Abstract
Microneedles applied to the skin create micropores, allowing transdermal drug delivery of skin-impermeable compounds. The first human study with this technique demonstrated delivery of naltrexone (an opioid antagonist) for two to three days. Rapid micropore closure, however, blunts the delivery window. Application of diclofenac (an anti-inflammatory) allows seven days of naltrexone delivery in animals.
Purpose
the purpose of the current work was to demonstrate delivery of naltrexone for seven days following one microneedle treatment in humans.
Methods
Human subjects were treated with microneedles, diclofenac (or placebo), and naltrexone. Impedance measurements were used as a surrogate marker to measure micropore formation, and plasma naltrexone concentrations were measured for seven days post-microneedle application.
Results
Impedance dropped significantly from baseline to post-microneedle treatment, confirming micropore formation. Naltrexone was detected for seven days in Group 1 (diclofenac + naltrexone, n = 6), vs. 72 hours in Group 2 (placebo + naltrexone, n = 2). At study completion, a significant difference in impedance was observed between intact and microneedle-treated skin in Group 1 (confirming the presence of micropores).
Conclusion
This is the first study demonstrating week-long drug delivery after one microneedle application, which would increase patient compliance and allow delivery of therapies for chronic diseases.
Keywords: microneedle, naltrexone, transdermal, diclofenac
Introduction
Transdermal drug delivery, through application of patches to the skin that passively deliver drugs into the underlying circulation, allows avoidance of first-pass metabolism through the liver, erratic bioavailability, and the pain of an injection. One substantial challenge, however, is that a very limited number of drugs can be successfully delivered via this route due to the strict physicochemical properties required to permeate the outermost skin layer, the stratum corneum (SC) (1).
Microneedles (MNs) represent a minimally invasive technique of temporarily breaching the SC to allow transdermal delivery for a larger number of molecules (1–5). The simplest technique is the “poke/press and patch” method, in which MNs are pressed gently into the skin and immediately removed, leaving a grid of micropores in the SC. A drug formulation is applied over the micropores, allowing for passive delivery into the underlying circulation (6). Two critical factors directly relate to the effectiveness of this method of MN-enhanced permeation: 1) sufficient creation of micropores, and 2) micropore lifetime. The micropores heal quickly (≤ two hours) when exposed to air, but this can be extended to approximately 48 – 72 hours under occlusion (7–11). The first pharmacokinetic study using this technique confirmed that one MN application permits transdermal delivery of naltrexone (NTX) for 48 – 72 hours in humans (11). Topical application of diclofenac, a non-specific cyclooxygenase (COX) inhibitor, prolongs micropore lifetime in hairless guinea pigs, allowing for seven day NTX delivery (8), and proof-of-concept human studies have demonstrated a similar trend with impedance spectroscopy as a surrogate marker (12). Prolonging micropore lifetime alone is not beneficial, however, unless an active drug moiety can be delivered through the micropores for a clinically relevant timeframe in humans (seven days, ideally). The objective of the present work was to collect pilot pharmacokinetic data in humans demonstrating seven day NTX delivery following one MN treatment and application of a NTX gel, with co-application of diclofenac or placebo gel.
Materials and Methods
Drug formulations
The following components were purchased through the University of Kentucky: Solaraze® gel (PharmaDerm, Melville, NY), naltrexone HCl (Covidian/Mallinckrodt, Hazelwood, MO), benzyl alcohol (Fisher Scientific, Hanover Park, IL), polyethylene glycol monomethyl ether (Dow Chemicals, Louisville, KY), and propylene glycol (VWR, Atlanta, GA). Hyaluronate sodium (Rita Corporation, Crystal Lake, IL) and hydroxyethylcellulose (Ashland Specialty Ingredients, Wilmington, DE) were gifts from the companies.
A commercially available preparation of diclofenac (Solaraze® gel, containing 3% diclofenac sodium, 2.5% hyaluronate sodium) was used to topically deliver diclofenac. The NTX gel was compounded as follows: 110 mg/ml NTX•HCl, propylene glycol (10% v/v), benzyl alcohol (1% v/v), water (89% v/v), and hydroxyethylcellulose (2% w/v). A 2.5% hyaluronate sodium placebo gel was compounded with polyethylene glycol monomethyl ether (20% v/v), benzyl alcohol (1% v/v), water (79% v/v), and hyaluronate sodium (2.5% w/v). All gels were compounded in the Investigational Drug Services Pharmacy.
Preparation of microneedles and occlusive patches
Stainless steel arrays of 50 MNs were assembled into adhesive patches with ARcare® 7717 (Adhesives Research, Inc., Glen Rock, PA) to enhance the contact between the rigid array and the flexible skin. Each MN was 800 μm long and 200 μm in width at the base; all arrays were ethylene oxide sterilized. Design and geometry of the arrays were specified by the Prausnitz lab at the Georgia Institute of Technology.
Blank patches were made to occlude the treatment sites and drug gels. A medical-grade rubber ring was fabricated with a drug-impermeable membrane on one side (Scotchpak 1109 SPAK 1.34 MIL heat-sealable polyester film; 3M, St. Paul, MN), secured to the ring with 3M double-sided medical tape. The other side of the ring had a layer of double-sided tape to hold the patches to the skin. The patches were further secured to the skin with waterproof Bioclusive® tape (Systagenix Wound Management, Quincy, MA).
Microneedle application
The same investigator applied all MN treatments (to eliminate inter-investigator variability). The array was pressed gently into the skin for 15 – 20 seconds and immediately removed, creating a grid of 50 micropores. A 2nd insertion was applied, rotating the array 45° to c reate a total of 100 non-overlapping micropores.
Clinical procedures
This was an open-label pharmacokinetic study carried out in healthy human volunteers with no history of dermatologic disease. All procedures were approved by the Institutional Review Board and complied with the principles set forth by the Declaration of Helsinki. All subjects provided written informed consent before enrolling. Suitability for the study was determined through baseline blood samples (including chemistries and cell counts), urine samples (urinalysis, pregnancy test if applicable, and drug of abuse screen), and a complete drug/medical history and physical exam. Subjects with a history of opioid or alcohol abuse or hepatitis were excluded.
Subjects were randomly assigned into 3 groups: MN + diclofenac + NTX (Group 1, n = 6), MN + placebo + NTX (Group 2, n = 2), and diclofenac + NTX, no MN treatment (Group 3, n = 2). Eight sites were marked on the upper arm (Groups 1 and 3), and two sites were marked for subjects in Group 2. All treatment sites, regardless of assigned group, were wiped with 70% isopropyl alcohol pads and allowed to dry. Subjects assigned to MN groups were treated with MN arrays at each site before gel patch application. Group 1 received 2×8 MN array insertions per subject, creating 800 total micropores for eight patch sites; Group 2 received 2×2 MN array insertions, creating 200 total micropores.
In Group 1, each MN site was treated with 3% diclofenac gel (100 μl, rubbed gently into the skin), followed by NTX gel (500 μl) and occlusion with a blank patch. For Group 2, placebo gel replaced the diclofenac gel. Group 3 received application of the same gels as Group 1, in the absence of MN treatments. In all groups, two non-MN treated control sites (opposite arm) received identical gel applications as the MN sites. For all groups, the NTX gel was applied immediately following the diclofenac or placebo gel treatment. Every 48 hours the patches were removed for visual inspection of the skin for irritation; the gels were then replenished and occluded with clean patches. Gels and patches were removed on the last day.
Calculation of patch number
In the first human MN pharmacokinetic study with NTX, four patches were applied to each subject (11). This was calculated based on the equation: A = Cl * Css * Jss where Cl is the systemic clearance of NTX (3.5 L/min), Css is the target steady state concentration (2 ng/ml) and Jss is steady state NTX flux (39.0 ± 13.1 nmol/cm2•hr). This yielded a mean (± SD) NTX plasma concentration of 2.5 ± 1.0 ng/ml. For the current study, the in vitro flux for diclofenac + NTX was 42.58 ± 7.88 nmol/cm2•hr, and 170.84 ± 32.12 nmol/cm2•hr for placebo gel + NTX. In order to target a higher plasma NTX concentration (4 ng/ml), eight MN sites were necessary for the subjects in Group 1, vs. two sites for Group 2.
Pharmacokinetic sampling schedule
On day 1, subjects came to the outpatient research unit in the Center for Clinical and Translational Science. An indwelling catheter was inserted into the antecubital vein and a single blood sample was drawn as a blank baseline. Following MN treatment and NTX patch administration, serial blood samples (four ml each) were obtained at 15, 30, 45, and 60 minutes and at 1.5, 2, 4, 6, and 8 hours. At the end of the day the catheter was removed from the arm and subjects went home. They returned to the clinic daily for the remainder of the study, and individual venipunctures were performed for all additional time points. For days 2 – 6, one sample was drawn every 24 hours. On days 7 and 8, samples were drawn at 24 and 30 hours following the previous points. Samples were immediately centrifuged at 1308 × g for 10 minutes; the plasma was pipetted off and stored at −80°C until analysis.
Plasma assay
The plasma extraction procedure was similar to that described previously (11, 13). All samples were analyzed on an LC-MS/MS system; positive mode atmospheric pressure chemical ionization was used for detection. Multiple reaction monitoring was carried out with the following parent to daughter ion transitions for NTX•HCl and NTXol•HCl: m/z 341.8→323.8, m/z 343.8→325.8, respectively. Plasma standards (range of 1 – 75 ng/ml of NTX•HCl and NTXol•HCl) were made by spiking blank human plasma with working standards, followed by the extraction. All standards displayed excellent linearity over the whole concentration range (R2 ≥ 0.97).
Impedance spectroscopy and micropore closure
Micropore formation and lifetime were assessed via impedance spectroscopy, as previously described (12). Measurements were made at baseline, post-MN, and on the last day. Gel Ag/AgCl measurement electrodes (S&W Healthcare Corporation, Brooksville, FL) were held to the skin by the thumb of the investigator; a large electrode with a conductive gel surface served as the reference (Superior Silver Electrode with PermaGel, Tyco Healthcare Unit-Patch, Wabasha, MN). Measurements were made by connecting lead wires to measurement and reference electrodes; the opposite ends were connected to an impedance meter (EIM-105 Prep-Check Electrode Impedance Meter; General Devices, Ridgefield, NJ). A low frequency alternating current was modified with a 200 kΩ resistor in parallel (IET labs, Inc., Westbury, NY). Three parallel pathways can be distinguished:
where Ztotal is the raw measurement, Zbox represents the 200 kΩ resistor in parallel, and Zskin is estimated from the intact skin sites. This allows for calculation of the impedance specifically at the micropores, assuming that they occupy approximately 2% of the total area under the electrode. This also allows for estimation of an “upper limit” at the 2% surface area, providing a reference point for evaluating micropore closure. On Day 1, each site’s own intact skin baseline was used to calculate the upper limit and the difference in pre- vs. post-MN Zpores values; at Day 8 the same process was followed (Zskin estimated from non-MN control sites). This method controls for the mismatch occurring from the differing skin hydration status from baseline to the end of the study (baseline measurements are made on dry skin, while end of study measurements are made on skin that has become hydrated under an occlusive patch).
An additional means of monitoring micropore formation is to calculate the permeable area (Apermeable) as follows (10):
where ρ represents the interstitial fluid electrical resistivity in the skin (~78 Ω-cm), L is the estimated length of the diffusional pathway in the SC (~15 μm), and Z is the absolute impedance. Calculating the Apermeable further allows for determination of each individual micropore radius, assuming a cylindrical shape and that each micropore occupies 1/100 of the total permeable area.
Data analysis
Plasma NTX and NTXol concentration vs. time profiles were assessed by fitting the data to a noncompartmental model with extravascular input (Phoenix™ WinNonlin®, version 6.3, Pharsight Corporation, Mountain View, CA). Peak concentration (Cmax), steady state concentration (Css), lag time (Tlag), and area under the plasma concentration time curve from 0 to 174 hours (AUC0 – 174 hr) were determined. Steady state NTX concentration was calculated as follows: Css = AUC0–t/time. Clast was the plasma concentration at the last time point. Statistical analysis was performed with Student’s t-tests (GraphPad Prism®, version 5.04).
Results
Nine studies were completed in ten subjects, mean (± SD) age of 26.4 ± 3.4 years (demographics in Table I). Six subjects enrolled in Group 1 (MN + diclofenac + NTX), and two subjects enrolled in each control group (Group 2: MN + placebo + NTX, Group 3: diclofenac + NTX). One subject completed the study twice in a crossover design, in Groups 1 and 3 (one week washout in between).
Table I.
Subject demographics across nine healthy human volunteers.
| Sex | 4 male 5 female |
| Mean age, years (SD) | 26.4 (3.4) (range 23 to 32) |
| Mean body mass index, kg/m2 (SD) | 27.9 (6.9) (range 18.5 – 42.2) |
| Race | 8 Caucasian 1 African American |
Micropore impedance
As a surrogate measure of micropore lifetime, micropore impedance (Zpores) and total micropore permeable area (Apermeable) were calculated (described below). In Groups 1 and 2, Apermeable was significantly higher post-MN treatment compared to baseline (p < 0.05, Student’s t-test), demonstrating the presence of new pathways for drug diffusion. The calculated micropore radii was within the range of previous reports (10): 1.8 ± 0.4 μm in Group 1 (n = 46 measurements in six subjects), and 1.3 ± 0.04 μm in Group 2 (n = 4 measurements in two subjects).
In all subjects treated with MNs, Zpores decreased significantly from baseline to post-MN (p < 0.05, Student’s t-test), confirming an adequate breach of the SC barrier (n = 50 measurements in eight subjects; two measurements thrown out as outliers). At study completion, Zpores was significantly lower than intact skin control sites (p < 0.05, Student’s t-test) for all subjects in Group 1 (n = 44 measurements in six subjects, four measurements thrown out as outliers), suggesting prolonged micropore lifetime. In contrast, MN-treated sites had reached their Zpores “upper limit” in both subjects in the placebo treatment group (n = 4 measurements in 2 subjects), demonstrating micropore closure. Representative impedance profiles are displayed in Figure 1.
Figure 1. Representative impedance profiles from one subject in Group 1 (MN + diclofenac + NTX) and one subject in Group 2 (MN + placebo + NTX).
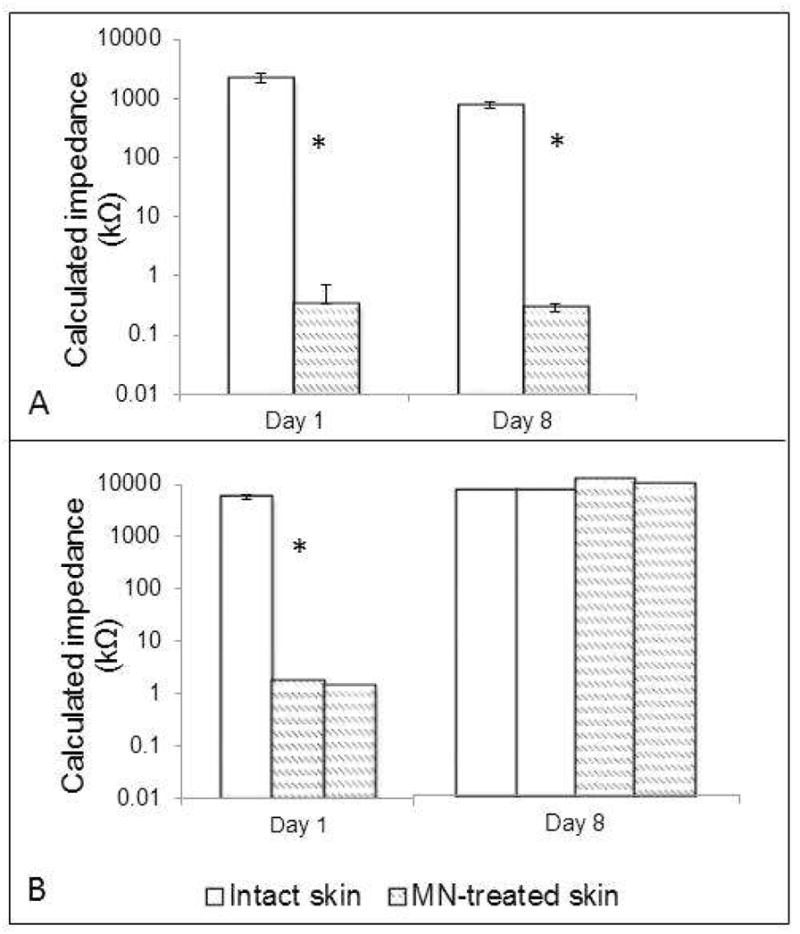
In both subjects, the Zpores decreased significantly from baseline to post-MN (signified by an asterisk), demonstrating a breach of the SC via the creation of micropores.
1A: Representative subject from Group 1. At the end of the study, a significant difference was still present between intact skin (white bars) and the MN-treated sites (hatched bars). All bars represent the mean of all 8 treatment sites.
1B: Represenative subject from Group 2. The Zpores had reached its upper limit by Day 8 in the subject from Group 2, confirming that the micropores had healed (thus preventing further drug delivery). On Day 1, the white bar (intact skin) represents the mean impedance of the 4 treatment sites; all other bars represent individual measurements at each site (the bars represent individual MN-treated sites, since subjects in this group only had 2 sites treated with MNs).
Pharmacokinetic parameters
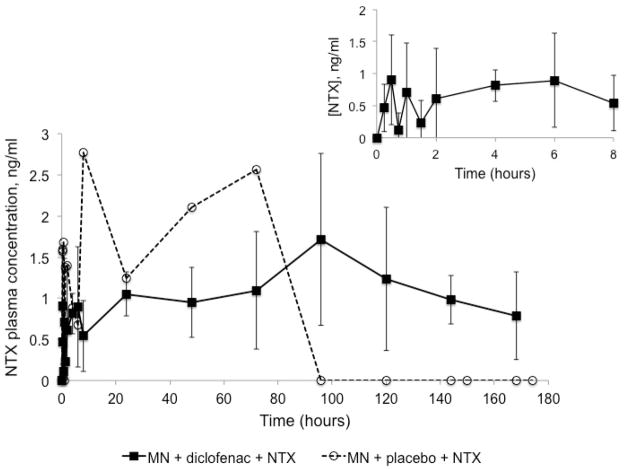
Pharmacokinetic data is displayed in Table II. No NTX was detected in Group 3 (no MN treatment). In contrast, NTX was delivered for the duration of the study for all subjects in Group 1 (n = 6), with a mean ± SD AUC0 – 174 hr of 196.5 ± 37.7 ng•h/ml, compared to the AUC0 – 174 hr of 188.1 ng•h/ml in one subject in Group 2 (the other subject in Group 2 did not have detectable levels). Lag time (Tlag) was short, at 0.4 ± 0.8 hours (Group 1) and 0 hours (Group 2). Cmax and Css were similar between groups: Cmax of 2.6 ± 0.7 ng/ml (Group 1) and 2.8 ng/ml (Group 2); Css values were 1.2 ± 0.3 ng/ml and 1.1 ng/ml, respectively. Tmax was notably different between groups. This parameter was variable amongst subjects in Group 1, with a mean of 112.0 ± 62.7 hours, compared to a Tmax of 8 hours in Group 2. In all subjects in Group 1, however, NTX was detectable until the end of the study; in contrast, NTX was not detectable after 72 hours in Group 2. Plasma NTX profiles can be seen in Figure 2.
Table II.
Pharmacokinetic parameters for naltrexone (NTX) and its active metabolite, 6-β-naltrexol (NTXol) in human plasma.
| NTX | NTXol | |||||
|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 | Group 1 | Group 2 | Group 3 | |
| Css, ng/ml | 1.2 (0.3) | 1.1 | NA | 2.0 (0.6) | NA | NA |
| Tlag, h | 0.4 (0.8) | 0 | NA | 2.8 (1.4) | 48.0 | NA |
| Cmax, ng/ml | 2.6 (0.7) | 2.77 | NA | 3.5 (1.3) | 0.49 | NA |
| Tmax, h | 112.0 (62.7) | 8 | NA | 126.0 (41.3) | 48.0 | NA |
| Clast, ng/ml | 1.4 (0.4) | 0 | NA | 1.89 (1.4) | 0 | NA |
| AUC0–174 hr ng•h/ml | 196.5 (37.7) | 188.1 | NA | 335.8 (103.6) | 18.98 | NA |
Group 1 received treatment with MN + diclofenac + NTX gel (Group 3 received the same treatment but with no MN application), and Group 2 was treated with placebo gel.
Figure 2. NTX plasma profiles following one-time MN treatment and application of diclofenac and NTX gel every 48 hours for 7 days post-MN.
NTX was detected to the end of the study for all subjects in Group 1 (MN + diclofenac + NTX, n = 6), with a mean AUC0 – 174 hr of 196.5 ± 37.7 ng•h/ml, which correlated with the impedance measurements that predicted micropore lifetime for approximately 3 – 7 days with application of diclofenac. Conversely, NTX was only detected until 72 hours in Group 2 (MN + placebo + NTX, n = 1).
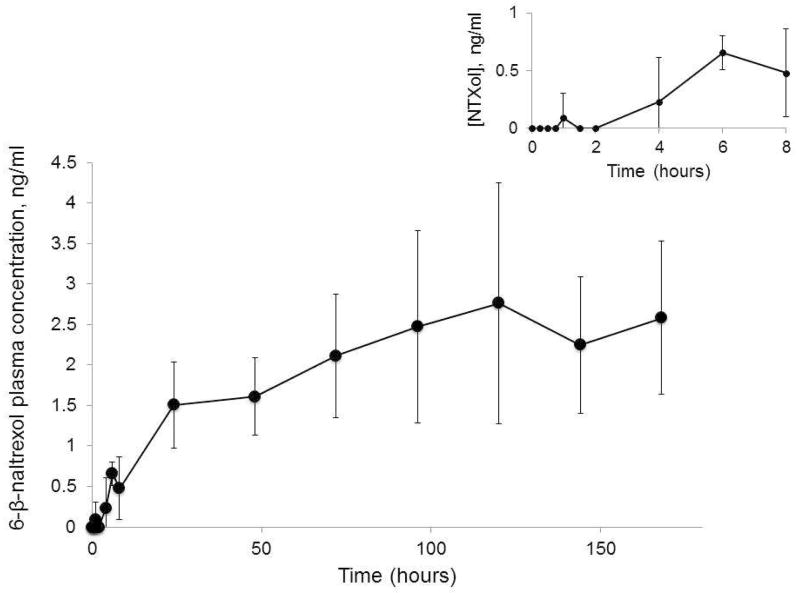
Plasma concentrations of the active metabolite, NTXol, were also quantified. NTXol was detectable at only one timepoint (48 hours) for the subject in Group 2. However, it was detected from one hour onward for subjects in Group 1, with a mean AUC0 – 174 hr of 335.8 ± 103.6 ng•h/ml. In contrast to NTX, a longer delay to max NTXol concentration was observed, with a Tmax of 126 ± 41.3 hours. NTXol profiles can be seen in Figure 3.
Figure 3.
NTXol plasma profiles following one time MN treatment and application of diclofenac and NTX gel every 48 hours for 7 days post-MN treatment (Group 1, n = 6 subjects).
Treatment tolerability
MN insertion and gel application were well tolerated. For most subjects, mild erythema was noted under the waterproof tape that secured the patches to the skin, but little (if any) redness was observed at the MN and gel treatment sites. The mild irritation from the tape resolved in all subjects over the course of hours to a few days. One subject developed mild dermatitis at the MN sites, with erythema and pruritus over the MN insertion grid; this subject was withdrawn a day early. The irritation and pruritus subsided after a short treatment course with 1% hydrocortisone cream.
The systemic adverse event profile was favorable. The most common side effects included nausea and general gastrointestinal upset, mild dysphoria or anxiety, and sleep disturbances (primarily vivid dreams). No subjects withdrew because of adverse events. These systemic adverse events were consistent with those observed in the previous NTX pharmacokinetic study in humans (11).
Discussion
Naltrexone is an FDA approved opioid antagonist used for the treatment of opioid and alcohol addiction, but several problems exist with currently available formulations of NTX. Extensive first-pass metabolism and hepatotoxicity are associated with the oral formulation (ReVia®), and the monthly injectable formulation (Vivitrol®) comes with a high cost and inconvenience for obtaining treatment. Furthermore, the depot injection presents significant challenges in emergency situations when acute pain treatment with opioids is necessary (removing the NTX depot from the skin to remove opioid blockage is both painful and difficult). These properties create significant challenges for chronic treatment, which is required for maintaining abstinence and preventing relapse. In light of the downfalls of the current NTX formulations, transdermal delivery would provide several key advantages, specifically: 1) decreased potential for hepatotoxicity, via avoidance of the first-pass effect; 2) simplicity of application and likely enhanced patient compliance; and 3) ease of removal in the event of an acute need for emergency pain relief. Despite the clear benefits of transdermal delivery, NTX does not possess ideal physicochemical characteristics to allow it to be delivered at a therapeutic rate through the SC via passive diffusion. It is not hydrophobic enough to passively traverse through the SC, but possesses sufficient hydrophilicity to partition into the interstitial fluid and aqueous environment in the deeper layers of the skin. For these reasons, NTX is a poor candidate for traditional transdermal delivery, but it is an ideal candidate for MN-enhanced delivery. The micropores create aqueous pathways permitting NTX to pass through the SC and be readily measured in the plasma. In fact, NTX was the model compound in the first pharmacokinetic study describing successful delivery of a drug to therapeutic concentrations with the “poke/press and patch” method in humans (11). While therapeutic concentrations were maintained during the study, variability increased between 48 – 72 hours post-MN, and not all subjects maintained therapeutic levels beyond 48 hours.
These results suggest that the micropores were beginning to re-seal and the skin was restoring its barrier properties. A similar delivery timeframe was observed when NTXol was administered to hairless guinea pigs with the same approach (9). The current study demonstrates that topical application of diclofenac to MN-treated skin can maintain micropore viability, allowing NTX delivery for seven days. The shapes of the pharmacokinetic profiles demonstrate sustained delivery over the whole timeframe, in stark contrast from the placebo condition. In that case, the Cmax was reached at 8 hours, followed by a sharp decline after 72 hours, demonstrating full closure of the micropores under the predicted timeframe of 2 – 3 days.
Predicting drug delivery timeframes
Impedance spectroscopy has been utilized previously to monitor micropore formation and lifetime (10, 12). This technique provides multiple options for monitoring micropore lifetime, through calculation of Zpores (impedance at the microporated skin), radii of individual micropores, and Apermeable (permeable area of the entire micropore grid). In both MN groups, adequate micropore formation was confirmed through a significant difference in Zpores and Apermeable from baseline values, and micropore radii were similar to previous reports (10).
A drug delivery window of ~2 – 3 days was predicted from Zpores calculations under placebo conditions, based on a micropore half-life of 0.76 days (12). This correlates precisely with placebo data in the current study, matches predictions from Gupta et al, and is in agreement with previous pharmacokinetic studies (8–11). Furthermore, our previous impedance data suggested that diclofenac prolongs drug delivery approximately 1.76 ± 0.62 times over placebo, resulting in a window of 3.4 – 7.1 days (12). The current data agrees with these values, as NTX was not detectable after 72 hours in the placebo subject, whereas subjects in the diclofenac + NTX group had drug delivery for seven days. These data demonstrate the capability of impedance spectroscopy to closely predict a drug delivery window; this valuable technique could also be extrapolated to other microporation physical enhancement techniques (thermal ablation, electroporation, etc.) to expand the possibilities of prolonging the skin’s re-sealing time after a one-time breach.
Naltrexone delivery considerations
In the first MN-assisted study with NTX, four patches were applied (400 micropores total, 100 micropores/patch) (11). Patch calculations were based on in vitro NTX flux of 39.0 ± 13.1 nmol/cm2•hr, with a target plasma NTX concentration of 2 ng/ml. The in vitro-in vivo correlation was very good, with an observed steady state concentration of 2.5 ± 1.0 ng/ml (11). In the current study, in vitro NTX flux in the presence of diclofenac was 42.58 ± 7.88 nmol/cm2•hr. In order to achieve higher plasma concentrations, a steady state NTX plasma concentration of 4 ng/ml was targeted. Thus, eight patches were required to achieve the target Css (800 total micropores). In contrast, only two MN-treated sites with patches were calculated for the placebo condition (200 micropores total), based on the in vitro flux of 170.84 ± 32.12 nmol/cm2•hr. This notable difference in NTX flux between the active and placebo conditions is most likely due to a chemical incompatibility between diclofenac and naltrexone that results from mixing an acidic and a basic molecule, respectively. This could potentially create a challenge for delivering other weak base compounds in the presence of diclofenac, though we have not specifically examined this in vitro or in vivo. While the ideal conditions would have allowed comparison of NTX flux through an equal number of micropores in the diclofenac and placebo groups, this would have resulted in 4 times more NTX delivery in the placebo group than is necessary to achieve the target plasma concentration. Therefore, for reasons of safety and tolerability we did not treat the subjects in Group 2 with 800 micropores, but rather applied MNs to create the appropriate number of micropores relative to the NTX flux.
In the MN treatment groups, NTX delivery was as predicted: seven days in the presence of diclofenac vs. no delivery after 72 hours with placebo. The sum of NTX and NTXol achieved a mean ± SD Css plasma concentration of 3.18 ± 0.7 ng/ml (Group 1), which is very close to the predicted value of 4 ng/ml. NTXol is the primary metabolite of NTX, with a negligible difference in molecular weight (341.8 and 343.8 for NTX and NTXol, respectively). Thus, the sum of NTX and NTXol can be compared directly to the predicted NTX concentration, assuming approximately 1:1 conversion of NTX to NTXol. This also represents the most accurate clinical scenario, as near zero-order delivery of NTX will result in the presence of the metabolite over the entire treatment course. Thus, the in vitro-in vivo correlation is 79.5%, which is excellent for human clinical studies.
One placebo subject did not have detectable plasma NTX levels during the study, but there are a few possibilities to explain this. This individual had a higher BMI and his blood volume may have been larger, requiring higher amounts of drug to reach detectable concentrations. He was the only African American subject, likely contributing to greater inter-subject variability. Recovery has been shown to be faster in darker skin following acute barrier perturbation, SC lipid content is higher in African Americans, and increased electrical resistance of the skin in African Americans suggests increased SC thickness (14). However, despite not having a plasma profile to compare to other subjects, his impedance data followed the expected trend. Zpores at MN-treated sites reached the upper limits at the end of the study, confirming micropore closure under placebo conditions.
NTX flux from this formulation through intact skin has been described as 8 nmol/cm2•hr, which is negligible for systemic delivery (15). To confirm this, 10 patches were applied to non-MN treated skin in two subjects. There was no detectable NTX in either subject, confirming the lack of flux without micropores. Thus, the plasma concentrations observed in the MN groups is attributable to delivery through the micropores, rather than passive diffusion. This was also confirmed by the crossover subject, who had detectable NTX levels following MN treatment, but not when patches were applied to intact skin.
Limitations
There are some limitations to this work. There was a large amount of variability in the data, especially after the first 48 hours. This could be the result of several factors, many of which are simple to correct. First, the study was conducted in a small sample size (n = 9 subjects) due to the proof-of-concept nature of the work. Substantial variability is not surprising in a small sample size, and we would expect the variation to be significantly reduced when the study is conducted in a larger sample. Second, the gel patch conditions were not ideal (600 μl, a reasonably large amount for a small patch), and may have caused the amount of NTX in contact with the micropores (and thus available for systemic delivery) throughout the entire study to further contribute to the variability. The patch system would be improved for a drug product.
The concern of high gel volume also highlights an additional limitation of this work, in that the application of two gels after MN treatment is not clinically reasonable for reasons of patient acceptance/compliance and regulatory concerns. Three steps for drug delivery (MN insertion plus two gel applications) is clearly not the ideal clinical situation. This would also present regulatory challenges, as both gel formulations and the MN delivery device would require approval prior to commercialization. However, ongoing research efforts will present some appealing means of overcoming these issues. Recent work from Ghosh et al. has demonstrated that diclofenac and NTX can be integrated into a codrug, which consists of diclofenac and NTX joined together with a covalent link (16). The codrug cleaves in the skin, allowing concurrent delivery of diclofenac and NTX together from one gel application. This codrug strategy removes the physical incompatibility of the two compounds, allowing co-delivery with one gel application. Integrating the codrug formulation into an extended-release patch would eliminate the concerns of the need to apply two separate gels, and would require regulatory approval of a new chemical entity patch formulation. Furthermore, larger pharmaceutical companies (i.e. 3M, Zosano) are developing MN delivery systems that provide MN insertion and patch application from one reusable device. This type of device, when combined with a diclofenac/NTX codrug patch, will provide the ideal one-step application process; which could be approved as one delivery system, rather than three distinct approvals combined for a single product.
The final limitation to this work is our incomplete understanding of the mechanisms by which diclofenac delays micropore closure. Previous work from Banks et al. and Brogden et al. (in addition to the current study) has demonstrated that diclofenac prevents micropores from closing in animals and humans, demonstrated by both pharmacokinetic analysis and impedance spectroscopy (8,12). All of these studies have been performed under the hypothesis that subclinical local inflammation contributes to the micropore closure process, as one of the first responses to a micron-scale breach in the skin. However, the exact mechanisms underlying micropore closure have not been elucidated (no research has specifically studied this), and it is possible that diclofenac may be exerting off-target effects beyond inhibiting local inflammation. It has been previously shown that diclofenac can modulate the skin immune system in vitro (17), and effects such as these need to be explored in the context of MN treatment to the skin. Furthermore, any effect of diclofenac on other barrier restoration processes in the skin (lipid synthesis, cytokine release, antimicrobial lipids and peptides) should be studied. A better understanding of the exact mechanisms of how diclofenac modulates micropore closure would allow for development of more targeted therapies to extend micropore lifetime, thus representing an exciting and novel direction for transdermal drug delivery techniques.
Conclusion
This is the first study in humans demonstrating that drug delivery can be achieved for a full week after one MN application, using the “poke/press and patch” method. Drug delivery windows were accurately predicted by impedance spectroscopy, allowing for extrapolation of the technique to other drug moieties and microporation techniques. These data demonstrate the immense potential of MN technologies for drug delivery over longer periods of time than what has been shown previously.
Table III.
Number of micropores created for each treatment group and the gels applied to the skin.
| Group | Total number of micropores | Gels applied | In vitro flux of NTX•HCl, nmol/cm2•hr | Predicted [NTX], plasma (ng/ml) | Actual [NTX], plasma (ng/ml) | Actual [NTX] + [NTXol], plasma (ng/ml) |
|---|---|---|---|---|---|---|
| 1 | 800 (8 patches) | Diclofenac + NTX | 42.58 ± 7.88 (n = 3) | 4 | 1.2 (0.3) | 3.18 (0.7) |
| 2 | 200 (2 patches) | Placebo + NTX | 170.84 ± 32.12 (n = 3) | 4 | 1.1 | 1.19 |
| 3 | NA | Diclofenac + NTX | NA | 0 | 0 | 0 |
Acknowledgments
We would like to acknowledge Dr. Mark Prausnitz and Dr. Vladimir Zarnitsyn (Georgia Institute of Technology) for their expertise regarding the MNs and impedance measurements, and the staff at the UK Center for Clinical and Translational Science for their assistance. This work was funded by the following NIH grants: CTSA 1UL1RR033173-01, R01DA13425, R42DA32191, 1F31DA029374, and the Clinical Loan Repayment Program. Other funding included the UK Center for Clinical and Translational Science Seed Grant.
Abbreviations
- COX
Cyclooxygenase
- MN
Microneedle
- NTX
Naltrexone
- NTXol
Naltrexol
- SC
Stratum corneum
Footnotes
Disclosure
Dr. Stinchcomb is the Chief Scientific Officer of AllTranz Inc., a specialty pharmaceutical company involved in the development of transdermal formulations for microneedle delivery; both she and Dr. Banks are significant shareholders in the company.
References
- 1.Prausnitz MR, Langer R. Transdermal drug delivery. Nat biotech. 2008 Nov;26(11):1261–8. doi: 10.1038/nbt.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arora A, Prausnitz MR, Mitragotri S. Micro-scale devices for transdermal drug delivery. Int J Pharm. 2008 Dec 8;364(2):227–36. doi: 10.1016/j.ijpharm.2008.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Therapeutic Delivery. 2010;1(1):109–31. doi: 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prausnitz MR. Microneedles for transdermal drug delivery. Adv Drug Deliv Rev. 2004 Mar 27;56(5):581–7. doi: 10.1016/j.addr.2003.10.023. Epub 2004/03/17. eng. [DOI] [PubMed] [Google Scholar]
- 5.Prausnitz MR, Mitragotri S, Langer R. Current status and future potential of transdermal drug delivery. Nat Rev Drug Discov. 2004 Feb;3(2):115–24. doi: 10.1038/nrd1304. Epub 2004/03/26.eng. [DOI] [PubMed] [Google Scholar]
- 6.Kim YC, Park JH, Prausnitz MR. Microneedles for drug and vaccine delivery. Adv Drug Deliv Rev. 2012 Nov;64(14):1547–68. doi: 10.1016/j.addr.2012.04.005. Epub 2012/05/12. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bal S, Kruithof AC, Liebl H, Tomerius M, Bouwstra J, Lademann J, et al. In vivo visualization of microneedle conduits in human skin using laser scanning microscopy. Laser Physics Letters. 2010;7(3):242–6. [Google Scholar]
- 8.Banks SL, Paudel KS, Brogden NK, Loftin CD, Stinchcomb AL. Diclofenac enables prolonged delivery of naltrexone through microneedle-treated skin. Pharm Res. 2011 May;28(5):1211–9. doi: 10.1007/s11095-011-0372-2. Epub 2011/02/09. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banks SL, Pinninti RR, Gill HS, Paudel KS, Crooks PA, Brogden NK, et al. Transdermal delivery of naltrexol and skin permeability lifetime after microneedle treatment in hairless guinea pigs. J Pharm Sci. 2009 Jul;99(7):3072–80. doi: 10.1002/jps.22083. Epub 2010/02/19. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta J, Gill HS, Andrews SN, Prausnitz MR. Kinetics of skin resealing after insertion of microneedles in human subjects. J Control Release. 2011 May 26; doi: 10.1016/j.jconrel.2011.05.021. Epub 2011/06/07. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wermeling DP, Banks SL, Hudson DA, Gill HS, Gupta J, Prausnitz MR, et al. Microneedles permit transdermal delivery of a skin-impermeant medication to humans. Proc Natl Acad Sci. 2008 Feb 12;105(6):2058–63. doi: 10.1073/pnas.0710355105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brogden NK, Milewski M, Ghosh P, Hardi L, Crofford LJ, Stinchcomb AL. Diclofenac delays micropore closure following microneedle treatment in human subjects. J Control Release. 2012 Oct 28;163(2):220–9. doi: 10.1016/j.jconrel.2012.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valiveti S, Nalluri BN, Hammell DC, Paudel KS, Stinchcomb AL. Development and validation of a liquid chromatography-mass spectrometry method for the quantitation of naltrexone and 6beta-naltrexol in guinea pig plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004 Oct 25;810(2):259–67. doi: 10.1016/j.jchromb.2004.08.016. Epub 2004/09/24. eng. [DOI] [PubMed] [Google Scholar]
- 14.Darlenski R, Fluhr JW. Influence of skin type, race, sex, and anatomic location on epidermal barrier function. Clinics in dermatology. 2012 May-Jun;30(3):269–73. doi: 10.1016/j.clindermatol.2011.08.013. Epub 2012/04/18. eng. [DOI] [PubMed] [Google Scholar]
- 15.Milewski M, Stinchcomb AL. Vehicle composition influence on the microneedle-enhanced transdermal flux of naltrexone hydrochloride. Pharm Res. 2011 Jan;28(1):124–34. doi: 10.1007/s11095-010-0191-x. Epub 2010/06/26. eng. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh P, Pinninti RR, Hammell DC, Paudel KS, Stinchcomb AL. Development of a codrug approach for sustained delivery across microneedle treated skin. J Pharm Sci. 2013 Jan; doi: 10.1002/jps.23469. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 17.Toebak MJ, de Rooij J, Moed H, Stoof TJ, von Blomberg BM, Bruynzeel DP, Scheper RJ, Gibbs S, Rustemeyer T. Differential suppression of dendritic cell cytokine production by anti-inflammatory drugs. Br J Dermatol. 2008;158:225–233. doi: 10.1111/j.1365-2133.2007.08297.x. [DOI] [PubMed] [Google Scholar]