Abstract
BACKGROUND
The purpose of this study was to characterize the cause of death in severely injured trauma patients in order to define potential responses to resuscitation.
METHODS
Prospective analysis of 190 critically-injured patients who underwent massive transfusion protocol activation (MTP) or received massive transfusion (MT; greater than 10 units of packed red blood cells (pRBC)/24 hours). Cause of death was adjudicated into one of four categories: 1) Exsanguination, 2) Early physiologic collapse, 3) Late physiologic collapse, and 4) Non-survivable injury.
RESULTS
190 patients underwent MT or MTP with 76 deaths (40% mortality) of which 72 deaths were adjudicated to one of four categories: 33.3% died from exsanguination, 16.6% died from early physiologic collapse, 11.1% died from late physiologic collapse, while 38.8% died from non-survivable injuries. Patients who died from exsanguination were younger and had the highest RBC:FFP ratio (2.97 ± 2.24), although the early physiologic collapse group survived long enough to use the most blood products (p<0.001). The late physiologic collapse group had significantly fewer penetrating injuries, was older, and had significantly more crystalloid use, but received a lower RBC:FFP ratio (1.50 ± 0.42). Those who were determined to have a non-survivable injury had a lower presenting GCS, fewer penetrating injuries, and higher initial blood pressure reflecting a preponderance of non-survivable traumatic brain injury. The average survival time for patients with potentially survivable injuries was 2.4 hrs versus 18.4 hours for non-survivable injuries (p<0.001).
CONCLUSIONS
Severely injured patients requiring MTP have a high mortality rate. However, no studies to date have addressed the cause of death after MTP. Characterization of cause of death will allow targeting of surgical and resuscitative conduct to allow extension of the physiologic reserve time therefore rendering previously non-survivable injury potentially survivable.
LEVEL OF EVIDENCE
Prognostic study, Level III
Keywords: Massive transfusion, Cause of Death
INTRODUCTION
Considerable interest and research exist regarding optimal resuscitation of the severely injured trauma patients. Multiple recent studies have shown that early usage of a balanced resuscitation via a massive transfusion protocol (MTP) decreases mortality in these patients. (1–6) Despite data that overwhelmingly supports these plasma based advances in resuscitation, there is considerable disparity in effectiveness from center to center and from study to study. Even in the most optimistic studies, the severely injured continue to have considerable mortality. (7, 8) This high mortality rate lends to a large population of potentially preventable deaths and the lack of data on the cause of early death after injury makes it difficult to determine the true effectiveness of balanced resuscitation protocols on patients with severe survivable injuries.
Indeed, the majority of epidemiologic studies have examined the mechanism of injury, rather than the cause of or physiology preceding death. It has been well described that trauma patients die at three distinct time points and that truncal hemorrhage is a leading cause of potentially preventable death. (9, 10) Despite these characterizations, identification of which patients die from anatomically non-survivable (and hence unresuscitatable) injury, versus which patients exsanguinate from anatomically survivable injury, versus which patients survive initially only to perish from late physiologic collapse has not been done. Hence, we hypothesized that the characterization of the precise cause of death would provide significant insight into not only epidemiologic reasons for mortality, but also elucidate reasons for the effectiveness of resuscitation protocols. Ultimately, this would help to more efficiently target resources and resuscitation of our injured warfighters and civilians that could allow extension of the physiologic reserve time, thereby rendering previously non-survivable injury potentially survivable.
METHODS
Data was prospectively collected from 190 critically-injured trauma patients who met the highest level of trauma activation criteria on arrival to the emergency department (ED) of San Francisco General Hospital from 2005 to 2011. Inclusion criteria consisted of all patients in whom the MTP was activated (see below for definition of MTP). Patients who were <18 years old, had >5% body surface area burns, received >2 liters of intravenous fluid prior to arrival, or were transferred from another institution were excluded. Admission blood samples were collected immediately upon arrival to the ED and processed as previously described elsewhere. (11) Standard laboratory, resuscitation, and outcomes data were prospectively collected in parallel. Consent was obtained as approved by the University of California Institutional Committee on Human Research.
Activation of the MTP was based on clinician judgment, immediately on arrival, or if there were ongoing transfusion requirements. Massive transfusion (MT) was defined as transfusion of ≥10 units of red blood cells within the first 24h of admission, in patients surviving to 24h; in order to account for survivor bias and to include patients who received high-volume transfusion but did not survive to 24h, scaled transfusion of ≥5 units in patients dying by 12h or ≥2.5 units in patients dying by 6h were also defined as MT. MTP activation releases 4 units of FFP and 6 units of pRBC from the blood bank with apheresis platelets and cryoprecipitate being released on orders. At time of study, neither tranexamic acid (TXA) nor Factor VII were part of the MTP.
The cause of death was codified and adjudicated by two independent trauma surgeons. The cause of death categories were defined as: 1.)Exsanguination–the patient died due to uncontrolled surgical hemorrhage; 2.)Early collapse–the patient died within 24 hours of arrival to the hospital from physiologic collapse, after adequate control of surgical bleeding; 3.)Late cardiopulmonary collapse –the patient received surgical and/or intensive care therapy, had adequate hemorrhage control, and died from cardiopulmonary failure later than 24 hours after admission; 4.)Non-survivable injury–For the purposes of this analysis, survivability was based on chart documentation of the attending trauma surgeon or neurosurgeon, in whose judgment the patient was deemed to have a non-survivable injury by at least one of the following: physical exam, diagnostic imaging (ie CT scan), cerebral blood flow imaging or operative findings. We then compared non-survivors in the three broad categories of exsanguination, early collapse, and late collapse to each other using ANOVA, Kruskal-Wallis, and chi-squared tests as appropriate. The corresponding data for survivors is provided for reference.
All data are presented as mean ± standard deviation, median (inter-quartile range [IQR]), or percentage; univariate comparisons were made using Student’s t-test for normally distributed data, Wilcoxon rank-sum testing for skewed data, and Fisher’s exact test for proportions. Kaplan-Meier time-to-event analysis and logrank testing were used to assess differences in 24-hour and in-hospital mortality between groups. An alpha of 0.05 was considered significant. All data analysis was performed by the authors using Stata version 12 (StataCorp; College Station, TX).
RESULTS
One hundred and ninety patients underwent MT or MTP, with 76 deaths (40% mortality) of which 72 deaths were adjudicated to: Exsanguination, Early cardiovascular collapse, Late cardiovascular collapse, or Non-survivable injury. Four deaths were excluded due to lack of available documentation that could clearly identify the cause of death. In the entire cohort of those patients that died, the average age was 38.4 +/− 18.5 years, with men constituting 75.3% of the population. Penetrating injuries were found in 50.5 %, and the average ISS was 28.3 +/−16.3.
Table 1 compares characteristics of survivors vs potentially survivable mortality vs anatomically non-survivable injury, of which 61% (n=44) died from a potentially survivable injury, while 38.8% (n=28) were deemed to have died from anatomically non-survivable injuries. Potential survivors had more penetrating injuries and a greater base deficit, were more hypotensive, acidotic, and coagulopathic than both the survivors and non-survivable injured. The percentage of patients who received the full definition of an MT is listed as percent massive transfusion (%MT). The physiologic parameters in the anatomically non-survivable group were consistent with those seen in patients with traumatic brain injury which was also identified by CT scan or physical exam in 96.4% of patients. (Table 5)
TABLE 1.
Demographics and resuscitation of those who survived compared to those who had a potentially survivable injury, but still died, and those with anatomically non-survivable injuries. Potential survivors had more penetrating injuries, were more hypotensive, more acidotic, had a greater base deficit, were more coagulopathic than both the survivors and non-survivable injured.
| Survivors (n = 114) | Potential survivors (n = 44) | Anatomically Non-survivable (n = 28) | P-value | |
|---|---|---|---|---|
| Age (years) | 35.8 ± 16.4 | 38.3 ± 17.8 | 45.7 ± 23.0 | 0.033 |
| % male | 74.6% | 84.1% | 64.3% | 0.167 |
| % penetrating | 53.5% | 61.4% | 28.6% | 0.021 |
| AIS-head | 2.2 ± 2.0 | 2.4 ± 2.0 | 2.9 ± 2.3 | 0.511 |
| ISS | 26.0 ± 14.9 | 31.5 ± 16.5 | 35.9 ± 19.1 | 0.009 |
| GCS | 15 (11 – 15) | 6 (3 – 12) | 3 (3 – 5.5) | <0.001 |
| Temperature (C) | 35.8 ± 0.8 | 35.1 ± 1.6 | 35.3 ± 1.2 | 0.016 |
| HR (BPM) | 106.7 ± 28.5 | 101.8 ± 45.9 | 99.4 ± 36.6 | 0.508 |
| SBP (mmHg) | 104.0 ± 32.6 | 88.2 ± 43.8 | 124.2 ± 52.3 | <0.001 |
| pH | 7.25 ± 0.15 | 7.04 ± 0.19 | 7.23 ± 0.13 | <0.001 |
| Base deficit | −8.6 ± 5.3 | −15.9 ± 7.2 | −8.8 ± 6.0 | <0.001 |
| Hgb (g/dL) | 12.0 ± 2.3 | 10.8 ± 2.6 | 11.3 ± 2.0 | 0.018 |
| INR | 1.3 (1.1 – 1.5) | 1.7 (1.4 – 2.3) | 1.3 (1.2 – 1.6) | <0.001 |
| PT | 15.4 (14.3 – 17.5) | 19.7 (17.3 – 24.5) | 15.9 (14.9 – 19.7) | <0.001 |
| PTT | 28.4 (24.9 – 32.9) | 40.0 (33.3 – 54.8) | 38.7 (31.5 – 55.0) | <0.001 |
| Platelets×103 | 276 ± 93 | 180 ± 90 | 243 ± 111 | <0.001 |
| WBC103/mm3 | 11.0 (8.0 – 14.2) | 8.3 (5.3 – 11.6) | 9.7 (6.4 – 13.6) | 0.006 |
| Creatinine(mg/dL) | 1.0 (0.8 – 1.3) | 1.3 (1.1 – 1.4) | 1.0 (0.9 – 1.3) | 0.002 |
| Prehospital IVF (mL) | 250 (50 – 500) | 100 (25 – 750) | 150 (25 – 1400) | 0.892 |
| 24h crystalloid (mL) | 6.3 (4.4 – 10.1) | 4.0 (2.0 – 9.0) | 8.3 (4.1 – 11.4) | 0.017 |
| 24h colloid(mL) | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 0) | 0.362 |
| 24h PRBC(units) | 10 (6 – 17) | 23.5 (13 – 38.5) | 13 (8.5 – 19) | <0.001 |
| 24h FFP(units) | 8 (4 – 12) | 13 (4.5 – 24) | 10 (4.5 – 14) | 0.035 |
| 24h platelets (apheresis unit) | 1 (0 – 2) | 1 (0 – 4) | 1 (0 – 2) | 0.572 |
| % MT | 56.1% | 86.4% | 67.9% | 0.001 |
| 24h RBC:FFP ratio | 1.47 ± 0.86 | 2.32 ± 1.76 | 1.56 ± 0.73 | <0.001 |
| Minutes to MTP | 46.5 (26 – 103.5) | 19.5 (15 – 25) | 67 (35 – 93) | <0.001 |
AIS = Abbreviated Injury Score; ISS = Injury Severity Score; GCS = Glascow Coma Score; HR = heart rate; BPM = Beats per Minute; SBP = Systolic blood pressure; Hgb = Hemoglobin; %MT = Percent of patients receiving full massive transfusion; pRBC = packed red blood cells; FFP = Fresh Frozen Plasma; IVF = Intravenous fluids Normally distributed data are reported in mean +/− SD; skewed data reported as median (Inter-quartile Range)
TABLE 5.
Demographics and resuscitation of those who were survivable injured, but still died versus those with anatomically non-survivable injuries identifies traumatic brain injury as driving force of anatomically non-survivable cohort.
| Potential Survivors (n = 44) | Anatomically Non-survivable (n = 28) | P-value | |
|---|---|---|---|
| Age (years) | 38.3 ± 17.8 | 45.7 ± 23.0 | 0.154 |
| % male | 84.1% | 64.3% | 0.086 |
| % penetrating | 61.4% | 28.6% | 0.008 |
| AIS-head | 2.4 ± 2.0 | 2.9 ± 2.3 | 0.462 |
| ISS | 31.5 ± 16.5 | 35.9 ± 19.1 | 0.367 |
| GCS | 6 (3 – 12) | 3 (3 – 5.5) | 0.018 |
| TBI | 20.5% | 96.4% | <0.001 |
| Temperature (C) | 35.1 ± 1.6 | 35.3 ± 1.2 | 0.695 |
| HR (BPM) | 101.8 ± 45.9 | 99.4 ± 36.6 | 0.807 |
| SBP (mmHg) | 88.2 ± 43.8 | 124.2 ± 52.3 | 0.004 |
| pH | 7.04 ± 0.19 | 7.23 ± 0.13 | <0.001 |
| Base deficit | −15.9 ± 7.2 | −8.8 ± 6.0 | <0.001 |
| Hgb (g/dL) | 10.8 ± 2.6 | 11.3 ± 2.0 | 0.360 |
| INR | 1.7 (1.4 – 2.3) | 1.3 (1.2 – 1.6) | 0.005 |
| PT | 19.7 (17.3 – 24.5) | 15.9 (14.9 – 19.7) | 0.036 |
| PTT | 40 (33.3 – 54.8) | 38.7 (31.5 – 55.0) | 0.801 |
| Platelets×103 | 180 ± 90 | 243 ± 111 | 0.019 |
| WBC103/mm3 | 8.3 (5.3 – 11.6) | 9.7 (6.4 – 13.6) | 0.073 |
| Creatinine(mg/dL) | 1.3 (1.1 – 1.4) | 1.0 (0.9 – 1.3) | 0.012 |
| Prehospital IVF (mL) | 100 (25 – 750) | 150 (25 – 1400) | 0.632 |
| 24h crystalloid (mL) | 4.0 (2 – 9) | 8.3 (4.1 – 11.4) | 0.033 |
| 24h colloid(mL) | 0 (0 – 0) | 0 (0 – 0) | 0.355 |
| 24h PRBC(units) | 23.5 (13 – 38.5) | 13 (8.5 – 19) | 0.001 |
| 24h FFP(units) | 13 (4.5 – 24) | 10 (4.5 – 14) | 0.133 |
| 24h platelets (apheresis unit) | 1 (0 – 4) | 1 (0 – 2) | 0.267 |
| % MT | 86.40% | 67.9% | 0.077 |
| 24h RBC:FFP ratio | 2.32 ± 1.76 | 1.56 ± 0.73 | 0.016 |
| Minutes to MTP | 19.5 (15 – 25) | 67 (35 – 93) | <0.001 |
We next compared the survivors (n=114) to those who had died despite potentially survivable injury (n=44) (Table 2) and found that these two groups were similar in age, mechanism, ISS, temperature and heart rate. However, those who died presented with lower GCS and systolic blood pressure (SBP) and were significantly more acidotic and coagulopathic than those who survived. Also, the potentially survivable cohort utilized less crystalloid, but significantly more blood products. The cause of death in potentially survivable patients was divided into either: 1) Exsanguination, 2)Early physiologic collapse, or 3)Late physiologic collapse, and compared to the non-survivable injured; survivor data is provided for reference (Table 3). 38.8% (n=28) of patients in this cohort died from anatomically non-survivable injury and 33.3% died from exsanguination (n=24). The percentage of deaths attributed to early physiologic collapse in this group was 16.6% (n=12), while late physiologic collapse comprised 11.1% (n=8).
TABLE 2.
Demographics and resuscitation of those who survived compared to those who had potentially survivable injuries but died. These groups were similar in age, percentage of penetrating mechanism, ISS, temperature and heart rate, but those who died presented with lower GCS and systolic blood pressure (SBP), and were significantly more acidotic and coagulopathic than those who survived.
| Survivors (n = 114) | Potential Survivors (n = 44) | P-value | |
|---|---|---|---|
| Age (years) | 35.8 ± 16.4 | 38.3 ± 17.8 | 0.426 |
| % male | 74.6% | 84.1% | 0.290 |
| % penetrating | 53.5% | 61.4% | 0.475 |
| AIS-head | 2.2 ± 2.0 | 2.4 ± 2.0 | 0.739 |
| ISS | 26.0 ± 14.9 | 31.5 ± 16.5 | 0.115 |
| GCS | 15 (11 – 15) | 6 (3 – 12) | <0.001 |
| Temperature (C) | 35.8 ± 0.8 | 35.1 ± 1.6 | 0.082 |
| HR (BPM) | 106.7 ± 28.5 | 101.8 ± 45.9 | 0.522 |
| SBP (mmHg) | 104.0 ± 32.6 | 88.2 ± 43.8 | 0.036 |
| pH | 7.25 ± 0.15 | 7.04 ± 0.19 | <0.001 |
| Base deficit | −8.6 ± 5.3 | −15.9 ± 7.2 | <0.001 |
| Hgb (g/dL) | 12.0 ± 2.3 | 10.8 ± 2.6 | 0.013 |
| INR | 1.3 (1.1 – 1.5) | 1.7 (1.4 – 2.3) | <0.001 |
| PT | 15.4 (14.3 – 17.5) | 19.7 (17.3 – 24.5) | <0.001 |
| PTT | 28.4 (24.9 – 32.9) | 40.0 (33.3 – 54.8) | <0.001 |
| Platelets×103 | 276 ± 93 | 180 ± 90 | <0.001 |
| WBC103/mm3 | 11.0 (8.0 – 14.2) | 8.3 (5.3 – 11.6) | 0.002 |
| Creatinine(mg/dL) | 1.0 (0.8 – 1.3) | 1.3 (1.1 – 1.4) | <0.001 |
| Prehospital IVF (mL) | 250 (50 – 500) | 100 (25 – 750) | 0.663 |
| 24h crystalloid (mL) | 6.3 (4.4 – 10.1) | 4.0 (2.0 – 9.0) | 0.008 |
| 24h colloid(mL) | 0 (0 – 0) | 0 (0 – 0) | 0.591 |
| 24h PRBC(units) | 10 (6 – 17) | 23.5 (13 – 38.5) | <0.001 |
| 24h FFP(units) | 8 (4 – 12) | 13 (4.5 – 24) | 0.013 |
| 24h platelets (apheresis unit) | 1 (0 – 2) | 1 (0 – 4) | 0.540 |
| % MT | 56.10% | 86.4% | <0.001 |
| 24h RBC:FFP ratio | 1.47 ± 0.86 | 2.32 ± 1.76 | 0.005 |
| Minutes to MTP | 46.5 (26 – 103.5) | 19.5 (15 – 25) | <0.001 |
TABLE 3.
Demographics and resuscitation by cause of death with Survivors shown for reference. There is a higher RBC:FFP ratio in the Exsanguination group as well as significantly higher crystalloid usage in the Late Collapse cohort. Additionally, all patients in the Early and Late Collapse cohorts received full MTP, while only 75% and 67.9% of exsanguination and non-survivable received full MTP.
| Cause of Death | ||||||
|---|---|---|---|---|---|---|
| Potential Survivors | Anatomically Non-Survivable | P value | ||||
| Survivors | Exsanguination | Early Collapse | Late Collapse | |||
| (n = 114) | (n = 24) | (n = 12) | (n = 8) | (n = 28) | ||
| Age (years) | 35.8 ± 16.4 | 32.8 ± 16.4 | 39.8 ± 16.3 | 52.6 ± 17.5 | 45.7 ± 23.0 | 0.039 |
| % male | 74.6% | 95.8% | 66.7% | 75.0% | 64.3% | 0.023 |
| % penetrating | 53.5% | 83.3% | 50.0% | 12.5% | 28.6% | <0.001 |
| AIS-head | 2.2 ± 2.0 | 1.7 ± 2.1 | 2.4 ± 2.3 | 3.3 ± 1.7 | 2.9 ± 2.3 | 0.440 |
| ISS | 26.0 ± 14.9 | 37.8 ± 19.5 | 31.4 ± 12.2 | 22.1 ± 11.4 | 35.9 ± 19.1 | 0.208 |
| GCS | 15 (11 – 15) | 4 (3 – 12) | 6 (3 – 14) | 11 (6 – 14) | 3 (3 – 5.5) | 0.035 |
| Temperature (C) | 35.8 ± 0.8 | 35.3 ± 1.6 | 35.6 ± 0.9 | 34.6 ± 2.1 | 35.3 ± 1.2 | 0.673 |
| HR (BPM) | 106.7 ± 28.5 | 93.8 ± 58.1 | 113.5 ± 30.4 | 105.4 ± 22.7 | 99.4 ± 36.6 | 0.623 |
| SBP (mmHg) | 104.0 ± 32.6 | 77.7 ± 44.7 | 94.9 ± 42.7 | 108.1 ± 38.1 | 124.2 ± 52.3 | 0.008 |
| pH | 7.25 ± 0.15 | 6.99 ± 0.20 | 7.05 ± 0.20 | 7.16 ± 0.15 | 7.23 ± 0.13 | <0.001 |
| Base deficit | −8.6 ± 5.3 | −18.1 ± 8.3 | −13.2 ± 6.2 | −14.4 ± 5.0 | −8.8 ± 6.0 | <0.001 |
| Hgb (g/dL) | 12.0 ± 2.3 | 10.9 ± 2.8 | 10.7 ± 1.6 | 10.5 ± 3.5 | 11.3 ± 2.0 | 0.817 |
| INR | 1.3 (1.1 – 1.5) | 1.9 (1.7 – 2.3) | 1.5 (1.2 – 3.0) | 1.8 (1.1 – 2.6) | 1.3 (1.2 – 1.6) | 0.018 |
| PT | 15.4 (14.3 – 17.5) | 20.7 (19.3 – 23.8) | 17.6 (16.2 – 30.4) | 20.8 (14.6 – 26.8) | 15.9 (14.9 – 19.7) | 0.121 |
| PTT | 28.4 (24.9 – 32.9) | 43.2 (39.0 – 60.9) | 41.2 (32.6 – 52.8) | 33.6 (27.4 – 78.5) | 38.7 (31.5 – 55.0) | 0.475 |
| Platelets×103 | 276 ± 93 | 171 ± 87 | 205 ± 100 | 158 ± 85 | 243 ± 111 | 0.072 |
| WBC103/mm3 | 11.0 (8.0 – 14.2) | 5.6 (5.0 – 8.4) | 8.6 (6.6 – 13.6) | 11.3 (9.2 – 14.7) | 9.7 (6.4 – 13.6) | 0.004 |
| Creatinine(mg/dL) | 1.0 (0.8 – 1.3) | 1.3 (1.2 – 1.4) | 1.4 (1.2 – 1.5) | 1.1 (0.8 – 1.3) | 1.0 (0.9 – 1.3) | 0.017 |
| Prehospital IVF (mL) | 250 (50 – 500) | 100 (0 – 750) | - | 100 (100 –100) | 150 (25 – 1400) | 0.892 |
| 24h crystalloid (mL) | 6.3 (4.4 – 10.1) | 3.0 (2 – 4.9) | 4.0 (2 – 7.5) | 16.0 (9 – 19.5) | 8.3 (4.1 – 11.4) | <0.001 |
| 24h colloid(mL) | 0 (0 – 0) | 0 (0 – 0) | 0 (0 – 0.5) | 0 (0 – 0.6) | 0 (0 – 0) | 0.141 |
| 24h PRBC(units) | 10 (6 – 17) | 18.5 (10 – 28.5) | 36.5 (19.5 – 65) | 23.5 (20 – 39) | 13 (8.5 – 19) | <0.001 |
| 24h FFP(units) | 8 (4 – 12) | 6.5 (3 – 13) | 25.0 (15 – 35.5) | 18.0 (14 – 23.5) | 10 (4.5 – 14) | <0.001 |
| 24h platelets (apheresis unit) | 1 (0 – 2) | 0 (0 – 0) | 3.5 (1 – 5) | 3 (2.5 – 4.5) | 1 (0 – 2) | <0.001 |
| % MT | 56.10% | 75.0% | 100.0% | 100.0% | 67.9% | 0.049 |
| 24h RBC:FFP ratio | 1.47 ± 0.86 | 2.97 ± 2.24 | 1.74 ± 0.63 | 1.50 ± 0.42 | 1.56 ± 0.73 | 0.004 |
| Minutes to MTP | 46.5 (26 – 103.5) | 19 (15 – 24) | 24 (14 – 47) | 21 (21 – 21) | 67 (35 – 93) | 0.002 |
Evaluation of the demographics of each group shows that the late physiologic collapse group was older and had significantly fewer penetrating injuries (p<0.02). Those who were determined to have a non-survivable injury had a lower presenting GCS, fewer penetrating injuries, and higher initial blood pressure (Table 3).
Analysis of the resuscitation of each group indicated that the late physiologic collapse group had significantly more 24 hour crystalloid use, but the RBC:FFP ratio was 1.50 ± 0.42. Patients who died from exsanguination were younger and had the highest RBC:FFP ratio, although the early physiologic collapse group survived long enough to use the most blood products (p<0.001).
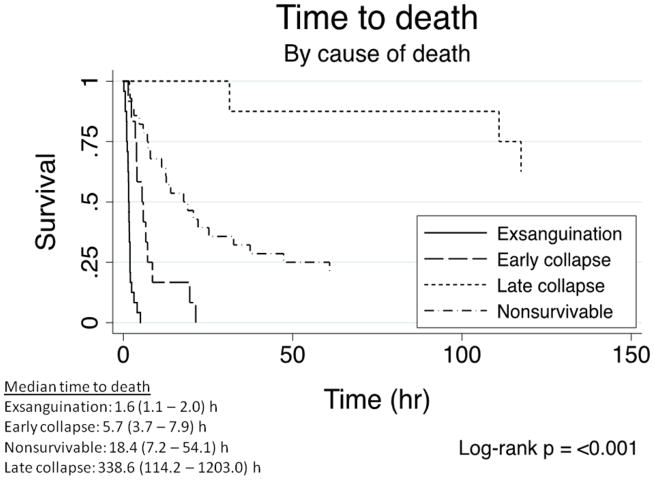
We then performed a Kaplan-Meier time-to-event analysis using log-rank testing to assess differences in 24-hour and in-hospital mortality between groups. The median survival time for patients who died from exsanguination was 1.6 hours vs 5.7 hours for those who died from early physiologic collapse. Those with non-survivable injuries died at a median of 18.4 hours after arrival, while those who died from late physiologic collapse had a median survival time to 338.6 hours (p<0.001; Figure 1).
FIGURE 1.
Time to death analysis. Those who died from exsanguination died the earliest at a median time of 1.6 hours, while those who died from late physiologic collapse died at a median time of about 2 weeks (338.6 hours).
We next evaluated those patients with anatomically non-survivable injury (n=28) and compared them to the rest of the study population at large (n=158), in order to identify predictive factors for anatomically non-survivable injuries (Table 4). Again, the non-survivable injured group had fewer penetrating injuries (p<0.013), significantly lower presenting GCS (p<0.001), and higher initial systolic blood pressure (p<0.023). There were no identifiable differences in the resuscitation of the two groups.
TABLE 4.
Demographics and resuscitation for all survivable injured patients versus those with anatomically non-survivable injuries to identify predictive factors for anatomically non-survivable injuries. There is a preponderance of blunt injury, depressed GCS, and elevated blood pressure in the non-survivable injured group.
| Survivable injured (n = 158) | Anatomically Non-survivable (n = 28) | P-value | |
|---|---|---|---|
| Age (years) | 36.5 ± 16.8 | 45.7 ± 23.0 | 0.052 |
| % male | 77.2% | 64.3% | 0.158 |
| % penetrating | 55.7% | 28.6% | 0.013 |
| AIS-head | 2.2 ± 2.0 | 2.9 ± 2.3 | 0.296 |
| ISS | 27.1 ± 15.4 | 35.9 ± 19.1 | 0.031 |
| GCS | 14 (6 – 15) | 3 (3 – 5.5) | <0.001 |
| Temperature (C) | 35.7 ± 1.1 | 35.3 ± 1.2 | 0.197 |
| HR (BPM) | 105.4 ± 33.9 | 99.4 ± 36.6 | 0.420 |
| SBP (mmHg) | 99.6 ± 36.6 | 124.2 ± 52.3 | 0.023 |
| pH | 7.20 ± 0.19 | 7.23 ± 0.13 | 0.268 |
| Base deficit | −10.3 ± 6.6 | −8.8 ± 6.0 | 0.236 |
| Hgb (g/dL) | 11.7 ± 2.4 | 11.3 ± 2.0 | 0.369 |
| INR | 1.3 (1.2 – 1.6) | 1.3 (1.2 – 1.6) | 0.895 |
| PT | 15.8 (14.5 – 18.7) | 15.9 (14.9 – 19.7) | 0.255 |
| PTT | 30.2 (26 – 38.1) | 38.7 (31.5 – 55.0) | 0.001 |
| Platelets×103 | 252 ± 101 | 243 ± 111 | 0.696 |
| WBC103/mm3 | 9.8 (7.5 – 13.4) | 9.7 (6.4 – 13.6) | 0.938 |
| Creatinine(mg/dL) | 1.1 (0.9 – 1.4) | 1.0 (0.9 – 1.3) | 0.416 |
| Prehospital IVF (mL) | 250 (50 – 500) | 150 (25 – 1400) | 0.910 |
| 24h crystalloid (mL) | 5.9 (3.1 – 9.8) | 8.3 (4.1 – 11.4) | 0.215 |
| 24h colloid(mL) | 0 (0 – 0) | 0 (0 – 0) | 0.187 |
| 24h PRBC(units) | 13 (7 – 21) | 13 (8.5 – 19) | 0.976 |
| 24h FFP(units) | 8 (4 – 17) | 10 (4.5 – 14) | 0.864 |
| 24h platelets (apheresis unit) | 1 (0 – 2) | 1 (0 – 2) | 0.396 |
| % MT | 64.6% | 67.9% | 0.832 |
| 24h RBC:FFP ratio | 1.71 ± 1.24 | 1.56 ± 0.73 | 0.381 |
| Minutes to MTP | 37 (19 – 77) | 67 (35 – 93) | 0.103 |
Finally, we compared only the patients that died, divided into those who had a potentially survivable injury but still succumbed to their injuries, versus those who were deemed to have an anatomically non-survivable injury (Table 5). This continued to show traumatic brain injury as the driving force in patients with non-survivable injury. The non-survivable injured patients had statistically significantly lower GCS, higher SBP, fewer penetrating wounds, less acid-base derangement, and less coagulopathy. The non-survivable injured did use more crystalloid, fewer pRBCs, and had a lower RBC:FFP ratio than those with potentially survivable injuries. There was also a significantly longer time to activation of the MTP in non-survivable injured patients of 67 minutes vs 19.5 minutes (p<0.001).
DISCUSSION
The cause of death confounds any discussion regarding the optimal resuscitation of severely injured patients. Whether these deaths result from the failure of surgical care and resuscitation or are the sequelae of overwhelming injury which is refractory to any resuscitative conduct remains an open question. To address this requires investigation into the causes of death and a classification of anatomically non-survivable versus potentially survivable injury. Hence, the aim of this paper was to codify the cause of death of severely injured trauma patients to learn more about the reasons for death and to better characterize which of our patients have a chance of survival and would potentially benefit from aggressive and progressive resuscitation.
Often patients are said to bleed to death; however, this description can be highly variable. Some patients bleed to death from giant holes in major vessels. In these cases, resuscitation and the biology of coagulation are irrelevant; overwhelmed by the severity of injury, these patients are in need of rapid transport to an operating theater for definitive surgical control of their injuries. Other patients are described as having succumbed to their injuries despite surgical control, with some dying from physiologic degradation in the operating room, while others slowly decompensate in the ICU. While these codifications seem intuitive, they have yet to be specifically described in the literature.
Since the landmark trauma epidemiology paper by Baker et al in 1980 (12), there have been multiple studies (10, 13–18) evaluating the epidemiology of trauma. These publications are roughly divided into examinations of mechanism of injury and physiologic cause of death. Although the vast majority of these epidemiology papers deal with the mechanism of injury for the utilization of regional trauma management and injury prevention programs, there are few which have focused on the reasons for death. To date, however, no studies have examined the potential survivability after injury and effects of resuscitation.
Previous studies that have evaluated the cause of death demonstrate that CNS injury and hemorrhage remain the two most significant causes of death, with each contributing 30–40% (10, 16, 19, 20) of the mortality in trauma patients. In our study, we saw a similar injury percentage rates to these previous reports, with exsanguination causing death in 33.3% of the patients and 38.8% having injuries that were deemed to be non-survivable (96.4% had brain injury).
Analysis of the cause of death in the recent military conflicts provides insight into potentially survivable versus non-survivable injuries (21–23). Similar to our study, these studies show that traumatic brain injury is a leading cause of non-survivable injury (22) while hemorrhage, and more specifically, truncal hemorrhage, remains as the leading cause of potentially survivable death in the military population (21–23). While these studies highlight the improvements in survival made by Tactical Combat Casualty Care, our study has increased the granularity of these military analyses by separating the potentially survivable cause of death into hemorrhage, early physiologic collapse, and late physiologic collapse.
The use of hemostatic resuscitation has been shown to improve survival in severely injured patients. However, the ability to identify early which patients need hemostatic resuscitation and the mathematical phenomenon of “decedent dropout” can hinder these studies. Early activation of an MTP has been demonstrated to improve survival (1–6) but can result in unnecessary MTP activation. We have attempted to address this by including the percent MT (%MT) to provide more accurate description of how many patients in each category achieved a “full MT” as defined in the methods section. Decedent dropout is the result of early death in trauma patients which results in an elevated RBC:FFP ratio. This elevated ratio is not necessarily reflective of the efficacy of the resuscitation, but instead, can indicate death prior to FFP being available for transfusion. Ho et al (24) addresses decedent dropout by utilizing a mathematical model to show the significant risk of survivorship bias in some observational studies comparing low and high FFP administration. Our comparison of cause of death identified the exsanguination group as having a statistically significant higher RBC:FFP ratio. We believe that this is not a failure of transfusion ratios, but more likely a lack of surgical control of hemorrhage from massive injury rendering resuscitative conduct moot. Those who died from early or late physiologic collapse had similar ratios to the survivors, raising interesting questions. Did the early physiologic collapse need ratios closer to 1:1? Were patients in the late physiologic collapse group over-resuscitated early, leading towards later collapse? The differences in transfusion ratios in our study calls for the codification of cause of death in clinical studies so that physiologic responses to resuscitation strategies can be accurately stratified.
The injured patient in whom surgical control has been obtained and a vigorous resuscitation has been utilized but who still succumbs to their injuries from early physiologic collapse proves to be a very frustrating cohort. Anecdotally these are the patients with significant injury who despite surgical control of the injury and an initial trajectory towards survival becomes refractory to resuscitation and enter a spiral of decompensation and die. These frustrating patients present with no predicting factors as compared to those who exsanguinate, despite having achieved surgical hemorrhage control. What effect our resuscitative efforts have on this cohort remains an open question. One hypothesis is that our better resuscitative conduct allows for correction of coagulopathy and restoration of appropriate physiology, providing more time for surgical bleeding control in a patient that would have previously exsanguinated. In a subset of these patients, large-volume blood product-based resuscitation may have merely prolonged survival past initial hemorrhage control in patients who were essentially non-survivable injured, shifting their cause of death to early physiologic collapse. Their significantly higher blood product usage, despite not reaching optimal ratios, hints at this. Another theory is that these patients have a survivable injury which, with better resuscitation, would not cross the threshold of physiologic collapse. In this case, efforts at better resuscitation would prevent exhaustion of physiologic reserve and ultimately lead to survival. This group utilizes the most blood products and still has a poor outcome. This intuitively survivable cohort in whom resuscitation practices may make the critical difference between survival and death should receive increased trauma resuscitation research.
The prolonged median time to death from late physiologic collapse indicates that we are improving in our ability to keep these patients alive longer than demonstrated in previous studies (10, 14, 16, 19, 20). The time to death analysis are comparable with older studies that (16, 20) reported 84.5% of their patients died within 48 hours and only 41 patients (6.5%) lived greater than 1 week. Our analysis showed 89% of patients died within 48 hours and 11% had a median time to death of two weeks. This is a clear indication that our critical care techniques are improving and that specific analysis of the cause of death in future studies will aid in identifying targeted methods to improve outcomes.
Our data shows that those patients who were deemed to have a non-survivable injury who still underwent a MTP did so with an average activation time of 67 minutes vs 19.5 minutes for those who were deemed to have a potentially survivable injury, but died anyway. It is interesting that there is such a long delay in these patients. This was not affected by withdrawal of care, as only three patients who had care withdrawn (data not shown). We hypothesize that this increase in lag time to activation of an MTP is secondary to the prominence of TBI in these patients which may delay MTP activation until there is progression of bleed, deterioration in clinical exam, etc. The earlier identification of these patients may be prudent to assist families in decision making and to allocate resources in a more efficient manner.
The choice of the cause of death categories is based on physiologic principles and the clinical observation that patients who die after undergoing MT fall into distinct general physiologic conditions. While we recognize that there are additional subsets of potential interest, such as the presence or absence of coagulopathy, our study is limited by the total number of patients in our dataset. The selection of these groups inherently affect some aspects of the study results (such as time to death), but the differences in physiologic parameters and resuscitation for each group identified here lends credence to these categories as clinically relevant.
As a result of our analysis, we believe that a staged approach to the goals of resuscitation should be considered in discussions of trauma epidemiology. Patients who die of uncontrolled hemorrhage are ultimately best served by injury prevention efforts and optimization of evacuation to definitive surgical treatment. Those who die from early physiologic collapse need better-targeted resuscitation. Those who die from late physiologic collapse need improvements in critical care; however, the early resuscitation of these patients may also play a critical role in their long-term trajectory.
CONCLUSION
This paper supports the need for a multi-institutional study and the start of a national databank that codifies the cause of death in trauma patients to specifically identify risk factors and better-targeted therapy for exsanguination, physiologic collapse, and non-survivable injuries. The inclusion of this data into Quality Improvement Programs could help identify areas of improvement in trauma resuscitation.
Footnotes
Contributions:
MWC, MEK, MJC contributed to the literature search, study design, data analysis and interpretation, writing and critical revision
AD, RCM, MDG, LMC, BJR, MFN contributed to the study design, data collection, and critical revision of the manuscript
Contributor Information
Michael W Cripps, Email: michael.cripps@utsouthwestern.edu.
Matthew E Kutcher, Email: matthew.kutcher@ucsfmedctr.org.
Aaron Daley, Email: daleya@sfghsurg.ucsf.edu.
Ryan C McCreery, Email: mccreeryr@sfghsurg.ucsf.edu.
Molly D Greenberg, Email: greenbergm@sfghsurg.ucsf.edu.
Leslie M Cachola, Email: cacholal@sfghsurg.ucsf.edu.
Brittney J Redick, Email: redickb@sfghsurg.ucsf.edu.
Mary F Nelson, Email: nelsonm@sfghsurg.ucsf.edu.
Mitchell Jay Cohen, Email: mcohen@sfghsurg.ucsf.edu.
References
- 1.Teixeira PG, Inaba K, Shulman I, Salim A, Demetriades D, Brown C, Browder T, Green D, Rhee P. Impact of plasma transfusion in massively transfused trauma patients. J Trauma. 2009;66(3):693–7. doi: 10.1097/TA.0b013e31817e5c77. Epub 2009/03/12. [DOI] [PubMed] [Google Scholar]
- 2.Holcomb JB, Wade CE, Michalek JE, Chisholm GB, Zarzabal LA, Schreiber MA, Gonzalez EA, Pomper GJ, Perkins JG, Spinella PC, et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann Surg. 2008;248(3):447–58. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 3.Borgman MA, Spinella PC, Perkins JG, Grathwohl KW, Repine T, Beekley AC, Sebesta J, Jenkins D, Wade CE, Holcomb JB. The ratio of blood products transfused affects mortality in patients receiving massive transfusions at a combat support hospital. J Trauma. 2007;63(4):805–13. doi: 10.1097/TA.0b013e3181271ba3. Epub 2007/12/20. [DOI] [PubMed] [Google Scholar]
- 4.Zehtabchi S, Nishijima DK. Impact of transfusion of fresh-frozen plasma and packed red blood cells in a 1:1 ratio on survival of emergency department patients with severe trauma. Academic emergency medicine: official journal of the Society for Academic Emergency Medicine. 2009;16(5):371–8. doi: 10.1111/j.1553-2712.2009.00386.x. Epub 2009/03/24. [DOI] [PubMed] [Google Scholar]
- 5.Spinella PC, Perkins JG, Grathwohl KW, Beekley AC, Niles SE, McLaughlin DF, Wade CE, Holcomb JB. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J Trauma. 2008;64(2 Suppl):S69–77. doi: 10.1097/TA.0b013e318160ba2f. discussion S-8. Epub 2008/04/11. [DOI] [PubMed] [Google Scholar]
- 6.Perkins JG, Cap AP, Spinella PC, Shorr AF, Beekley AC, Grathwohl KW, Rentas FJ, Wade CE, Holcomb JB. Comparison of platelet transfusion as fresh whole blood versus apheresis platelets for massively transfused combat trauma patients (CME) Transfusion. 2011;51(2):242–52. doi: 10.1111/j.1537-2995.2010.02818.x. Epub 2010/08/28. [DOI] [PubMed] [Google Scholar]
- 7.MacLeod JB, Lynn M, McKenney MG, Cohn SM, Murtha M. Early coagulopathy predicts mortality in trauma. J Trauma. 2003;55(1):39–44. doi: 10.1097/01.TA.0000075338.21177.EF. [DOI] [PubMed] [Google Scholar]
- 8.Maegele M, Lefering R, Yucel N, Tjardes T, Rixen D, Paffrath T, Simanski C, Neugebauer E, Bouillon B. Early coagulopathy in multiple injury: an analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. Epub 2007/01/12. [DOI] [PubMed] [Google Scholar]
- 9.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62(1):142–6. doi: 10.1097/01.ta.0000251558.38388.47. Epub 2007/01/12. [DOI] [PubMed] [Google Scholar]
- 10.Demetriades D, Murray J, Charalambides K, Alo K, Velmahos G, Rhee P, Chan L. Trauma fatalities: time and location of hospital deaths. Journal of the American College of Surgeons. 2004;198(1):20–6. doi: 10.1016/j.jamcollsurg.2003.09.003. Epub 2003/12/31. [DOI] [PubMed] [Google Scholar]
- 11.Brohi K, Cohen MJ, Ganter MT, Matthay MA, Mackersie RC, Pittet JF. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann Surg. 2007;245(5):812–8. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. American journal of surgery. 1980;140(1):144–50. doi: 10.1016/0002-9610(80)90431-6. Epub 1980/07/01. [DOI] [PubMed] [Google Scholar]
- 13.Potenza BM, Hoyt DB, Coimbra R, Fortlage D, Holbrook T, Hollingsworth-Fridlund P. The epidemiology of serious and fatal injury in San Diego County over an 11-year period. The Journal of trauma. 2004;56(1):68–75. doi: 10.1097/01.TA.0000101490.32972.9F. Epub 2004/01/30. [DOI] [PubMed] [Google Scholar]
- 14.Evans JA, van Wessem KJ, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World journal of surgery. 2010;34(1):158–63. doi: 10.1007/s00268-009-0266-1. Epub 2009/11/03. [DOI] [PubMed] [Google Scholar]
- 15.Cothren CC, Moore EE, Hedegaard HB, Meng K. Epidemiology of urban trauma deaths: a comprehensive reassessment 10 years later. World journal of surgery. 2007;31(7):1507–11. doi: 10.1007/s00268-007-9087-2. Epub 2007/05/17. [DOI] [PubMed] [Google Scholar]
- 16.Sauaia A, Moore FA, Moore EE, Moser KS, Brennan R, Read RA, Pons PT. Epidemiology of trauma deaths: a reassessment. The Journal of trauma. 1995;38(2):185–93. doi: 10.1097/00005373-199502000-00006. Epub 1995/02/01. [DOI] [PubMed] [Google Scholar]
- 17.Pang JM, Civil I, Ng A, Adams D, Koelmeyer T. Is the trimodal pattern of death after trauma a dated concept in the 21st century? Trauma deaths in Auckland 2004. Injury. 2008;39(1):102–6. doi: 10.1016/j.injury.2007.05.022. Epub 2007/09/21. [DOI] [PubMed] [Google Scholar]
- 18.Stewart RM, Myers JG, Dent DL, Ermis P, Gray GA, Villarreal R, Blow O, Woods B, McFarland M, Garavaglia J, et al. Seven hundred fifty-three consecutive deaths in a level I trauma center: the argument for injury prevention. The Journal of trauma. 2003;54(1):66–70. doi: 10.1097/00005373-200301000-00009. discussion -1. Epub 2003/01/25. [DOI] [PubMed] [Google Scholar]
- 19.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. The Journal of trauma. 2006;60(6 Suppl):S3–11. doi: 10.1097/01.ta.0000199961.02677.19. Epub 2006/06/10. [DOI] [PubMed] [Google Scholar]
- 20.Shackford SR, Mackersie RC, Holbrook TL, Davis JW, Hollingsworth-Fridlund P, Hoyt DB, Wolf PL. The epidemiology of traumatic death. A population-based analysis. Arch Surg. 1993;128(5):571–5. doi: 10.1001/archsurg.1993.01420170107016. Epub 1993/05/01. [DOI] [PubMed] [Google Scholar]
- 21.Kelly JF, Ritenour AE, McLaughlin DF, Bagg KA, Apodaca AN, Mallak CT, Pearse L, Lawnick MM, Champion HR, Wade CE, et al. Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003–2004 versus 2006. J Trauma. 2008;64(2 Suppl):S21–6. doi: 10.1097/TA.0b013e318160b9fb. discussion S6–7. Epub 2008/04/11. [DOI] [PubMed] [Google Scholar]
- 22.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, et al. Death on the battlefield (2001–2011): implications for the future of combat casualty care. The journal of trauma and acute care surgery. 2012;73(6 Suppl 5):S431–7. doi: 10.1097/TA.0b013e3182755dcc. Epub 2012/12/05. [DOI] [PubMed] [Google Scholar]
- 23.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetjen-Gerdes L, Champion HR, Lawnick M, Farr W, Rodriguez S, et al. Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001–2004. Ann Surg. 2007;245(6):986–91. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho AM, Dion PW, Yeung JH, Joynt GM, Lee A, Ng CS, Chang A, So FL, Cheung CW. Simulation of survivorship bias in observational studies on plasma to red blood cell ratios in massive transfusion for trauma. The British journal of surgery. 2012;99 (Suppl 1):132–9. doi: 10.1002/bjs.7732. Epub 2012/03/28. [DOI] [PubMed] [Google Scholar]