SUMMARY
Oxidative stress, an imbalance between reactive oxygen species production and antioxidative defense activity, is believed to have a role in the development and pathogenesis of nasal polyps (NPs). Based on this assumption, several known oxidants and antioxidants have been studied in patients with NPs. The purpose of this study was to evaluate the association between oxidative stress parameters with a more valid and reliable method in patients with NPs. Seventy-three patients with NPs, septal deviations and middle concha hypertrophies were recruited. Patients were divided into two groups; group 1 (n = 38) consisted of patients with NPs, and group 2 (n = 35) included patients with septal deviations and middle concha hypertrophies. Polyp specimens were taken from all patients who underwent endoscopic surgery for NPs. Control specimens were obtained from patients who underwent an operation for septoplasty or middle concha hypertrophy. Blood and tissue samples were obtained to assess total oxidant status (TOS), total antioxidant status (TAS) and oxidative stress index (OSI). Compared to group 2, group 1 had significantly higher TOS and OSI and lower TAS levels both in serum and tissue samples (p < 0.001 for all). In group 1, tissue TOS and OSI levels were significantly higher, and TAS levels were significantly lower than in serum (p < 0.001 for all), whereas no significant difference was found in TOS, OSI and TAS levels either in serum or tissue samples in group 2 (p = 1.0; p = 1; p = 0.208, respectively). In group 1, serum OSI levels were significantly correlated with age (r = 0.442, p = 0.005). Our study demonstrated that oxidative stress, both in serum and tissues in patients with NPs, was higher than in patients without NPs. Our study differs from previous studies in that we used a more reliable method that measures both TOS and TAS.
KEY WORDS: Nasal polyp, Reactive oxygen species, Oxidative stress, Total antioxidant status, Total oxidant status, Aging, Pathogenesis
RIASSUNTO
Lo stress ossidativo cellulare, risultato dell'equilibrio tra produzione ed eliminazione di specie reattive dell'ossigeno, è considerato un agente patogenetico di rilievo coinvolto nello sviluppo della poliposi nasale (NPs). Per tale ragione diversi fattori ossidanti e i relativi antiossidanti sono stati oggetti di studio nei pazienti affetti da poliposi naso-sinusale. Scopo del nostro studio è stato quello di esaminare l'associazione tra gli indici di stress ossidativo cellulare in pazienti affetti da poliposi nasale attraverso metodiche affidabili e standardizzate. Sono stati arruolati complessivamente 73 pazienti affetti da poliposi nasosinusale, deviazione del setto nasale e ipertrofia dei turbinati medi con concha bullosa. Tali pazienti sono stati divisi in due gruppi: nel gruppo 1 (n = 38) sono stati inseriti pazienti affetti da poliposi nasale, nel gruppo 2 (n = 35) sono stati inclusi, invece, i pazienti con deviazione del setto e ipertrofia dei turbinati medi con concha bullosa. I campioni di polipi nasali sono stati raccolti e analizzati dai pazienti sottoposti a chirurgia endoscopica funzionale nasale durante l'intervento chirurgico. I campioni di controllo, invece, sono stati raccolti ed analizzati nei pazienti sottoposti ad intervento chirurgico di settoplastica e riduzione di concha bullosa. I prelievi ematici e istopatologici sono stati utilizzati per la valutazione di Status Ossidativo Totale (TOS), Status Antiossidativo Totale (TAS) e Indice di Stress Ossidativo (OSI). Nel gruppo 1 rispetto al gruppo 2 sono stati misurati, sia a livello sierico che tissutale, più alti livelli di TOS e OSI e più bassi livelli di TAS con una differenza in tutti casi statisticamente significativa (p < 0,001). Inoltre nel gruppo 1 i livelli tissutali di TOS e OSI erano significativamente più alti rispetto a quelli sierici mentre i livelli di TAS erano significativamente più bassi (p < 0.0001) mentre nessuna differenza significativa è stata osservata fra i livelli di TOS, OSI e TAS tissutale e sierici nel gruppo 2. Nel gruppo 1, i livelli serici di OSI risultavano correlati in maniera significativa con l'età (r = 0,442, p = 0,005). In conclusione il nostro studio dimostra che lo stress ossidativo cellulare è più elevato in pazienti affetti da poliposi nasale rispetto ai controlli, sia a livello sistemico che localmente a livello tissutale. A differenza di altri studi precedenti è stata utilizzata una metodica di determinazione di TOS e TAS più affidabile e standardizzata.
Introduction
Nasal polyp (NP) is a chronic inflammatory disease of the upper respiratory tract; however, its pathophysiology remains poorly understood. There are no aetiological factors that explain the pathogenesis of NPs, though inflammation continues to be a major factor 1 2. Many studies have noted that histopathological abnormities of NP are closely related to the infiltration of inflammatory cells. As a result of inflammation, neutrophils activate and migrate to the inflammatory area and exert their bactericidal effects by producing reactive oxygen species (ROS) 3-7. Once the balance between ROS production and antioxidative defense activity is disrupted, oxidative stress can occur that may result in cell injury or death, subsequent tissue damage and, finally, chronic disease 8-10.
Studies investigating the role of ROS and antioxidants in nasal polyposis have found strong evidence for the involvement of oxidative stress in the pathogenesis of the condition 7 11-23. However, several of the oxidants and antioxidant enzymes that were evaluated in these studies may not be sufficient to accurately quantify oxidative stress. To clarify the results of these studies, we examined patients who underwent endoscopic surgery for NPs and assessed oxidative stress using a more valid and reliable method that measures total oxidant status (TOS) and total antioxidant status (TAS) both in serum and tissue.
Material and methods
Study design and patients
This cross-sectional study was conducted at the Otolaryngology Head and Neck Surgery Department of Harran University School of Medicine, Sanliurfa, Turkey. Prior to subject recruitment, the study protocol was reviewed and approved by the University's ethics committee, in accordance with the ethical principles for human investigations, as outlined by the Second Declaration of Helsinki. Written informed consent was obtained from all patients. From May 2010 to March 2011, 73 consecutive patients with NPs, septal deviations and middle concha hypertrophies were recruited for the study.
Patients were divided in two groups; group 1 (n = 38) consisted of patients with NPs, and group 2 (n = 35) was composed of patients with septal deviations and middle concha hypertrophies with computed tomography (CT) scores comparable to those of the study population. The diagnosis was based on anterior rhinoscopy, endoscopic examination and paranasal sinus CT. Findings on preoperative CT were graded according to the Lund–Mackay system. The mucosal abnormalities were graded as 0 (no abnormality), 1 (partial opacification), or 2 (total opacification) for each sinus group. The ostiomeatal complexes were scored bilaterally as 0 (not occluded) or 2 (occluded). The maximal CT grading score was 24 24. Polyp specimens were taken from all patients who underwent endoscopic surgery for NPs, and control specimens (mucosal punctates from lateral lamella of the middle turbinate) were acquired from patients who underwent an operation for septoplasty or middle concha hypertrophy.
The exclusion criteria included the following: recent acute infectious illness; any evidence of liver, kidney, or respiratory disease; diabetes mellitus; malignancy; any inflammatory or infiltrative disorder; recent use (within 2 weeks) of any systemic or local drug with antioxidant properties; preoperative drug use such as parenteral or oral corticosteroids and antibiotics for at least 4 weeks prior to sampling; and regular alcohol use or alcohol use within the previous 48 hours. No patient had a history of nasal allergy, asthma, or acetylsalicylic acid sensitivity. All showed negative results to a skin prick test with common airborne allergens, which was evaluated by the same experienced dermatologist to prevent interobserver variability. During interventions, tissue samples from polyps and turbinates were collected, fixed in formalin and frozen (-80° C) until the date of analysis.
Biochemical analysis
All blood samples were drawn after a 12-hour overnight fasting from a large antecubital vein without interruption of venous flow using a 19-gauge butterfly needle connected to a plastic syringe. Twenty ml of blood was drawn, with the first few ml being discarded. Ten ml were used for baseline routine laboratory tests. The residual content of the syringe was transferred immediately to polypropylene tubes, which were then centrifuged at 3,000 rpm for 10 min at 10 to 18°C. Supernatant plasma samples were stored in plastic tubes at –80°C until assayed. As serum markers of oxidant stress, TOS was measured and the oxidative stress index (OSI) was calculated. TAS was measured as an indicator of antioxidant status. These parameters were also studied and calculated in tissues. Tissues were homogenized in saline solution using a homogenizer. After centrifugation at 10,000 g for approximately 60 min, the clear supernatant was taken. TAS and TOS were measured in this fraction and OSI was calculated.
Measurement of total oxidant status
Serum TOS was measured using a novel automated method developed by Erel 25. Oxidants present in the sample oxidize the ferrous ion-o-dianisidine complex to ferric ion. The oxidation reaction is enhanced by glycerol molecules, which are abundantly present in the reaction medium. The ferric ion generates a colored complex with xylenol orange in an acidic medium. The colour intensity, which can be measured spectrophotometrically (V-530; Jasco®, Tokyo, Japan), is related to the quantity of oxidant molecules present in the sample. The assay is calibrated with hydrogen peroxide, and the results are expressed in terms of micromolar hydrogen peroxide equivalents per liter (µmol H2O2 equiv/l).
Measurement of total antioxidant status
Serum TAS was measured using a novel automated method developed by Erel 26. In this technique, hydroxyl radical, the most potent biological radical, is produced. In the assay, the ferrous ion solution in reagent 1 is mixed with the hydrogen peroxide present in reagent 2. Sequentiallyproduced radicals, such as the hydroxyl radical-produced brown-colored dianisidinyl radical cation, are also potent radicals. This method allows the measurement of the sample's antioxidative effect against potent free radical reactions that are initiated by the hydroxyl radical. The assay has excellent precision values of more than 97%. The results are expressed as mmol Trolox equiv/l.
Oxidative stress index
The OSI was defined as the ratio of TOS to TAS level. A standardized value does not exist for OSI levels, which were used only for comparisons. For the calculation, TAS units were changed to mmol/l, and the OSI value was calculated according to the following formula: OSI (arbitrary unit) = TOS (µmol H2O2 equiv./l)/TAS (mmol Trolox equiv/l) 25 26.
Other variables
Serum urea, creatinine, fasting blood glucose, aspartate aminotransferase, alanine aminotransferase, triglycerides, total cholesterol, and high-density and low-density lipoprotein cholesterol levels were determined using commercially available assay kits (Abbott®, Abbott Park, North Chicago, Illinois, USA) with an auto-analyzer (Abbott®, Abbott Park, North Chicago, Illinois, USA).
Statistical analysis
All statistical analyses were performed using SPSS for Windows version 17.0 (SPSS, Chicago, IL, USA). The Kolmogorov-Smirnov test was used to test the normality of data distribution. The data were expressed as arithmetic means and standard deviations. The chi-square test was used to compare categorical variables between groups. The independent sample T-test and Mann Whitney-U tests were used to compare continuous variables between the two groups. One-way ANOVA with a post-hoc Bonferroni test was used in normally distributed continuous data between all groups. Pearson's and Spearman's rank correlation analyses were used to examine the association of demographic and biochemical variables with oxidative stress parameters in group 1. A two-sided p value < 0.05 was considered statistically significant.
Results
The biochemical and demographic characteristics of all patients are presented in Table I. There were no significant differences in gender, age or biochemical values in the two groups (p > 0.05 for all). Renal and the liver function tests, cholesterol, triglyceride levels, and the CT scores were similar between the two groups.
Table I.
Comparison of demographic and biochemical characteristics.
| Group 1 (n = 38) |
Group 2 (n = 35) |
p | |
|---|---|---|---|
| Gender, male/female | 17/21 | 16/19 | NSa |
| Age, years | 38.68 ± 8.44 | 36.26 ± 8.48 | NSb |
| Glucose, mg/dL | 82.20 ± 10.20 | 79.00 ± 9.76 | NSb |
| CT scores | 13.37 ± 2.85 | 12.29 ± 2.55 | NSb |
| Urea, mg/dL | 25.24 ± 6.18 | 23.49 ± 4.46 | NSb |
| Creatinine, mg/dL | 0.68 ± 0.25 | 0.71 ± 0.22 | NSb |
| ALT, U/mL | 24.03 ± 4.54 | 25.17 ± 3.75 | NSb |
| AST, U/mL | 26.15 ± 5.48 | 25.43 ± 5.88 | NSb |
| Total cholesterol, mg/dL | 202.13 ± 24.54 | 194 ± 36.22 | NSc |
| LDL cholesterol, mg/dL | 131.70 ± 36.93 | 141.70 ± 32.82 | NSc |
| Triglyceride level, mg/dL | 205.16 ± 55.46 | 197.32 ± 61.37 | NSc |
All measurable values are given as the mean ± standard deviation. NS: non-significant, CT: computed tomography, ALT: alanine aminotransferase, AST: aspartate aminotransferase, LDL: low-density lipoprotein
Chi-squarea, independent sample T testb and Mann Whitney-Uc tests were used
Table II.
Comparison of oxidative stress parameters in all patients.
| Group 1 (n = 38) |
Group 2 (n = 35) |
p | ||
|---|---|---|---|---|
| TOS, µmol H2O2 equiv/l | Serum Tissue p |
21.34 ± 5.98 28.59 ± 3.98 < 0.001 |
17.38 ± 4.14 16.72 ± 2.49 > 0.05 |
< 0.001 < 0.001 |
| TAS, mmol Trolox equiv/l | Serum Tissue p |
0.94 ± 0.14 0.13 ± 0.03 < 0.001 |
1.10 ± 0.14 1.16 ± 0.12 > 0.05 |
< 0.001 < 0.001 |
| OSI, arbitrary unit | Serum Tissue p |
2.33 ± 0.65 3.12 ± 0.43 < 0.001 |
1.89 ± 0.45 1.76 ± 0.29 > 0.05 |
< 0.001 < 0.001 |
All measurable values are given as the mean ± standard deviation.
TOS: total oxidant status, TAS: total antioxidant status, OSI: oxidative stress index
One-way ANOVA with a post-hoc Bonferroni* test was used
Comparison of total antioxidant status levels
Compared to group 2, group 1 had significantly lower TAS levels both in serum and tissues (both p < 0.001). In group 1, tissue TAS levels were significantly lower than in serum (p < 0.001). In group 2, there was no significant difference in TAS levels either in serum or tissues (p = 0.208).
Comparison of total oxidant status and oxidative stress index levels
Compared to group 2, group 1 had significantly higher TOS and OSI levels both in serum and tissues (both p < 0.001). In group 1, the TOS and OSI levels were significantly higher in tissue than in serum (p < 0.001 for all). In group 2, there was no significant difference in the TOS or OSI levels either in serum or tissues (both p = 1.0). All OSI levels in serum and tissue are presented in Figure 1.
Fig. 1.
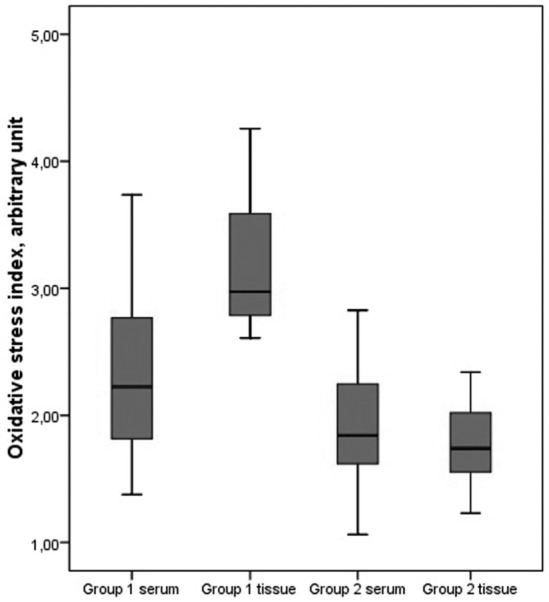
Oxidative stress index levels in serum and tissue.
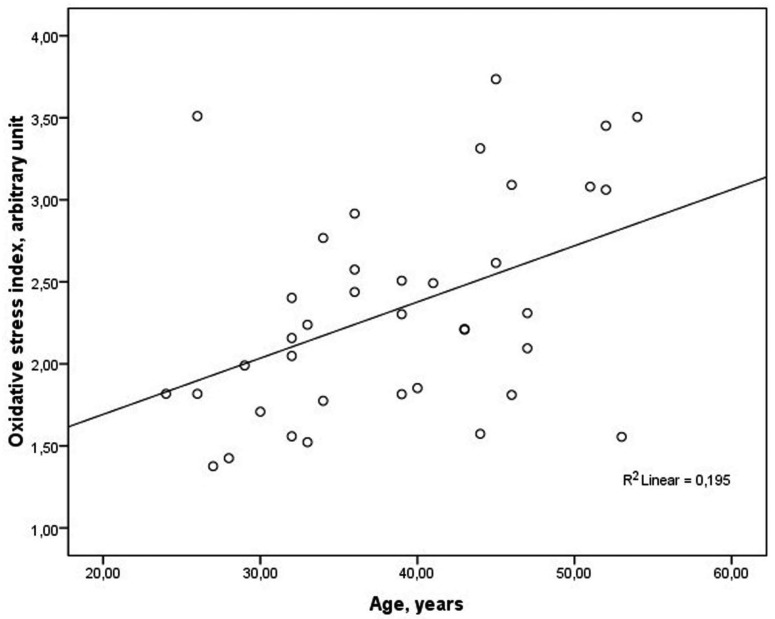
In bivariate analysis, the serum OSI levels were significantly correlated with age in group 1 (r = 0.442, p = 0.005) (Fig. 2). However, no significant correlations were observed between oxidative stress parameters and gender, serum fasting glucose, lipid parameters, urea, creatinine, AST or ALT levels (p > 0.05 for all).
Fig. 2.
Relationship between serum oxidative stress index and age (r = 0.442, p = 0.005).
Discussion
The main findings of this study are summarized as follows: (i) oxidative stress both in serum and tissues of patients with NPs was higher than in patients without NPs, (ii) serum OSI levels were significantly correlated with age and (iii) our study was able to provide important new insights and data because it assessed oxidative stress parameters using a more reliable method that measures both TOS and TAS.
Measuring different oxidant and antioxidant molecules is impractical, and oxidant and antioxidant effects are additive. Because there are numerous oxidants and antioxidants in the body, measuring total oxidant-antioxidant status is more valid and reliable. When only a few parameters are measured, levels may remain unchanged or decrease, even though the actual oxidant status increased or vice versa 25-27. In the light of this information, we used TOS and TAS levels in our study.
ROS are suspected to have a major function in the pathogenesis of NPs 7 11-23. Studies have reported similar results, showing an increase in oxidative stress markers and deterioration in antioxidant scavenging systems. These studies provide strong evidence that oxidative stress plays a role in the pathogenesis of NPs 12. Dogru et al. and Uneri et al. investigated the role of ROS in the development of NPs and reported that ROS may play a significant role in the pathogenesis of NPs 7 13. Dagli et al. compared 31 patients and 19 control subjects to investigate the role of ROS and antioxidants in NPs, and showed that the increase in MDA was higher in NP tissue and the blood of patients with NPs than in a concha bullosa group 18. According to their results, in polyp tissue, the levels of an oxidant (malondialdehyde) were increased, and the levels of antioxidants (glutathione and α-tocopherol) were decreased compared with control tissues from inferior turbinates. In another study performed by Okur et al., the investigators demonstrated the production of ROS in the neutrophils of NPs and determined a very high ratio of ROS production in NPs 19. Uneri at al. also reported high ROS levels in NP samples and showed a strong relationship between tissue damage and NP. Moreover, Cheng et al. studied the expression of superoxide dismutase 1 and 3 and found higher levels in polyp tissues. Such investigations indicate that ROS may be important in the pathogenesis of NP 13 14.
Recent studies have also confirmed the role of oxidative stress in the development of NPs. Park et al. suggested the possibility that [6]-gingerol may play an important role in inhibiting the production of the extracellular matrix in the development of NPs through an antioxidant effect 28. The same authors performed another study and showed that nicotinamide adenine dinucleotide phosphate oxidase and ROS have a role in myofibroblast differentiation and collagen production of transforming growth factor-β1-induced nasal polyp-derived fibroblasts, and that these processes are inhibited by the elimination of ROS 29. Moon et al. have also demonstrated the ROS derived from NADPH oxidases, and transforming growth factor-β-1 have been implicated in the pathogenesis of hypoxia-induced NPs 30. As confirmation, Jeanson et al. have shown the existence of an unfolded protein response in nasal epithelial cells that is linked to oxidative stress leading to interleukin-8 and leukotriene-B4 secretions. They concluded that these mechanisms may participate in chronic inflammation in NPs 31. In our study, oxidative stress both in serum and tissue in patients with NPs was higher than in patients without NPs. Our study results on tissue and serum samples support the previous studies as it provides a more reliable quantification of oxidative stress parameters.
The frequency of NPs increases with age and seems to occur more often in men. The prevalence of this condition increases with age in both sexes to reach a peak in those aged 50 years or older. In the aging process, ROS production increases, while degradation is impaired, and thus oxidative stress accumulates 32-34. In our study, serum OSI levels were significantly correlated with age but not correlated with gender in patients with NPs. Although not definitive, our study suggests that the increase in oxidative stress inherent in the aging process may contribute to the formation of NPs.
Conclusions
The findings of the present study demonstrate that oxidative stress is increased in patients with NPs. The important therapeutic potential to repair the harmful effects of oxidative stress on nasal tissues with antioxidant agents will only emerge with an improved understanding of the role of oxidative stress in NPs. The sample size was relatively small, and the design of the study was cross-sectional which are limitations of the present study. Therefore, large, prospective cohort studies are needed to address this issue.
Acknowledgements
This study was conducted at the Otolaryngology Head and Neck Surgery, Internal Medicine, Dermatology and Biochemistry Departments of Harran University School of Medicine, Sanliurfa. This study was approved by the Institutional Review Board.
References
- 1.Watkins DN, Lewis RH, Basclain KA, et al. Expression and localization of the inducible isoform of nitric oxide synthase in nasal polyp epithelium. Clin Exp Allergy. 1998;28:211–219. doi: 10.1046/j.1365-2222.1998.00215.x. [DOI] [PubMed] [Google Scholar]
- 2.Sarisoy BA, Eken M, Oktay AZ, et al. Myeloperoxydase expression in the pathogenesis of nasal polyps. Indian J Otolaryngol Head Neck Surg. 2011;63:260–263. doi: 10.1007/s12070-011-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bachert C, Gevaert P, Holtappels G, et al. Nasal polyposis: from cytokines to growth. Am J Rhinol. 2000;14:279–290. doi: 10.2500/105065800781329573. [DOI] [PubMed] [Google Scholar]
- 4.Norlander T, Brönnegård M, Stierna P. The relationship of nasal polyps, infection, and inflammation. Am J Rhinol. 1999;13:349–355. doi: 10.2500/105065899781367537. [DOI] [PubMed] [Google Scholar]
- 5.Nelson HS. Advances in upper airway diseases and allergen immunotherapy. J Allergy Clin Immunol. 2004;113:635–642. doi: 10.1016/j.jaci.2004.01.741. [DOI] [PubMed] [Google Scholar]
- 6.Shukla GK, Mahajan A, Pandey S, et al. A study of free radicals and scavenging enzyme in tonsillitis. Boll Chim Farm. 1996;135:653–655. [PubMed] [Google Scholar]
- 7.Doğru H, Delibaş N, Döner F, et al. Free radical damage in nasal polyp tissue. Otolaryngol Head Neck Surg. 2001;124:570–572. doi: 10.1067/mhn.2001.115086. [DOI] [PubMed] [Google Scholar]
- 8.Döner F, Delibaş N, Doğru H, et al. Malondialdehyde levels and superoxide dismutase activity in experimental maxillary sinusitis. Auris Nasus Larynx. 1999;26:287–291. doi: 10.1016/s0385-8146(98)00078-9. [DOI] [PubMed] [Google Scholar]
- 9.Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann Intern Med. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 10.Halliwell B, Gutteridge JM, Cross CE. Free radicals, antioxidants, and human disease: where are we now? J Lab Clin Med. 1992;119:598–620. [PubMed] [Google Scholar]
- 11.Taysi S, Uslu C, Yilmaz A, et al. Lipid peroxidation and some antioxidant enzymes in nasal polyp tissue. Cell Biochem Funct. 2006;24:461–465. doi: 10.1002/cbf.1303. [DOI] [PubMed] [Google Scholar]
- 12.Sagit M, Erdamar H, Saka C, et al. Effect of antioxidants on the clinical outcome of patients with nasal polyposis. J Laryngol Otol. 2011;125:811–815. doi: 10.1017/S0022215111001149. [DOI] [PubMed] [Google Scholar]
- 13.Uneri C, Ozturk O, Polat S, et al. Determination of reactive oxygen species in nasal polyps. Rhinology. 2005;43:185–189. [PubMed] [Google Scholar]
- 14.Cheng YK, Hwang GY, Lin CD, et al. Altered expression profile of superoxide dismutase isoforms in nasal polyps from nonallergic patients. Laryngoscope. 2006;116:417–422. doi: 10.1097/01.MLG.0000199738.37455.55. [DOI] [PubMed] [Google Scholar]
- 15.Cekin E, Ipcioglu OM, Erkul BE, et al. The association of oxidative stress and nasal polyposis. J Int Med Res. 2009;37:325–330. doi: 10.1177/147323000903700206. [DOI] [PubMed] [Google Scholar]
- 16.Cheng YK, Tsai MH, Lin CD, et al. Oxidative stress in nonallergic nasal polyps associated with bronchial hyperresponsiveness. Allergy. 2006;61:1290–1298. doi: 10.1111/j.1398-9995.2006.01228.x. [DOI] [PubMed] [Google Scholar]
- 17.Karlidağ T, Ilhan N, Kaygusuz I, et al. Roles of free radicals, nitric oxide, and scavenging enzymes in nasal polyp development. Ann Otol Rhinol Laryngol. 2005;114:122–126. doi: 10.1177/000348940511400207. [DOI] [PubMed] [Google Scholar]
- 18.Dagli M, Eryilmaz A, Besler T, et al. Role of free radicals and antioxidants in nasal polyps. Laryngoscope. 2004;114:1200–1203. doi: 10.1097/00005537-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Okur E, Inanc F, Yildirim I, et al. Malondialdehyde level and adenosine deaminase activity in nasal polyps. Otolaryngol Head Neck Surg. 2006;134:37–40. doi: 10.1016/j.otohns.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 20.Giannessi F, Ursino F, Fattori B, et al. Expression of 3-nitrotyrosine, a marker for peroxynitrite, in nasal polyps of nonatopic patients. Med Sci Monit. 2010;16:172–179. [PubMed] [Google Scholar]
- 21.Woo HJ, Bae CH, Song SY, et al. Expression of glutaredoxin- 1 in nasal polyps and airway epithelial cells. Am J Rhinol Allergy. 2009;23:288–293. doi: 10.2500/ajra.2009.23.3318. [DOI] [PubMed] [Google Scholar]
- 22.Bugdayci G, Kaymakci M. Nitrite/nitrate and malondialdehyde levels in nasal polyp. Cell Mol Biol (Noisy-le-grand) 2008;54(Suppl):1043–1045. [PubMed] [Google Scholar]
- 23.Kang BH, Huang NC, Wang HW. Possible involvement of nitric oxide and peroxynitrite in nasal polyposis. Am J Rhinol. 2004;18:191–196. [PubMed] [Google Scholar]
- 24.Lund VJ, Kennedy DW. Staging for rhinosinusitis. Otolaryngol Head Neck Surg. 1997;117:35–40. doi: 10.1016/S0194-59989770005-6. [DOI] [PubMed] [Google Scholar]
- 25.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Erel O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin Biochem. 2004;37:112–119. doi: 10.1016/j.clinbiochem.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Ulas T, Buyukhatipoglu H, Kirhan I, et al. The effect of day and night shifts on oxidative stress and anxiety symptoms of the nurses. Eur Rev Med Pharmacol Sci. 2012;16:594–599. [PubMed] [Google Scholar]
- 28.Park SA, Park IH, Cho JS, et al. Effect of [6]-gingerol on myofibroblast differentiation in transforming growth factor beta 1-induced nasal polyp-derived fibroblasts. Am J Rhinol Allergy. 2012;26:97–103. doi: 10.2500/ajra.2012.26.3736. [DOI] [PubMed] [Google Scholar]
- 29.Park IH, Park SJ, Cho JS, et al. Role of reactive oxygen species in transforming growth factor beta1-induced alpha smooth-muscle actin and collagen production in nasal polyp- derived fibroblasts. Int Arch Allergy Immunol. 2012;159:278–286. doi: 10.1159/000337460. [DOI] [PubMed] [Google Scholar]
- 30.Moon YM, Kang HJ, Cho JS, et al. Nox4 mediates hypoxiastimulated myofibroblast differentiation in nasal polyp-derived fibroblasts. Int Arch Allergy Immunol. 2012;159:399–409. doi: 10.1159/000337658. [DOI] [PubMed] [Google Scholar]
- 31.Jeanson L, Kelly M, Coste A, et al. Oxidative stress induces unfolding protein response and inflammation in nasal polyposis. Allergy. 2012;67:403–412. doi: 10.1111/j.1398-9995.2011.02769.x. [DOI] [PubMed] [Google Scholar]
- 32.Shimosawa T. [[Increasing oxidative stress in aging]]. Nihon Rinsho. 2005;63:994–999. [PubMed] [Google Scholar]
- 33.Settipane GA, Chafee FH. Nasal polyps in asthma and rhinitis. A review of 6,037 patients. J Allergy Clin Immunol. 1977;59:17–21. doi: 10.1016/0091-6749(77)90171-3. [DOI] [PubMed] [Google Scholar]
- 34.Lund VJ. Diagnosis and treatment of nasal polyps. BMJ. 1995;311:1411–1414. doi: 10.1136/bmj.311.7017.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]