Abstract
Background
Although chronic kidney disease (CKD) is a highly prevalent condition among older adults with diabetes, the associations between health-related quality of life (HRQOL) and severity of CKD in this population are not well understood. The objective of this study was to assess HRQOL and depressive symptoms across estimated GFR (eGFR) stages.
Study Design
Cross-sectional.
Setting & Participants
Participants included 5,805 members of Kaiser Permanente Northern California age 60 or older with diabetes from the 2005–2006 Diabetes Study of Northern California (DISTANCE) survey.
Predictor
eGFR categories were defined as ≥90 (referent category), 75–89, 60–74, 45–59, 30–44 or ≤29 ml/min/1.73m2.
Outcomes
HRQOL was measured using the modified Short Form 8 Physical Component Summary (PCS) and Mental Component Summary (MCS) scores. Depressive symptoms were measured using the Patient Health Questionnaire 8.
Results
In unadjusted linear regression analyses, physical (PCS) and mental (MCS) HRQOL scores were significantly lower with worsening eGFR level. However, after adjustment for sociodemographics, diabetes duration, obesity, and cardiovascular comorbidities, and taking into account interactions with proteinuria, none of the eGFR categories were significantly or substantively associated with PCS or MCS score. In both unadjusted and adjusted analyses, higher risk of depressive symptoms was observed among respondents with eGFR ≤29 ml/min/1.73m2 (relative risk, 2.02; 95% CI,1.10–3.71; p<0.05) compared with the referent group. However, this eGFR-depression relationship was no longer significant after adjusting for hemoglobin levels.
Limitations
Participants are part of a single health care delivery system.
Conclusions
Our findings suggest the need for greater attention to and potential interventions for depression in patients with reduced eGFR.
Chronic kidney disease (CKD) is a recognized public health concern with significant associated morbidity and mortality. 1 Nearly one third of community-dwelling adults older than age 70 meet the criteria for CKD when defined as an estimated glomerular filtration rate (eGFR) less than 60ml/min/1.73m2.2 Older adults also experience higher rates of having eGFR <30ml/min/1.73m2 than younger persons in part due to higher rates of diabetes and hypertension; further, older adults are the fastest growing population of incident dialysis patients.3
Decisions about how to manage CKD and when to initiate dialysis are clinically challenging, especially for older patients with diabetes who are more likely to have CKD compounded by high levels of co-morbidity, including depression, cognitive impairment, physical disability, and frailty.4–7 Maintaining or improving quality of life should be an important consideration in guiding CKD treatment decisions.
Only a small number of studies have described health-related qualify of life (HRQOL) or depression in patients with CKD. One previous study by Mujais, et al., showed that in advanced CKD (stages 3–5), physical HRQOL is more often affected than mental HRQOL, and patients with advanced CKD have lower HRQOL scores than the general population.8 Prior studies that have evaluated depressive symptoms among earlier stages of CKD have shown a high prevalence, some exceeding 50% of the population.9–12 While these studies have been valuable, the results have limited generalizability due to single center populations, small sample sizes, or a focus on patients in specialized clinics.
We performed a cross-sectional assessment of HRQOL and depressive symptoms across eGFR categories among older adults with diabetes. This research was conducted as part of The Diabetes and Aging Study, an ancillary study to the Diabetes Study of Northern California (DISTANCE) survey. The Diabetes and Aging Study is a collaborative investigation to address significant gaps in existing knowledge regarding the natural history, service use and self-care of a multi-ethnic population of older adults with diabetes.
Methods
Study Design
In 2005–2006, the DISTANCE survey collected information about demographics, health behaviors, social support, and patient-provider interactions from a race-stratified, random sample of members of the diabetes registry of Kaiser Permanente Northern California, an integrated healthcare delivery system. The diabetes registry consists of all patients within the Kaiser Permanente Northern California health system with diabetes identified from automated databases of pharmacy data, laboratory data, hospitalization records, and outpatient diagnoses as having diabetes using standardized criteria. The DISTANCE survey yielded 20,188 respondents (62% response rate) based on a standard algorithm from the Council of American Survey Research Organization.13 Data collection methods have been described in detail previously.14,15 Survey data were linked to respondents’ electronic medical records including laboratory results and medical diagnoses. All laboratory tests were conducted by a single regional laboratory and eGFR was calculated using isotope-dilution mass spectrometry (IDMS) standardized measurements of creatinine.16 In this study, we included DISTANCE survey respondents who were age 60 years or older at the time of the survey (baseline) and had at least one eGFR value in the 15 months preceding baseline.
Exposure of Interest
The exposure of interest was eGFR stage as calculated by the CKD-EPI (KD Epidemiology Collaboration) creatinine equation,17 adjusted for gender and race. Estimated GFR categories were defined as ≥90, 75–89, 60–74, 45–59, 30–44, and ≤−29 (not receiving dialysis) ml/min/1.73m2. The referent group was defined as eGFR ≥90 ml/min/1.73m2. Where possible, the eGFR category was assigned using the last two consecutive eGFR values (separated by at least 3 months) within the 15 months preceding survey response (n=4,255 [73%]). If the two eGFR values were discrepant in terms of category, we assigned the respondent to the higher value eGFR category. If only one eGFR value was available, it alone was used to define eGFR stage (n=1,550 [27%]).
Outcomes of Interest
Health-related quality of life
HRQOL was assessed using an instrument based on the Short Form 8 (SF-8) that includes one question for each domain of the Medical Outcomes Study 36-Item Short Form Health Survey (SF-36). The eight domains are: 1) physical functioning, 2) role limitations due to physical problems; 3) pain; 4) general health; 5) vitality; 6) emotional well-being; 7) role limitations due to emotional problems; and 8) social function.18 Responses to each question were transformed into SF-36 equivalent scores and overall Physical Component Summary (PCS) and Mental Component Summary (MCS) HRQoL scores were calculated.19 This instrument is normalized so that in the general population the mean PCS and MCS scores are each 50 points with a standard deviation (SD) of 10 points. In adults 65–74 years of age, the mean PCS is 44.7 +/− 9.3 (SD) and MCS is 52.8 +/− 8.3 points.20 At least a one-point difference in SF-36 scores is considered the minimal cutoff point for clinical significance,21 but differences of 3–5 points have also been suggested.22 These differences in scores can be illustrated using changes to the first SF-8 question, “Overall, how would you rate your health during the past 4 weeks?” for which the response options are “excellent, very good, good, fair, poor, very poor.” Holding the responses to other questions constant, the difference in score for a response of “excellent” vs. “very good” is approximately one point; the difference in score for a response of “excellent” vs. “good” is approximately three points. For this study, continuous (means) and categorical (lowest quartiles) PCS and MCS scores were the outcomes of interest.
Depressive symptoms
Depressive symptoms were assessed using the Patient Health Questionnaire (PHQ-8), an eight-item survey previously validated for use in the primary care setting based on the DSM-IV diagnostic criteria for major depressive disorder.23,24 The PHQ-8 score ranges from 0 to 24 with scores ≥10 consistent with depression.24,25
Covariates
Covariates for this study included self-reported survey data: age, gender, race/ethnicity, marital status, household income, education level and duration of diabetes.15 For other covariates, we used diagnostic and procedure codes from outpatient and inpatient medical records measures within 15 months of each participant’s survey date. We included obesity categories, defined as underweight (body mass index [BMI], <18.5 kg/m2, normal (BMI, 18.5–24.9 kg/m2), overweight (BMI, 25.0–29.9 kg/m2), mildly obese (BMI, 30.0–34.9 kg/m2), or moderately/extremely obese (BMI, ≥35.0 kg/m2).26 A history of cardiovascular disease was defined by cerebrovascular disease (ICD-9 codes: 430–438.9), myocardial infarction (codes 410–410.92 and 412), congestive heart disease (codes 428–428.9), or peripheral vascular disease (codes 440.20–440.24, 440.31–440.32, 440.8, 440.9, 443.9, 441–441.9, 785.4, V43.4, and v43.4). Duration of diabetes was defined as time in years since diagnosis at the time of the survey. The presence of proteinuria was defined either as urinary protein-creatinine ratio ≥200 mg/g or as 24-hour urinary protein ≥150 mg per total volume or urinary albumin-creatinine ratio ≥300 mg/g.
Statistical Methods
The DISTANCE survey used a stratified random sampling design that over-sampled minority patients to provide adequate power for racial/ethnic contrasts. To account for this sampling design, we weighted analyses using expansion weights (reciprocal of the non-proportional sampling fractions for each ethnic group) in all multivariable models. Additionally, we addressed survey non-response bias in the analysis using the Horvitz-Thompson approach 27 which entailed fitting a model which predicted response to the DISTANCE survey, then creating individual weights (reciprocal of the probability of the observed response) and applying the weights to our statistical models.28
We specified multivariable linear regression models to evaluate the association between eGFR category and mean PCS or MCS, and calculated the adjusted means at each eGFR category, keeping all other covariates at their mean values. We then specified modified Poisson regression models with a log link function and robust error variance to directly estimate the 1) relative risk (RR) of scoring in the lowest quartile of PCS or MCS and 2) RR of reporting depressive symptoms as measured by the PHQ-8 (score ≥10).29 Starting with a base (unadjusted) model, we sequentially introduced blocks of covariates to evaluate their effects on the main exposure variable, eGFR category. Model 1 was unadjusted; Model 2 was adjusted for respondent sociodemographics (age, gender, race/ethnicity, marital status, household income, and education level); Model 3 was adjusted for sociodemographics, obesity, duration of diabetes and proteinuria; and Model 4 was further adjusted for cardiovascular comorbidities. Model 5 was a sensitivity analysis to determine if hemoglobin level accounted for any significant effects observed between eGFR category and HRQOL or depressive symptoms from the fully adjusted Model 4. Finally, we specified models with an added interaction term (eGFR × proteinuria) to assess whether the potential effect of eGFR differed for those with versus without proteinuria. When interactions were significant, we then specified stratified models (separate models for those with and without proteinuria).
Results
Patient Characteristics
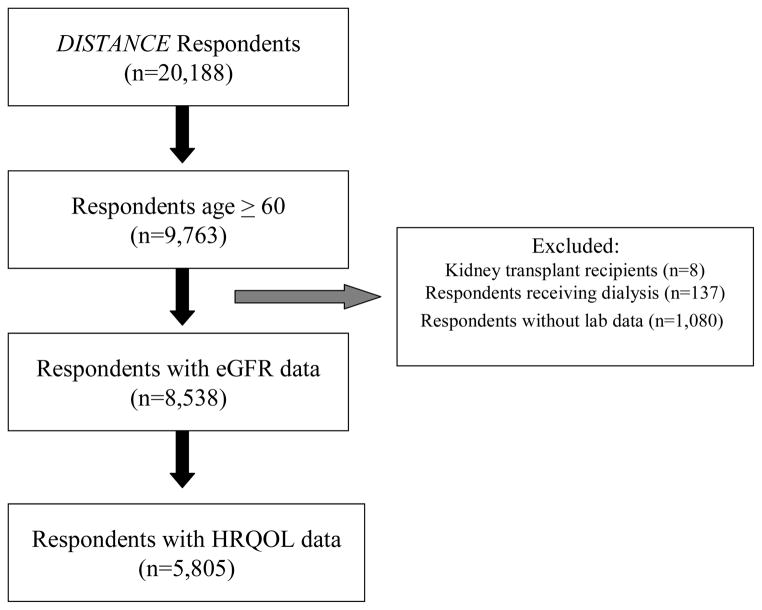
There were 5,805 respondents aged 60 years or older who had an identifiable eGFR measurement and who completed the HRQOL questionnaire. [Figure 1] The distribution of patients in each eGFR category (all in ml/min/1.73m2) was as follows: eGFR ≥90 (referent group), 1,222 (21.1%); eGFR 75–89, 1,513 (26.1%); eGFR 60–74, 1,602 (27.5%); eGFR 45–59, 959 (16.5%); eGFR 30–44, 392 (6.8%); and eGFR ≤29, 117 (2.0%) [Table 1]. The mean age was 67.2 ± 4.8 (SD) years, with the youngest mean age among respondents in the referent group. Lower eGFR categories were associated with higher proportions of women and lower household income. Respondents in lower eGFR categories had longer durations of diabetes, higher prevalences of cardiovascular disease and proteinuria, and lower mean hemoglobin levels.
Figure 1.
Flowchart of respondents
Table 1.
Characteristics of older adults with diabetes who participated in the DISTANCE survey, 2005–2006
| eGFR Category (mL/min/1.73 m2) | p-value | ||||||
|---|---|---|---|---|---|---|---|
| ≥90 (n=1,222) | 75–89 (n=1,513) | 60–74 (n=1,602) | 45–59 (n=959) | 30–44 (n=392) | ≤29 (n=117) | ||
| Age (y)* | 64.2± 3.7 | 67.2 ±4.2 | 68.1 ±5.0 | 68.7 ±4.7 | 69.2 ±4.5 | 68.2 ±4.7 | < 0.001 |
| Female sex | 653 (53.4) | 664 (43.9) | 724 (45.2) | 512 (53.4) | 220 (56.1) | 73 (62.4) | < 0.001 |
| Race/ethnicity | < 0.001 | ||||||
| Black | 218 (17.8) | 268 (17.7) | 296 (18.5) | 195 (20.3) | 94 (24.0) | 33 (28.2) | |
| Asian | 163 (13.3) | 226 (14.9) | 206 (12.9) | 117 (12.2) | 40 (10.2) | 9 (7.7) | |
| White | 264 (21.6) | 402 (26.6) | 500 (31.2) | 291 (30.3) | 120 (30.6) | 31 (26.5) | |
| Filipino | 136 (1.1) | 143 (9.5) | 154 (9.6) | 121 (12.6) | 39 (10.0) | 15 (12.8) | |
| Latino | 232 (19.0) | 255 (16.9) | 222 (13.9) | 110 (11.5) | 52 (13.3) | 14 (12.0) | |
| Multiracial | 159 (13.0) | 166 (11.0) | 164 (10.2) | 93 (9.7) | 40 (10.2) | 13 (11.1) | |
| Other | 50 (4.1) | 53 (3.5) | 60 (3.8) | 32 (3.3) | 7 (1.8) | 2 (1.7) | |
| Education level | < 0.001 | ||||||
| No degree | 184 (15.4) | 256 (17.2) | 238 (15.2) | 163 (17.4) | 65 (16.9) | 25 (21.7) | |
| High school or GED | 316 (26.5) | 398 (26.7) | 454 (29.0) | 293 (31.3) | 141 (36.6) | 40 (34.8) | |
| Associates degree or technical | 336 (28.1) | 358 (24.0) | 357 (22.8) | 220 (23.5) | 85 (22.1) | 22 (19.1) | |
| College, masters, doctorate | 358 (30.0 | 477 (32.0) | 515 (32.9) | 261 (27.9) | 94 (24.4) | 28 (24.4 | |
| Income | < 0.001 | ||||||
| <$25,000 | 248 (22.8) | 332 (24.9) | 348 (24.8) | 224 (26.7) | 107 (30.3) | 31 (30.4) | |
| $25–49,000 | 325 (29.9) | 430 (32.3) | 415 (29.6) | 294 (35.0) | 128 (36.3) | 38 (37.3) | |
| $50–79,000 | 262 (24.1) | 286 (21.5) | 329 (23.5) | 177 (21.1) | 77 (21.8) | 20 (19.6) | |
| −≥$80,000 | 251 (23.1) | 284 (21.3) | 310 (22.1) | 144 (17.2) | 41 (11.6) | 13 (12.8) | |
| Married | 825 (68.1) | 1,095 (72.8) | 1,108 (69.5) | 632 (66.5) | 257 (65.9) | 68 (58.1) | <0.001 |
| Obesity category | 0.001 | ||||||
| Underweight/normal | 198 (17.4) | 275 (19.5) | 265 (17.5) | 139 (15.2) | 46 (12.2) | 16 (14.2) | |
| Overweight | 391 (34.3) | 518 (36.7) | 547 (36.2) | 323 (35.3) | 109 (28.8) | 39 (34.5) | |
| Mildly obese | 278 (24.4) | 319 (22.6) | 383 (25.4) | 232 (25.4) | 115 (30.4) | 31 (27.4) | |
| Moderate/extreme obesity | 274 (24.0) | 300 (21.3) | 316 (20.9) | 220 (24.1) | 108 (28.6) | 27 (23.9) | |
| Duration of diabetes (y)** | 10.2 ±8.8 | 10.8 ±9.1 | 11.8 ±9.6 | 12.8 ±10.0 | 14.8 ±10.5 | 18.6 ±10.0 | < 0.001 |
| Cerebrovascular disease | 58 (4.8) | 93 (6.2) | 110 (6.9) | 83 (8.7) | 44 (11.2) | 17 (14.5) | < 0.001 |
| Myocardial infarction | 67 (5.5) | 89 (5.9) | 131 (8.2) | 94 (9.8) | 65 (16.6) | 21 (18.0) | < 0.001 |
| Peripheral vascular disease | 49 (4.0) | 68 (4.5) | 113 (7.1) | 77 (8.0) | 46 (11.7) | 21 (18.0) | < 0.001 |
| Congestive heart failure | 62 (5.1) | 71 (4.7) | 134 (8.4) | 119 (12.4) | 99 (25.3) | 40 (34.2) | < 0.001 |
| Proteinuria | 56 (5.2) | 79 (6.0) | 111 (7.9) | 134 (16.1) | 107 (31.0) | 60 (60.6) | < 0.001 |
| Hemoglobin (g/dL)^ | 13.7 ±1.4 | 13.8 ±1.5 | 13.5 ±1.5 | 13.0 ±1.7 | 12.5 ±1.5 | 12.0 ±1.4 | < 0.001 |
Note: N=5,805. Values for categorical variables are given as number (percentage); values for continuous variables, as mean ± standard deviation.
Abbreviations: DISTANCE, Diabetes Study of Northern California; eGFR, estimated glomerular filtration rate; GED, General Education Development.
Range, 60–77 y.
Range, 0–72 y.
Range, 7.6–19.4 g/dL.
Physical HRQOL
In unadjusted analyses (Model 1), physical HRQOL scores (PCS) were significantly lower with each eGFR category (except eGFR 75–89 ml/min/1.73m2) compared with the referent group of eGFR ≥90 ml/min/1.73m2 [Table 2]. This association persisted after adjusting for sociodemographics, duration of diabetes, obesity and proteinuria (Model 3) for respondents with eGFR less than 45 (referent group adjusted mean, 43.97; 95% CI, 43.11–44.83); eGFR 30–44 (adjusted mean, 42.21; 95% CI, 40.98–43.43) and eGFR ≤29 ml/min/1.73m2 (adjusted mean, 40.93; 95% CI, 38.73–43.14). However, in the fully adjusted model (Model 4) that additionally adjusted for cardiovascular disease, these differences in adjusted means were no longer significant. The RR of being in the lowest 25th percentile of physical HRQOL compared with the referent group was not significantly different in the adjusted models (data not shown). We also detected significant (p<0.001) interactions between eGFR × proteinuria, however the lack of relationship between eGFR and PCS persisted in those models with and without proteinuria (data not shown).
Table 2.
Adjusted Mean PCS score by eGFR category
| eGFR category | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| ≥90 mL/min/1.73 m2 ^ | 44.04 (43.39–44.68) | 45.75 (44.98, 46.53) | 43.97 (43.11, 44.83) | 44.16 (43.31, 45.0) | 43.37 (42.35, 44.39) |
| 75–89 mL/min/1.73 m2 | 44.97 (44.43, 45.51)* | 46.19 (45.51, 46.87) | 44.73 (43.95, 45.51) | 44.54 (43.78, 45.30) | 43.72 (42.83, 44.62) |
| 60–74 mL/min/1.73 m2 | 45.00 (44.49, 45.51)* | 46.43 (45.75, 47.11) | 44.88 (44.12, 45.64) | 44.86 (44.11, 45.61) | 44.02 (43.14, 44.91) |
| 45–59 mL/min/1.73 m2 | 42.32 (41.66, 42.97)* | 44.02 (43.21, 44.82)* | 43.07 (42.19, 43.96) | 43.37 (42.50, 44.24) | 42.82 (41.82, 43.83) |
| 30–44 mL/min/1.73 m2 | 40.41 (39.39, 41.42)* | 42.55 (41.41, 43.69)* | 42.21 (40.98, 43.43)* | 43.00 (41.78, 44.22) | 43.40 (42.01, 44.79) |
| ≤29 mL/min/1.73 m2 | 40.02 (38.20, 41.85)* | 41.22 (39.24, 43.19)* | 40.93 (38.73, 43.14)* | 42.56 (40.37, 44.75) | 43.27 (41.02, 45.53) |
Indicates statistical significance (p<0.05) compared to the reference group.
Reference.
Note: Values are given as mean PCS score (95% confidence interval). Multivariable linear models included the following: in model 1, unadjusted regression analysis; in model 2, patient demographics (age, gender, race/ethnicity, marital status, household income, and education level); in model 3, additional adjustment for demographics and obesity, duration of diabetes and proteinuria; in model 4, fully-adjusted, including medical conditions and cardiovascular comorbidities: cerebrovascular disease, myocardial infarction, peripheral vascular disease, and coronary heart failure; and in model 5, mediation model with additional adjustment for baseline hemoglobin value.
PCS, physical component summary; eGFR, estimated glomerular filtration rate;
Mental HRQOL
In unadjusted analyses (Model 1), mental HRQOL scores (MCS) were significantly lower only for respondents in the lowest eGFR category of ≤29 ml/min/1.73m2 (adjusted mean, 48.18; 95% CI, 46.68–49.68)) compared with the referent group eGFR category of ≥90 ml/min/1.73m2 (adjusted mean, 50.71; 95% CI, 50.18–51.24; p<0.05) while respondents with eGFR 60–74 ml/min/1.73m2 had significantly higher MCS scores (adjusted mean, 51.47; 95% CI, 51.05–51.89; p<0.05) [Table 3]. In fully adjusted analyses including sociodemographics, duration of diabetes, obesity, proteinuria and cardiovascular disease (Model 4) only those respondents with lowest eGFR level (≤29 ml/min/1.73m2) demonstrated significantly lower mean MCS score (48.58; 95% CI, 46.61–50.54; p<0.05) compared to the referent group adjusted mean (50.96; 95% CI, 50.20–51.72; p<0.05), but these findings were not substantive. The RR of being in the lowest quartile of mental HRQOL compared with the referent group in fully adjusted analyses was not significant (data not shown). We also detected significant (p<0.04) interactions between eGFR × proteinuria, however the lack of relationship between eGFR and MCS persisted in those models with and without proteinuria (data not shown).
Table 3.
Adjusted Mean MCS score by eGFR category
| eGFR category | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| ≥90 mL/min/1.73 m2^ | 50.71 (50.18, 51.24) | 51.23 (50.58, 51.88) | 50.91 (50.15, 51.68) | 50.96 (50.20, 51.72) | 50.48 (49.54, 51.41) |
| 75–89 mL/min/1.73 m2 | 51.32 (50,88, 51.77) | 51.06 (50.49, 51.64) | 50.90 (50.21, 51.59) | 50.80 (50.12, 51.49) | 50.64 (49.82, 51.47) |
| 60–74 mL/min/1.73 m2 | 51.47 (51.05, 51.89)* | 51.25 (50.68, 51.83) | 50.50 (49.82, 51.17) | 50.44 (49.77, 51.12) | 50.41 (49.60, 51.22) |
| 45–59 mL/min/1.73 m2 | 50.90 (50.36, 51.45) | 50.57 (49.88, 51.24) | 50.58 (49.80, 51.37) | 50.77 (49.99, 51.56) | 50.99 (50.07, 51.91) |
| 30–44 mL/min/1.73 m2 | 51.04 (50.21, 51.88) | 51.10 (50.14, 52.07) | 50.65 (49.56, 51.74) | 51.19 (50.10, 52.28) | 51.50 (50.22, 52.77) |
| ≤29 mL/min/1.73 m2 | 48.18 (46.68, 49.68)* | 48.12 (46.45, 49.78)* | 47.52 (45.56, 49.48)* | 48.58 (46.61, 50.54)* | 49.04 (46.98, 51.11) |
Indicates statistical significance (p<0.05) compared to the reference group.
Reference.
Note: Values are given as mean MCS score (95% confidence interval). Multivariable linear models included the following: in model 1, unadjusted regression analysis; in model 2, patient demographics (age, gender, race/ethnicity, marital status, household income, and education level); in model 3, additional adjustment for demographics and obesity, duration of diabetes and proteinuria; in model 4, fully-adjusted, including medical conditions and cardiovascular comorbidities: cerebrovascular disease, myocardial infarction, peripheral vascular disease, and coronary heart failure; and in model 5, mediation model with additional adjustment for baseline hemoglobin value.
MCS, mental component summary; eGFR, estimated glomerular filtration rate;
Depressive Symptoms
In the unadjusted analyses (Model 1), we found that only respondents with the lowest eGFR category (≤29 ml/min/1.73m2) had a significantly increased risk for depressive symptoms (RR, 2.02; 95% CI, 1.10–3.71; p<0.05) [Table 4]. In the fully adjusted model (Model 4), those respondents with eGFR ≤29 ml/min/1.73m2 remained at significantly increased risk of reporting depressive symptoms compared with the referent group with eGFR ≥90 ml/min/1.73m2 (RR, 2.04; 95% CI, 1.06–3.91; p<0.05). We detected no significant effect eGFR × proteinuria interactions, and thus this strong relationship between eGFR and depression was consistent across those with and without proteinuria.
Table 4.
Relative Risk of Depressive Symptoms by eGFR category
| eGFR category | Model 1 | Model 2 | Model 3 | Model 4 | Model 5 |
|---|---|---|---|---|---|
| ≥90 mL/min/1.73 m2 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 75–89 mL/min/1.73 m2 | 0.90 (0.66, 1.23) | 1.04 (0.74, 1.46) | 1.03 (0.71, 1.50) | 1.09 (0.75, 1.57) | 1.05 (0.70, 1.57) |
| 60–74 mL/min/1.73 m2 | 0.84 (0.62, 1.14) | 0.97 (0.69, 1.35) | 1.08 (0.76, 1.54) | 1.12 (0.79, 1.58) | 1.09 (0.74, 1.59) |
| 45–59 mL/min/1.73 m2 | 1.05 (0.75, 1.47) | 1.28 (0.88, 1.87) | 1.32 (0.88, 1.97) | 1.29 (0.87, 1.90) | 1.23 (0.81, 1.87) |
| 30–44 mL/min/1.73 m2 | 1.25 (0.82, 1.90) | 1.26 (0.80, 1.99) | 1.32 (0.81, 2.14) | 1.21 (0.75, 1.96) | 1.02 (0.62, 1.68) |
| ≤29 mL/min/1.73 m2 | 2.02 (1.10, 3.71)* | 2.39 (1.27, 4.48)* | 2.19 (1.16, 4.13)* | 2.04 (1.06, 3.91)* | 1.72 (0.89, 3.33) |
indicates statistical significance (p<0.05 versus reference group)
Note: Modified Poisson regression model. Values are given as relative risk (95% confidence interval). Depressive symptoms defined as Patient Health Questionnaire 8 score 10. Multivariable poisson regression models included the following: in model 1, unadjusted regression analysis; in model 2, patient demographics (age, gender, race/ethnicity, marital status, household income, and education level); in model 3, additional adjustment for demographics and obesity, duration of diabetes and proteinuria; in model 4, fully-adjusted, including medical conditions and cardiovascular comorbidities: cerebrovascular disease, myocardial infarction, peripheral vascular disease, and coronary heart failure; and in model 5, mediation model with additional adjustment for baseline hemoglobin value.
eGFR, estimated glomerular filtration rate;
Mediation Models
To test the effect of hemoglobin as a potential mediator of any observed relationships between eGFR and HRQOL or depressive symptoms, we added hemoglobin level to the fully adjusted models for MCS and PHQ-8 scores. As expected, after adding hemoglobin to the model for PCS (Table 2, Model 5), there were no significant differences in PCS mean scores for any eGFR categories compared with the referent group, which was not different from the results for the fully adjusted Model 4 (adjusted for sociodemographics, obesity, duration of diabetes, cardiovascular comorbidities and proteinuria). When adding hemoglobin as a covariate for MCS (Table 3, Model 5), we found that respondents with eGFR ≤29 ml/min/1.73m2 no longer had significantly lower mean MCS scores (49.04; 95% CI, 46.98–51.11) compared to the referent group with eGFR ≥90 ml/min/1.73m2 (mean score, 50.48; 95% CI, 49.54–51.41; p=0.3). When hemoglobin was added to the model for depressive symptoms (Table 4, Model 5), we found that although respondents with eGFR ≤29 ml/min/1.73m2 remained at increased risk for depressive symptoms compared with the referent group (RR, 1.72; 95% CI, 0.89–3.33; p=0.1), this result was no longer statistically significant.
Discussion
This study assessed the relationship between eGFR and HRQOL and depressive symptoms in a population of over 5,800 community-dwelling, insured older adults with diabetes enrolled in Kaiser Permanente Northern California. In this study, we found that after adjustment, there was no substantive relationship between eGFR and physical or mental HRQoL as a whole, or separately among those with and without proteinuria. However, we also found that respondents with the lowest eGFR values (eGFR ≤29 ml/min/1.73m2) had double the risk of depressive symptoms when compared to respondents with the highest eGFR (≥90 ml/min/1.73m2) in fully adjusted analyses. This strong relationship was observed in those with and without proteinuria. We also found that the relationship between eGFR and depressive symptoms was partially attributable to variation in hemoglobin level.
Our results differ from prior studies that have examined HRQoL among CKD patients and have shown a stronger effect of lower eGFR based on CKD stage for physical HRQOL compared with mental HRQOL.8,30 Our results are particularly important as they represent a large outpatient primary care population with diabetes, a population not previously evaluated in the literature but a population that carries a high burden of kidney disease. These results, which show no significant relationship between eGFR and physical HRQOL after adjustment for cardiovascular comorbidities, suggest that it may be the increased burden of cardiovascular disease present at lower eGFR levels rather than the kidney function itself that impairs physical function. The negative effects of cardiovascular disease on HRQOL may be due to cardiovascular deconditioning, the side-effects of medications used to treat cardiovascular disease, or the burden of polypharmacy.15,31
Our results suggest an independent association between decreased kidney function and an increased risk of depressive symptoms among those with the worst kidney function (eGFR ≤29 ml/min/1.73m2) even in the presence of other common comorbidities including diabetes and cardiovascular disease among older adults. It is possible that those in the lowest eGFR category are experiencing symptoms more directly related to their kidney disease including dyspnea, poor appetite, or fatigue, which might affect depressive symptoms. Moreover, this relationship was partially mediated by hemoglobin level. These results indicates that lower hemoglobin levels, as a result of worsened kidney function (lower eGFR), may account for symptoms such as dyspnea or fatigue, that could in turn influence mood. In addition to symptoms associated with poor kidney function, mediated by lower hemoglobin levels, respondents with low eGFR levels may also experience uncertainty and anxiety about potential impending kidney failure and the need to consider renal replacement therapy that may also affect mood. Depression in CKD has important clinical implications. Young et al, have previously reported that major depression was common among stage 5 CKD patients with diabetes and was associated with an almost tripling in mortality rates.32
There are several limitations of this study. First, this is a cross-sectional study of patients with diabetes in a large managed care organization and the findings may be the result of specific care patterns of this health delivery system. For example, all patients had uniform access to primary care with integrated nephrology consult services available. On the other hand, this is a large study of nearly 6,000 insured patients who received care within an integrated health care system. Second, we did not have more than a single eGFR value (in the baseline time period prior to survey response) for about one quarter of our sample. The lack of multiple eGFR records was due to this being a sample of primary care outpatient sample in which laboratory values were obtained during routine clinical care. Therefore, we can only describe our results in terms of eGFR level rather than stage of CKD. In an effort to reduce the likelihood that our sample contained persons with acute kidney injury rather than CKD, we did exclude all respondents with laboratory values obtained in the in-patient or emergency room settings during the baseline period. The laboratory data presented in this study is representative of that for patients with diabetes in general primary care and therefore, these results have relevance for many patients. Also, we cannot confirm the direction of the relationships between eGFR category, comorbidities, and HRQOL, although reverse causality (poorer HRQOL significantly reducing eGFR) is unlikely. Future studies that evaluate HRQOL in patients prospectively would be able to better define these relationships. Finally, the HRQOL measures in this study did not include kidney disease-specific items, such as those validated in the KDQoL-SF (Kidney Disease Quality of Life) questionnaire or the CHOICE (Choices for Healthy Outcomes in Caring for ESRD) health experience questionnaire, because the original study was aimed at general HRQoL among persons with diabetes.33–35 However, it is not clear if disease-specific measures would alter significantly our results since such kidney disease-specific questionnaires are validated for use in patients with advanced CKD (stage 4) and not the general population with CKD. The measures used in this study were validated for a population of primary care patients with diabetes.36 Despite these limitations, we believe that the results of this study provide additional information in an area of great need, the understanding of the relationship between kidney disease, eGFR, HRQOL, and depression.
In summary, among older adults with diabetes, lower eGFR, with and without the presence of proteinuria, was associated with a markedly increased risk of depressive symptoms after accounting for cardiovascular comorbidities. However we found no evidence to support previous reports suggesting a relationship between eGFR and physical or mental HRQOL. Our findings argue for the need for greater attention toward identifying and addressing depressive symptoms among those with decreased kidney function.
Acknowledgments
Support: Dr Campbell is supported by a T. Franklin Williams Scholars Award. Funding was provided by Atlantic Philanthropies, Inc; the American Geriatrics Society Foundation for Health in Aging; the John A. Hartford Foundation; and the Association of Specialty Professors. The Diabetes and Aging Study was supported by funding from the National Institute of Diabetes and Digestive and Kidney Diseases (grants R01DK081796, R01DK080726, R01DK65664). Investigators were also supported by the Centers for Diabetes Translation Research at Kaiser Permanente and University of California, San Francisco (P30 DK092924) and the University of Chicago (P30 DK092949). The sponsors played no role in the design or interpretation of the study. Dr. Young’s contribution is supported by resources from the VA Puget Sound Health Care System, Seattle, Washington and by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (DK079745). None of the aforementioned funders had input into the content of this manuscript, and did not report any potential conflicts of interest relevant to this article.
Footnotes
Financial Disclosure: The authors declare that they have no other relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004 Sep 23;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. Jama. 2007 Nov 7;298(17):2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 3.USRDS. Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes, Digestive and Kidney Disorders; 2010. [Google Scholar]
- 4.Kurella M, Yaffe K, Shlipak MG, Wenger NK, Chertow GM. Chronic kidney disease and cognitive impairment in menopausal women. Am J Kidney Dis. 2005 Jan;45(1):66–76. doi: 10.1053/j.ajkd.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 5.Fried LF, Lee JS, Shlipak M, et al. Chronic kidney disease and functional limitation in older people: health, aging and body composition study. J Am Geriatr Soc. 2006 May;54(5):750–756. doi: 10.1111/j.1532-5415.2006.00727.x. [DOI] [PubMed] [Google Scholar]
- 6.Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009 Oct 15;361(16):1539–1547. doi: 10.1056/NEJMoa0904655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Altintepe L, Levendoglu F, Okudan N, et al. Physical disability, psychological status, and health-related quality of life in older hemodialysis patients and age-matched controls. Hemodial Int. 2006 Jul;10(3):260–266. doi: 10.1111/j.1542-4758.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 8.Mujais SK, Story K, Brouillette J, et al. Health-related quality of life in CKD Patients: correlates and evolution over time. Clin J Am Soc Nephrol. 2009 Aug;4(8):1293–1301. doi: 10.2215/CJN.05541008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Odden MC, Whooley MA, Shlipak MG. Depression, stress, and quality of life in persons with chronic kidney disease: the Heart and Soul Study. Nephron Clin Pract. 2006;103(1):c1–7. doi: 10.1159/000090112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen SD, Patel SS, Khetpal P, Peterson RA, Kimmel PL. Pain, sleep disturbance, and quality of life in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2007 Sep;2(5):919–925. doi: 10.2215/CJN.00820207. [DOI] [PubMed] [Google Scholar]
- 11.Shidler NR, Peterson RA, Kimmel PL. Quality of life and psychosocial relationships in patients with chronic renal insufficiency. Am J Kidney Dis. 1998 Oct;32(4):557–566. doi: 10.1016/s0272-6386(98)70017-4. [DOI] [PubMed] [Google Scholar]
- 12.Hedayati SS, Minhajuddin AT, Afshar M, Toto RD, Trivedi MH, Rush AJ. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010 May;303(19):1946–1953. doi: 10.1001/jama.2010.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Standard Definitions: final dispositions of case codes and outcome rates for surveys. 2011. [Google Scholar]
- 14.Moffet HH, Adler N, Schillinger D, et al. Cohort Profile: The Diabetes Study of Northern California (DISTANCE)--objectives and design of a survey follow-up study of social health disparities in a managed care population. Int J Epidemiol. 2009 Feb;38(1):38–47. doi: 10.1093/ije/dyn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laiteerapong N, Karter AJ, Liu JY, et al. Correlates of quality of life in older adults with diabetes: the diabetes & aging study. Diabetes Care. 2011 Aug;34(8):1749–1753. doi: 10.2337/dc10-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skali H, Uno H, Levey AS, Inker LA, Pfeffer MA, Solomon SD. Prognostic assessment of estimated glomerular filtration rate by the new Chronic Kidney Disease Epidemiology Collaboration equation in comparison with the Modification of Diet in Renal Disease Study equation. Am Heart J. 2011 Sep;162(3):548–554. doi: 10.1016/j.ahj.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992 Jun;30(6):473–483. [PubMed] [Google Scholar]
- 19.Ware JE, Kosinski M, Bayliss MS, McHorney CA, Rogers WH, Raczek A. Comparison of methods for the scoring and statistical analysis of SF-36 health profile and summary measures: summary of results from the Medical Outcomes Study. Med Care. 1995 Apr;33(4 Suppl):AS264–279. [PubMed] [Google Scholar]
- 20.Ware JE, Kosinski M, Dewey JE, Gandek B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8 Health Survey. Lincoln, RI: Quality Metric Inc; 2001. [Google Scholar]
- 21.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–413. doi: 10.1023/a:1012588218728. discussion 415–420. [DOI] [PubMed] [Google Scholar]
- 22.Farivar SS, Liu H, Hays RD. Half standard deviation estimate of the minimally important difference in HRQOL scores? Expert Rev Pharmacoecon Outcomes Res. 2004 Oct;4(5):515–523. doi: 10.1586/14737167.4.5.515. [DOI] [PubMed] [Google Scholar]
- 23.Spitzer RL, Kroenke K, Williams JB. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. Primary Care Evaluation of Mental Disorders. Patient Health Questionnaire. JAMA. 1999 Nov;282(18):1737–1744. doi: 10.1001/jama.282.18.1737. [DOI] [PubMed] [Google Scholar]
- 24.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009 Apr;114(1-3):163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Lin EH, Rutter CM, Katon W, et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010 Feb;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO. Report of WHO Expert Committee. Geneva: World Health Organization; 1995. Physical Status: The Use and Interpretation of Anthropometry. [PubMed] [Google Scholar]
- 27.Horvitz D. A Generalization of Sampling Without Replacement From a Finite Universe. American Statistical Association Stable. 1952;47:663–685. [Google Scholar]
- 28.Parker MM, Moffet HH, Schillinger D, et al. Ethnic Differences in Appointment-Keeping and Implications for the Patient-Centered Medical Home-Findings from the Diabetes Study of Northern California (DISTANCE) Health Serv Res. 2011 Oct; doi: 10.1111/j.1475-6773.2011.01337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004 Apr;159(7):702–706. doi: 10.1093/aje/kwh090. [DOI] [PubMed] [Google Scholar]
- 30.Han SS, Kim KW, Na KY, et al. Quality of life and mortality from a nephrologist’s view: a prospective observational study. BMC Nephrol. 2009;10:39. doi: 10.1186/1471-2369-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu YW, Teitelbaum I, Misra M, de Leon EM, Adzize T, Mehrotra R. Pill burden, adherence, hyperphosphatemia, and quality of life in maintenance dialysis patients. Clin J Am Soc Nephrol. 2009 Jun;4(6):1089–1096. doi: 10.2215/CJN.00290109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Young BA, Von Korff M, Heckbert SR, et al. Association of major depression and mortality in Stage 5 diabetic chronic kidney disease. Gen Hosp Psychiatry. 2010 Mar-Apr;32(2):119–124. doi: 10.1016/j.genhosppsych.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unruh M, Benz R, Greene T, et al. Effects of hemodialysis dose and membrane flux on health-related quality of life in the HEMO Study. Kidney Int. 2004 Jul;66(1):355–366. doi: 10.1111/j.1523-1755.2004.00738.x. [DOI] [PubMed] [Google Scholar]
- 34.Mapes DL, Bragg-Gresham JL, Bommer J, et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004 Nov;44(5 Suppl 2):54–60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Wu AW, Fink NE, Cagney KA, et al. Developing a health-related quality-of-life measure for end-stage renal disease: The CHOICE Health Experience Questionnaire. Am J Kidney Dis. 2001 Jan;37(1):11–21. doi: 10.1053/ajkd.2001.20631. [DOI] [PubMed] [Google Scholar]
- 36.Turner-Bowker DM, Bayliss MS, Ware JE, Jr, Kosinski M. Usefulness of the SF-8 Health Survey for comparing the impact of migraine and other conditions. Qual Life Res. 2003 Dec;12(8):1003–1012. doi: 10.1023/a:1026179517081. [DOI] [PubMed] [Google Scholar]