13):2187–2197. doi: 10.1242/dev.030544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okubo T, Pevny LH, Hogan BL. Sox2 is required for development of taste bud sensory cells. Genes Dev. 2006;20(19):2654–2659. doi: 10.1101/gad.1457106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Liu HX, Mistretta CM. Bone morphogenetic proteins and noggin: inhibiting and inducing fungiform taste papilla development. Dev Biol. 2006;297(1):198–213. doi: 10.1016/j.ydbio.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Nie X. Apoptosis, proliferation and gene expression patterns in mouse developing tongue. Anat Embryol (Berl) 2005;210(2):125–132. doi: 10.1007/s00429-005-0009-5. [DOI] [PubMed] [Google Scholar]
- 6.Jung HS, Oropeza V, Thesleff I. Shh, Bmp-2, Bmp-4 and Fgf-8 are associated with initiation and patterning of mouse tongue papillae. Mech Dev. 1999;81(1–2):179–182. doi: 10.1016/s0925-4773(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim JY, Mochizuki T, Akita K, Jung HS. Morphological evidence of the importance of epithelial tissue during mouse tongue development. Exp Cell Res. 2003;290(2):217–226. doi: 10.1016/s0014-4827(03)00319-7. [DOI] [PubMed] [Google Scholar]
- 8.Kim JY, Lee MJ, Cho KW, Lee JM, Kim YJ, Jung HI, et al. Shh and ROCK1 modulate the dynamic epithelial morphogenesis in circumvallate papilla development. Dev Biol. 2009;325(1):273–280. doi: 10.1016/j.ydbio.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 9.Ota MS, Kaneko Y, Kondo K, Ogishima S, Tanaka H, Eto K, et al. Combined in silico and in vivo analyses reveal role of Hes1 in taste cell differentiation. PLoS Genet. 2009;5(4):e1000443. doi: 10.1371/journal.pgen.1000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wojcik SM, Longley MA, Roop DR. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J Cell Biol. 2001;154(3):619–630. doi: 10.1083/jcb.200102079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong P, Colucci-Guyon E, Takahashi K, Gu C, Babinet C, Coulombe PA. Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. J Cell Biol. 2000;150(4):921–928. doi: 10.1083/jcb.150.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hirao T, Saga T, Kusukawa J, Yamaki K. Angiogenesis and developmental expression of vascular endothelial growth factor in rat lingual papillae. Kurume Med J. 2007;54(1–2):9–24. doi: 10.2739/kurumemedj.54.9. [DOI] [PubMed] [Google Scholar]
- 13.Nie X, Luukko K, Kettunen P. BMP signalling in craniofacial development. Int J Dev Biol. 2006;50(6):511–521. doi: 10.1387/ijdb.052101xn. [DOI] [PubMed] [Google Scholar]
- 14.Tucker AS, Matthews KL, Sharpe PT. Transformation of tooth type induced by inhibition of BMP signaling. Science. 1998;282(5391):1136–1138. doi: 10.1126/science.282.5391.1136. [DOI] [PubMed] [Google Scholar]
- 15.Kim JY, Cho SW, Lee MJ, Hwang HJ, Lee JM, Lee SI, et al. Inhibition of connexin 43 alters Shh and Bmp-2 expression patterns in embryonic mouse tongue. Cell Tissue Res. 2005;320(3):409–415. doi: 10.1007/s00441-005-1091-y. [DOI] [PubMed] [Google Scholar]
- 16.Guha U, Gomes WA, Kobayashi T, Pestell RG, Kessler JA. In vivo evidence that Bmp signaling is necessary for apoptosis in the mouse limb. Dev Biol. 2002;249(1):108–120. doi: 10.1006/dbio.2002.0752. [DOI] [PubMed] [Google Scholar]
- 17.Ohazama A, Johnson EB, Ota MS, Choi HY, Choi HJ, Porntaveetus T, et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS One. 2008;3(12):e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jung HS, Akita K, Kim JY. Spacing patterns on tongue surface-gustatory papilla. Int J Dev Biol. 2004;48(2–3):157–161. doi: 10.1387/ijdb.15272380. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki S, Yoshizawa H, Aoyagi H. Immunohistochemical expression of keratins 13 and 14 in the lingual epithelium of rats during the morphogenesis of filiform papillae. Arch Oral Biol. 2006;51(5):416–426. doi: 10.1016/j.archoralbio.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa K, Shinozaki M, Hara T, Furuya T, Miyazono K. Two major Smad pathways in TGF-beta superfamily signalling. Genes Cells. 2002;7(12):1191–1204. doi: 10.1046/j.1365-2443.2002.00599.x. [DOI] [PubMed] [Google Scholar]
- 21.Miyazono K. Positive and negative regulation of TGF-beta signaling. J Cell Sci. 2000;113(Pt 7):1101–1109. doi: 10.1242/jcs.113.7.1101. [DOI] [PubMed] [Google Scholar]
- 22.ten Dijke P, Korchynskyi O, Valdimarsdottir G, Goumans MJ. Controlling cell fate by bone morphogenetic protein receptors. Mol Cell Endocrinol. 2003;211(1–2):105–113. doi: 10.1016/j.mce.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 23.Söderberg SS, Karlsson G, Karlsson S. Complex and context dependent regulation of hematopoiesis by TGF-beta superfamily signaling. Ann N Y Acad Sci. 2009;1176:55–69. doi: 10.1111/j.1749-6632.2009.04569.x. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki S, Aoyagi H, Yoshizawa H. Immunohistochemical detection of the expression of keratin 14 in the lingual epithelium of rats during the morphogenesis of filiform papillae. Arch Oral Biol. 2003;48(8):605–613. doi: 10.1016/s0003-9969(03)00118-3. [DOI] [PubMed] [Google Scholar]
- 25.Godwin AR, Capecchi MR. Hoxc13 mutant mice lack external hair. Genes Dev. 1998;12:11–20. doi: 10.1101/gad.12.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jonker L, Kist R, Aw A, Wappler I, Peters H. Pax9 is required for filiform papilla development and suppresses skin-specific differentiation of the mammalian tongue epithelium. Mech Dev. 2004;121(11):1313–1322. doi: 10.1016/j.mod.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Guha U, Gomes WA, Samanta J, Gupta M, Rice FL, Kessler JA. Target-derived BMP signaling limits sensory neuron number and the extent of peripheral innervation in vivo. Development. 2004;131(5):1175–1186. doi: 10.1242/dev.01013. [DOI] [PubMed] [Google Scholar]
- 28.Oakley B, Wu LH, Lawton A, DeSibour Neural control of ectopic filiform spines in adult tongue. Neuroscience. 1990;36:831–838. doi: 10.1016/0306-4522(90)90026-z. [DOI] [PubMed] [Google Scholar]
- 29.Sollars SI. Chorda tympani nerve transection at different developmental ages produces differential effects on taste bud volume and papillae morphology in the rat. J Neurobiol. 2005;64:310–320. doi: 10.1002/neu.20140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nosrat CA, Blomlof J, ElShamy WM, Ernfors P, Olson L. Lingual deficits in BDNF and NT3 mutant mice leading to gustatory and somatosensory disturbance, respectively. Development. 1997;124:1333–1342. doi: 10.1242/dev.124.7.1333. [DOI] [PubMed] [Google Scholar]
- 31.Plikus MV, Zeichner-David M, Mayer JA, Reyna J, Bringas P, Thewissen JGM, et al. Morphoregulation of teeth: modulating the number, size, shape and differentiation by tuning Bmp activity. Evol Dev. 2005;7:440–457. doi: 10.1111/j.1525-142X.2005.05048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]