SUMMARY
Mucopolysaccharidoses (MPSs) are lysosomal storage disorders caused by deficiency of enzymes involved in the degradation of glycosaminoglycans (GAGs). These disorders are associated with the accumulation of GAGs in tissues with organomegaly, mental retardation and short stature. Otologic and upper respiratory tract pathologies are among the earliest clinical manifestations. We analyzed 20 patients (13 male and 7 female, median age at the beginning of the observation 6 years) with MPS (35% type I, 30% type II, 20% type III, 5% type IV, 10% type VI), focusing on their otorhinolaryngologic problems and the impact of surgery on quality of life. We found ear, nose and throat manifestations in all types of MPS; in particular, recurrent otitis media was present in 30% of cases, hearing loss in 75% (mixed in 43.33%, conductive in 43.33%, sensorineural in 13.33%), adenotonsillar hypertrophy in 75%, frequent infections of the upper airway in 75% and obstructive sleep apnoea syndrome in 45% of cases. Fifty percent of patients required surgical therapy (adenotonsillectomy, adenoidectomy with insertion of middle ear ventilation tubes, tonsillectomy, tracheotomy and exeresis of vocal cord polyps). In our experience the ENT surgery reduced the frequency and severity of ear infections and relieved symptoms related to upper airway obstruction, thereby improving the quality of life in affected patients.
KEY WORDS: Mucopolysaccharidoses, Adenotonsillectomy, Obstructive sleep apnoea syndrome
RIASSUNTO
Le mucopolisaccaridosi (MPS) sono patologie da accumulo lisosomiale causate da carenza di enzimi coinvolti nella degradazione dei glicosaminoglicani (GAGs) che si associano ad organomegalia, ritardo mentale e ridotta statura. Le patologie a carico dell'orecchio e delle alte vie respiratorie sono tra le più precoci manifestazioni cliniche. Abbiamo analizzato 20 pazienti (13 maschi e 7 femmine, età mediana all'inizio dell'osservazione 6 anni) con MPS (35% tipo I, 30% tipo II, 20% tipo III, 5% tipo IV, 10% tipo VI) focalizzando l'attenzione sui problemi ORL e sugli effetti della chirurgia sulla qualità della vita. Abbiamo riscontrato manifestazioni ORL in tutti i tipi di MPS; in particolare l'otite media era presente nel 30% dei casi, l'ipoacusia nel 75% (mista nel 43.33%, trasmissiva nel 43.33% e neurosensoriale nel 13.33%), ipertrofia adenotonsillare nel 75%, frequenti infezioni delle alte vie aeree nel 75%, sindrome da apnea notturna nel 45% dei casi. Nel 50% dei pazienti è stato necessario ricorrere alla terapia chirurgica (adenotonsillectomia, adenoidectomia con timpanocentesi e apposizione di drenaggio trans-timpanico, tonsillectomia, tracheotomia ed exeresi di polipo cordale). Nella nostra casistica la chirurgia ORL riduce la frequenza e la severità delle infezioni auricolari e attenua i sintomi da ostruzione delle alte vie respiratorie migliorando la qualità di vita dei pazienti affetti da mucopolisaccaridosi.
Introduction
Mucopolysaccaridoses (MPSs) are a heterogeneous group of autosomal-recessive lysosomal storage disorders (except for MPS II, which is X-linked-recessive) characterized by deficiency of one of the lysosomal enzymes involved in the breakdown of glycosaminoglycans (GAGs). Seven types of one enzymatic defects have been described to date 1. This metabolic block leads to the accumulation of GAGs in lysosomes, resulting in cell, tissue and organ dysfunction 2. The ubiquitous nature of GAGs in the body's connective tissues gives rise to a wide phenotypic spectrum usually characterized by coarse facial features, liver and spleen enlargement, bone deformities with subsequent reduction of joint mobility, variable mental retardation and cardiac and ophthalmologic involvement 1 3.
Ear, nose and throat (ENT) disorders are extremely frequent, mostly in MPS I, II and VI, and are often the earliest clinical manifestations of these diseases 4. Indeed, MPS patients display an increased risk of otitis media with effusion (OME) due to the pathologic deposition of GAGs in the post-nasal space, eustachian tubes and middle ear 5. Sensorineural hearing loss, whose aetiology remains unclear, is believed to result from infiltration of the cochlear duct, stria vascularis and cochlear nerve afferents 4. Nevertheless, in most MPS VI patients deficits are conductive in nature 6. Other common ENT disorders are: adenotonsillar hypertrophy, almost universal in MPS 5, chronic recurrent rhinitis and persistent copious nasal discharge 7. These conditions, in addition to nasal dysmorphism, mandibular abnormalities, tracheomalacia, thickened vocal cords, macroglossia and redundant tissue in the upper airway (UA) can contribute to UA complications and to obstructive sleep apnoea (OSA) 6-8.
Although patients with MPS may improve airway obstruction with more conservative treatment approaches including positive airway pressure devices (CPAP/BIPAP), management often requires early adenotonsillectomy and in extreme cases tracheostomy to ensure a patent airway in the short- or long-term 5. Before the advent of haematopoietic stem cell transplantation (HSCT) and especially enzyme replacement therapy (ERT), the main focus of treatment of MPS I, II, and VI was prevention and management of complications. Recently, much progress has been achieved in the treatment of MPS, and HSCT has been used in patients with MPS to correct the enzyme deficiency. Although many studies reveal that HSCT can change the natural course of the disease, increasing life expectancy and improving many systemic abnormalities 9 10, it is a high-risk procedure with high morbidity/ mortality. Moreover, its indication depends on the type of MPS, patient's clinical picture, age and presence of neurological impairment 11-13. ERT, currently considered an efficient therapeutic method, is based on the periodic replacement of the defective enzyme, leading to higher GAG degradation in tissues and organs and promoting significant improvement in some clinical features. However, the influence of ERT on pathological manifestations is still not well understood, and long-term data on its efficacy is not yet available 14 15.
We designed an observational study in order to define the incidence of ENT problems in patients with MPSs and to establish the impact of surgical treatment. We also observed the role of surgery in improving the clinical phenotype and the quality of life of patients affected by MPSs.
Material and methods
The present study includes 20 patients with MPS (7 [35%] MPS I, 6 [30%] MPS II, 4 [20%] MPS III, 1 [5%] MPS IV and 2 [10%] MPS VI), 13 male and 7 female (median age at the beginning of the observation 6 years, ranging from 1 to 16), observed at the Department of Paediatric Medicine of Federico II University in Naples from June 1999 to June 2009. The median age at diagnosis of MPS was 3 years and the median age of presentation to an otolaryngologist was 12 months. The average length of follow-up was 8.4 years.
We performed flexible fibre otoscopy, rhinopharyngolaryngoscopy, tympamograms, audiograms in patients able to cooperate (n = 15) in addition to polysomnography.
The following was considered:
The number of OME and upper respiratory tract infections (URTIs) episodes per year.
Adenoid hypertrophy (Grade 1: adenoid tissue occupies only the upper segment in the rhinopharyngeal cavity; Grade 2: adenoid tissue is confined to the upper half of the rhinopharyngeal cavity; Grade 3: adenoid tissue is extended over the rhinopharynx with obstruction of choanal openings and partial closure of tube ostium; Grade 4: tube ostium and lower choanal border could not be observed) 16.
Tonsillar hypertrophy (0: tonsils entirely within the tonsillar fossa; 1: tonsils occupying less than 25% of the lateral dimension of the oropharynx as measured between the anterior tonsillar pillars; 2: tonsils occupying less than 50% of the lateral dimension of the oropharynx; 3: tonsils occupying less than 75% of the lateral dimension of the oropharynx; 4: tonsils occupying 75% or more of the lateral dimension of the oropharynx).
Audiometric test.
Type of tympanograms (Type A: static compliance [SA] ≥ 0.2 cm3, tympanic peak pressure [TPP] -200 → +100 mm H2O, type B: SA < 0.2 cm3, type C: SA ≥ 0.2 cm3, TPP ≤ 200 mm H2O).
OSAS (number of obstructive apnoea and hypopnoea events per hour of sleep: apnoea-hypopnoea index AHI and oxygen saturation: Sat % O2).
Eight (40%) of our patients (3 MPS I, 4 MPS II and 1 MPS VI) were treated with ERT; of these, 4 patients (1 MPS I, 2 MPS II and 1 MPS VI) underwent surgery, in 2 cases before starting enzyme therapy and in the remaining 2 cases one year after initiating therapy.
In 10 patients (50%) surgical therapy was required (Table I): adenotonsillectomy in 5 patients (25%), adenoidectomy in 3 patients (15%), tonsillectomy in 2 patients (10%), insertion of middle ear ventilation tubes in 3 patients (15%), tracheotomy in 1 patient (5%) and exeresis of vocal cord polyps in 1 patient (5%). Flexible fibre-optic laryngoscopy was performed before surgery to familiarize the anaesthesiologist with the anatomy that would be encountered.
Table I.
Surgical procedures performed.
| Patients | 1 | 5 | 6 | 8 | 11 | 12 | 14 | 16 | 19 | 20 |
|---|---|---|---|---|---|---|---|---|---|---|
| Adenoidectomy | X | X | X | |||||||
| Tonsillectomy | X | X | ||||||||
| Adenotonsillectomy | X | X | X | X | X | |||||
| Insertion of middle ear ventilation tubes | X | X | X | |||||||
| Tracheotomy | X | |||||||||
| Exeresis of polyps of the vocal cords | X |
We finally considered the role of ENT surgery on improvement in quality of life, estimated through parental opinion and clinical manifestations. In particular, the severity of respiratory tract and/or otological infections was evaluated according to an infection score system considering the type of infection, presence of systemic symptoms, impairment of daily activities, improvement of instrumental date, need for therapy and/or hospital admission and duration of disease (Table II).
Table II.
Total score ≤ 5: mild respiratory tract and/or otological infection; score 6-11: moderate respiratory tract and/or otological infection; score ≥ 12: severe respiratory tract and/or otological infection.
| Score | ||
|---|---|---|
| Type of infection | Rhinitis | 0 |
| Rhinitis + otitis and/or tonsillitis | 1 | |
| Pneumonia | 2 | |
| Systemic symptoms | Absent | 0 |
| Slight fever and/or some aches | 1 | |
| Definite elevation of temperature | 2 | |
| Daily activity | Not limited | 0 |
| Some limitation | 1 | |
| Severely incapacitated | 2 | |
| Therapy | Local | 0 |
| Systemic (oral administration) | 1 | |
| Systemic (intravenous administration) | 2 | |
| Hospitalization | No | 0 |
| Single entry followed by home therapy | 1 | |
| Admission | 2 | |
| Resolution | < 7 days | 0 |
| 7-10 days | 1 | |
| > 10 days | 2 |
A visual analogue scale (VAS) is a psychometric response scale used for subjective characteristics or attitudes that cannot be directly measured. A VAS is usually a horizontal line, 10 cm in length, anchored by picture descriptors. Patients indicate the line point that they feel best represents perception of their current state (pain, ability to do daily activity) before and after surgery.
Results
18 of 20 patients (90%) showed at least one ENT manifestations (Table III). In particular, 6 patients (30%) had a history of chronic and recurrent OME ≥ 5 episodes in a year. Hearing loss was demonstrated by audiogram and ABR in 15/20 (75%) patients: in 6 (43.33%) patients mixed hypoacusia was present, conductive hypoacusia in 6 (43.33%) and sensorineural hypoacusia in 3 (13.33%) cases. We found type A tympanograms in 6 patients, type B in 10 and type C in 4 patients. Adenoid hypertrophy (degree ≥ 2) was present in 15 of 20 (75%) cases; tonsillar hypertrophy (degree ≥ 1) was present in 17 of the 20 patients; UA obstruction and OSA was present in 8 cases. 15 patients (75%) had a history of URTIs, such as rhinosinusitis, pharyngotonsillitis and laryngitis. In 9 patients (45%), OSA was documented by positive results of polysomnnography that demonstrated apnoea-hypopnea indexes (AHI) ranging from 18 to 31, and an oxygen saturation ranging from 74% to 85%.
Table III.
Otorhinolaryngological manifestations.
| Pt | MPS | Otitis media No. of episodes per year |
Hypoacusia | Tympanograms | Degree adenoid hypertrophy |
Degree tonsillar hypertrophy |
(URTIs) No. of episodes per year |
OSAS AHI |
|---|---|---|---|---|---|---|---|---|
| 1 | I | 7 | Conductive | B | 4 | 2 | 6 | 31 |
| 2 | I | 2 | Mixed | C | 2 | 1 | 5 | |
| 3 | I | 2 | Mixed | B | 2 | 1 | 5 | |
| 4 | I | 2 | Sensorineural | A | 2 | 1 | 5 | |
| 5 | I H/S | 6 | Conductive | B | 3 | 2 | 6 | 22 |
| 6 | I S | 3 | Mixed | B | 3 | 2 | 6 | |
| 7 | I | 0 | Sensorineural | A | 2 | 0 | 2 | |
| 8 | II | 3 | Mixed | C | 1 | 3 | 5 | 25 |
| 9 | II | 1 | - | A | 2 | 0 | 3 | |
| 10 | II | 2 | Sensorineural | A | 2 | 2 | 5 | |
| 11 | II | 4 | Mixed | B | 3 | 3 | 6 | 28 |
| 12 | II | 6 | Conductive | B | 4 | 4 | 7 | 29 |
| 13 | II | 2 | - | C | 1 | 1 | 5 | |
| 14 | III B | 7 | Conductive | B | 3 | 3 | 6 | 18 |
| 15 | III B | 0 | - | A | 1 | 2 | 2 | 19 |
| 16 | III B | 7 | Conductive | B | 4 | 3 | 7 | 26 |
| 17 | III B | 1 | - | A | 1 | 0 | 2 | |
| 18 | IV A | 1 | - | C | 2 | 1 | 3 | |
| 19 | VI | 3 | Mixed | B | 1 | 3 | 6 | |
| 20 | VI | 8 | Conductive | B | 3 | 3 | 7 | 21 |
Ten patients underwent surgical treatment, and none required prolonged intubation. Figure 1 shows the comparison between ENT manifestations before and after surgery.
Fig. 1.
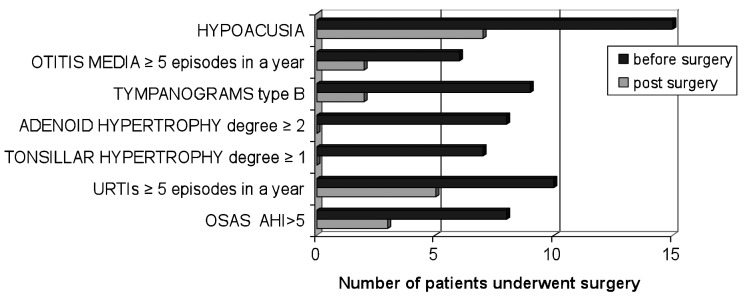
Comparison between otorhinolaryngological manifestations before and after surgery.
At least 30% (3 patients) of the patients obtained an improvement in quality of life. Indeed, VAS scores after surgery compared to VAS score before surgery significantly improved (median value 7.0 ± 1.5 vs. 3.6 ± 1.6). The severity of respiratory and ENT infections, measured with the infection score system also showed improvement (median infection score before surgery 13.7 ± 4.6 vs. 3.9 ± 2.4 after surgery). One patient (10%) still presented OSA after adenotonsillectomy because of macroglossia and dysmorphism. All patients are still alive at the time of writing.
Discussion
MPSs are lysosomal storage disorders with deficiency of enzymes involved in the degradation of GAGs. The structures of the head and neck are nearly always involved in MPSs, often at an early age, and as a result, otolaryngologists are commonly the first clinicians to whom these individuals present. Our study confirms the importance of ENT management in the multidisciplinary approach to these patients. Because of the high incidence of ear disorders, and their effects on language development and quality of life, routine otologic and audiologic evaluation must be obtained 17. Hearing loss affects almost all MPS patients, which is characterized by both conductive and sensorineural involvement. In fact, in the present study, hearing loss was present in 75% of cases, in agreement with literature data, in which the percentage varies from 59.7% to 89% 18. While it is reported that conductive hearing loss can be improved after adenoidectomy and insertion of middle ear ventilation tubes 19, amplification is typically required to overcome what is generally permanent sensorineural loss. As mentioned above, our data confirm these observations as about 60% of cases experienced improvement in hearing after surgical treatment.
While otologic manifestations have an effect on quality of life, upper-airway obstruction contributes more to morbidity and mortality. The accumulation of mucopolysaccharides can cause alterations of normal airway function, so prompt diagnosis is crucial. Changes in soft tissues including tonsils, adenoids, tongue and lingual tonsils are responsible for most respiratory problems. As the disease progresses, pharyngomalacia and tracheomalacia may develop and become severe, leading to significant airway obstruction 20.
Upper airway obstruction may range from varying degrees of OSA to life-threatening airway emergencies. However, airway evaluation is very difficult, typically non-uniform among different providers and varies from case to case 21. In the present study, we found upper airway obstruction in 75% of cases while literature data describes percentages varying from 38% 22 to 48% 18 to 92% 23. Currently, treatment of airway obstruction is controversial, but the accumulation of GAGs in the adenoids and tonsils, with resulting hypertrophy, makes these structures frequent targets of surgical intervention. Therefore, in more severe forms, such as purulent, recurrent and chronic cases, adenoidectomy should be performed without delay 16. OSA initially can be helped by tonsillectomy and adenoidectomy, but many patients require nocturnal oxygen treatment later 24 or, in more severe cases, tracheotomy. In the light of the elevated anaesthetic risk of this population due to copious secretions, temporomandibular joint arthritis, difficult or failed intubations, macroglossia, abnormal laryngeal anatomy and subglottic narrowing 15, in our centre, it is common practice to perform and record a bronchoscopy examination with a flexible fibre-optic bronchoscope before surgery 5 6 to evaluate the extent and severity of airway infiltration.
Among 20 MPS patients, 10 (50%) underwent surgery (Table I): adenotonsillectomy (5 patients, 25%), adenoidectomy (3 patients, 15%), tonsillectomy (2 patients, 10%), insertion of middle ear ventilation tubes (3 patients, 15%), tracheotomy (1 patient, 5%) and exeresis of vocal cord polyps (1 patient, 5%).
OSA evaluation should begin with history and physical examination, but the degree of pre and post-operative obstruction should be studied with polysomnography and rhino-oro-laryngoscopy 23 25. For these reasons, we performed a pre- and post-operative sleep study that demonstrated improvement of AHI ranging from 7 to 15 after surgery, with oxygen saturation ranging from 81% to 87% compared to preoperative results (AHI ranging from 18 to 31, and oxygen saturation ranging from 74% to 85%). One patient (10%), in contrast, still presented OSA after adenotonsillectomy because of his macroglossia and dysmorphism.
The study was also designed to examine the quality of life of patients affected by MPSs using a VAS score, measured before and after surgery, which demonstrated a significant improvement after treatment from score 5 (60%) and score 4 (40%) to score 3 (75%) and score 2 (25%).
Conclusions
In conclusion, because of the high incidence of ENT manifestations in MPS, otolaryngologists have a crucial role in the multidisciplinary approach to diagnosis and management of many subjects with this disorder. Moreover, despite the fact that ERT has made an important contribution to improving the quality of life of these patients, ENT surgery remains a fundamental therapeutic procedure for reducing the frequency and severity of ear infections and for relieving the symptoms of upper airway obstruction, even if these interventions are not definitive.
References
- 1.Neufeld EF, Muenzer J. The mucopolysaccharidoses. In: Scriver CR, Beaudet AL, Sly WS, editors. The metabolic and molecular bases of inherited disease. 8th ed. New York: McGraw-Hill; 2001. pp. 3421–3452. [Google Scholar]
- 2.Muenzer J. The mucopolysaccharidoses: a heterogeneous group of disorders with variable pediatric presentations. J Pediatr. 2004;144(Suppl 5):27–34. doi: 10.1016/j.jpeds.2004.01.052. [DOI] [PubMed] [Google Scholar]
- 3.Wenger DA. Insights into the diagnosis and treatment of lysosomal storage disorders. Arch Neurol. 2003;60:322–328. doi: 10.1001/archneur.60.3.322. [DOI] [PubMed] [Google Scholar]
- 4.Ruckenstein MJ, Macdonald RE, Clarke JTR, et al. The management of otolaryngological problems in the mucopolysaccharidoses: a retrospective review. J Otolaryngol. 1991;20:177–183. [PubMed] [Google Scholar]
- 5.Simmons MA, Bruce IA, Penney S, et al. Otorhinolaryngological manifestations of the mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2005;69:589–595. doi: 10.1016/j.ijporl.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Giugliani R, Harmatz P, Wraith JE. Management guidelines for mucopolysaccharidosis VI. Pediatrics. 2007;120:405–418. doi: 10.1542/peds.2006-2184. [DOI] [PubMed] [Google Scholar]
- 7.Wraith JE, Scarpa M, Beck M, et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nashed A, Al-Saleh S, Gibbons J, et al. Sleep-related breathing in children with mucopolysaccharidosis. J Inherit Metab Dis. 2009;32:544–550. doi: 10.1007/s10545-009-1170-4. [DOI] [PubMed] [Google Scholar]
- 9.Vellodi A, Young EP, Cooper A, et al. Bone marrow transplantation for mucopolysaccharidosis type I: experience of two British centres. Arch Dis Child. 1997;76:92–99. doi: 10.1136/adc.76.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wraith JE, Scarpa M, Beck M, et al. Mucopolysaccharidosis type II (Hunter syndrome): a clinical review and recommendations for treatment in the era of enzyme replacement therapy. Eur J Pediatr. 2008;167:267–277. doi: 10.1007/s00431-007-0635-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mckinnis EJ, Sulzbacher S, Rutledge JC, et al. Bone marrow transplantation in Hunter syndrome. J Pediatr. 1996;129:145–148. doi: 10.1016/s0022-3476(96)70202-0. [DOI] [PubMed] [Google Scholar]
- 12.Aldenhoven M, Boelens JJ, Konong TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Muenzer J, Beck M, Eng CM, et al. Multidisciplinary management of Hunter syndrome. Pediatrics. 2009;124:1228–1239. doi: 10.1542/peds.2008-0999. [DOI] [PubMed] [Google Scholar]
- 14.Viana GM, Lima NO, Cavaleiro R, et al. Mucopolysaccharidoses in northern Brazil: Targeted mutation screening and urinary glycosaminoglycan excretion in patients undergoing enzyme replacement therapy. Genet Mol Biol. 2011;34:410–415. doi: 10.1590/S1415-47572011005000025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wold SM, Derkay CS, Darrow DH, et al. Role of the pediatric otolaryngologist in diagnosis and management of children with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2010;74:27–31. doi: 10.1016/j.ijporl.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 16.Cassano P, Gelardi M, Cassano M, et al. Adeniod tissue rhinopharyngeal obstruction grading based on fiberendoscopic findings: a novel approach to therapeutic management. Int J Pediatr Otorhinolaryngol. 2003;67:1303–1309. doi: 10.1016/j.ijporl.2003.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Motamed M, Thorne S, Narula A. Treatment of otitis media with effusion in children with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2000;53:121–124. doi: 10.1016/s0165-5876(00)00320-7. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz IVD, Ribeiro MD, Mota JG, et al. A clinical study of 77 patients with mucopolisaccharidosis type II. Acta Paediatrica. 2007;96:63–70. doi: 10.1111/j.1651-2227.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 19.Peck JE. Hearing loss in Hunter's syndrome - mucopolysaccharidosis II. Ear and Hearing. 1984;5:243–246. doi: 10.1097/00003446-198407000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Pelley CJ, Kwo J, Hess DR. Tracheomalacia in an adult with respiratory failure and Morquio syndrome. Respir Care. 2007;52:278–282. [PubMed] [Google Scholar]
- 21.Yeung AH, Cowan MJ, Horn B, et al. Airway management in children with mucopolysaccharidoses. Arch Otolaryngol Head Neck Surg. 2009;135:73–79. doi: 10.1001/archoto.2008.515. [DOI] [PubMed] [Google Scholar]
- 22.Bredenkamp JK, Smith ME, Dudley JP, et al. Otolaryngologic manifestations of the mucopolysaccharidoses. Ann Otol Rhinol Laryngol. 1992;101:472–478. doi: 10.1177/000348949210100605. [DOI] [PubMed] [Google Scholar]
- 23.Leighton SE, Papsin B, Vellodi A, et al. Disordered breathing during sleep in patients with mucopolysaccharidoses. Int J Pediatr Otorhinolaryngol. 2001;58:127–138. doi: 10.1016/s0165-5876(01)00417-7. [DOI] [PubMed] [Google Scholar]
- 24.Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santamaria F, Andreucci MV, Parenti G, et al. Upper airway obstructive disease in mucopolysaccharidoses: polysomnography, computed tomography and nasal endoscopy findings. J Inherit Metab Dis. 2007;30:743–749. doi: 10.1007/s10545-007-0555-5. [DOI] [PubMed] [Google Scholar]


