Abstract
Background
Our previous work suggested that there was no significant association between plasma steroid hormone levels and prostate cancer (CaP) tumor grade at diagnosis. In this study, we systematically tested the hypothesis that inherited variations in the androgen and estrogen metabolic pathways may be associated with plasma levels of steroid hormones, or CaP aggressiveness at diagnosis.
Methods
Plasma hormone levels including total testosterone, total estradiol and sex hormone-binding globulin were measured in a cohort of 508 patients identified with localized CaP. D’Amico risk classification at diagnosis was also determined. 143 single nucleotide polymorphisms (SNPs) from 30 genes that are involved in androgen and estrogen metabolism were selected for analysis. The global association of genotypes with plasma hormone levels and CaP aggressiveness (D’Amico risk classification) was statistically analyzed. Q-values were estimated to account for multiple testing.
Results
We observed significant associations between plasma testosterone level and SNPs in HSD17B2 (rs1424151), HSD17B3 (rs9409407) and HSD17B1 (rs12602084), with P values of 0.002, 0.006 and 0.006, respectively. We also observed borderline significant associations between prostate aggressiveness at diagnosis and SNPs in AKR1C1 (rs11252845; P = 0.005), UGT2B15 (rs2045100; P = 0.007) and HSD17B12 (rs7932905; P = 0.008). No individual SNP was associated with both clinical variables.
Conclusions
Genetic variants of genes in hormone metabolic pathways may influence plasma androgen levels or CaP aggressiveness. However, it appears that the inherited variations affecting plasma hormone levels differ from those affecting disease aggressiveness.
Keywords: Prostate cancer, Hormone metabolism, Single-nucleotide Polymorphisms
Introduction
Sex steroid hormones influence the development, maturation and maintenance of the prostate, affecting both the proliferation and differentiation status of the luminal epithelium (1-3). Androgens are synthesized primarily in the testes or adrenal glands, and secondarily in peripheral tissues, including the prostate (2). Testosterone (T) is the principal androgen present in the blood, mostly bound to sex hormone-binding globulin (SHBG) and albumin. Intracellularly, T is enzymatically converted to the more potent metabolite dihydrotestosterone (DHT). The complex of androgen (T or DHT) and androgen receptor (AR) regulates the expression of a variety of genes that are involved in the growth, survival and differentiation of prostate cells by binding to androgen response elements present in the regulatory regions of these genes (2).
The relationship between androgens and the development of prostate cancer (CaP) is complex. Some early studies demonstrated that plasma T levels might be correlated with Gleason grade or time to progression after orchiectomy of CaP (3,4). However, other studies indicated no evidence of any association between plasma T, estradiol (E2), SHBG with Gleason score at the time of diagnosis (5, 6). Thus, there is insufficient evidence to support the hypothesis that higher blood levels of steroid hormones are associated with CaP development or progression. One explanation for this lack of association is the uncertain correlation between the plasma concentrations of androgens with the intraprostatic hormone levels an essential component in the tumor microenvironment that potentially affects tumor growth (2, 7, 8). Another explanation is that a single determination of plasma androgens in middle or older age may not be representative of time-averaged or maximum levels over the etiologically relevant time of life (9). Similarly, the relationship between estrogen levels and CaP is unclear. There is some evidence that estrogen metabolites functioning as genotoxins, can increase risk for CaP (10). It also has been suggested that a decrease in the androgen/estrogen ratio with aging could be responsible in part for prostate carcinogenesis (10).
Therapeutically, blocking androgen production has been one of the most commonly used treatments for advanced CaP and is often successful until castration resistant growth is acquired (2, 11, 12). In addition, estrogens are recognized therapies for CaP. Oral estradiol administration may be useful for treatment of advanced castration-resistant CaP (10). This therapy also has side effects and that the effects of estrogens are mediated by two different receptors, ERα and ERβ (10).
Genetic polymorphisms of genes involved in metabolic pathways of sex steroid hormones have been investigated as candidates for CaP risk in many association studies (13-16). Data from these case-control studies suggest that inter-individual variation in genes involved in sex hormone metabolic pathway could be related to the risk of CaP incidence (13-16). However, few studies have examined the association of these polymorphisms with blood hormone levels in CaP (17-19). In the NCI-Breast and Prostate Cancer Cohort Consortium study, SNPs in SHBG gene were found to be associated with circulating SHBG and testosterone levels in Caucasian men in both cases and controls. They also identified relating SNPs in ESR1 and SRD5A2 with testosterone and 3α-androstanediol-glucuronide, respectively (19).
We present here a systematic analysis of 143 common polymorphisms in 30 genes that are involved in the sex steroid hormones metabolic pathways for their potential associations with plasma total testosterone (TT), total estradiol (TE) and SHBG, or with CaP aggressiveness in an established cohort of 508 patients with newly diagnosed localized CaP.
Materials and Methods
Study population and Laboratory Assays
The study cohort and the measurement of hormone levels have been previously described in detail (5, 20). Briefly, a total of 539 patients with localized CaP evaluated at the Dana-Farber Cancer Institute from 2001 to 2005, who were enrolled in a prospective sample banking protocol were eligible for this study. The majority of clinical data was abstracted from the CRIS database, as described (21). Since mixed ethnicity could confound the association results, we removed 31 patients of non-Caucasian race or with indeterminate ancestry from this study. A total of 508 individuals were analyzed. These individuals either self-reported as European American or were determined to be of European ancestry based on genotyping of ancestry-informative markers. Using the D’Amico risk classification criteria, newly diagnosed CaP patients were classified as low, intermediate or high risk of clinical recurrence after primary therapy (22). Briefly, three risk groups were established based on serum PSA level, biopsy Gleason score, and clinical stage at diagnosis as described elsewhere (22). All the blood samples were drawn at around the time of diagnosis. Stored plasma was processed for the measurement of TT, TE, and SHBG levels using enzyme-linked immuno-absorbent assays, as previously described (5).
Single Nucleotide Polymorphism Selection and Genotyping
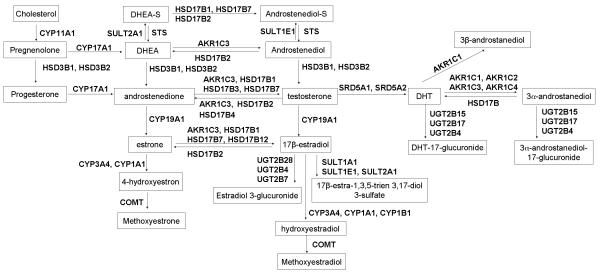
Candidate genes were identified from review of the sex hormone biosynthesis and metabolic pathway (23-25) and cross-referenced with the Gene Ontology database to confirm pathway information. The 30 candidate genes that are included in this study and their roles in sex hormone synthesis and metabolic pathway are demonstrated in Figure 1. Single Nucleotide Polymorphisms (SNPs) that were selected from each gene are of a minor allele frequency (MAF) was greater than 0.05 in the CEU population. The CEU HapMap population Phase II genotyping data was used to select tagging SNPs. We ran the program Tagger and selected tags to capture the unmeasured variants r2 more than 0.8. All non-synonymous SNPs with a MAF more than 0.02 were also included (Table 2 and Supplemental Table S1). One hundred sixty-one SNPs in 30 genes were initially selected for analysis on the basis of their known participation in hormone synthesis and metabolism. All selected SNPs were included in the design. After genotyping, SNPs that did not conform to Hardy-Weinberg equilibrium (P <0.01) or had genotyping call rates below 80% were omitted from further analysis (N = 18). Thus, a total of 143 SNPs in 30 genes were included for final statistical analyses. For quality control, about 5% random selected duplicates were included. Discrepancy rate was less than 0.1% between duplicates in overall genotyping data.
Figure 1. Candidate genes involved in androgen and estrogen biosynthesis and metabolism process.
This diagram show how the 30 metabolic genes analyzed in this study are involved in different steps of androgen and estrogen metabolism.
Table 2. List of genes and number of polymorphisms evaluated.
| Genes catalyze androgens and estrogens synthesis and bioactivation |
Genes catalyze the synthesis of catecholestrogens |
Genes metabolically inactive androgen and estrogens |
|||
|---|---|---|---|---|---|
|
| |||||
| Gene | No. of polymorphisms |
Gene | No. of polymorphisms |
Gene | No. of polymorphisms |
| AKR1C1 | 4 | CYP1A1 | 3 | SULT1A1 | 2 |
| AKR1C2 | 7 | CYP1B1 | 5 | SULT1E1 | 6 |
| AKR1C3 | 5 | CYP3A4 | 4 | SULT2A1 | 4 |
| AKR1C4 | 8 | UGT2B15 | 3 | ||
| COMT | 6 | UGT2B17 | 4 | ||
| CYP11A1 | 4 | UGT2B28 | 2 | ||
| CYP17A1 | 4 | UGT2B4 | 5 | ||
| CYP19A1 | 6 | UGT2B7 | 3 | ||
| HSD17B1 | 3 | ||||
| HSD17B2 | 4 | ||||
| HSD17B3 | 8 | ||||
| HSD17B4 | 5 | ||||
| HSD17B7 | 6 | ||||
| HSD17B12 | 8 | ||||
| HSD3B1 | 7 | ||||
| HSD3B2 | 3 | ||||
| SRD5A1 | 6 | ||||
| SRD5A2 | 5 | ||||
| STS | 3 | ||||
|
| |||||
| Total: | 143 | ||||
Statistical Analysis
Descriptive statistics were used to characterize the study population in terms of frequency and percent, or median and interquartile range. Plasma hormone levels were evaluated as continuous and D’Amico risk was dichotomized as Low vs Intermediate/High, the latter denoting aggressive disease. The Pearson Chi-square (χ2) test and the Wilcoxon rank sum test were used to evaluate global associations of SNP genotype with hormone levels and D’Amico risk, respectively. Relative Risk (RR) and 95% confidence intervals (CIs) were estimated using a generalized linear model (GLM) for binomial data with a log link rather than a logit link function. The two-sided P-values of SNPs that were considered as statistically significant were based on an alpha of < 0.05. Statistical analyses were performed using the SAS software version 9.1 (SAS Institute Inc. Cary, NC USA). As we were testing 143 different SNPs, we reported q-values, a measure of significance in terms of the false-discovery rate as proposed by Storey and Tibshirani for multiple comparisons (26). The q-value represents the expected proportion of false-positive results when announcing a test significant. Q-values were estimated using R q-value package on the basis of the observed distribution of P values from the global association test for 143 individual SNPs. We considered a q-value less than 25% to be significant.
Results
Cohort Patients Characteristics
The characteristics of the current study cohort are shown in Table 1. In brief, patients had low- or intermediate-risk, early-stage localized disease. The median PSA at diagnosis is 5.1 ng/dL. Seventy-nine percent patients were diagnosed with T0 or T1 stage tumor. Fifty-three percent of patients’ tumor Gleason Score were equal to or less than 6. In this cohort, 492 patients had sufficient information for criteria of D’Amico risk classification; and among them, 233 (47%) patients were low-risk, 202 (41%) patients intermediate-risk and 57 (12%) patients high-risk. The majority of patients (82%) were overweight (BMI > 25) at the time of diagnosis. The average plasma TT in this cohort was 440 ng/dL, the average plasma level of TE was 9.2 ng/dL, and the average plasma SHBG level was 51 nM. The average TE to TT ratio was 2.1.
Table 1. Characteristics of Patients and Disease at Diagnosis.
| Characteristic | Frequency | Percent (%) |
|---|---|---|
| Age * | 60 (55-67) years | |
| Race | ||
| Caucasian | 508 | 100.0 |
| PSA at Diagnosis (ng/dL) | ||
| Median | 5.1 | |
| ≤4 | 110 | 22.7 |
| 4-10 | 318 | 65.7 |
| 10-20 | 40 | 8.3 |
| >20 | 16 | 3.3 |
| Tumor stage | ||
| T0 | 61 | 12.2 |
| T1 | 335 | 66.8 |
| T2 | 100 | 20.0 |
| T3 | 5 | 1.0 |
| Gleason Score | ||
| <= 6 | 271 | 53.4 |
| =7 | 190 | 37.4 |
| >= 8 | 47 | 9.2 |
| D’Amico Risk Class | ||
| Low | 233 | 47.4 |
| Medium | 202 | 41.0 |
| High | 57 | 11.6 |
| BMI (kg/m2) | ||
| < 25 | 87 | 17.6 |
| 25-30 | 271 | 55.0 |
| > 30 | 135 | 27.4 |
| Hormone Levels* | ||
| Total Testosterone (ng/dL) | 440 (336- 573) | |
| Total Estradiol (ng/dL) | 9.2 (7.8-10.7) | |
| SHBG (nM) | 51 (36-70) | |
| Estradiol-to-testosterone ratio | 2.1 (1.6-2.8) |
Data presented as median, with IQR in parentheses.
IQR, Interquartile range; BMI, body mass index; PSA, prostate specific antigen; SHBG, sex hormone-binding globulin.
Association of SNPs with plasma sex steroid hormone levels
We analyzed the association of plasma hormone levels and disease aggressiveness by using D’Amico criteria with 143 SNPs from 30 genes that catalyze androgens and estrogens synthesis and bioactivation, catalyze the synthesis of catecholestrogens and that metabolically inactivate androgens and estrogens (Figure1 and Table 2).
Among the 143 SNPs, three SNPs in HSD17B2 (rs1424151), HSD17B3 (rs9409407) and HSD17B1 (rs12602084), respectively, were found to be significantly associated with the level of plasma TT (Table 3). The median TT level in patients of the rs1424151 AG or GG genotype in HSD17B2 (371.6 ng/dL) was 17.4% less (P = 0.002), compared to patients with the rs1424151 AA genotype (448.9 ng/dL). Similar results were observed for intronic SNP rs9409407 in HSD17B3, rs9409407 GT or TT genotype carriers have ~19.2 % decrease in their median TT (361.5 ng/dL), compared with the rs9409407 GG genotype carriers (447.6 ng/dL; P = 0.006). Individuals homozygous for rs12602084 in HSD17B1 gene had a median TT (383.0 ng/dL), which is 14.5% less (P = 0.006) compared to those in carriers of the rs12602084 CC genotype (448.8 ng/dL) or carriers of the rs12602084 CT genotype (447.0 ng/dL). The q-values for all three SNPs were approximately 0.133.
Table 3. Genotyping Frequencies and Association of Genotype with Patients’ Total Testosterone.
| SNP | Associated Gene |
Location | Genotype | No. | Median of TT (ng/dL) |
Q1, Q3 | P Wilcox | P adj * | q-values |
|---|---|---|---|---|---|---|---|---|---|
| rs1424151 | HSD17B2 | Intron 4 | AA | 448 | 448.9 | 337.0, 578.1 | 0.002 | 0.003 | 0.133 |
| AG/GG | 30 | 371.6 | 278.1, 423.0 | ||||||
| rs9409407 | HSD17B3 | Intron 2 | GG | 385 | 447.6 | 342.2, 575.8 | 0.006 | 0.006 | 0.133 |
| GT/TT | 26 | 361.5 | 310.8, 439.3 | ||||||
| rs12602084 | HSD17B1 | 3′ downstream | CC | 199 | 448.8 | 313.1, 579.1 | 0.006 | 0.008 | 0.133 |
| CT | 194 | 447.0 | 355.0, 577.6 | ||||||
| TT | 90 | 383.0 | 296.3, 494.9 |
TT: total testosterone; Q1, Q3: 25%, 75%
: Pearson chi-square P value, adjusted by age and body mass index
We also analyzed the association between the 143 SNPs and TE (Suppl. Table 1) or SHBG (data not shown) plasma levels. No significant association was observed.
Association of SNPs with D’Amico risk criteria at diagnosis
We correlated the SNP genotyping data with the D’Amico risk categories at diagnosis in the study cohort. Of the 143 SNPs analyzed, 17 SNPs (12%) had an association with the risk of having an intermediate or high D’Amico risk class at diagnosis, with an unadjusted P-value less than 0.05. Among them, 3 SNPs had q-values which were 0.248 and also had clear genotype-dose effect (Table 4). Individuals who carried the minor allele rs11252845 (CT or TT genotype), a variant which is located in the upstream region of AKR1C1 gene, were found to have a reduced risk of having a intermediate or high D’Amico risk class at the time of diagnosis, compared to the rs11252845 wild genotype (CC genotype) carriers (RR of 0.62, 95% CI 0.40-0.88; P = 0.005). Similarly, individuals with the AT or AA genotype of rs2045100 in the first intron of UGT2B15, had a ~50% reduced risk of being diagnosed with a high risk CaP (RR of 0.54, 95%CI 0.28-0.87; P = 0.007), compared to rs2045100 TT genotype carriers. We also found that individuals who were homozygote for the minor allele genotype (GG genotype) of the intronic SNP, rs7932905, in HSD17B12, had a decreased risk of being diagnosed with a CaP of intermediate or high D’Amico risk class (RR of 0.65, 95% CI 0.48-0.86; P = 0.008), when compared with individuals carrying the rs7932905 AA genotype. Individuals carrying the rs7932905-AG genotype did not show a higher likelihood of having higher risk cancer at the time of diagnosis (RR of 0.89, 95% CI 0.74-1.07).
Table 4. Genotyping Frequencies and Association of Genotype with Patients’ D’Amico Risk Class.
| SNP | Associated Gene |
Location | Genotype | No. | Intermediate /High |
RR | 95% CI | P Chisq | FDR | Median of TT (ng/dL) |
Q1, Q3 | P Wilcox |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs11252845 | AKR1C1 | 5′ upstream | CC | 438 | 250 (55.0%) | 1 | 0.005 | 0.248 | 444.4 | 336.9, 578.6 | 0.124 | |
| CT/TT | 52 | 17 (34.0%) | 0.62 | 0.40-0.88 | 400.5 | 303.6, 545.3 | ||||||
|
| ||||||||||||
| rs2045100 | UGT2B15 | Intron 1 | TT | 395 | 212 (55.4%) | 1 | 0.007 | 0.248 | 431.1 | 323.0, 565.1 | 0.259 | |
| AT/AA | 31 | 9 (30.0%) | 0.54 | 0.28-0.87 | 466.9 | 349.2, 606.7 | ||||||
|
| ||||||||||||
| rs7932905 | HSD17B12 | Intron 1 | AA | 140 | 85 (59.9%) | 1 | 0.008 | 0.248 | 432.6 | 308.3, 564.6 | 0.743 | |
| AG | 245 | 133 (53.2%) | 0.89 | 0.74-1.07 | 442.4 | 351.1, 579.8 | ||||||
| GG | 93 | 41 (39.1%) | 0.65 | 0.48-0.86 | 427.9 | 337.1, 555.6 | ||||||
RR, relative risk; CI, confidential level; TT: total testosterone; Q1, Q3: 25%, 75%
Discussion
We previously showed in this cohort the correlation of hormone levels with age and body mass index (BMI) (5). Weak associations were found between SHBG and BMI and age, and no relationship was observed among BMI, age and sex steroid hormone levels (5). CaP aggressiveness related inherited variants have been studied in several genetic association studies but have not been studied in sex steroid hormones metabolic pathway genes (27-30). Several association studies also tried to identify quantitative trait loci predicting sex steroid levels in men includes one recent large cohort study (17-19). The current study sought to determine whether polymorphisms in genes involved in steroid hormone metabolism may impact plasma steroid hormone levels at the time of diagnosis in CaP patients and the D’Amico risk CaP at diagnosis.
In this study, we observed that three non-coding SNPs, rs1424151 in HSD17B2, rs9409407 in HSD17B3 and rs12602084 in HSD17B1, were associated with significantly reduced plasma TT level in newly diagnosed CaP patients. Intriguingly, some investigators have associated prostate cancer grade with low serum testosterone risk (31). The human HSD17B gene family encodes the 17β-hydroxysteroid dehydrogenases that play a pivotal role in the production of steroid hormones (32, 33). HSD17B1 selectively catalyzes the transformation of androestendione to testosterone and estrone (E1) to E2. HSD17B2 catalyzes the conversion of testosterone into androestenedione, converts 5-diol into DHEA and converts E2 into E1 (34, 35). HSD17B3 plays an important role in the conversion from androstenedione to testosterone (35). Some studies indicated that changes of HSD17B1, HSD17B2 or HSD17B3 activity resulted in changes of the androgen level (33, 35). It is possible that these genetic polymorphisms in HSD17B1, HSD17B2 or HSD17B3 affect the expression of their encoded enzymes, consequently affecting plasma androgen level. This speculation remains to be determined.
We identified three SNPs related to circulating testosterone that differed from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3) (19). Explanations for such discrepancies could include variability in sample size, diagnostic criteria, or ethnic background of the sample studied etc. In comparing the current study with the NCI-BPC3, there are a few such issues worth notice. First, the average age and BMI of the two populations are quite different. The median age of our cohort is 60 years, while the median age of NCI-BPC3 cohort is approximately 65 years. In our study, the majority (86%) of patients have a BMI >25, whereas, in NCI-BPC3 cohort, only 58% had a BMI >25 (19). Indeed, many studies have shown that age and BMI are tightly associated with circulating sex steroid levels (36, 37). Secondly, although case-control status in NCI-BPC3 study seemed not related to steroid levels, there was heterogeneity in measured sex steroid levels between different sub-cohorts, maybe due to the use of multiple laboratories. Our cohort consisted of only newly diagnosed early stage CaP patients, with a homogenous European ancestry. All our hormones levels were performed in the same laboratory. Nonetheless, our findings need to be validated in other independent cohorts.
We did not observe any impact of these 143 SNPs on of the plasma TE level. Since our subjects are CaP patients and TE level in men is relatively low, any potentially subtle changes of TE level in men, caused by the genetic polymorphisms in hormone metabolic pathway genes may be difficult to identify.
This study of SNPs in metabolic pathway genes and CaP aggressiveness identified three tagging SNPs, rs11252845 in ARK1C1, rs2045100 in UGT2B15 and rs7932905 in HSD17B12, borderline significantly associated with CaP patients’ D’Amico risk categories. Interestingly, both AKR1C1 and UGT2B15, involved in catabolic processes, were previously found highly expressed in androgen independent CRPC tissues (38). AKR1C1 facilitates the conversion of DHT to 3α-androstanediol or 3β-androstanediol, a possible endogenous ligand for estrogen receptor β in prostate (38). UGT2B15, in conjunction with UGT2B17, mediates glucouridination of DHT metabolites (40). HSD17B12 is a multifunctional isoenzyme functioning in the conversion of E1 to E2, and elongation of long-chain fatty acids, in particular the conversion of palmitic to archadonic (AA) acid, the precursor of sterols and the inflammatory mediator, prostaglandin E. Its over-expression together with that of COX-2 in breast carcinoma is associated with a poor prognosis (41). The rs11252845 is located in the 5′ flanking region of the AKR1C1 and may potentially be involved in the regulation of AKR1C1 expression. The rs2045100 and rs7932905 are intronic SNPs. These three SNPs did not affect plasma levels of TT or TE. The possibility that they may influence intratumoral androgen is not excluded and remains to be determined.
In summary, of the 143 polymorphisms in the 30 genes involved in the steroid hormone metabolic pathways, we identified SNPs in 3 genes (HSD17B1, HSD17B2 and HSD17B3) which were associated with plasma TT level, whereas SNPs in another 3 genes (AKR1C1, UGT2B15, HSD17B12) might correlate with the risk of aggressive CaP at diagnosis. Our study did not find SNPs that are significantly associated with both plasma hormone levels and CaP aggressiveness. These results are consistent with our previous work finding no association between plasma steroid hormone levels and CaP tumor grades.
Supplementary Material
Acknowledgments
This work was supported by a SPORE in Prostate Cancer 2 P50 CA090381-06 and a Prostate Cancer Foundation Challenge Grant. T.S is supported by a DoD Prostate Cancer Training Award W81XWH-09-1-0372.
Glossary
Abbreviations
- T
testosterone
- TT
total testosterone
- TE
total estradiol
- E1
estrone
- E2
estradiol
- SHBG
sex hormone-binding globulin
- DHT
dihydrotestosterone
- AR
androgen receptor
- SNPs
single nucleotide polymorphisms
- CIs
confidence intervals
- RR
relative risk
- CaP
prostate cancer
Footnotes
Competing interest statement: The authors declare no conflict of interests.
References
- 1.Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28:4038–44. doi: 10.1200/JCO.2009.27.4290. [DOI] [PubMed] [Google Scholar]
- 2.Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: history and current clinical relevance. Urology. 2008;72:247–54. doi: 10.1016/j.urology.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, et al. Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:86–91. doi: 10.1158/1055-9965.EPI-05-0633. [DOI] [PubMed] [Google Scholar]
- 4.Platz EA, Giovannucci E. The epidemiology of sex steroid hormones and their signaling and metabolic pathways in the etiology of prostate cancer. J Steroid Biochem Mol Biol. 2004;92:237–53. doi: 10.1016/j.jsbmb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Sher DJ, Mantzoros C, Jacobus S, Regan MM, Lee GS, Oh WK. Absence of relationship between steroid hormone levels and prostate cancer tumor grade. Urology. 2009;73:356–61. doi: 10.1016/j.urology.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 6.Imamoto T, Suzuki H, Utsumi T, Endo T, Takano M, Yano M, et al. Association between serum sex hormone levels and prostate cancer: effect of prostate cancer on serum testosterone levels. Future Oncol. 2009;5:1005–13. doi: 10.2217/fon.09.82. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Hsing AW, et al. Endogenous sex hormones and the risk of prostate cancer: a prospective study. Int J Cancer. 2008;122:2345–50. doi: 10.1002/ijc.23326. [DOI] [PubMed] [Google Scholar]
- 8.Isbarn H, Pinthus JH, Marks LS, Montorsi F, Morales A, Morgentaler A, et al. Testosterone and prostate cancer: revisiting old paradigms. Eur Urol. 2009;56:48–56. doi: 10.1016/j.eururo.2009.03.088. [DOI] [PubMed] [Google Scholar]
- 9.Crawford ED. Understanding the epidemiology, natural history, and key pathways involved in prostate cancer. Urology. 2009;73:S4–10. doi: 10.1016/j.urology.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 10.Carruba G. Estrogen and prostate cancer: an eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 11.Morgentaler A, Schulman C. Testosterone and prostate safety. Front Horm Res. 2009;37:197–203. doi: 10.1159/000176054. [DOI] [PubMed] [Google Scholar]
- 12.Hsing AW, Chu LW, Stanczyk FZ. Androgen and prostate cancer: is the hypothesis dead? Cancer Epidemiol Biomarkers Prev. 2008;17:2525–30. doi: 10.1158/1055-9965.EPI-08-0448. [DOI] [PubMed] [Google Scholar]
- 13.Beuten J, Gelfond JA, Franke JL, Weldon KS, Crandall AC, Johnson-Pais TL, et al. Single and multigenic analysis of the association between variants in 12 steroid hormone metabolism genes and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 2009;18:1869–80. doi: 10.1158/1055-9965.EPI-09-0076. [DOI] [PubMed] [Google Scholar]
- 14.Mononen N, Schleutker J. Polymorphisms in genes involved in androgen pathways as risk factors for prostate cancer. J Urol. 2009;181:1541–9. doi: 10.1016/j.juro.2008.11.076. [DOI] [PubMed] [Google Scholar]
- 15.Cunningham JM, Hebbring SJ, McDonnell SK, Cicek MS, Christensen GB, Wang L, et al. Evaluation of genetic variations in the androgen and estrogen metabolic pathways as risk factors for sporadic and familial prostate cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:969–78. doi: 10.1158/1055-9965.EPI-06-0767. [DOI] [PubMed] [Google Scholar]
- 16.Lindström S, Zheng SL, Wiklund F, Jonsson BA, Adami HO, Bälter KA, et al. Systematic replication study of reported genetic associations in prostate cancer: Strong support for genetic variation in the androgen pathway. Prostate. 2006;66:1729–43. doi: 10.1002/pros.20489. [DOI] [PubMed] [Google Scholar]
- 17.Travis RC, Schumacher F, Hirschhorn JN, Kraft P, Allen NE, Albanes D, et al. CYP19A1 genetic variation in relation to prostate cancer risk and circulating sex hormone concentrations in men from the Breast and Prostate Cancer Cohort Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:2734–44. doi: 10.1158/1055-9965.EPI-09-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boger-Megiddo I, Weiss NS, Barnett MJ, Goodman GE, Chen C. V89L polymorphism of the 5alpha-reductase Type II gene (SRD5A2), endogenous sex hormones, and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:286–91. doi: 10.1158/1055-9965.EPI-07-0238. [DOI] [PubMed] [Google Scholar]
- 19.Ahn J, Schumacher FR, Berndt SI, Pfeiffer R, Albanes D, Andriole GL, et al. Quantitative trait loci predicting circulating sex steroid hormones in men from the NCI-Breast and Prostate Cancer Cohort Consortium (BPC3) Hum Mol Genet. 2009;18:3749–57. doi: 10.1093/hmg/ddp302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sher DJ, Oh WK, Jacobus S, Regan MM, Lee GS, Mantzoros C. Relationship between serum adiponectin and prostate cancer grade. Prostate. 2008;68:1592–8. doi: 10.1002/pros.20823. [DOI] [PubMed] [Google Scholar]
- 21.Oh WK, Hayes J, Evan C, Manola J, George DJ, Waldron H, et al. Development of an integrated prostate cancer research information system. Clin Genitourin Cancer. 2006;5:61–6. doi: 10.3816/CGC.2006.n.019. [DOI] [PubMed] [Google Scholar]
- 22.D’Amico AV, Schultz D, Loffredo M, Dugal R, Hurwitz M, Kaplan I, et al. Biochemical outcome following external beam radiation therapy with or without androgen suppression therapy for clinically localized prostate cancer. JAMA. 2000;284:1280–3. doi: 10.1001/jama.284.10.1280. [DOI] [PubMed] [Google Scholar]
- 23.Miller WL. Steroidogenic enzymes. Endocr Dev. 2008;13:1–18. doi: 10.1159/000134751. [DOI] [PubMed] [Google Scholar]
- 24.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Rev. 2004;25:947–70. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 25.Luu-The V, Bélanger A, Labrie F. Androgen biosynthetic pathways in the human prostate. Best Pract Res Clin Endocrinol Metab. 2008;22:207–21. doi: 10.1016/j.beem.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zabaleta J, Su LJ, Lin HY, Sierra RA, Hall MC, Sartor AO, et al. Cytokine genetic polymorphisms and prostate cancer aggressiveness. Carcinogenesis. 2009;30:1358–62. doi: 10.1093/carcin/bgp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun T, Lee GS, Werner L, Pomerantz M, Oh WK, Kantoff PW, et al. Inherited variations in AR, ESR1, and ESR2 genes are not associated with prostate cancer aggressiveness or with efficacy of androgen deprivation therapy. Cancer Epidemiol Biomarkers Prev. 2010;19:1871–8. doi: 10.1158/1055-9965.EPI-10-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penney KL, Salinas CA, Pomerantz M, Schumacher FR, Beckwith CA, Lee GS, et al. Evaluation of 8q24 and 17q risk loci and prostate cancer mortality. Clin Cancer Res. 2009;15:3223–30. doi: 10.1158/1078-0432.CCR-08-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun T, Lee GS, Oh WK, Freedman ML, Pomerantz M, Pienta KJ, et al. Inherited variants in the chemokine CCL2 gene and prostate cancer aggressiveness in a Caucasian cohort. Clin Cancer Res. 2011;17:1546–52. doi: 10.1158/1078-0432.CCR-10-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schatzl G, Madersbacher S, Thurridl T, Waldmüller J, Kramer G, Haitel A, et al. High-grade prostate cancer is associated with low serum testosterone levels. Prostate. 2001;47(1):52–8. doi: 10.1002/pros.1046. [DOI] [PubMed] [Google Scholar]
- 32.Labrie F, Luu-The V, Lin SX, Labrie C, Simard J, Breton R, et al. The key role of 17 beta-hydroxysteroid dehydrogenases in sex steroid biology. Steroids. 1997;62:148–158. doi: 10.1016/s0039-128x(96)00174-2. [DOI] [PubMed] [Google Scholar]
- 33.Mindnich R, Moller G, Adamski J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol Cell Endocrinol. 2004;218:7–20. doi: 10.1016/j.mce.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Wang JH, Tuohimaa P. Regulation of 17beta-hydroxysteroid dehydrogenase type 2, type 4 and type 5 by calcitriol, LXR agonist and 5alpha-dihydrotestosterone in human prostate cancer cells. J Steroid Biochem Mol Biol. 2007;107:100–5. doi: 10.1016/j.jsbmb.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 35.Soronen P, Laiti M, Törn S, Härkönen P, Patrikainen L, Li Y, et al. Sex steroid hormone metabolism and prostate cancer. J Steroid Biochem Mol Biol. 2004;92:281–6. doi: 10.1016/j.jsbmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22:110–6. [PubMed] [Google Scholar]
- 37.MacDonald AA, Herbison GP, Showell M, Farquhar CM. The impact of body mass index on semen parameters and reproductive hormones in human males: a systematic review with meta-analysis. Hum Reprod Update. 2010;16:293–311. doi: 10.1093/humupd/dmp047. [DOI] [PubMed] [Google Scholar]
- 38.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66:2815–25. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 39.Steckelbroeck S, Jin Y, Gopishetty S, Oyesanmi B, Penning TM. Human cytosolic 3alpha-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3beta-hydroxysteroid dehydrogenase activity: implications for steroid hormone metabolism and action. J Biol Chem. 2004;279:10784–95. doi: 10.1074/jbc.M313308200. [DOI] [PubMed] [Google Scholar]
- 40.Hum DW, Bélanger A, Lévesque E, Barbier O, Beaulieu M, Albert C, et al. Characterization of UDP-glucuronosyltransferases active on steroid hormones. J Steroid Biochem Mol Biol. 1999;69:413–23. doi: 10.1016/s0960-0760(99)00061-8. [DOI] [PubMed] [Google Scholar]
- 41.Visus C, Ito D, Dhir R, Szczepanski MJ, Chang YJ, Latimer JJ, et al. Identification of Hydroxysteroid (17β) dehydrogenase type 12 (HSD17B12) as a CD8(+) T-cell-defined human tumor antigen of human carcinomas. Cancer Immunol Immunother. 2011;60:919–29. doi: 10.1007/s00262-011-1001-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.