Abstract
Atopic dermatitis (AD) is the most common chronic inflammatory skin disease of humans, affecting approximately 17% of children. AD patients are especially susceptible to cutaneous bacterial and viral infections, and may develop severe or fatal herpes simplex virus (HSV) infection (eczema herpeticum, EH), requiring intensive antiviral therapy. However, even though a majority of adults show serologic evidence of previous HSV exposure, EH occurs in less than 3% of AD patients. The unexpected rarity of AD patients with EH (ADEH+) suggests that multiple host factors play a role in the clinical expression of this complex phenotype. Recent studies comparing ADEH+ versus ADEH− patients reveal that patients prone to ADEH+ have more severe AD skin disease, biomarkers associated with Th2 helper cell responses (reduced interferon levels, circulating eosinophil counts, increased serum IgE and allergen sensitization) and decreased epidermal expression of filaggrin and antimicrobial peptides. ADEH+ subjects are also more likely to have a history of food allergy or asthma, early onset of AD and a history of other cutaneous infections with S. aureus or molluscum contagiosum.
Keywords: Atopic dermatitis, herpes simplex virus, eczema herpeticum
Atopic dermatitis (AD) is a highly pruritic, complex genetic skin disease (Table 1) that often precedes the development of food allergy, asthma and allergic rhinitis (Boguniewicz and Leung, 2011; Guttman-Yassky et al., 2011; Table 1). It is the most common chronic inflammatory skin disease in the general population affecting approximately 17% of children in the United States (Laughter et al., 2000). Recent findings in the pathobiology of AD point to an important role of genetic abnormalities in epidermal barrier function, environmental exposures as well as immune dysregulation in contributing to the severity of this complex genetic skin disease (Fig. 1; Barnes, 2010).
Table 1.
Features of Atopic Dermatitis
| Major Characteristics |
| Pruritus |
| Eczematoid rash on face and/or extensors in young children |
| Lichenification in flexural areas in older children and adults |
| Chronic or chronically relapsing dermatitis |
| Personal or family history of atopic disease such as food allergy, asthma, allergic rhinitis |
| Other Findings |
| Dryness of skin and barrier dysfunction |
| Allergic shiners (darkening beneath the eyes) |
| Keratosis pilaris |
| Ichthyosis vulgaris |
| Hyperlinearity of palms and soles |
| Elevated serum immunoglobulin E |
| Immediate skin test reactivity to allergens |
| Recurrent bacterial and viral skin infections |
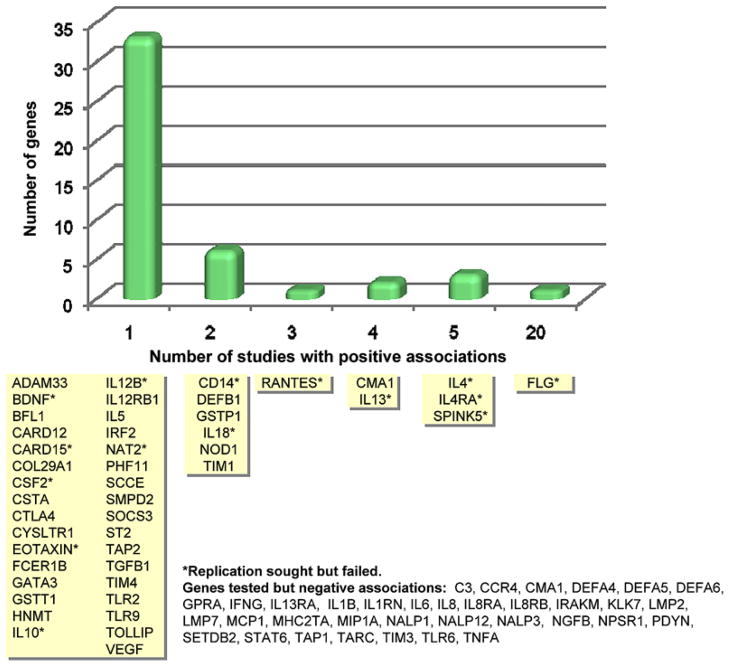
Figure 1.
Genes associated with AD in at least 1 published study. Genes are grouped according to how many positive association studies have been reported. The y-axis indicates the number of genes (corresponding to the yellow boxes) for each time that a positive association was reported (From: Barnes, K.C. 2010).
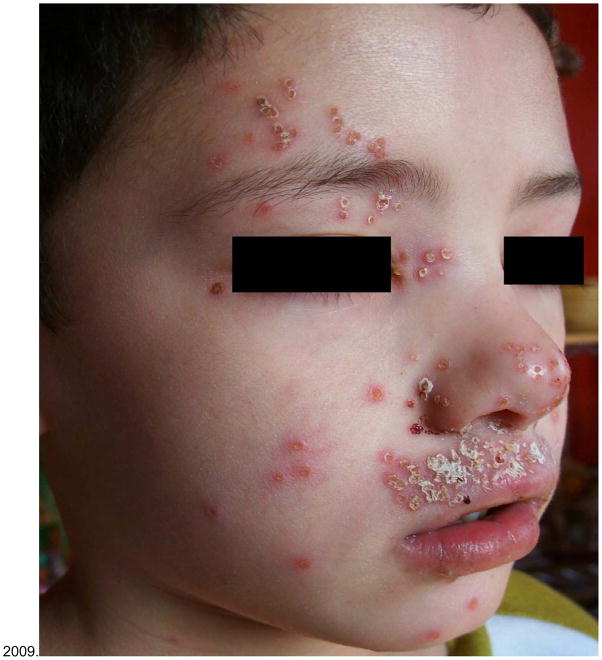
AD is complicated by recurrent bacterial and viral skin infections (Boguniewicz and Leung, 2010). Eczema herpeticum (EH) is a particularly serious complication of AD that requires prompt and effective anti-viral therapy (Wollenberg, 2012). EH presents as a disseminated eruption of dome-shaped monomorphic vesicles that contain multi-nucleated giant cells infected with herpes simplex virus (HSV) (Fig. 2). It is associated with viremia, fever, malaise, lymphadenopathy and significant systemic complications including keratoconjunctivitis that can result in blindness, as well as multi-organ involvement leading to meningitis and encephalitis. Prior to effective anti-viral therapy, death was not uncommon in patients suffering from acute EH.
Figure 2.
A Child with atopic dermatitis complicated by eczema herpeticum. From Boguniewicz and Leung, 2009.
The primary requisite for development of EH is HSV-1 exposure. HSV is ubiquitous in the general population e.g., approximately 20% of children and over 60% of adults are seropositive (Xu, et al. 2006) however, only approximately 3% of AD patients develop disseminated cutaneous HSV infections (ADEH+) despite the high likelihood of HSV-1 exposure (Beck, et al. 2009). This suggests that ADEH+ is not a function of environmental exposure alone, and host factors play an important role in its development. Recent studies indicate that a combination of immunologic and genetic determinants make a small subset of AD patients especially vulnerable to severe viral infections such as EH. We speculate that this complex phenotype that increases propensity of ADEH+ likely also accounts for the infrequent occurrence of eczema vaccinatum following smallpox vaccination (Reed, et al. 2011; Kempe, 1968). However, it is very difficult to study mechanisms of EV since smallpox vaccination is contraindicated in patients with AD.
Multi-center studies by The Atopic Dermatitis Research Network funded by The National Institute of Allergy and Infectious Diseases (NIAID) have played a key role in establishing a database of clinical information and repository of biological samples for the study of this rare ADEH+ phenotype allowing considerable progress to be made in our understanding of mechanisms underlying this condition (Beck, et al. 2009). A comparison of the clinical features of ADEH+ versus ADEH− subjects reveal that patients who are ADEH+ have more severe AD (Fig. 3A,B), an earlier onset of skin disease and a higher prevalence of associated allergic diseases such as food allergy or asthma. Nearly 50% of the ADEH+ group had more than one episode of EH and 4.5% reported greater than 5 episodes. ADEH+ subjects more frequently reported a history of cutaneous infections with S. aureus than ADEH− patients. Interestingly, staphylococcal toxins have been found to increase viral replication in skin cells (Bin, et al. 2012) suggesting S. aureus colonization or infection may increase propensity to viral skin infections.
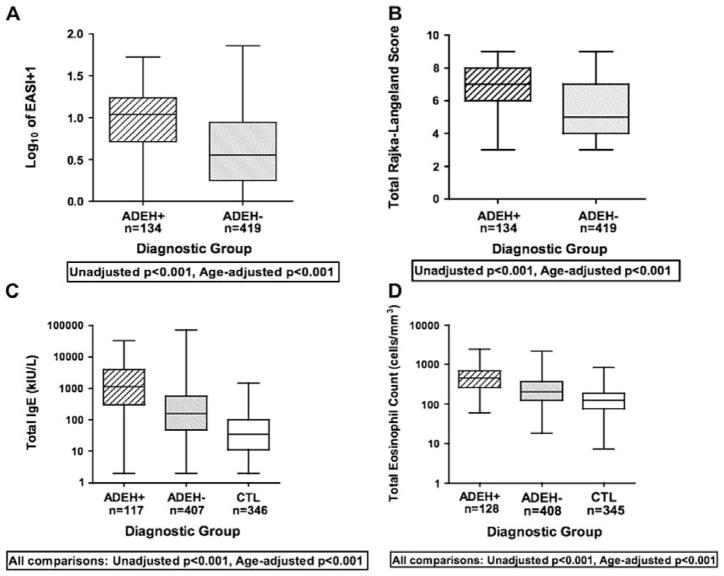
Figure 3.
Boxplot graphs of AD severity scores [EASI (A) and Rajka and Langeland (B)] and biomarkers indicative of Th2 polarity[serum total IgE (C) and total eosinophil counts (D)]. From: Beck, L.A., et al, 2009.
Consistent with its increased association with other allergic diseases, ADEH+ subjects have significantly higher serum total IgE and circulating total eosinophil counts compared to ADEH− subjects and healthy controls (Fig. 3C,D) suggesting a higher level of Th2 polarity in their immune responses (Beck, et al 2009; Wollenberg, et al. 2003). The high total serum IgE values in ADEH+ compared to ADEH− group was reflected in significant differences in allergen specific sensitization between these 2 subsets of AD. ADEH+ subjects demonstrated significantly higher levels of IgE directed to inhalant, food and staphylococcal toxins. Beck et al (2009) also assessed TARC/CCL17, a chemokine which binds to CCR4 that is highly expressed on skin-homing Th2 lymphocytes and found serum TARC to be significantly increased in ADEH+, as compared to ADEH− subjects.
The finding that ADEH+ subjects have increased serum total IgE, IgE directed to multiple allergens and TARC/CCL17 as well as eosinophilia suggests that their clinical phenotype stems from polarized Th2 immune responses. Th2 cytokines, such as IL-4, and IL-13, are known to play a key role in driving serum IgE synthesis. More importantly, these cytokines have been found to dampen host anti-viral immune responses on the basis of their inhibitory actions on the expression of antimicrobial proteins in the skin, epidermal barrier proteins and cell-mediated immunity. Keratinocyte expression of cationic peptides (beta-defensin [HBD-3] and cathelicidins [LL-37]) have been shown to exert antiviral activity (Howell, et al. 2006a). AD lesions have increased levels of the Th2 cytokines, IL-4 and IL-13, and these cytokines reduce expression of beta-defensins (HBD-2 and -3) and LL-37 by epidermal keratinocytes (Ong, et al. 2002). A correlation has been reported between serum IgE level and expression of LL-37, with the lowest levels of LL-37 found in the skin of ADEH+ subjects (Howell, et al. 2006b; Hata et al. 2010). Increased IL-4 is found in both clinically involved and uninvolved AD skin. This may explain why EH can develop in both symptomatic and asymptomatic AD subjects. Importantly, gene variants of thymic stromal lymphopoietin (TSLP), a cytokine that markedly enhances Th2 cell differentiation, are strongly associated with the ADEH+ phenotype (Gao, et al. 2010). The actions of IL-4 and IL-13 are mediated by STAT6. Therefore the important role of Th2 cytokines in driving the ADEH+ phenotype is strengthened by the observation that STAT6 transgenic mice have increased eczema, their skin supports enhanced viral replication and there is an association of STAT6 gene variants with ADEH+ subjects (Howell, et al. 2011).
Global transcriptional differences in peripheral blood mononuclear cells (PBMCs) from ADEH+ subjects, compared to ADEH− and non-atopic control participants following stimulation with vaccinia virus vs. sham treatment have been carried out. Expression analysis of 38,500 genes demonstrated significant association of ADEH+ with transcriptomics of the interferon (IFN) superfamily (Leung, et al. 2011). Participants with the ADEH+ phenotype were found to have significantly decreased gene expression of IFN-gamma (IFN-γ), as well as the receptors for IFN-γ and alpha IFN. Consistent with this finding, IFN-γ protein generation was reported to be significantly decreased in PBMCs from ADEH+ participants, as compared to ADEH− participants and non-atopic individuals, after stimulation with HSV ex vivo. These findings are highly relevant to development of the ADEH+ phenotype because IFN-γ plays critical roles in the innate and acquired immune responses by activating macrophages, enhancing natural killer cell activation, and promoting anti-viral T cell differentiation (Koluman, et al. 2005). Animal model studies have demonstrated that genetic disruption of IFN-γ or neutralization of IFN-γ increases the susceptibility of mice to HSV infection. The addition of IFN-γ in an epidermal explants model significantly reduced the number and size of viral cytopathic plaques (Mikloska, et al. 2001). Therefore deficient IFN-γ induction can contribute to increased susceptibility to HSV infection in ADEH+ patients.
The mechanism for reduced IFN-γ generation in ADEH+ subjects is complex and related to both genetic, immunologic responses and environmental exposures including allergen and infection. One mechanism by which enhanced IL-4 responses may promote HSV infections is by inhibiting the release of anti-viral interferons (IFNs). AD subjects also have an impaired recruitment of plasmacytoid dendritic cells (DC) to skin (Wollenberg, et al. 2002). This DC subpopulation is recognized for its ability to produce type 1 IFNs, that are important for antiviral immunity. Finally, there have been several reports of pattern recognition receptor (PRR) polymorphisms, especially in toll-like receptor (TLR)2, to be associated with AD. Interestingly, TLR2 recognizes S. aureus and HSV, which are pathogens causing skin infections in AD patients. Moreover, patients with TLR2 polymorphisms have been reported to have more severe AD (Ahmad-Nejad et al., 2004), a condition that predisposes to ADEH+ (Beck et al., 2009). These data are consistent with the concept that defective IFN-γ responses in PBMCs from ADEH+ patients could result from aberrant PRR signaling in antigen presenting cells.
Recent studies have postulated that IgE mediated allergen sensitization in AD subjects occurs primarily following allergen penetration through the skin where engagement of IgE+ Langerhans cells in the presence of TSLP leads to increased Th2 responses. This process is enhanced by epidermal barrier defects and suggests that such defects could also be risk factors for EH. The skin has 2 epidermal barrier structures: the first is represented by the stratum corneum, which is widely accepted to be dysfunctional in AD (Broccardo, et al. 2011). This was initially ascribed to mutations in the gene (FLG) encoding filaggrin, a critical protein involved in the aggregation of keratins in the stratum corneum and critical for maintenance of skin barrier function (Irvine, et al. 2011). FLG mutations, have been consistently associated with risk of AD and related traits, including asthma, hay fever, rhinoconjunctivitis, and high level allergen-specific IgE production.
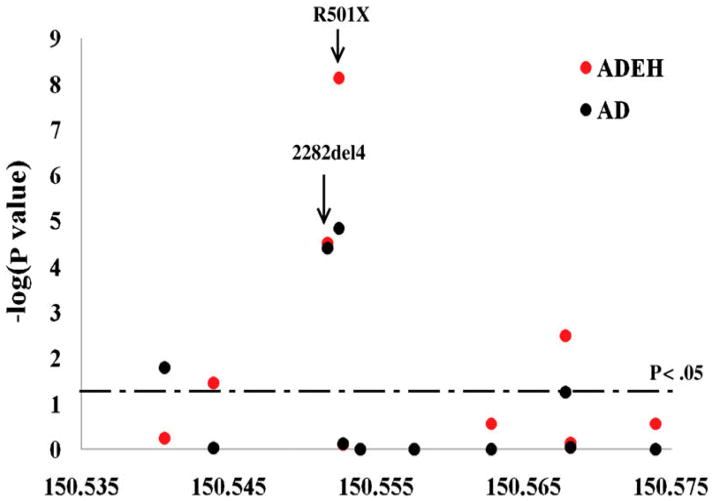
FLG gene mutations represent the strongest and most replicated genetic risk factor for AD. Highly significant associations (Gao, et al. 2009) have been reported between FLG gene mutations and ADEH+ subjects (odds ratio=10.1) at significance levels greater than observed for FLG gene mutations (Fig. 4) and ADEH− subjects (odds ratio= 3.4). FLG mutations, however, account for only 50% of severe AD subjects. Importantly, enhanced Th2 cytokine responses affecting the majority of AD subjects also inhibit the production of skin epidermal barrier proteins, including filaggrin and involucrin (Howell, et al. 2007). FLG deficient mice have enhanced allergen penetration through their skin, increased serum IgE and demonstrates reduced capacity to control skin viral replication (Oyoshi, et al. 2009). Filaggrin breakdown products have also been found to reduce S. aureus proliferation. Since staphylococcal products promote viral replication and reduce skin barrier function, intact filaggrin may reduce viral skin infections (Miajlovic, et al. 2010; Leung, et al. unpublished observations).
Figure 4.
Summary of association tests among European American subjects showing significance for each FLG mutation. The −log10-transformed P values on the y axis for all genotyped markers are plotted against their genomic position in NCBI Build 35 in megabase along the x axis. Red circles indicate associations for ADEH; green circles indicate associations for AD. The horizontal dotted line indicates nominal significance P < .05 (Gao, et al. 2009).
Tight junctions (TJs) are a second skin barrier structure (De Benedetto, et al. 2011a). They are found on opposing membranes of keratinocytes in the stratum granulosum, directly below the stratum corneum. TJs consist of a complex of adhesive proteins controlling the passage of water, and solutes via the paracellular pathway in the skin. The susceptibility of human keratinocytes to HSV-1 infection is inversely related to the degree of cell-cell contact and confluency (De Benedetto, et al. 2011a). Healthy junctional complexes are a key deterrent to the spread of HSV-1 from one keratinocyte to another. Gene knockdown of epidermal CLDN1 has been found to enhance HSV-1 infectivity of human keratinocytes. The AD epidermis has bioelectric abnormalities indicative of a TJ defect thought to be the consequence of reduced levels of claudin-1 (CLDN1), a key TJ adhesive protein (De Benedetto, et al. 2011b). In AD, an inverse correlation was found between CLDN1 expression and markers of TH2 polarity (total eosinophil counts and serum total IgE). Preliminary studies also suggest that genetic variations in CLDN1 contribute to risk of EH in AD subjects. Furthermore, excluding subjects with a FLG mutation strengthened the association of CLDN1 mutations with susceptibility to widespread HSV skin infections in subjects with AD. Overall these data suggest that both stratum corneum and TJ epidermal barrier defects participate in mechanisms that increase the susceptibility of subjects with ADEH+ to widespread cutaneous infections with HSV, i.e. EH.
In conclusion, despite the high prevalence of AD in the general population and common HSV exposure, EH is unexpectedly rare. This is likely because ADEH+ is a complex phenotype requiring a multitude of environmental and host factors, each alone being necessary but not sufficient. These include an impaired physical skin barrier involving the stratum corneum (e.g. filaggrin expression) and stratum granulosum (e.g. decreased claudin), reduced chemical barrier function (e.g. abnormal induction of antimicrobial peptides), decreased viral recognition receptors (e.g. decreased TLR2 impairing innate immune response), increased severity of skin disease, polarity of Th2 responses and reduced anti-viral Th1 responses (lack of IFN generation). In contrast, expression of milder forms of AD may only require 1–2 of these factors, with reduced skin barrier function potentially being the only necessary factor in mild AD since eczema would be elicited following allergen penetration of the skin. Identification of genetic variants and biomarkers for AD subsets at risk for serious disseminated skin infections including EH, molluscum contagiosum and eczema vaccinatum will be critical in protecting this group of patients against live viral vaccines and the development of more effective anti-viral strategies in preventing this devastating complication of AD.
Highlights.
Atopic dermatitis is a common skin disease.
Exposure to herpes simplex virus is frequent.
Eczema herpeticum occurs rarely as a complication of atopic dermatitis.
EH is associated with polarized Th2 helper cell responses and decreased filaggrin.
Acknowledgments
Funding: The Atopic Dermatitis Research Network NIH/NIAID contract HHSN272201000020C and R01 AR41256.
I would like to acknowledge Shih-Yun Lyman for her assistance in the preparation of this manuscript. The investigator’s work described in this manuscript was supported by NIH/NIAID contract HHSN272201000020C and NIH/NIAMS R01 AR41256.
Abbreviations
- AD
atopic dermatitis
- ADEH+
AD with a history of eczema herpeticum
- ADEH
AD without a history of eczema herpeticum
- CLDN
claudin
- EH
eczema herpeticum
- FLG
gene encoding filaggrin
- HSV
herpes simplex virus
- IFN
interferon
- PBMC
peripheral blood mononuclear cells
- TJ
tight junctions
- TLR
toll like receptor
Footnotes
Conflicts of Interest: None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad-Nejad P, Mrabet-Dahbi S, Breuer K, Klotz M, Werfel T, Herz U, Heeg K, Neumaier M, Renz H. The toll-like receptor 2 R753Q polymorphism defines a subgroup of patients with atopic dermatitis having severe phenotype. J Allergy Clin Immunol. 2004;113:565–7. doi: 10.1016/j.jaci.2003.12.583. [DOI] [PubMed] [Google Scholar]
- Barnes KC. An update on the genetics of atopic dermatitis: Scratching the surface in 2009. J Allergy Clin Immunol. 2010;125:16–29. doi: 10.1016/j.jaci.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck LA, Boguniewicz M, Hata TR, Schneider LC, Hanifin JM, Gallo RL, Paller AS, Lieff S, Reese J, Zaccaro D, Milgrom H, Barnes KC, Leung DYM. Phenotype of atopic dermatitis: Subjects with a history of eczema herpeticum. J Allergy Clin Immunol. 2009;124:260–9. doi: 10.1016/j.jaci.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bin L, Brauweiler A, Goleva E, Streib J, Yinduo J, Schilevert PM, Leung DYM. Staphylococcus aureus α-toxin modulates skin host response to viral infection. J Allergy Clin Immunol. 2012;130:683–691. e2. doi: 10.1016/j.jaci.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boguniewicz M, Leung DY. Recent insights into atopic dermatitis and implications for management of infectious complications. J Allergy Clin Immunol. 2010;125:4–13. doi: 10.1016/j.jaci.2009.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broccardo CJ, Mahaffey S, Schwarz J, Wruck L, David G, Schlievert PM, Reisdorph NA, Leung DY. Comparative proteomic profiling of patients with atopic dermatitis based on history of eczema herpeticum infection and Staphylococcus aureus colonization. J Allergy Clin Immunol. 2011;127:186–93. 93e1–11. doi: 10.1016/j.jaci.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Rafaels NM, McGirt LY, Ivanov AI, Georas SN, Cheadle C, Berger AE, Zhang K, Vidyasagar S, Yoshida T, Boguniewicz M, Hata T, Schneider LC, Hanifin JM, Gallo RL, Novak N, Weidinger S, Beaty TH, Leung DY, Barnes KC, Beck LA. Tight junction defects in patients with atopic dermatitis. J Allergy Clin Immunol. 2011a;127:773–86. doi: 10.1016/j.jaci.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedetto A, Slifka MK, Rafaels NM, Kuo IH, Georas SN, Boguniewicz M, Hata TR, Schneider LC, Hanifin JM, Gallo RL, Johnson DC, Barnes KC, Leung DY, Beck LA. Reductions in Claudin-1 may enhance susceptibility to HSV-1 infections in atopic dermatitis. J Allergy Clin Immunol. 2011b;128:242–6. doi: 10.1016/j.jaci.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PS, Rafaels NM, Hand T, Murray T, Boguniewicz M, Hata T, Schneider L, Hanifin JM, Gallo RL, Gao L, Beaty TH, Beck LA, Barnes KC, Leung DY. Filaggrin mutations that confer risk of atopic dermatitis confer greater risk for eczema herpeticum. J Allergy Clin Immunol. 2009;124:507–13. doi: 10.1016/j.jaci.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao PS, Rafaels NM, Mu D, Hand T, Murray T, Boguniewicz M, Hata T, Schneider L, Hanifin JM, Gallo RL, Gao L, Beaty TH, Beck LA, Leung DY, Barnes KC. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol. 2010;125:1403–1407. doi: 10.1016/j.jaci.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis--part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127:1110–8. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- Hata TR, Kotol P, Boguniewicz M, Taylor P, Paik A, Jackson M, Nguyen M, Kabigting F, Miller J, Gerber M, Zaccaro D, Armstrong B, Dorschner R, Leung DY, Gallo RL. History of eczema herpeticum is associated with the inability to induce human beta-defensin (HBD)-2, HBD-3 and cathelicidin in the skin of patients with atopic dermatitis. Br J Dermatol. 2010;163:659–61. doi: 10.1111/j.1365-2133.2010.09892.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gao PS, Kim BE, Lesley LJ, Streib JE, Taylor PA, Zaccaro DJ, Boguniewicz M, Beck LA, Hanifin JM, Schneider LC, Hata TR, Gallo RL, Kaplan MH, Barnes KC, Leung DYM. The signal transducer and activator of transcription 6 gene (STAT6) increases propensity of patient with atopic dermatitis patients toward disseminated viral skin infections. J Allergy Clin Immunol. 2011;128:1006–14. doi: 10.1016/j.jaci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Kim BE, Gao P, Grant AV, Boguniewicz M, Debenedetto A, Schneider L, Beck LA, Barnes KC, Leung DY. Cytokine modulation of atopic dermatitis filaggrin skin expression. J Allergy Clin Immunol. 2007;120:150–5. doi: 10.1016/j.jaci.2007.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, Leung DY. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006a;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Howell MD, Wollenberg A, Gallo RL, Flaig M, Streib JE, Wong C, Pavicic T, Boguniewicz M, Leung DYM. Cathelicidin deficiency predisposes to eczema herpeticum. J Allergy Clin Immunol. 2006b;117:836–41. doi: 10.1016/j.jaci.2005.12.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine AD, McLean WHI, Leung DYM. Filaggrin mutations: Associations with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Kempe CH. Smallpox vaccination of eczema patients with attenuated live vaccinia virus. Yale J Biol Med. 1968;41:1–12. [PMC free article] [PubMed] [Google Scholar]
- Koluman GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughter D, Istvan JA, Tofte SJ, Hanifin JM. The prevalence of atopic dermatitis in Oregon schoolchildren. J Am Acad Dermatol. 2000;43:649–655. doi: 10.1067/mjd.2000.107773. [DOI] [PubMed] [Google Scholar]
- Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, Taylor PA, Boguniewicz M, Canniff J, Armstrong B, Zaccaro DJ, Schneider LC, Hata TR, Hanifin JM, Beck LA, Weinberg A, Barnes KC. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73. e1–5. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miajlovic H, Fallon Padraic G, Irvine AD, McLean WHI, Foster TJ. Effect of filaggrin breakdown products on growth of and protein expression by Staphylococcus aureus. J Allergy Clin Immunol. 2010;126:1184–90. doi: 10.1016/j.jaci.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikloska Z, Cunningham AL. Alpha and gamma interferons inhibit herpes simplex virus type 1 infection and spread in epidermal cells after axonal transmission. J Virol. 2001;75:11821–6. doi: 10.1128/JVI.75.23.11821-11826.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, Gallo RL, Leung DY. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Oyoshi MK, Murphy GF, Geha RS. Filaggrin-deficient mice exhibit TH17-dominated skin inflammation and permissiveness to epicutaneous sensitization with protein antigen. J Allergy Clin Immunol. 2009;124:485–93. doi: 10.1016/j.jaci.2009.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JL, Scott ES, Bray M. Eczema Vaccinatum. Clin Infect Dis. 2012;54:832–40. doi: 10.1093/cid/cir952. [DOI] [PubMed] [Google Scholar]
- Wollenberg A. Eczema herpeticum. Chem Immunol Allergy. 2012;96:89–95. doi: 10.1159/000331892. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Zoch C, Wetzel S, Plewig G, Przybilla B. Predisposing factors and clinical features of eczema herpeticum: a retrospective analysis of 100 cases. J Am Acad Dermatol. 2003;49:198–205. doi: 10.1067/s0190-9622(03)00896-x. [DOI] [PubMed] [Google Scholar]
- Wollenberg A, Wagner M, Gunther S, Towarowski A, Tuma E, Moderer M. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J Invest Dermatol. 2002;119:1096–102. doi: 10.1046/j.1523-1747.2002.19515.x. [DOI] [PubMed] [Google Scholar]
- Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE. Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA. 2006;296:964–73. doi: 10.1001/jama.296.8.964. [DOI] [PubMed] [Google Scholar]