Abstract
Nuc1 is a spontaneous rat mutant resulting from a mutation in the Cryba1 gene, coding for βA3/A1-crystallin. Our earlier studies with Nuc1 provided novel evidence that astrocytes, which express βA3/A1-crystallin, have a pivotal role in retinal remodeling. The role of astrocytes in the retina is only beginning to be explored. One of the limitations in the field is the lack of appropriate animal models to better investigate the function of astrocytes in retinal health and disease. We have now established transgenic mice that overexpress the Nuc1 mutant form of Cryba1, specifically in astrocytes. Astrocytes in wild type mice show normal compact stellate structure, producing a honeycomb-like network. In contrast, in transgenics over-expressing the mutant (Nuc1) Cryba1 in astrocytes, bundle-like structures with abnormal patterns and morphology were observed. In the nerve fiber layer of the transgenic mice, an additional layer of astrocytes adjacent to the vitreous is evident. This abnormal organization of astrocytes affects both the superficial and deep retinal vascular density and remodeling. Fluorescein angiography showed increased venous dilation and tortuosity of branches in the transgenic retina, as compared to wild type. Moreover, there appear to be fewer interactions between astrocytes and endothelial cells in the transgenic retina than in normal mouse retina. Further, astrocytes overexpressing the mutant βA3/A1-crystallin migrate into the vitreous, and ensheath the hyaloid artery, in a manner similar to that seen in the Nuc1 rat. Together, these data demonstrate that developmental abnormalities of astrocytes can affect the normal remodeling process of both fetal and retinal vessels of the eye and that βA3/A1-crystallin is essential for normal astrocyte function in the retina.
Keywords: βA3-A1-crystallin, astrocyte, retina, transgenic mice, hyaloid and retinal vasculature
INTRODUCTION
Crystallins are highly abundant proteins of the ocular lens that are essential to its transparency and refractive properties (Piatigorsky, 2007). It is believed that crystallins were evolutionarily recruited to the lens from other tissues, mainly to perform such a structural function (Wistow, 1995). In recent years, several laboratories have shown that crystallins expressed outside of the lens may have other functions (Bhat, 2004; Andley, 2007).
Over the past several years, our laboratory has been studying the non-lens function of one of the β-crystallin family members, βA3/A1-crystallin (Sinha et al., 2008; Ma et al., 2011; Zigler et al., 2011; Zhang et al., 2011). In all mammalian lenses, members of three major families of crystallins, α, β, and γ, are expressed. βA3/A1-crystallin is unique among crystallins in that two polypeptides are translated from a single mRNA by utilizing alternate start sites (Werten et al., 1999). By utilizing the Nuc1 spontaneous mutant rat, in which the βA3/A1-crystallin gene has undergone mutation, we have shown that this crystallin is required by astrocytes, and our studies suggest that βA3/A1-crystallin may be important in the retina during vascular remodeling (Sinha et al., 2005; Gehlbach et al., 2006; Sinha et al., 2008; Parthasarathy et al., 2011; Zhang et al., 2011). We have also reported that this crystallin is expressed by retinal pigmented epithelial (RPE) cells where it is necessary for normal processes of phagocytosis and autophagy (Zigler et al., 2011). Our recent data suggest that βA3/A1-crystallin is a novel regulator of both life and death decisions in ocular astrocytes (Ma et al., 2011).
We are now generating both loss-of-function and gain-of-function genetically engineered mice to further understand the “non-crystallin” function of βA3/A1-crystallin. Here, we report the effects of overexpressing the mutant (Nuc1) βA3/A1-crystallin protein specifically in astrocytes of transgenic mice. Our data suggest that such overexpression affects astrocyte template formation and remodeling of the retinal vessels.
MATERIALS AND METHODS
Transgenic mice
We have used transgenic technology to target expression of the Nuc1 mutant βA3/A1-crystallin specifically to mouse astrocytes, under transcriptional control of the human GFAP promoter (gfa2 segment). The plasmid pGfa2-cLac, a kind gift from Dr. Michael Brenner, University of Alabama, Birmingham, contained the gfa2 promoter, the lacZ gene, and a portion of the mouse protamine 1 gene, which supplies splice sites and polyadenylation signal. This hGFAP promoter has been used successfully by a large number of investigators to express transgenes in astroglia in vitro and astrocytes in vivo (Brenner, 1994; Delaney et al., 1996; Segovia et al., 1998; Vandier et al., 1998). The coding region of the rat Nuc1 mutant βA3/A1-crystallin cDNA was RT-PCR amplified from Nuc1 lens RNA with the following primers: cctcggatccACCAGATGGAGACCCAGACTGTG and ccttggatccGTAAGTTTGCATGCTTGAGG, which also added BamH I sites flanking the cDNA. The PCR product and pGfa2-cLac were both digested with BamH I, and the Nuc1 cDNA was ligated into the plasmid. PCR and restriction analyses were used to select clones with the cDNA in the correct orientation, and resulting clones were sequenced to ensure fidelity of the PCR amplification. The final plasmid construct was digested with EcoR I to release the 3.4 kb transgene, which was then microinjected into the pronuclei of C57BL/6 fertilized oocytes to produce transgenic founders. Experiments were performed using postnatal transgenic and wild type mice, in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy Press) and were approved by the Animal Care and Use Committee of Johns Hopkins University.
Western analysis
Freshly dissected retinae from wild type and transgenic mice were rinsed in PBS and homogenized in lysis buffer (150 mM NaCl, 1% NP-40, 5 mM EDTA, 1 mM DTT, 25% glycerol, 50 mM Tris, pH 8.0) with 1% protease inhibitor cocktail (Sigma-Aldrich, St Louis, MO). Samples were incubated on a rotator at 4°C for 30 minutes followed by centrifugation at 13,000 g for 15 minutes. Approximately 25 μg of protein from the supernatant was mixed with 2X LDS sample buffer (Invitrogen, Carlsbad, CA) and then heated in a boiling water bath for 2 minutes. Each sample was loaded onto a 4–12% Bis-Tris Nu-PAGE gel and run with MES Buffer (Invitrogen). The gels were stained with Colloidal Coomassie Brilliant Blue. For western blotting, proteins were electrophoretically transferred to nitrocellulose membranes (Invitrogen) and then blocked with 3% BSA in TTBS (Tris-buffered saline, 0.1% Tween-20) overnight at 4°C. Blots were incubated with primary antibodies to βA3/A1-crystallin (1:8000) for 1 hour at room temperature followed by four washes of 10 minutes each. Blots were incubated with HRP-conjugated secondary antibodies (Kirkegaard and Perry Laboratories, Gaithersburg, MD) for 1 hour at room temperature at a dilution of 1:20,000 followed by four washes of 10 minutes each. ECL western blotting detection reagents (GE Healthcare, Piscataway, NJ) were used for detection with varying exposure times. The βA3/A1-crystallin antibody was raised against a synthetic peptide sequence common to both βA3- and βA1-crystallin and was produced at Spring Valley Laboratories, Woodbine, MD. The peptide sequence is from the N-terminal region of the protein and is not affected by the mutation in the C-terminal region; the antibody therefore reacts with both normal and mutant forms of both βA3- and βA1-crystallin (Zigler et al., 2011).
Immunofluorescence and Confocal microscopy
For preparation of frozen sections, freshly enucleated eyes were fixed in 2% paraformaldehyde, 5% sucrose in 0.1N phosphate buffer, pH 7.4 for 3 hours at room temperature. The eyes were then transferred to 20% sucrose in PBS and kept at 4°C until embedding in O.C.T. compound (Sakura Finetek USA, Torrence, CA). Sections (10μm) were cut on a cryostat and mounted on Superfrost Plus slides. The primary antibody used on cryosections was polyclonal rabbit anti-GFAP (glial fibrillary acidic protein) (DAKO, Carpinteria, CA; 1:1000) Sections were incubated with primary antibody overnight at 4°C, washed with PBS and incubated with Alexa 488 labeled goat anti-rabbit IgG (Molecular Probes, Eugene, OR; 1:300) for 1 hour at room temperature. The sections were then counterstained with 4’,6-diamidino-2-phenylindole (DAPI) (Invitrogen, Carlsbad, CA; 1:1000) and mounted with DAKO fluorescent mounting medium (DAKO). Fluorescent images were taken with a Leica 6000 fluorescent microscope fitted with a 40X objective lens and a Leica DFC 310FX digital camera.
Eyes to be used for flat mounts were fixed for one hour in 4% paraformaldehyde, transferred to PBS and stored at 4°C until needed. To isolate the retina, the anterior segment was removed and the retina teased away from the sclera using a fine camels hair brush. The whole retina was then washed/blocked in D-PBS with Ca2+ and Mg2+ (Quality Biologicals Inc., Gaithersburg, MD) plus 5% normal goat serum for 24 hours at 4°C on a oscilating platform. The blocking solution was removed and the samples were incubated overnight in primary antibody (polyclonal rabbit anti-GFAP, DAKO; 1:1000 or polyclonal rabbit anti-rat aldehyde dehydrogenase 1 family, member L1 (Aldh1L1); 1:1000, a kind gift from Dr. Sergey Krupenko, Medical University of South Carolina, Charleston) diluted in D-PBS plus 2% blocking serum at 4°C on a rotating platform. Samples were then washed 3X for 15 minutes each and once for 1 hour in D-PBS at 4°C. The retinas were then incubated for 4 hours in secondary antibody at 4°C. Typically the following mixture prepared in D-PBS plus 2% blocking serum was used: Alexa 568 labeled goat anti-rabbit IgG (1:300) and Alexa 488 labeled isolectin (1:100) (both from Molecular Probes, Eugene, OR). Samples were subsequently washed as for the secondary antibody and mounted with the ganglion cell layer up on superfrost slides using DAKO fluorescent mounting medium (DAKO). Relaxing cuts were used to make the retinas lay flat on the slide. Cover slips were applied and the edges sealed with clear nail polish. For double labeling of astrocytes (GFAP) and blood vessels (Isolectin B-4) fluorescent digital images were taken with a Leica 6000 fluorescent microscope as indicated above for frozen sections. Astrocytes were analyzed with a confocal microscope (model 510META; Carl Zeiss Microimaging Inc., Thornwood, NY) using a 40X oil objective lens.
Quantification of vessel density
Quantitative assessment of isolectin-stained superficial and deep plexus vessels of the retina was performed by computer-assisted digital image analysis using Image J (NIH). In order to compare the blood vessel parameters in wild type and transgenic retinal flat mounts the vessel width was normalized by creating the binary images after application of a common threshold value. The binary images were converted into skeletonized images using NIH Image J software (http://rsbweb.nih.gov/ij/) and later quantified using the ‘analyze skeleton’ plug-in of Image J. The analysis was performed as described (McKay et al., 2008; Valapala et al., 2011). Quantifications were performed in 3 wild type and 5 transgenic animals and 30 different 40x magnification images were used per animal. For analysis, all data are representative of experiments performed at least three times in triplicate. The data are represented as mean±S.E. For statistical analysis, Student’s t-test was performed and a P-value of <0.05 was considered statistically significant.
Fluorescein Angiography
Fluorescein angiography was performed on wild type and transgenic animals anesthetized with 0.15 ml/kg ketamine/Xylazine (10 mg/ml ketamine and 2 mg/ml Xylazine) IP. Pupils were dilated with 1 drop of topical 1:1:1:1 solution of 1% Mydriacyl, 1% Atropine, 1% Cyclogel, and 10% Phenylephrine. The retina was observed with a Micron III retinal imaging microscope for mice and rats (Phoenix Research Lab, Inc.). The mice were administered 0.1 ml of 10% sodium fluorescein via the tail vein, and retinal fluorescein angiograms were taken. The entire procedure was performed in 15 minutes. When experiments were complete, the animals were euthanized by an overdose of anesthetic.
RESULTS AND DISCUSSION
Targeted expression of mutant (Nuc1) βA3/A1-crystallin in mouse astrocytes affects the normal honeycomb-patterning of the astrocyte template in the developing retina
We have shown that retinal astrocytes express βA3/A1-crystallin, and that in Nuc1 homozygous rats, the astrocytes are abnormal, both structurally and functionally (Sinha et al., 2008). To test the hypothesis that βA3/A1-crystallin is crucial for astrocyte template formation in the developing retina, we have generated transgenic mice that express mutant βA3/A1-crystallin specifically in astrocytes.
Three lines of transgenic mice were established using the construct shown in Figure 1A. Western blot analysis reveals robust expression of the mutant protein in transgenic mouse retina (Figure 1C). Attempts to achieve overexpression of the wild type Cryba1, using the same strategy as described above, led to expression of the transgene and increased mRNA level for Cryba1, as compared to normal littermates. However, there was no increase in βA3/A1-crystallin protein in these transgenic mice, compared to endogenous levels in normal mice. Moreover, we did not find any changes in the astrocyte template formation in the developing retinae of transgenic mice overexpressing the wild type message, compared to normal mice. It is highly likely that, in vivo, there are biological controls, such as silencing by RNAi, that do not allow expression of the normal βA3/A1-crystallin protein in the astrocytes at levels higher than that present in normal mice.
Figure 1.
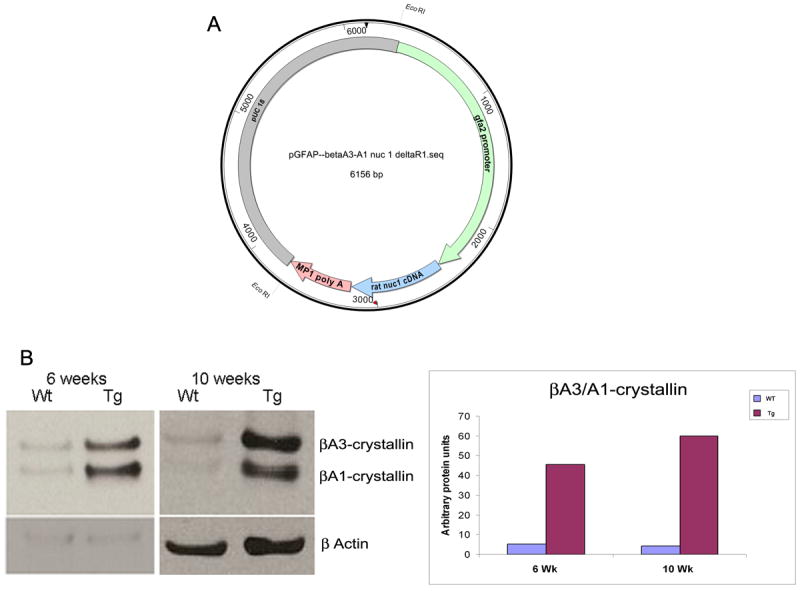
A: Schematic diagram of the construct made to generate transgenic mice. The human GFAP (hGFAP) promoter shown in green (a kind gift from Dr Michael Brenner, University of Alabama, Birmingham) was used to direct overexpression of the rat Nuc1 mutant protein (blue) specifically in astrocytes. The transcription termination signal is a 3’ fragment of mouse protamine-1 (MP-1) gene containing the functional polyadenylation signal (pink). B: Western blot of 6 and 10 week old wild type and transgenic mouse retina with targeted overexpression of mutant (Nuc1) βA3/A1-crystallin in astrocytes, probed with antibody specific for βA3/A1-crystallin. The upper band is βA3-crystallin and the lower is βA1-crystallin. There is a significant increase in the expression of mutant βA3/A1-crystallin in the transgenic mice at both ages tested.
Confocal microscopy of retinal flat mounts from wild type mice with astrocytes immunolabelled with anti-GFAP, show cells with a normal compact stellate structure, producing a typical honeycomb-like network (red, Figure 2A). In contrast, in transgenics over-expressing the mutant (Nuc1) βA3/A1-crystallin, astrocytes form bundle-like structures with abnormal patterns and short, thickened processes (Figure 2B). Abnormal organization of the developing astrocytes in the retinas of the transgenic animals is apparent. In addition, the mutant transgenic mice show an additional layer of astrocytes in the nerve fiber layer (Figure 2D-arrows), not present in wild type mice (Figure 2C).
Figure 2.
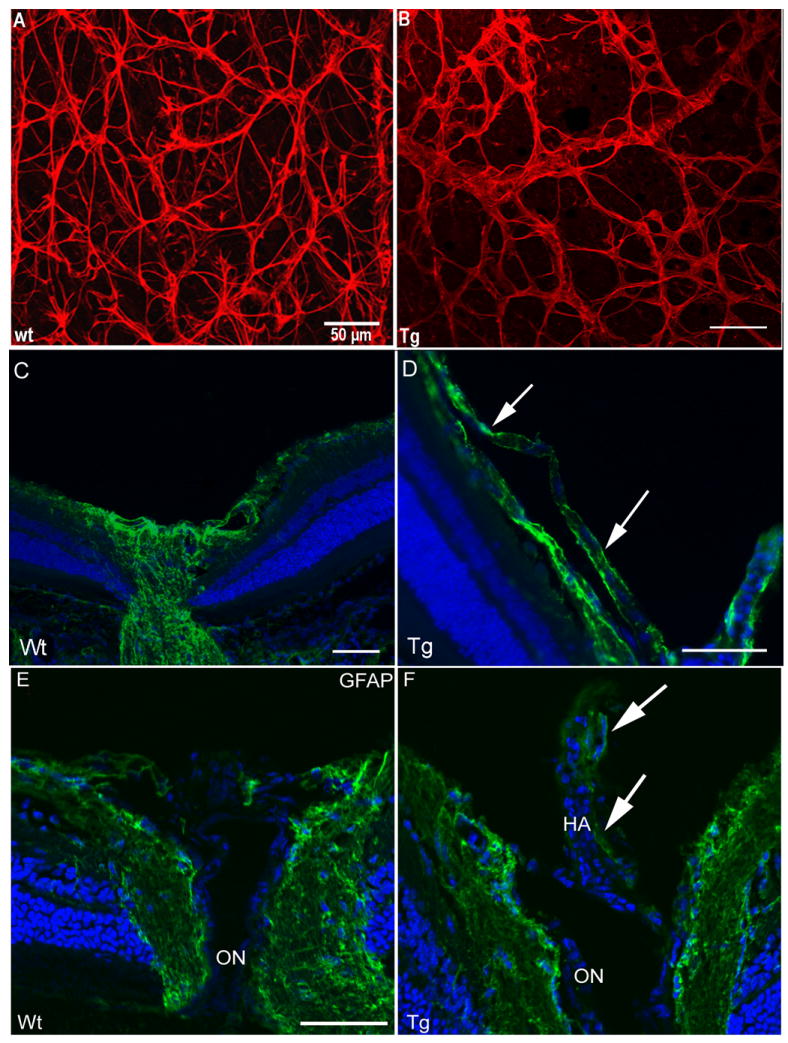
Analysis of retinas from transgenic mice overexpressing Nuc1 mutant βA3/A1-crystallin in astrocytes, and wild type littermates. Panels A and B show confocal microscopy of P21 retinal flat mounts with astrocytes immunolabelled with anti- GFAP (red). The left image (A) is from a wild type littermate. The astrocytes show a normal compact stellate structure producing a typical honeycomb-like network. In contrast, the image on the right (B), from a transgenic overexpressing the mutant (Nuc1) βA3/A1-crystallin in astrocytes, shows bundle-like structures with abnormal patterning and short, thickened processes. In panels C-F, frozen sections from eyes of wild type and transgenic mice are shown. An additional layer of astrocytes (GFAP stained green) from the hyaloid artery along the surface of the nerve fiber layer is present in transgenic mice (D, arrows), but not present in wild type mice (C). Panels E and F show GFAP (green) immunostaining of the optic nerve head from P49 wild type and transgenic mice. In wild type optic nerve head (E), GFAP-positive astrocytes are seen on the surface and inside the optic nerve head. In the transgenic mouse expressing the mutant protein (F), GFAP-positive astrocytes are present on the optic nerve head and on the surface of the retained hyaloid artery (HA, arrows). Scale bar=50 μm, n=7 wild type, 7 transgenic mutant mice.
Aberrant remodeling of hyaloid vasculature in transgenic mice compared to wild type
During development of the mammalian eye, nourishment of the immature lens, inner retina and vitreous is provided by the hyaloid vascular system (Saint-Geniez and D’Amore, 2004). One of the congenital, developmental disorders of the human eye, persistent fetal vasculature (PFV), results from the complete or partial failure of this vascular regression (Goldberg, 1997). Knowledge of the cellular and molecular mechanisms by which hyaloid vascular regression fails is very limited. Our studies have provided novel evidence that abnormalities in astrocytes during retinal development can inhibit regression of the hyaloid artery (Zhang et al., 2005; Zhang et al., 2011). Astrocytes ensheath the hyaloid artery in human PFV disease (Zhang et al., 2005; Zhang et al., 2011) and in many other mouse models (Reneker and Overbeek, 1996; Hurskainen et al., 2005; Edwards et al., 2011).
Astrocytes in the transgenic mice overexpressing the mutant βA3/A1-crystallin, migrate into the vitreous, and ensheath the hyaloid artery (Figure 2F), in a manner similar to that seen in the Nuc1 rat (Zhang et al., 2005; Zhang et al., 2011). In the case of our transgenic mice, PFV is not as severe or frequent as in the Nuc1 rat (Zhang et al., 2005; Zhang et al., 2011). Although it is technically difficult to visualize PFV in mice, we have detected abnormal retention of the hyaloid artery in 8 mutant (Nuc1) transgenic mice out of the 14 tested. In the Nuc1 homozygous rats, where PFV involves complete retention (i.e. of all parts of the fetal vasculature), the lens capsule ruptures before birth (Sinha et al., 2005), potentially releasing VEGF (Vascular Endothelial Growth Factor) and various other factors into the vitreous. In contrast, in our transgenic mice the lens is not affected, so abnormal release of such factors probably does not occur. In an ELISA based VEGF assay, we found the VEGF concentration to be 5-fold higher in the vitreous of Nuc1 homozygous rats than in wild type rats (360 pg/ml vs. 70 pg/ml). Although astrocytes produce VEGF during the critical time of hyaloid regression, the lens is likely the major source of intravitreal VEGF in Nuc1 rats. We believe that the difference in severity of PFV may be due to the fact that in the transgenic mice the lens is unaffected, whereas in the Nuc1 homozygous rats, it ruptures before birth (Sinha et al., 2005). Thus, the transgenic mice closely resemble Nuc1 heterozygous rats, which have nuclear cataracts at birth, but with normal appearing cortex and intact lens capsule until adulthood (Sinha et al., 2005). The heterozygous rats show only mild features of PFV (unpublished observation). Together, these data demonstrate that developmental abnormalities of astrocytes leading to abnormal migration and association with the hyaloid artery, may inhibit the normal programmed regression of the fetal vasculature and thereby be involved in the pathogenesis of PFV disease (Goldberg, 1997).
Retinal blood vessel abnormalities in transgenic mice overexpressing mutant (Nuc1) βA3/A1-crystallin in astrocytes
Astrocytes are closely associated with retinal blood vessels. Avascular retinas contain no astrocytes (Stone and Dreher, 1987; Schnitzer, 1988). Retinas that are diffusely vascularized contain diffusely distributed astrocytes (Stone and Dreher, 1987; Schnitzer, 1988), and those that are vascularized in a restricted region contain astrocytes only in that region (Stone and Dreher, 1987; Schnitzer, 1988). In fetal humans, astrocyte differentiation occurs in association with fetal retinal vasculature (Chan-Ling et al., 2004).
Retinal flatmounts show that the aberrant astrocyte development in the transgenic retina overexpressing the mutant (Nuc1) βA3/A1-crystallin is associated with abnormal retinal vascular patterning and with destabilized astrocyte-blood vessel interactions (Figure 3). This intimate relationship is disrupted in the transgenic mice with astrocyte-specific overexpression of the mutant βA3/A1-crystallin protein (Figure 3C, yellow-arrows). By labeling astrocytes with anti-Aldh1L1, a second specific marker for astrocytes (Krupenko and Oleinik, 2002; Cahoy et al., 2008), it is apparent that astrocytes overexpressing the mutant (Nuc1) βA3/A1-crystallin are abnormal in morphology and are less dense (Figure 3H) than astrocytes in normal retina (Figure 3G). It is important to note that our previous studies with cultured brain astrocytes from neonatal Nuc1 homozygous rats, also showed larger cells with striking morphological abnormalities compared to wild type (Sinha et al., 2008). Interestingly, as shown in our previous studies with the Nuc1 homozygous rats (Gehlbach et al., 2006; Sinha et al., 2008), our transgenic mouse data confirms that βA3/A1-crystallin is absolutely necessary in the formation of a normal astrocyte template and for blood vessel patterning and remodeling in the developing retina. In transgenic mice overexpressing the mutant βA3/A1-crystallin, the decreased density of astrocytes correlates with a reduced number of branches, junctions and endpoints in both the superficial and deep retinal vessels, as compared to wild type (Figure 3I and J). Astrocyte-derived VEGF has been shown to be an important factor in the stabilization of the retinal vasculature (Scott et al., 2010). Our recent studies do indicate that loss of function of βA3/A1-crystallin in retinal astrocytes inhibits the normal secretory levels of VEGF from astrocytes (Valapala et al., manuscript in preparation). Therefore, βA3/A1-crystallin plays a pivotal role in maintaining the normal homeostasis of astrocytes in the retina.
Figure 3.
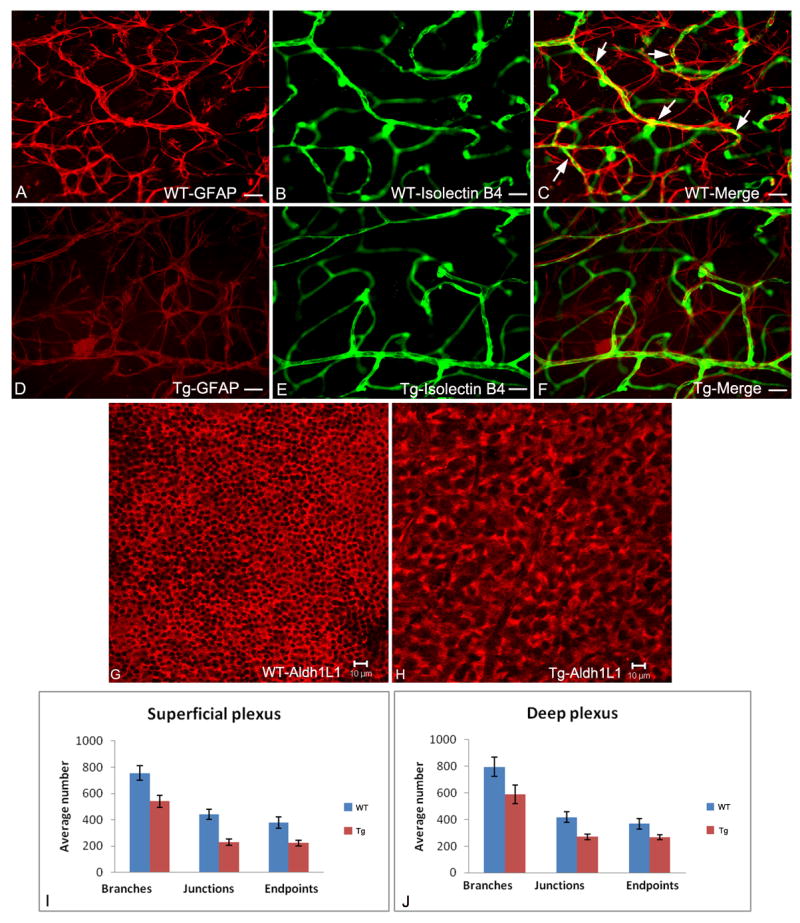
Fluorescent microscopy of retinal flat mounts from 3 week old wild type and transgenic mice with astrocytes immunolabelled with anti-GFAP (red; A, D); blood vessels immunolabelled with isolectin-B4 (green; B, E) with merged images shown in C, F. The compact stellate structure and honeycomb template of wild type astrocytes (A) is disrupted in the transgenic animals (D). The capillary plexus pattern is less dense in the transgenic (E) compared to normal (F) retina. In wild type retina (C), the astrocyte processes enwrap the blood vessels (yellow, arrows); this is not seen in the transgenic retinas (F). Confocal microscopy of retinal flat mounts from 3 week old mice with astrocytes immunolabelled with anti-Aldh1L1 (red channel) clearly shows abnormal morphology and less dense astrocytes in transgenic mice overexpressing the mutant (Nuc1) βA3/A1-crystallin (H) compared to astrocytes in normal template of the retina (G). Quantification of the superficial (I) and deep plexus (J) in wild type and transgenic mice was performed by scoring the vascular architecture using the digital analysis software image J (NIH). The major vascular parameters were scored by counting the number of branches, junctions and endpoints per 30 different microscope fields (40X) taken from the same region in multiple animals (n=3 wild type; n=5 transgenic). Binary images were created, skeletonized and analyzed using the ‘analyze skeleton plugin of image J. The retinal vasculature of transgenic animals showed a reduction in the number of branches, junctions and endpoints in the superficial (I) and deep plexus (J) compared to their wild type controls. Scale bar: 25μm (A-F) and 10μm (G&H). Data represented as mean + SE. Pvalue for superficial and deep plexus is < 0.01.
Fluorescein angiograms taken from the transgenic mice overexpressing mutant βA3/A1-crystallin showed significant vascular disorders compared to the wild type animals. This included increased dilation of the veins (Figure 4). We also noticed branching tortuosity as early as 1 month in the transgenic mice compared to their wild type counterparts (Figure 4). No obvious arterial abnormalities were observed in the transgenic mice.
Figure 4.
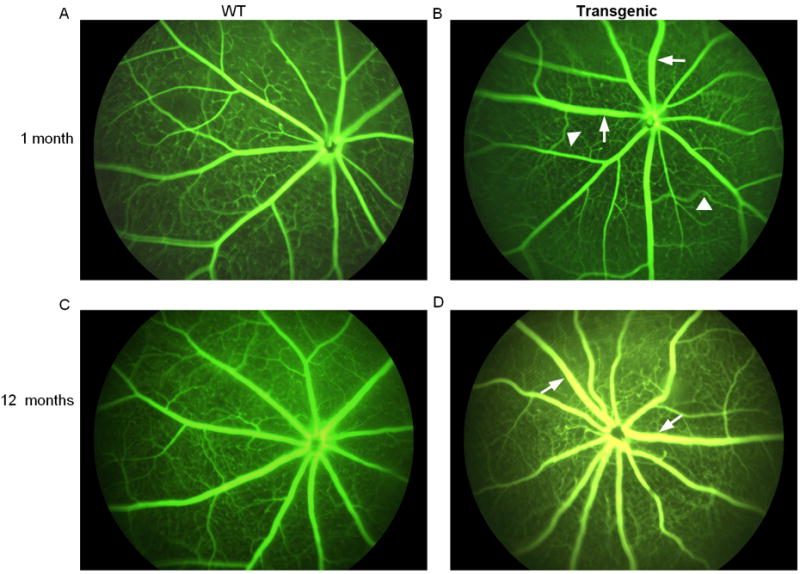
Fluorescein angiography of the wild type and βA3/A1-crystallin mutant (Nuc1) transgenic animals at 1 month and 12 months of age. Increased tortuosity of branches, as indicated by arrowheads, was apparent in the transgenic animals as compared to their wild type counterparts. There was also a suggestion of increased vessel dilation in the transgenic (arrows). n= 3 wild type; 7 transgenic mutant mice.
The only other crystallin that is known to be expressed in astrocytes is αB-crystallin, a small heat shock protein (Horwitz, 2003; Cahoy et al., 2008). The expression of αB-crystallin has been shown to be increased in astrocytes under stress condition and also in degenerative states of the retina (Jones et al.,1998; Salvador-Silva et al., 2001). It has been shown that αB-crystallin is inducible by hypoxia/reoxygenation and by TGFβ1 and TGFβ2 treatments of astrocytes cultured from the human lamina cribrosa (Yu et al., 2007). αB-crystallin has been shown to play a crucial role in the regulation of the angiogenic response in models of ocular angiogenesis (Kase et al., 2010).
Conclusion
Our studies, both with the Nuc1 rat and transgenic mice, as described here, suggest that βA3/A1-crystallin is essential for astrocyte template formation in the developing retina and for the subsequent patterning and remodeling of the retinal vasculature. Experiments under way in our laboratory using the Cre-loxP system to delete the βA3/A1-crystallin gene selectively from retinal astrocytes, should help elucidate the mechanisms whereby βA3/A1-crystallin is involved in the regulation of these vital processes.
Acknowledgments
DS is thankful to the National Eye Institute for support of his IPA. This work was also supported by National Institutes of Health: EY018636 (DS), EY019037 (DS), EY019037-S (DS), Wilmer Pooled Professors Fund (DS), Helena Rubinstein Foundation (DS), EY01765 (Wilmer Imaging Core), Intramural Research Program, National Eye Institute (EFW and JSZ), and Research to Prevent Blindness (an unrestricted grant to The Wilmer Eye Institute). We also wish to thank the Norman Raab Foundation and the Wilmer Pooled Professors Fund for purchasing the Micron III imaging system. We thank Steven Lee and Carl Haugen for their technical help with the transgenic mouse production and to the Staff Members at Spring Valley Laboratories, Woodbine, MD, for taking care of the experimental animals. We thank Drs. Morton F. Goldberg and Bhaja K. Padhi for critical reading and discussion regarding this manuscript.
References
- Andley UP. Crystallins in the eye: Function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bhat SP. Transparency and non-refractive functions of crystallins-a proposal. Exp Eye Res. 2004;79:809–816. doi: 10.1016/j.exer.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Brenner M. Structure and transcriptional regulation of the GFAP gene. Brain Path. 1994;4:245–257. doi: 10.1111/j.1750-3639.1994.tb00840.x. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christpherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28:264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Ling T, McLeod DS, Hughes S, Baxter L, Chu Y, Hasegawa T, Lutty GA. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest Ophthalmol Vis Sci. 2004;45:2020–32. doi: 10.1167/iovs.03-1169. [DOI] [PubMed] [Google Scholar]
- Delaney CL, Brenner M, Messing A. Conditional ablation of cerebellar astrocytes in postnatal transgenic mice. J Neurosci. 1996;6:6908–6918. doi: 10.1523/JNEUROSCI.16-21-06908.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MM, McLeod DS, Grebe R, Heng C, Lefebvre O, Lutty GA. Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of the fetal vasculature, and epiretinal membrane formation in mice. BMC Dev Biol. 2011;11(1):60. doi: 10.1186/1471-213X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlbach P, Hose S, Lei B, Zhang C, Cano M, Arora M, Neal R, Barnstable C, Goldberg MF, Zigler JS, Jr, Sinha D. Developmental abnormalities in the Nuc1 rat retina: a spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137:447–61. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- Goldberg MF. Persistant fetal vasculature (PFV): an integrated interpretation of signs and symptoms associated with persistent hyperplastic primary vitreous (PHPV) LIV Edward Jackson Memorial Lecture. Amer J Ophthalmol. 2007;124:587–626. doi: 10.1016/s0002-9394(14)70899-2. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Experimental Eye Research. 2003;76(2):145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Hurskainen M, Eklund L, Hägg PO, Fruttiger M, Sormunen R, Ilves M, Pihlajaniemi T. Abnormal maturation of the retinal vasculature in type XVIII collagen/endostatin deficient mice and changes in retinal glial cells due to lack of collagen types XV and XVIII. FASEB J. 2005;19:1564–1566. doi: 10.1096/fj.04-3101fje. [DOI] [PubMed] [Google Scholar]
- Jones SE, Jomary C, Grist J, Thomas MR, Neal MJ. Expression of αB-crystallin in a mouse model of inherited retinal degeneration. Neuroreport. 1998;9:4161–4165. doi: 10.1097/00001756-199812210-00030. [DOI] [PubMed] [Google Scholar]
- Kase S, He S, Sonoda S, Kitamurra M, Spee C, Wawrousek E, Ryan SJ, Kannan R, Hinton K. αB-crystallin regulation of angiogenesis by modulation of VEGF. Blood. 2010;115:3398–3406. doi: 10.1182/blood-2009-01-197095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupenko SA, Oleinik NV. 10-formyltetrahydrofolate dehydrogenase, one of the major folate enzymes, is down-regulated in tumor tissues and possesses suppressor effects on cancer cells. Cell Growth Differ. 2002;13(5):227–236. [PubMed] [Google Scholar]
- Ma B, Sen T, Asnaghi L, Valapala M, Yang F, Hose S, McLeod DS, Lu Y, Eberhart C, Zigler JS, Jr, Sinha D. βA3/A1-crystallin controls anoikis-mediated cell death in astrocytes by modulating PI3K/AKT/mTOR and ERK survival pathways via the PDK/Bit1 signaling axis. Cell Death and Disease. 2011;2:e217. doi: 10.1038/cddis.2011.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay TL, Gedeon DJ, Vickerman MB, Hylton AG, Ribita D, Olar HH, Kaiser PK, Parsons-Wingerter P. Selective inhibition of angiogenesis in small blood vessels and decrease in vessel diameter throughout the vascular tree by triamcinolone acetonide. Invest Ophthalmol Vis Sci. 2008 Mar;49(3):1184–90. doi: 10.1167/iovs.07-1329. [DOI] [PubMed] [Google Scholar]
- Parthasarathy G, Ma B, Zhang C, Gongora C, Zigler JS, Jr, Duncan MK, Sinha D. Expression of βA3/A1-crystallin in the developing and adult rat eye. J Mol Histol. 2011;42:59–69. doi: 10.1007/s10735-010-9307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piatigorsky J. Gene sharing and evolution: the diversity of protein function. Harvard University Press; Cambridge, MA: 2007. [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A in Transgenic mice results in retinal astrocytic hamartomas. Invest Ophthalmol Vis Sci. 1996;37(12):2455–2466. [PubMed] [Google Scholar]
- Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- Salvador-Silva M, Ricard CS, Agapora OA, Yang P, Hernandez MR. Expression of small heat shock proteins and intermediate filaments in the human optic nerve head astrocytes exposed to elevated hydrostatic pressure in vitro. J Neurosci Res. 2001;66:59–73. doi: 10.1002/jnr.1197. [DOI] [PubMed] [Google Scholar]
- Schnitzer J. Astrocytes in the guinea pig, horse, and monkey retina: their occurrence coincides with the presence of blood vessels. Glia. 1988;1:74–89. doi: 10.1002/glia.440010109. [DOI] [PubMed] [Google Scholar]
- Scott A, Powner MB, Gandhi P, Clarkin C, Gutmann DH, Johnson RS, Ferrara N, Fruttiger M. Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. Plos ONE. 2010;5(7):e1 1863. doi: 10.1371/journal.pone.0011863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segovia J, Vergara P, Brenner M. Astrocyte-specific expression of tyrosine hydroxylase after intracerebral gene transfer induces behavioral recovery in experimental parkinsonism. Gene Ther. 1998;5:1650–1655. doi: 10.1038/sj.gt.3300776. [DOI] [PubMed] [Google Scholar]
- Sinha D, Hose S, Zhang C, Neal R, Ghosh M, O’Brien TP, Sundin O, Goldberg MF, Robison WG, Jr, Russell P, Lo WK, Zigler JS., Jr A rat spontaneous mutation affects programmed cell death during the early development of the eye. Exp Eye Res. 2005;80:323–335. doi: 10.1016/j.exer.2004.09.014. [DOI] [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-Llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RN, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JS., Jr βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol Cell Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J, Dreher Z. Relationship between astrocytes, ganglion cells and vasculature of the retina. J Comp Neurol. 1987;255:35–49. doi: 10.1002/cne.902550104. [DOI] [PubMed] [Google Scholar]
- Valapala M, Thamake SI, Vishwanatha JK. A competitive hexapeptide inhibitor of annexin A2 prevents hypoxia-induced angiogenic events. J Cell Sci. 2011 May 1;124(Pt 9):1453–64. doi: 10.1242/jcs.079236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandier D, Rixe O, Brenner M, Gouyette A, Besnard F. Selective killing of glioma cell lines using an astrocyte-specific expression of the herpes simplex virus-thymidine kinase gene. Cancer Res. 1998;58:4577–4580. [PubMed] [Google Scholar]
- Werten PJL, Stege GJJ, de Jong WW. The short 5’ untranslated region of the βA3/A1-crystallin mRNA is responsible for leaky ribosomal scanning. Mol Biol Reports. 1999;26:201–205. doi: 10.1023/a:1007046926233. [DOI] [PubMed] [Google Scholar]
- Wistow G. Molecular biology and evolution of crystallins: gene recruitment and multifunctional proteins in the eye lens. Springer; New York: 1995. [Google Scholar]
- Yu AL, Fuchshofer R, Birha M, Priglinger SG, Eibl KH, Kampik A, Bloemendal H, Welge-Lussen U. Hypoxia/reoxygenation and TGF-β increase αB-crystallin expression in human optic nerve head astrocytes. Experimental Eye Research. 2007;84:694–706. doi: 10.1016/j.exer.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Zhang C, Gehlbach P, Gongora C, Cano M, Fariss R, Hose S. A potential role for β- and γ-crystallins in the vascular remodeling of the eye. Dev Dyn. 2005;234:36–47. doi: 10.1002/dvdy.20494. [DOI] [PubMed] [Google Scholar]
- Zhang C, Asnaghi L, Gongora C, Patek B, Hose S, Ma B, Fard MA, Brako L, Singh K, Goldberg MF, Handa JT, Lo WK, Eberhart CG, Zigler JS, Jr, Sinha D. A developmental defect in astrocytes inhibits programmed regression of the hyaloid vasculature in the mammalian eye. Eur J Cell Biol. 2011;90:440–448. doi: 10.1016/j.ejcb.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, McLeod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the βA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J Cell Sci. 2011;124:523–531. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]


