Summary
Dengue is among the most prevalent and important arbovirus diseases of humans. In order to effectively control this rapidly spreading disease, control of the vector mosquito and a safe and efficacious vaccine are critical. Despite considerable efforts, the development of a successful vaccine has remained elusive. Multiple factors have complicated the creation of a successful vaccine, not the least of which are the complex, immune-mediated responses against four antigenically distinct serotypes necessitating a tetravalent vaccine providing long lasting protective immunity. Despite the multiple impediments, there are currently many promising vaccine candidates in pre-clinical and clinical development. Here we review the recent advances in dengue virus vaccine development and briefly discuss the challenges associated with the use of these vaccines as a public health tool.
Keywords: Dengue virus, vaccine, dengue fever, dengue hemorrhagic fever, antibody-dependent enhancement, immunopathogenesis
Background
Dengue virus (DENV) causes a common self-limited illness, dengue fever (DF), and a less common syndrome manifested variably by organ failure, hemorrhage, capillary leakage, shock and death (severe dengue, DHF/DSS). DENV is a globally important human pathogen. Roughly two-fifths of the world’s population lives in areas that are at risk for DENV transmission [1–3]. According to the World Health Organization (WHO), “In 2012, dengue ranks as the most important mosquito-borne viral disease with an epidemic potential in the world. There has been a 30-fold increase in the global incidence of dengue during the past 50 years, and its human and economic costs are staggering.” [4]. A large proportion of those affected by DENV infection are children, and it is a leading cause of serious illness and death in some Asian and Latin American countries [5]. An estimated 294 million asymptomatic infections and 96 million symptomatic DENV infections of any severity occurred in 2010 [6]. An enormous economic burden is associated with DENV infections [1,7,8]. While asymptomatic infections do not result in a direct burden on the health care system, these infected individuals contribute to DENV transmission.
Despite concerted efforts for the past four decades, there is no currently licensed vaccine available to protect against DENV infection. This review will focus on recent advances in the development of vaccines against DENV and the impediments facing these vaccines.
Dengue virus
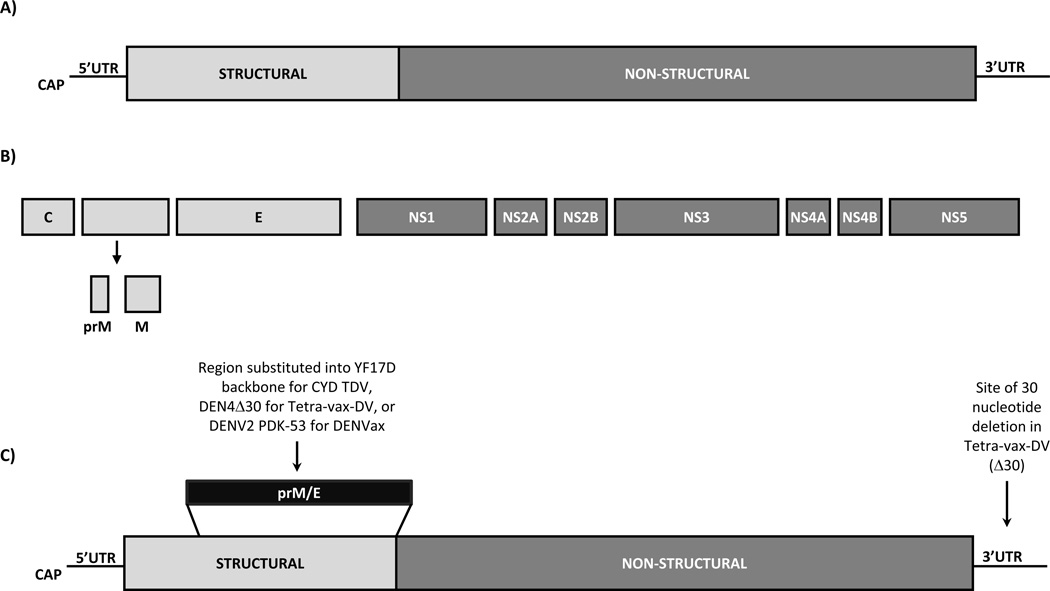
The dengue viruses are a group of mosquito-borne flaviviruses composed of four antigenically distinct serotypes (DENV1–4) that co-circulate throughout Southeast Asia, Africa, and the Americas [9,10]. The Flavivirus genus includes many human pathogens of clinical significance, including mosquito-borne viruses such as yellow fever virus (YFV), Japanese encephalitis virus (JEV), and West Nile virus (WNV), as well as tick-borne viruses such as tick-borne encephalitis virus (TBEV). The flaviviruses are single-stranded, positive-sense RNA viruses with a genome of ~11 kilobases. The genome consists of a single open reading frame flanked by 5’ and 3’ untranslated regions (UTR). The open reading frame encodes a polyprotein which is processed by both virus-encoded and host proteases resulting in three structural proteins (capsid (C), membrane (M), and envelope (E)) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (Figure 1 A & B) [11].
Figure 1.
Organization of the flavivirus genome. A) RNA genome including the 5’ and 3’ untranslated regions (UTR) and coding regions for the structural and non-structural proteins. B) Polyprotein processed by both virus-encoded and host proteases resulting in three structural proteins (capsid (C), membrane (M), and envelope (E)) and seven non-structural (NS) proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). C) Diagram indicating the alterations to the DENV genome for generation of several live attenuated virus vaccine candidates. See text for a brief description of the vaccines mentioned in this panel.
The E protein is the major surface protein of the flaviviruses and has been extensively characterized. The crystal structures for the E protein of TBEV, WNV, and DENV have been determined [12–18]. The E protein is composed of three distinct domains (DI, DII, and DIII). DIII is exposed on the virion surface and has been implicated in binding to the host cell surface receptor [19]. Furthermore, DIII is known to contain multiple type-specific neutralizing epitopes [20]. Because of its importance as an immunogen, the E protein is a major component of DENV vaccines.
Clinical Disease
Infection with any of the four serotypes of DENV may range from asymptomatic infection, classical DF or more severe clinical manifestations, including dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS) [21]. Previous WHO classifications included DF and DHF/DSS, the latter being the sole representative of severe dengue [22]. DF is characterized as a febrile illness with two or more of the following: myalgia, arthralgia, headache, retro-orbital pain, rash, leukopenia, and/or hemorrhagic manifestations, plus supportive serology or occurrence at the same location and time as other confirmed cases of DF. The case definition of DHF requires the presence of fever or history of acute fever, hemorrhagic tendencies, thrombocytopenia (platelet count 100,000 cells per mm3 or less), and evidence of plasma leakage due to increased vascular permeability, and DSS is characterized by rapid, weak pulse with narrowing of the pulse pressure or hypotension [22]. Recently, the WHO developed a new classification system that includes DF with or without warning signs for the development of severe dengue and severe dengue itself [21]. “Dengue without warning signs” is characterized by fever plus any two of the following: nausea/vomiting, rash, aches/pains, leukopenia, and/or a positive tourniquet test. Based on the WHO assessment/treatment algorithm, patients meeting the criteria for “dengue without warning signs” may safely be managed at home. “Dengue with warning signs” includes the above definition plus one or more of the following: abdominal pain/tenderness, persistent vomiting, clinical fluid accumulation, mucosal bleeding, lethargy/restlessness, liver enlargement >2 cm, and/or laboratory testing showing increased hematocrit (>20% above patient’s baseline value or age-specific population value if the patient’s baseline is not available) with concurrent rapid decrease in platelet count. Patients meeting the “dengue with warning signs” criteria should be referred for in-hospital care. The final category, severe dengue, requires emergency treatment and includes patients with any of the following: severe plasma leakage leading to shock or fluid accumulation with respiratory distress, severe bleeding as evaluated by the clinician, or severe organ involvement [21].
Recent evaluation of the traditional and revised WHO dengue classification schemes identified increased sensitivity of the revised classification system for detecting severe disease which may be useful for clinicians in determining treatment [23]. However, because the classifications of “severe dengue” and “dengue with warning signs” are quite broad and are less precise, they may fail to adequately categorize the pathophysiology of immunopathogenesis in vaccine trials. For this reason, comparison of studies that used traditional versus revised classification systems must be evaluated with caution.
Impediments to Vaccine Development
Multiple difficulties have hindered the development of a successful DENV vaccine. These include: (1) the epidemiology of the four DENV serotypes, (2) the complex and incompletely understood immunoprotective and/or immunopathogenic responses following natural infection or vaccination, and (3) a lack of validated animal models of disease. These impediments are discussed below.
Epidemiology
DENV is arguably the most significant human arboviral disease with an excess of 2.5 billion people at risk world-wide [5,9]. Although accurately determining the number of cases is complicated by underreporting and lack of surveillance in some regions, an estimated 50–100 million DENV infections occur annually resulting in 2.3 million cases of DF reported to the WHO in 2010 [5]. Furthermore, DENV is responsible for approximately 500,000 cases of severe disease requiring hospitalization each year [9]. DENV is endemic in most tropical and subtropical regions of the world with the highest burden of disease in Asia and the Americas; however, DENV transmission has also been reported in Africa and the Eastern Mediterranean region [24,25]. Additionally, due to changes in climate, travel, and urbanization, DENV continues to spread to new areas and intensify in endemic areas.
A major factor in the re-emergence of DENV is the re-infestation of many parts of the world with Aedes aegypti mosquitos. DENV is transmitted primarily by the urban mosquito Ae. aegypti, and, less efficiently, by Ae. albopictus mosquitos. The geographic range of both mosquito vectors continues to expand as a consequence of environmental factors and decreased mosquito control [9,26]. Additionally, endemicity of DENV has increased as a result of rapid urbanization in regions of Asia and Latin America that provide both increased population density and an abundance of vector breeding sites [9,26].
Although there was global distribution of DENV throughout the tropics prior to World War II, most regions had only one or two serotypes co-circulating and only sporadic epidemics were reported [27]. Following the transport of troops and war materials associated with World War II, DENV spread dramatically and many countries in Asia became hyperendemic (co-circulation of all four serotypes) [27]. Severe DENV infection subsequently became a leading cause of hospitalization and death among children in Southeast Asia and the majority of deaths from severe dengue continue to occur there [25,27]. Although DENV infection has typically been considered a disease of childhood in most Asian countries, there are increasing reports of infection in adolescents and adults in Asia and the Americas [28,29].
Mosquito eradication programs in the Americas initially led to a dramatic decrease in mosquito-borne diseases. However, following the completion of these programs in the 1970s, there was re-infestation of the Americas with Ae. aegypti resulting in rapid re-expansion of all four DENV serotypes on this continent [30,31]. As DENV continues to spread through Latin America, changes in the epidemiological profile (increased severe disease in children and young adults) have been noted in some regions [29,31]. Expansion of DENV into new geographic regions, re-introduction of DENV serotypes following significant time lapses, and travel to endemic areas have all led to increased infections in adults [5,9].
Although all four DENV serotypes have been reported in Africa, there is no reliable prevalence or incidence data, likely due to poor surveillance/detection systems and the heavy burden of other infectious diseases like malaria [24]. Additionally, there is evidence that African ancestry is protective against DHF/DSS and DENV may therefore be circulating in Africa without causing epidemics or even sporadic cases of DHF/DSS [32,33].
DENV exhibits a complex epidemiology with co-circulation of multiple serotypes in a given geographic location and an unpredictable predominance of different serotypes at different time points. Although mathematical and epidemiological models have been developed in an attempt to predict DENV epidemics, there is no single model that takes into account the many factors that can affect DENV transmission. These include vector specific factors (including vector density, biting rates, and vector competence), as well as host factors (including population density, immune status, and travel), both of which are affected by climactic, environmental, and socioeconomic variables [34,35].
The unpredictable nature of DENV transmission and epidemics makes the design of vaccine trials difficult. Because no reliable way of accurately predicting the circulation of a specific serotype currently exists, determining protective efficacy for all serotypes requires multiple trial sites over long time periods with large numbers of volunteers. An additional concern is how genetic variation within a given serotype may impact vaccine trials. Each DENV serotype can be sub-divided into multiple genotypes. For example, the ability of sera from patients infected with DENV3 to neutralize DENV3 viruses of distinct genotypes is not equivalent in vitro [36]. However, this has not been confirmed in animal models, and recent studies in non-human primates indicate that antibodies generated in response to a tetravalent live-attenuated DENV vaccine are able to neutralize a broad range of DENV isolates from multiple genotypes [36]. The effect of such genetic differences on vaccine efficacy in humans remains to be determined. To summarize, co-circulation of multiple DENV serotypes in the same geographic location for many years generates the possibility of complex immune-mediated cross-protection as well as immune-mediated enhancement that must be considered in clinical trial design [9].
Immunology
Despite extensive studies, our understanding of the immune responses to DENV infection is incomplete. Following primary infection, there is initially serotype cross-reactive protective immunity; however, this wanes after a few months leaving the host susceptible to infection with heterologous serotypes [37]. Human challenge studies performed in a small number of individuals by Sabin in 1944 and published in 1952 are, to our knowledge, the only experimental evidence of the presence and duration of serotype cross-protective immunity in humans [37]. Studies of the natural history of dengue in endemic areas support these findings as primary infections are typically followed by several months of broad protection [20]. Although secondary DENV infection is a significant risk factor for severe disease, only a small percentage of individuals experiencing secondary DENV infection are severely ill, suggesting cross-protection in some persons may persist for a considerable period of time [38].
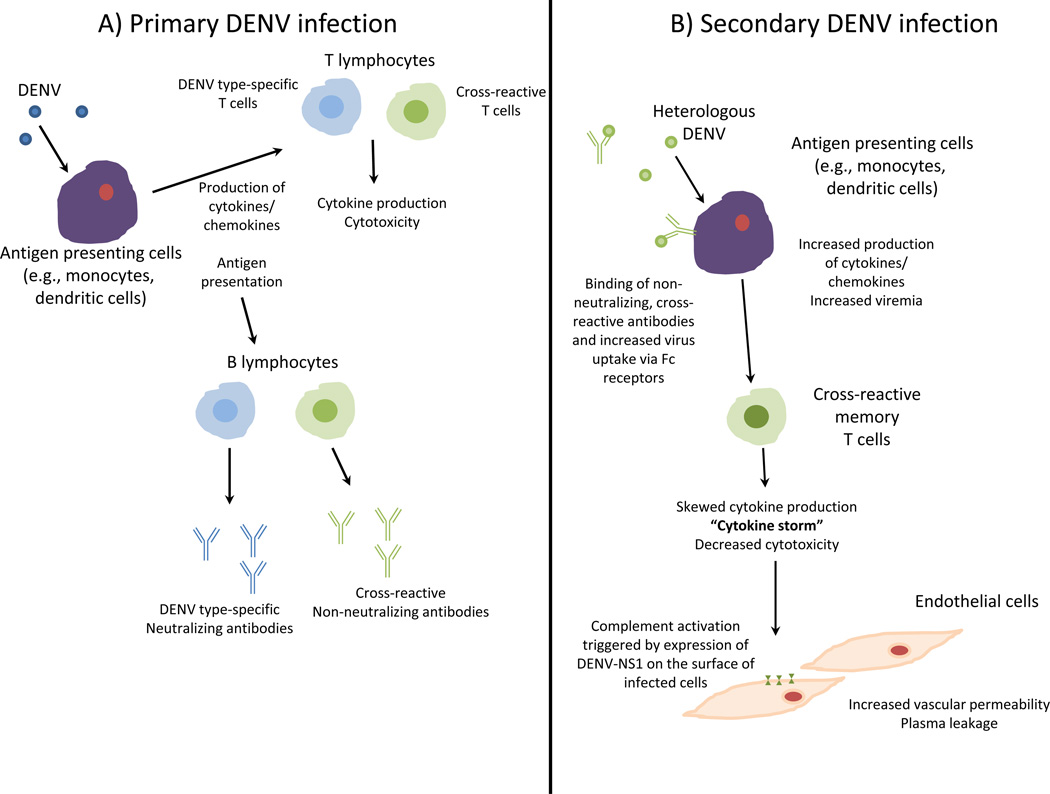
Epidemiologically, heterologous secondary infection in children and adults is associated with higher risk of severe disease (DHF/DSS) [39]. Additionally, infants with waning maternal antibody are more susceptible to severe disease during primary infection, which may be due to the presence of heterologous maternal antibodies mimicking secondary infection [40]. It has been hypothesized that immune responses to heterologous infection play a direct role in the pathogenesis of DHF/DSS (summarized in Figure 2).
Figure 2.
Potential mechanisms for immune mediated enhancement of DENV infection. A) Immune responses following primary DENV infection include infection of monocytes and other antigen presenting cells which produce cytokines/chemokines and present antigen to lymphocytes. Type-specific and cross-reactive B and T lymphocytes are activated. B lymphocytes produce serotype-specific, neutralizing antibodies as well as cross-reactive, weakly or non-neutralizing antibodies. B) Following secondary heterologous infection, uptake of DENV is facilitated through binding of cross-reactive, non-neutralizing antibodies from a previous infection (or maternally derived antibodies in the case of primary infection in infants). Additionally, there is activation of cross-reactive T cells that produce skewed cytokine responses and demonstrate decreased cytotoxicity for DENV infected cells. Massive quantities of pro-inflammatory cytokines (“cytokine storm”) result in increased vascular permeability and plasma leakage. Furthermore, expression or release of DENV-NS1 by infected cells may mediate complement activation resulting in increased vascular permeability.
Viral factors such as peak viremia and serotype are also associated with severe disease [41]. Thus, a combination of host immunological mechanisms and viral factors likely contribute to the plasma leakage associated with severe disease. The precise interplay between host and viral factors remains to be elucidated.
The potential threat of disease enhancement due to incomplete DENV immunity necessitates the development of a tetravalent DENV vaccine (TDV) that induces long-lasting protective immunity against all four serotypes simultaneously. Furthermore, unpredictable circulation of the four serotypes also necessitates a TDV. This protective response should ideally be achieved over a short time period to prevent the theoretical possibility of disease potentiation after an infective mosquito bite during the series of primary vaccinations.
Multiple non-mutually exclusive mechanisms for enhanced disease severity due to heterologous immune responses have been proposed including antibody dependent enhancement (ADE), cell-mediated immunity (CMI), e.g., the generation of cross-reactive T cells, as well as complement activation [40,42–48]. Despite extensive efforts, there is no conclusive in vivo data implicating the causative role of these responses in the severe manifestations (plasma leakage) of DENV infection.
Antibody Dependent Enhancement (ADE)
One proposed mechanism for the increased disease severity after a second heterotypic DENV infection is that of ADE. In addition to serotype-specific, protective antibody responses, DENV infection also induces non-neutralizing or weakly neutralizing, cross-reactive antibodies. Binding of these antibodies to a heterotypic DENV facilitates viral entrance into Fc-receptor bearing cells such as monocytes and macrophages [20,40,44]. Evidence to support a role for ADE in severe DENV infection includes observations that 1) secondary DENV infection leads to higher serum viremia and greater risk of severe disease [41], 2) the risk of severe disease is increased following primary infection in infants with waning maternal antibody titers [40], and 3) temporal associations between in vitro DENV enhancing activity of plasma and the epidemiology of age-related severe disease exist [49,50]. More recently, it has been suggested that in addition to increasing infection by augmenting the number of susceptible cells, Fc receptor signaling inhibits antiviral responses within the infected cells, thus contributing to increased viral replication associated with ADE [48,51]. It is important to note, however, that in a study of secondary DENV2 and 3 infection, no correlation between pre-illness enhancing activity and disease severity or viremia was identified [52]. Additionally, a recent study found no association between ADE activity and disease severity during primary DENV3 infection in infants [53]. Caution must be used in extrapolating in vitro mechanisms of immunopathogenesis to in vivo scenarios.
Cell-mediated immunity (CMI)
Another proposed mechanism of immune-mediated pathogenesis is that of a “cytokine storm” during which high levels of pro-inflammatory cytokines are released in response to heterologous DENV infection contributing to vascular leakage and severe dengue disease [42,43,54]. Increased activation and production of cytokines/chemokines such as interferon (IFN)-γ and tumor necrosis factor (TNF)-α by DENV-specific T cells have been detected [43]. Additionally, higher levels of inflammatory cytokines have been identified in sera of patients with DHF compared to DF [42,43,49,54–57]. A leading hypothesis for the “cytokine storm” is that cross-reactive memory T cells drive pathogenic responses to heterologous secondary DENV infection [54]. Prospective studies have identified T cell response patterns that may be associated with differential risk for severe disease [43,57]. Furthermore, an association between the magnitude of CD8+ T cell response during acute infection and disease severity has been identified [58]. It has also been suggested that secondary responses (to heterologous virus) may be dominated by cross-reacting memory T cells induced by primary infection that may be of lower affinity for the heterologous antigen, thus resulting in skewed cytokine responses and/or inefficient cytotoxicity [58]. Although skewed T cell responses may contribute to immunopathogenesis, the anti-viral effects of cytokines are also critical for protection. In a human challenge model of DENV infection, IFN-γ production was associated with protection from illness indicating that the quality of the T cell response may play a role in protection versus pathogenesis [56].
Complement activation
Complement activation is also thought to potentially play a role in the pathogenesis of severe DENV infection. Patients with DSS were observed to have accelerated consumption of complement, and high plasma levels of terminal complement, C5b-9, was associated with increased disease severity [46]. Anaphylotoxins, such as C5b-9, have been shown to promote plasma leakage and could thus play an important role in the pathophysiology of severe dengue [59].
Correlates of protection
There are currently no established correlates of protection against DENV infection. As already discussed above, the E protein is a major immunogen and the site of many serotype-specific neutralizing antibody epitopes. There have been extensive studies characterizing mouse monoclonal antibody epitopes of DENV, particularly to the E protein and more specifically to DIII (reviewed in [20]). The applicability of these epitopes in human immune responses remains unclear. More recent studies of human antibodies induced by natural DENV infection or by human monoclonal antibodies generated by Epstein Barr virus (EBV)-transformed B cells from DENV immune volunteers, indicate that neutralizing human antibodies recognizing epitopes on E DIII represent only a minority of the antibody spectrum. Some neutralizing human monoclonal antibodies bind to the E protein on the surface of the virion but not to recombinant E protein. These findings implicate a role for structural epitopes that may rely on the E protein configuration on the virion surface in serotype-specific neutralization [20,60,61]. In addition to serotype-specific antibodies, cross-reactive non-neutralizing or weakly neutralizing antibodies are also a significant part of the response against DENV infection. Much work remains to be done before the most relevant epitopes which correlate with protection from exposure to wild-type DENV are identified. These findings will have a great impact in accelerating vaccine development,
In general, neutralizing antibody titers are believed to be associated with protection; however, defined levels of neutralizing antibodies (e.g., 50% plaque reduction, PRNT50) have not been correlated with protection for any serotype [62]. Guidelines to standardize PRNT assays for DENV vaccine trials have been developed by the WHO [63]. A considerable drawback to traditional PRNT is the labor and time intensive nature of this assay. Microneutralization tests may provide a high throughput alternative, but remain to be validated and standardized for use in clinical DENV vaccine trials [64]. Additionally, recent studies have indicated differences in neutralization when Fcγ -receptor-transformed cells are used to perform PRNT. The use of Fcγ -receptor-transformed cells may better reflect the in vivo situation in which enhancement of infectivity and neutralization presumably occurs via antibody-virus interactions with Fcγ-receptor-bearing cells [64,65].
The relationship between neutralizing antibodies and protection is not straightforward. In a recent human challenge study, 10 volunteers immunized with a live-attenuated TDV and 4 DENV naïve volunteers were subsequently challenged with either DENV1 or 3. Five of 5 vaccinated volunteers were protected against DENV1 challenge despite the fact that one of the protected volunteers had no detectable neutralizing antibody against DENV1. Three of 5 volunteers challenged with DENV3 developed clinical disease and 2 of the unprotected volunteers had low but detectable anti-DENV3 neutralizing antibodies (reciprocal PRNT50 titers 19 and 16). All four DENV naïve volunteers developed clinical dengue [66]. Furthermore, protective titers may vary among serotypes as evidenced by recent results from a phase 2b trial in which protection against DENV2 was not obtained despite neutralizing antibody titers that were higher than those against other serotypes [67].
Identification of a reliable correlate of protection, either mechanistic or non-mechanistic [68], would allow measurement of a vaccine candidate’s probable efficacy without the need to establish protective efficacy against naturally acquired infection with each of the four serotypes. Thus the sample size required for clinical trials could be reduced and/or the requirement for formal protective efficacy trials might even be eliminated. Furthermore, a well-defined correlate of protection would foster non-inferiority trials once a DENV vaccine is licensed. This would dramatically reduce trial costs and effort needed to develop additional DENV vaccines.
Animal models
Because DENV is primarily a human pathogen, identification of an animal system that accurately models the human immune response to DENV and disease pathogenesis has been elusive. Historical studies investigating intra-abdominal and/or intracerebral inoculation of infant mice, hamsters, newborn and adult guinea pigs, cotton rats, rabbits, and rhesus monkeys with serum or whole blood with proven infectivity for humans did not identify clinical signs of infection [37]. Currently available animal models possess significant limitations which necessitate careful selection of the appropriate model for specific studies and cautious interpretation of results. Models that have been used for DENV vaccine development in recent years include mice, non-human primates (NHP), rabbits, and miniature swine [69–71], as described below.
Mouse models
Immunocompetent mice do not generally develop clinical signs of DENV infection reflective of human disease (fever, rash, and thrombocytopenia). Following inoculation with DENV, most immunocompetent mice demonstrate low levels of viral replication and may present with neurotropic manifestations such as paralysis following intraperitoneal or intravenous inoculation, which is not typical of human disease [70,72,73]. While not typical, it is important to note that neurologic manifestations including coma, convulsions, and spastic paraparesis have been associated with human DENV infections [74]. There are also limited reports of histopathological liver injury and/or clinical signs of hemorrhage more consistent with human disease following inoculation of immunocompetent mice with DENV [75,76]. Additionally, adaptation of DENV strains to the mouse model by serial passage has been reported to result in clinical and histopathologic manifestations of liver injury, hemorrhage, and death [72,77]. Finally, despite the lack of clinical manifestations of disease or viremia, BALB/c mice have been shown to develop T cell responses against DENV [78]. In preclinical vaccine development, immunocompetent mice have been used to demonstrate the generation of neutralizing antibodies and in some cases protection against intracerebral challenge [79].
Due to the limitations of immunocompetent mice as a model for clinical manifestations of DENV infection, multiple immunodeficient mouse models have been developed. Severe combined immunodeficiency (SCID) mice, lacking humoral and cellular immune responses, engrafted with human tumor cells have been shown to support DENV replication [70,80–82]. Infection in these models is, however, predominantly of the engrafted tumor limiting their utility for studies of virus tropism and host immunity to DENV infection.
IFN receptor deficient mice (AG129) have also been utilized as models for DENV infection. In this model, all four DENV serotypes are capable of replication; however, in order to recapitulate clinical signs of severe DENV2 infection, virus adaptation is required [71,83,84]. This model has been extensively studied and reviewed in [84]. In AG129 mice, DENV infects the same target cells as in humans and can induce both T cell and antibody responses [71,84]; however, the lack of IFN receptors limit the spectrum of immune responses that can be studied using this model. AG129 mice are used extensively in vaccine studies to document attenuation of vaccine candidates and generation of neutralizing antibodies/protection from challenge.
Multiple strains of humanized mice have been utilized for studies of DENV pathogenesis; however, to our knowledge, there are no reports of DENV vaccine studies in humanized mice [71,85,86].
NHP models
Although NHP can be naturally infected by sylvatic strains of DENV in the wild, NHP do not develop overt clinical disease following DENV infection [71]. Viremia can be detected, albeit at lower levels than in humans, and protection from infection is shown by reduction or absence of viremia following subsequent DENV infection [70]. NHP also develop neutralizing antibody responses following DENV infection [71]. Partial protection in NHP results in reduced or undetectable viremia but the presence of an anamnestic antibody response, reflecting viral replication. Complete protection in NHP is demonstrated by both the absence of viremia and lack of an anamnestic antibody response [87,88]. Increased viremia following administration of non-neutralizing antibodies has been documented in NHP providing a partial model for ADE [89]. However, the lack of severe disease manifestations in NHP limits the utility of this model for vaccine studies and the study of effector immune responses. In spite of these limitations, prevention of viremia following DENV challenge in the NHP model has been used to estimate vaccine efficacy [70,71,90–97].
Other models
Although mice and NHP are currently the primary animal model systems being utilized for vaccine studies, other animal systems including rabbits and miniature swine are also under investigation [69,71].
Vaccine Candidates in Pre-clinical and Clinical Development
Initial efforts to develop a DENV vaccine began in the 1920s and involved attenuating DENV in blood with ox-bile or grinding DENV infected Ae. aegypti mosquitoes in a salt solution and chemically pure phenol and formalin [98,99]. In 1952, Sabin and Schlesinger developed an attenuated strain of DENV1 by serial passage in mouse brain. This vaccine was protective in 16 volunteers subjected to the bite of infected mosquitoes [37]. The ability to cultivate DENV in tissue culture (1970s) and more recently, recombinant DNA technology have contributed to considerable advances in DENV vaccine development.
Below we discuss many of the DENV vaccine candidates in advanced pre-clinical and clinical trials. It is important to note that for a multitude of reasons there are limitations in the comparison of vaccine candidates from one trial to another. Some of these limitations include: differences in trial design (e.g. volunteer numbers, prior flavivirus experience, volunteer age, inclusion of a placebo or active control group, length of follow-up, geographic location and intensity of DENV transmission during the trial), use of different immune assays, and non-standardized reporting of adverse events and serological results. Furthermore, for vaccines in phase 1 and 2 trials, only comparisons of the degree of reactogenicity and immunogenicity can be performed. Currently, the recently completed Phase 2b trial of CYD TDV is the only clinical trial reporting vaccine efficacy data.
Live attenuated vaccines (LAV)
Safe and effective LAV vaccines for flaviviruses, including YFV and JEV, are licensed [12,100,101] indicating that LAV may also be effective for DENV. LAV candidates are the furthest along in the development pipeline to include multiple phase 1–2 trials of monovalent and tetravalent formulations and most recently, completion of a phase 2b trial of a tetravalent DENV vaccine candidate (Table 1) [67,91,95,102–108]. These vaccine candidates utilize distinct methods of attenuation including directed mutagenesis (Figure 1C) and serial passage.
Table 1.
Dengue virus vaccine candidates in clinical trials. Clinical trials to evaluate DENV vaccine candidates registered on ClinicalTrials.gov are shown. We have not listed earlier studies that do not have a ClinicalTrials.gov identifier. Primary completion dates for the studies shown range from 2005-present. The primary completion/estimated primary completion date refers to the final data collection date for the primary outcome measure and is listed as provided by ClinicalTrials.gov. The enrollment is also that provided by ClinicalTrials.gov.
| Vaccine type | Phase | Valent | Title | NCT Number | Vaccine name | Sponsor/Collaborators | Age Groups | Enrollment | Primary Completion/Estimated primary completion |
References |
|---|---|---|---|---|---|---|---|---|---|---|
| Live Attenuated Virus | Phase 1 | monovalent DENV1 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN1delta30) in Healthy Adults | NCT00089908 | rDEN1delta30 | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 28 | November 2005 | Durbin 2006 [92] |
| monovalent DENV2 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN2/4delta30[ME]) in Healthy Adults | NCT00094705 | rDEN2/4delta30(ME) | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 28 | April 2006 | |||
| monovalent DENV1 or DENV2 | Safety of and Immune Response to Two Different Dengue Virus Vaccines in Individuals Previously Immunized Against Dengue Virus | NCT00458120 | rDEN1delta30 or rDEN2/4delta30(ME) | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 36 | August 2008 | |||
| monovalent DENV3 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN3/4delta30[ME]) in Healthy Adults | NCT00375726 | rDEN3/4delta30(ME) | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 58 | Septmeber 2008 | |||
| monovalent DENV4 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN4delta30–4995) in Healthy Adults | NCT00322946 | rDEN4delta304995 | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 84 | August 2009 | |||
| monovalent DENV3 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN3- 3'Ddelta30) in Healthy Adults | NCT00712803 | rDEN3–3'Ddelta30 | NIAID | Adult | 29 | September 2009 | |||
| monovalent DENV4 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN4delta30–200,201) in Healthy Adults | NCT00270699 | rDEN4delta30–200,201 | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 56 | December 2009 | |||
| monovalent DENV2 | Safety and Immune Response to Two Doses of rDEN2/4delta30 Dengue Vaccine | NCT00920517 | rDEN2/4delta30(ME) | NIAID | Adult | 25 | January 2010 | |||
| monovalent DENV1 | Safety of and Immune Response of a 2-dose Regimen of rDEN1delta30 Dengue Virus Vaccine | NCT00473135 | rDEN1delta30 | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 60 | January 2010 | Durbin 2011 [103] | ||
| monovalent DENV4 | Safety of and Immune Response to DEN4 Vaccine Component Candidate for Dengue Virus | NCT00919178 | rDEN4delta30 | NIAID | Adult | 70 | May 2010 | |||
| monovalent DENV2 | Safety and Immune Response to an Investigational Dengue Type 2 Vaccine | NCT01073306 | rDEN2/4delta30 | NIAID | Adult | 18 | June 2010 | |||
| monovalent DENV1 | Evaluation of the Safety and Immune Response to an Investigational Dengue Type 1 Vaccine | NCT01084291 | rDEN1d30 | NIAID | Adult | 18 | June 2010 | |||
| monovalent DENV3 | Safety of and Immune Response to a Dengue Virus Vaccine (rDEN3delta30/31–7164) in Healthy Adults | NCT00831012 | rDEN3delta30/31–7164 | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 56 | June 2011 | |||
| Phase 2 | bivalent (DENV1 & 3 or DENV2 & 4) AND tetravalent | Safety and Immunogenicity of Formulations of Dengue Vaccines in Healthy Flavivirus-Naïve Adults | NCT00740155 | CYD | Sanofi | Adult | 154 | October 2009 | ||
| Phase 1 | tetravalent | Safety and Immunogenicity Study to Assess DENVax, a Live Attenuated Tetravalent Vaccine for Prevention of Dengue Fever | NCT01224639 | DENVax | Inviragen Inc. | Adult | 112 | June 2011 | ||
| tetravalent | Tetravalent Chimeric Dengue Vaccine Trial | NCT01110551 | DENVax | NIAID | Adult | 72 | April 2012 | |||
| tetravalent | Evaluation of the Safety and Immune Response of Five Admixtures of a Tetravalent Dengue Virus Vaccine | NCT01072786 | TetraVax-DV | NIAID/Johns Hopkins Bloomberg School of Public Health | Adult | 141 | June 2012 | |||
| tetravalent | A Comparison of the Safety and Immunogenicity of Various Schedules of Dengue Vaccine in Healthy Adult Volunteers | NCT01542632 | DENVax | Inviragen Inc. | Adult | 152 | May 2013 | |||
| tetravalent | Impact of SQ vs IM Administration of DENVax on Safety and Immunogenicity | NCT01728792 | DENVax | Inviragen Inc./WRAIR | Adult | 80 | September 2013 | |||
| tetravalent | Phase 1b Study Investigating Safety & Immunogenicity of DENVax Given Intradermally by Needle or Needle Free PharmaJet Injector | NCT01765426 | DENVax | Inviragen Inc./NIH | Adult | 96 | November 2013 | |||
| tetravalent | Evaluating the Safety and Immune Response to Two Admixtures of a Tetravalent Dengue Virus Vaccine | NCT01436422 | TetraVax-DV | NIAID | Adult | 112 | May 2014 | |||
| tetravalent | Evaluating the Safety and Immune Response to Two Admixtures of a Tetravalent Dengue Virus Vaccine | NCT01506570 | TetraVax-DV | NIAID | Adult | 112 | May 2015 | |||
| Phase 1/2 | tetravalent | A Phase I/II Trial of a Tetravalent Live Attenuated Dengue Vaccine in Flavivirus Antibody Naïve Children | NCT00384670 | not listed | WRAIR/United States Army Medical Materiel Development Activity/GSK | Child | 10 | May 2004 | Simasthien 2008 [105] | |
| tetravalent | Follow-Up Study of Thai Children From Dengue-003 and Evaluation of a Booster Dose of Dengue Vaccine | NCT00318916 | F17 | WRAIR/U.S. Army Office of the Surgeon General/GSK | Child | 7 | March 2006 | |||
| tetravalent | A Phase I/II Trial of Tetravalent Live Attenuated Dengue Vaccine in Flavivirus Antibody Naive Infants | NCT00322049 | F17 | U.S. Army Medical Research and Materiel Command/GSK | Child | 51 | June 2009 | Watanaveeradej 2011 [107] | ||
| Phase 2 | tetravalent | A Trial of a Walter Reed Army Institute of Research (WRAIR) Live Attenuated Virus Tetravalent Dengue Vaccine in Healthy US Adults | NCT00239577 | not listed | GSK | Adult | 132 | June 2007 | ||
| tetravalent | Study of ChimeriVax™ Dengue Tetravalent Vaccine in Adult Subjects | NCT00730288 | Chimerivax | Sanofi | Adult | 35 | August 2007 | |||
| tetravalent | A Phase II Trial of a Walter Reed Army Institute of Research (WRAIR) Live Attenuated Virus Tetravalent Dengue Vaccine in Healthy Adults in Thailand | NCT00370682 | T-DEN F17 & T-DEN F19 | U.S. Army Medical Research and Materiel Command/GSK | Adult | 120 | February 2008 | |||
| tetravalent | A Phase II Trial of a WRAIR Live Attenuated Virus Tetravalent Dengue Vaccine in Healthy US Adults | NCT00350337 | not listed | WRAIR/GSK | Adult | 88 | July 2008 | |||
| tetravalent | Immunogenicity and Safety of Three Formulations of Dengue Vaccines in Healthy Adults Aged 18 to 45 Years in the US | NCT00617344 | Chimerivax | Sanofi | Adult | 260 | December 2009 | |||
| tetravalent | A Study of Two Doses of WRAIR Dengue Vaccine Administered Six Months Apart to Healthy Adults and Children | NCT00468858 | F17 & F19 | U.S. Army Medical Research and Materiel Command/GSK | Child|Adult | 720 | April 2010 | |||
| tetravalent | Study of ChimeriVax™ Tetravalent Dengue Vaccine in Healthy Peruvian Children Aged 2 to 11 Years | NCT00788151 | Chimerivax | Sanofi | Child | 300 | August 2010 | Lanata 2012 [104] | ||
| tetravalent | Immunogenicity and Safety of Sanofi Pasteur's CYD Dengue Vaccine in Healthy Children and Adolescents in Latin America | NCT00993447 | CYD | Sanofi | Child | 600 | September 2011 | |||
| tetravalent | A Study of Dengue Vaccine in Healthy Toddlers Aged 12 to 15 Months in the Philippines | NCT01064141 | CYD | Sanofi | Child | 210 | September 2012 | |||
| tetravalent | Study of CYD Dengue Vaccine in Healthy Children and Adolescents in South America | NCT01187433 | CYD | Sanofi | Child | 150 | December 2012 | |||
| tetravalent | Study of a Tetravalent Dengue Vaccine in Healthy Adult Subjects Aged 18 to 45 Years in India | NCT01550289 | CYD | Sanofi | Adult | 189 | December 2013 | |||
| tetravalent | Efficacy and Safety of Dengue Vaccine in Healthy Children | NCT00842530 | Chimerivax | Sanofi | Child | 4002 | December 2013 | Sabchareon 2012 [67] | ||
| tetravalent | Study to Investigate the Safety and Immunogenicity of a Tetravalent Chimeric Dengue Vaccine in Healthy Volunteers Between the Ages of 1.5 – 45 Years | NCT01511250 | DENVax | Inviragen Inc. | Child|Adult | 344 | December 2013 | |||
| tetravalent | Study of ChimeriVax™ Tetravalent Dengue Vaccine in Healthy Subjects | NCT00875524 | Chimerivax | Sanofi | Child|Adult | 180 | April 2014 | |||
| tetravalent | Phase II Trial to Evaluate Safety and Immunogenicity of a Dengue 1,2,3,4 (Attenuated) Vaccine | NCT01696422 | TetraVax-DV | Butantan Institute/Banco Nacional de Desenvolvimento Economico e Social/Fundação de Amparo a Pesquisa do Estado de Sao Paulo/Butantan Foundation | Adult | 300 | May 2014 | |||
| tetravalent | Immune Response to Different Schedules of a Tetravalent Dengue Vaccine Given With or Without Yellow Fever Vaccine | NCT01488890 | CYD | Sanofi | Adult | 390 | June 2014 | |||
| tetravalent | Study of Sanofi Pasteur's CYD Dengue Vaccine in Healthy Subjects in Singapore | NCT00880893 | CYD | Sanofi | Child|Adult | 1200 | October 2014 | Sin Leo 2012 [112] | ||
| Phase 3 | tetravalent | Study of a Tetravalent Dengue Vaccine in Healthy Adults in Australia | NCT01134263 | CYD | Sanofi | Adult | 715 | November 2012 | ||
| tetravalent | Study of Yellow Fever Vaccine Administered With Tetravalent Dengue Vaccine in Healthy Toddlers | NCT01436396 | CYD | Sanofi | Child | 792 | January 2013 | |||
| tetravalent | Study of a Tetravalent Dengue Vaccine in Healthy Children Aged 2 to 11 Years in Malaysia | NCT01254422 | CYD | Sanofi | Child | 250 | January 2013 | |||
| tetravalent | Study of a Booster Injection of Pentaxim™ Vaccine Administered With Dengue Vaccine in Healthy Toddlers | NCT01411241 | CYD | Sanofi | Child | 732 | April 2013 | |||
| tetravalent | Study of a Novel Tetravalent Dengue Vaccine in Healthy Children Aged 2 to 14 Years in Asia | NCT01373281 | CYD | Sanofi | Child | 10278 | July 2014 | |||
| tetravalent | Study of a Novel Tetravalent Dengue Vaccine in Healthy Children and Adolescents Aged 9 to 16 Years in Latin America | NCT01374516 | CYD | Sanofi | Child | 20875 | August 2016 | |||
| DNA | Phase 1 | monovalent DENV1 | Safety Study of a Dengue Virus DNA Vaccine | NCT00290147 | D1ME | WRAIR/U.S. Army Office of the Surgeon General/U.S. Army Me dical Research and Materiel Command | Adult | 24 | December 2006 | Beckett 2011 [139] |
| tetravalent | Evaluation of the Safety and the Ability of a DNA Vaccine to Protect Against Dengue Disease | NCT01502358 | TVDV | U.S. Army Medical Research and Materiel Command/Vical/WR AIR/Naval Medical Research Center | Adult | 40 | December 2012 | |||
| Purified Inactivated | Phase 1 | monovalent DENV1 | Safety Study of a Vaccine (DENV-1 PIV) to Prevent Dengue Disease | NCT01502735 | DENV-1 PIV | U.S. Army Medical Research and Materiel Command | Adult | 20 | September 2012 | |
| tetravalent | A Two-dose Primary Vaccination Study of a Tetravalent Dengue Virus Purified Inactivated Vaccine vs. Placebo in Healthy Adults | NCT01666652 | TDENV-PIV | U.S. Army Medical Research and Materiel Command/GSK/WRAI R | Adult | 100 | December 2012 | |||
| tetravalent | A Two-dose Primary Vaccination Study of a Tetravalent Dengue Virus Purified Inactivated Vaccine vs. Placebo in Healthy Adults (in Puerto Rico) | NCT01702857 | TDENV-PIV | U.S. Army Medical Research and Materiel Command/GSK/WRAI R | Adult | 100 | December 2015 | |||
| Subunit | Phase 1 | monovalent DENV1 | Study of HBV-001 D1 in Healthy Adults | NCT00936429 | DEN1–80E | Hawaii Biotech, Inc. | Adult | 16 | July 2010 | |
| tetravalent | Study of a Dengue Vaccine (V180) in Healthy Adults (V180-001 AM1) | NCT01477580 | V180 | Merck | Adult | 120 | December 2013 |
Sanofi Pasteur CYD TDV
Currently, CYD TDV (chimeric YF17D- DENV tetravalent dengue vaccine) produced by Sanofi Pasteur is most advanced in clinical testing having recently completed a phase 2b clinical trial [67]. Currently, phase 3 trials of CYD TDV in more than 30,000 volunteers in 10 countries are underway with an expected completion in 2016 (Table 1: NCT01374516 and NCT01373281) [67]. This vaccine is composed of chimeric viruses which encode the prM and E regions of DENV1–4 in the backbone of YFV vaccine strain 17D [109,110]. The preclinical and early clinical development of CYD TDV has been described in detail by Guy et al [95]. Early clinical studies of CYD TDV indicated that CYD TDV was safe and immunogenic in children and adults, including those with previous DENV or YFV exposure [104,111,112]. Furthermore, balanced neutralizing antibody responses against all four serotypes were demonstrated in healthy adults with seroconversion rates of 70–100% after 2 doses and 100% seroconversion after 3 doses of CYD TDV [113]. In a phase 2b trial in Thailand, CYD TDV showed a disappointing overall vaccine efficacy of only 30.2%; however, differences in serotype-specific vaccine efficacy were identified [67]. Vaccine efficacy, greater than 28 days after the third immunization, was 55.6%, 9.2%, 75.3%, and 100% for DENV1, 2, 3, and 4 respectively. Neutralizing antibody responses 28 days after the third immunization were 146, 310, 405, and 155 (reciprocal geometric mean PRNT50 antibody titers) against DENV1, 2, 3, and 4 respectively. Despite PRNT50 antibody titers against DENV2 that were comparable to the other serotypes, no significant protection against DENV2 was observed. However, the vaccine was safe and non-reactogenic, consistent with profiles reported in prior studies [95,102,104,112,114]. The reasons for the unexpected failure against DENV2 are under intensive investigation.
Walter Reed Army Institute of Research (WRAIR) and GlaxoSmithKline (GSK) TDV
A TDV prepared from four monovalent DENV vaccines (DENV1–4) attenuated by serial passage in primary dog kidney (PDK) cells was tested in infants, children, and adults in Phase 1 and 2 clinical trials (Table 1) [105–107,115]. The vaccine was found to be safe and immunogenic in all age groups (12 months – 45 years). In adults, tetravalent neutralizing antibody responses ranged from 36–63% following 2 doses of 3 different tetravalent formulations [106]. In contrast to adults, seroconversion was seen in 100% of flavivirus naïve children following 2 doses (n=7) [105]. In the above trials, lyophilized monovalent vaccines were combined into a tetravalent preparation at the time of administration. Most recently, a phase 2 trial in adults to evaluate a re-derived tetravalent vaccine prepared from vaccine strains with three additional passages in fetal rhesus lung (FRhL) cells was completed [116]. The monovalent bulk vaccines were formulated with a carbohydrate stabilizer rather than human serum albumin and the final product was lyophilized as a tetravalent vaccine [116]. The primary sign encountered in the vaccine groups compared to placebo was a non-specific, self-limited rash, which occurred in 13.6–31.8% of vaccine recipients depending on formulation. The febrile response was similar in vaccinees and placebo-inoculated volunteers. The tetravalent antibody response following the second dose in DENV naïve individuals was 60–66.7% and a third dose (5–12 months after the second dose) did not increase tetravalent antibody responses [116].
DENVax
A LAV DENV vaccine candidate, produced by the Center for Disease Control (CDC) and Inviragen and based on the DENV2 attenuated vaccine PDK-53, is also under investigation. DENV2 PDK-53 was attenuated by serial passage in PDK cells and has been studied in clinical trials as a monovalent vaccine candidate as well as in multivalent formulations (reviewed in [97]). A chimeric DENV2 PDK-53 based tetravalent vaccine (DENVax) has been developed by substituting the prM and E genes of DENV2 PDK-53 with those of wild-type DENV1, 3, or 4 [117]. Seed lots of DENVax have been produced under good manufacturing practices (GMP) by Shantha, Biotechnics, Ltd., Hyderabad, India and tested in AG129 mice [118]. All formulations tested were immunogenic and elicited neutralizing antibodies against all four serotypes; however, neutralizing antibody titers were not equivalent suggesting differences in immunogenicity and/or virus interference among the vaccine strains [118]. A phase 2 trial in children and adults is currently underway (Table 1) (http://www.clinicaltrials.gov).
Tetra-Vax-DV
Many LAV DENV vaccine candidates, developed by the National Institute of Allergy and Infectious Diseases (NIAID) have been produced using directed mutagenesis. Specifically, deletion of 30 nucleotides from the 3’ UTR of DENV1 and DENV4 resulted in satisfactory attenuation. In contrast, DENV2 and 3 were not sufficiently attenuated using the same methodology [119–121]. Several of these monovalent DENV vaccine candidates have been tested in phase 1 and 2 clinical trials to identify those best suited for combination into TDV formulations [91].
Following encouraging results in preclinical studies, an attenuated DENV4 candidate (rDEN4Δ30) was tested in clinical trials and found to be safe and immunogenic. The most common adverse events were an asymptomatic rash (>50% of vaccinees) and neutropenia (~20% of vaccinees). Additionally, elevated serum alanine aminotransferase (ALT) levels were identified in several volunteers [90,93]. Liver enzyme elevation appeared to be related to dose of virus administration [93]. Seroconversion (≥ 4 fold rise in neutralizing antibody titers) against DENV4 was 95–100%. Further mutations were also introduced into the rDEN4Δ30 vaccine candidate in attempts to reduce reactogenicity/hepatotoxicity while maintaining immunogenicity (rDEN4Δ30–4995 with an amino acid substitution at residue 158 of NS3 and rDEN4Δ30–200,201 with mutations at amino acid positions 200 and 201 in the NS5 polymerase gene) [94,108]. rDEN1Δ30 was also tested in clinical trials and found to be safe and immunogenic. This vaccine candidate was also associated with asymptomatic rash in 24–40% of vaccinees and neutropenia in 42–48% [92,103].
Due to insufficient attenuation of rDEN2Δ30 in preclinical trials, a chimeric DENV2/DENV4 vaccine candidate, rDEN2/4Δ30(ME), was produced and tested in volunteers [122]. It was found to be safe and immunogenic with similar adverse events as rDEN4Δ30 and rDEN1Δ30 including asymptomatic rash (45%), mild neutropenia (35%), and elevated ALT (15%) (n=20). There was a 100% seroconversion rate against DENV2 after a single dose [122].
Results of a phase 1 clinical trial of tetravalent formulations (Tetra-Vax-DV) comparing 4 admixtures of rDEN1Δ30, rDEN2/4Δ30(ME), rDEN3–3’D4Δ30 or rDEN3Δ30/31–7164, and rDEN4Δ30 or rDEN4Δ30–200,201 were recently published [123]. Tri- or tetravalent neutralizing antibody responses after a single dose were obtained in 75–90% of volunteers depending on the formulation. Additionally, the responses were generally well balanced among serotypes with the geometric mean PRNT60 titers ranging from 32 to 154. Asymptomatic rash in 64.2% of vaccinees was the only adverse event that was significantly higher in vaccine recipients.
Interestingly, the incidence of vaccine viremia and seroconversion in Black volunteers was significantly reduced compared to non-Black volunteers. This may be in part due to protective genetic factors associated with Black race which have been previously described [32,33]. The formulation which induced the most balanced antibody responses (less than two-fold difference between the mean PRNT60 to each serotype) induced a trivalent response in 90% of volunteers, but of these, only 45% had a complete tetravalent response. This formulation, admixture TV003 (rDEN3Δ30/31, rDEN4Δ30, rDEN1Δ30, and rDEN2/4Δ30), has been proposed for further evaluation and a phase 2 trial by the Butantan Institute in Brazil (Table 1) (http://www.clinicaltrials.gov) [123].
Purified inactivated vaccines- WRAIR/GSK
Inactivated whole virus vaccines against DENV have been shown to induce neutralizing antibody responses in animal models [124,125]. There are disadvantages and advantages of inactivated and subunit DENV vaccines compared to LAV candidates. Inactivated vaccines are frequently more expensive to manufacture than LAV. Inability to replicate within the host may be disadvantageous from the perspective of duration of immune responses, and more doses may be required. However, inactivated vaccines do not pose a risk of reversion to virulence or transmission via infected mosquitos. Furthermore, inactivated vaccines are generally safe in immunosuppressed individuals, such as those with HIV infection. Additionally, there is no interference among the four serotypes for infectivity, although immunological interference remains a potential problem. Previously, it was not possible to grow DENV to high enough titer in cell culture to make inactivated whole virus vaccines practical; however, methods have been optimized to allow replication of adequate titers of DENV in cell culture for purification and inactivation [124,125]. Preclinical studies of a formalin inactivated DENV2 vaccine candidate showed protection in a mouse model. In NHP, the vaccine induced neutralizing antibody, but only partial protection was obtained with 11 of 17 vaccinated NHP developing detectable viremia following challenge, although the duration of viremia was shorter than in control animals [124]. Currently, two phase 1 trials are underway evaluating a purified inactivated DENV1 vaccine candidate and a tetravalent purified inactivated DENV vaccine candidate (Table 1) (http://www.clinicaltrials.gov).
Recombinant subunit vaccines- Hawaii Biotech/Merck
Recombinant subunit vaccine candidates, primarily based on the E protein of DENV, are in various stages of pre-clinical and early clinical development. Advantages of recombinant subunit vaccines are similar to those for purified inactivated vaccines, as discussed above. Certainly, the possibility of an accelerated administration schedule makes these attractive candidates [126]. In attempts to identify a reliable, affordable source for production of large quantities of recombinant proteins in their native conformation, multiple expression systems for the production of recombinant E protein have been employed. Among these are E. coli, Saccharomyces cerevisciae, Pichia pastoris, Chinese Hamster Ovary DHFR system, Vaccinia expression in mammalian cells, Baculovirus in Sf9 or High five cells, and stably transformed Drosophila S2 cells (reviewed in [126]). Stably transformed Drosophila S2 cells are capable of expressing high levels of truncated DENV E protein (80E) in its native conformation [127,128]. Tetravalent formulations of 80E subunits (with or without recombinant NS1) were evaluated as potential vaccine candidates in mice and NHP [127]. A balanced immune response was obtained and protection was demonstrated in a small NHP trial when tetravalent formulations were given with ISCOMATRIX® adjuvant [127]. Currently a Phase 1 trial of a tetravalent subunit vaccine candidate (V180, previously referred to as 80E) is sponsored by Merck using either alhydrogel or ISOCOMATRIX® as adjuvants compared to vaccine without adjuvant (Table 1) (http://www.clinicaltrials.gov).
Virus vectored and viral like particle (VLP)-based vaccines
Through the use of VLP and virus vectors, it is possible to more closely approximate the natural presentation of DENV surface antigens. Several vaccine candidates utilizing these platforms are currently under investigation.
VLP-based vaccines utilize carriers which self-assemble into VLP and display the antigen of interest on the surface of the particle. This has proven to be a successful strategy with the licensure of the human papillomavirus (HPV) vaccine [129]. The hepatitis B virus (HBV) core antigen is a carrier capable of forming VLP and expressing surface antigens from multiple pathogens [130]. DENV E DIII was detected on the surface of VLP using recombinant HBV core antigen, but it only induced modest DENV antibody responses in mice [131,132].
Adenoviral vectors have been extensively studied as carriers for vaccine antigens [133]. Two tetravalent DENV vaccine candidates utilizing adenovirus type 5 (AdV5) vectors have been described. The first includes AdV5 encoding tetravalent DENV E DIII, which induces both humoral and cellular immune responses against all four serotypes in mice [134]. Additionally, immune responses against DENV were not decreased by pre-existing anti-AdV5 immunity. In fact, pre-existing AdV5 antibodies appeared to facilitate entry of the vector into human monocytic cells [134]. The second vector is a combination of two bivalent AdV5/DENV constructs [135]. Immunized rhesus monkeys were protected from viremia when challenged with DENV1 or 3 and had a significant reduction in the number of viremic days when challenged with DENV2 or 4 [135].
Recently, a Venezuelan equine encephalitis (VEE) virus replicon was employed as a vector for a TDV candidate [136]. Initial studies in NHP indicate that a VEE replicon expressing a C terminally truncated segment of 85% of the E protein of DENV3 showed robust neutralizing antibody responses following 2 doses administered 6 weeks apart [136]. Additionally, a tetravalent formulation induced neutralizing antibody responses against all 4 serotypes in 16 of 16 rhesus macaques. Following challenge with wild-type virus, there was no breakthrough viremia from DENV3 or 4, and significantly reduced viremia from DENV1 and 2. Furthermore, a significant boost in neutralizing antibody responses followed challenge, indicating this platform might be useful in a prime-boost strategy with LAV as the booster.
DNA vaccines
DNA vaccines have been in development since the early 1990s. They consist of a selected gene sequence cloned into a plasmid backbone.
The plasmid is injected allowing DNA to be taken up by antigen-presenting cells, which then express the plasmid-encoded genes to generate the target antigen(s) [137]. DNA vaccines against DENV have focused on the E protein as the target antigen. Preclinical studies in mice identified a tetravalent vaccine candidate composed of four different plasmids encoding the prM/E genes of each DENV serotype [137]. Very recent evaluation of DNA DENV vaccine candidates encoding DENV2 prM/E and NS1 show some protective efficacy in a mouse model [138]. In a phase 1 trial, a DENV1 monovalent DNA vaccine was found to be safe at low and high doses with the most commonly solicited signs/symptoms being local mild pain/tenderness, mild swelling at the vaccination site, muscle pain, and fatigue [139]. The vaccine was found to be moderately immunogenic with 5 of 12 (41.6%) high dose vaccine recipients producing low but measurable neutralizing antibody titers ranging from 1:11 to 1:135 PRNT50 following 2–3 doses. Additionally, CMI was measured by IFN-γ ELISPOT and was positive in 83.3% of high dose recipients (n=12) and 50% of low dose recipients (n=8). Currently, a phase 1 clinical trial of a TDV is underway (Table 1) (http://www.clinicaltrials.gov).
Heterologous prime-boost strategies
Given the importance of rapidly inducing tetravalent protective immunity to avoid potential enhancement after natural infection and to rapidly protect during epidemic outbreaks, it is critical to develop strategies that improve immunogenicity and/or reduce the time-frame for DENV immunization. Heterologous prime-boost strategies, in which initial immunization with one type of vaccine is followed by a boost with a second, heterologous type of vaccine (e.g., DNA vaccine followed by LAV), have shown improved immunogenicity for multiple pathogens (human immunodeficiency virus, tuberculosis, rabies, influenza, malaria) (reviewed in [140]). However, various factors such as order of administration of the prime-boost and the specific antigens used may be of critical importance in efficacy necessitating careful evaluation of these strategies in clinical trials [140,141].
Studies in NHP using non-replicating tetravalent DENV vaccines (either purified inactivated virus or DNA) to prime followed by a boost with tetravalent LAV DENV vaccines demonstrated protection from viremia for all serotypes. This strategy allowed a shorter interval between the first and second immunization while maintaining protective efficacy against all four serotypes [142]. Preliminary investigations in mice indicate that different patterns of alternating prime-boost with DNA vaccines against the E protein, adenovirus and/or vaccinia vectored vaccines (expressing the E protein) can elicit differential responses [141]. Additionally, pre-clinical studies are underway investigating the use of DNA vaccine priming as a means to “redirect” the immune response to avoid enhancing antibody responses [143].
Challenges to Vaccine Implementation
Generation and licensure of a safe and effective tetravalent DENV vaccine is only the first hurdle toward full implementation in a public health setting. Vaccine cost and stability, long-term surveillance for potential adverse events, and compatibility with current vaccine schedules will challenge implementation of the vaccine in the field.
Cost is a major determinant of the successful use of any vaccine, particularly in developing countries. A survey of policymakers in Cambodia, Indonesia, Philippines, and Vietnam indicated there would be public demand for a DENV vaccine and suggested that governments would likely be willing to pay $0.50-$1.00 per dose [144]. It is likely that the private market would tolerate vaccine costs 10–30 times higher. Given constraints in the availability of a “cold chain” in some endemic regions, a stable vaccine that does not require refrigeration would be ideal. Also, due to the potential for rare adverse events following any vaccine, DENV included, long-term post-licensure follow up will be necessary. In the case of DENV, with the theoretical possibility of potentiation of severe disease following natural infection, phase 4 studies will be critical [145]. This will add cost to vaccine development. A recent economic analysis for cost of production of a LAV TDV in Brazil suggested that with an estimated annual vaccine production of 15–60 million doses per year, production costs would likely be $0.20-$0.65 per dose in 10-dose vials and $0.70-$1.75 for single-dose vials [146]. Even if a successful DENV vaccine is licensed, considerable efforts to improve the vaccine profile may still be required. Developing second-generation DENV vaccines with shorter intervals between immunizations or fewer required doses, more cost-effective manufacturing, and improved immunogenicity would be preferred. It is generally estimated that >80% protective efficacy will be necessary for public consideration and to confer “herd-immunity” by blocking mosquito transmission [144]. Therefore, this has been the target for vaccine developers. Consideration of the intended use (routine immunization of young children, “catch-up” campaigns, management of epidemics, or use for travellers) will affect the desired qualities of the vaccine. For example, a vaccine for travellers to endemic countries should induce rapid effective protection, but it might be costly ([147]). By contrast, a less costly vaccine intended for routine immunization in endemic areas should be compatible with existing Expanded Program on Immunization (EPI) and national dosing schedules. Finally, the possibility of immune interference or enhanced reactogenicity engendered by any DENV vaccine when given in combination with other flavivirus vaccines (i.e. yellow fever or Japanese encephalitis) must be investigated.
Strategies for the Introduction of DENV Vaccines in Resource Limited Countries
Current vaccine efforts are targeted primarily toward development of a pediatric DENV vaccine for use in endemic areas. Resource limitations in DENV endemic regions engender obstacles impeding implementation of DENV vaccines as an effective public health tool. Such countries must decide which vaccines to prioritize for public immunization programs based on consideration of the disease burden, public perception of disease impact, the safety and efficacy of the vaccine, it’s cost and the economic resources available [148]. Additional considerations for most vaccines include an adequate cold chain and long term vaccine availability which must be thoroughly assessed prior to vaccine implementation. Disease surveillance following vaccine implementation is important for the introduction of all vaccines, but it is critical for DENV vaccines because of the theoretical risk of disease enhancement following incomplete or ineffective immunization.
Expert Commentary
DENV continues to be a rapidly spreading threat to global health. Re-infestation of many regions of the world with Aedes mosquito vectors, global travel, massive urbanization and climate change ensure this threat will only increase unless dramatic measures are taken. DENV control will require a multi-faceted approach including vector control and an effective vaccine.
Further studies of immune responses to natural DENV infection and to vaccine candidates are necessary to improve our understanding of the contributions of enhanced complement system activation, ADE, and CMI responses, which are likely to play significant roles in the immunopathogenesis of severe dengue. Racial differences in response to vaccines could affect the use of vaccines in geographic areas with distinct racial profiles. Additionally, identification of immune correlates of protection would greatly facilitate future development and licensure of second-generation DENV vaccines.
Given the limitations of current animal models to recapitulate human disease, consideration of human challenge studies for the evaluation of vaccine efficacy should be considered. Recently, volunteers previously immunized with multiple formulations of TDV [106,115] submitted to challenge with near wild-type DENV1 or 3 [66]. This study highlights the utility and safety of human challenge studies in the evaluation of DENV vaccine candidates. There has been much debate regarding the use of human challenge studies in this context due to the potential for severe disease; however, if performed properly, these studies can be safe and provide vital information [149].
Recent advances in development of DENV vaccines are extremely promising; however, there is considerable need for further financial and political commitment to ensure the successful licensure and implementation of a DENV vaccine. Continued development of alternative vaccine platforms that may result in second-generation vaccines is also crucial. The theoretical risk of immune-mediated enhancement as a result of immunization necessitates stringent post-licensure evaluation and long-term follow-up of vaccines. The follow-up required will far exceed that possible in a clinical trial and will require surveillance systems for severe dengue in coordination with a database of vaccine recipients to enable tracking of serious adverse events remote from the immunization. It is, however, encouraging that in the completed trials summarized above (some with follow-up out to 2 years) there has been no evidence of disease enhancement or unanticipated severe or serious adverse events.
The unexpected failure of CYD TDV to protect against DENV2 in a recent phase 2b trial leaves many unanswered questions. Particularly since neutralizing antibody responses against DENV2 were within the range of what would be considered protective. It is unclear what role the heterologous nature of the non-structural proteins from YF17D may play. Although the E protein is considered to be an extremely important immunogen for the generation of neutralizing antibodies, CMI responses against NS proteins are also a major component of the immune response against DENV. It is also unclear whether or not viral genetics may affect vaccine efficacy. While NHP studies seem to indicate that CYD TDV is broadly cross-reactive against many genotypes of DENV2, it is still possible that the circulating DENV2 strain during the trial was sufficiently different to reduce vaccine efficacy. The failure of CYD TDV to protect against DENV2 presents a troublesome set-back to DENV vaccine development; however, ongoing phase 3 trials with CYD TDV may help answer some of these questions. This failure also highlights the importance of continuing to support the development of additional vaccine candidates, and to expand basic research focused on understanding mechanisms of immunopathogenesis and protection against dengue and other flaviviruses.
Five-year View
With several LAV candidates currently in advanced clinical trials, the next 5–10 years are likely to see the licensure of a tetravalent DENV vaccine. Continued efforts to confirm the vaccine profile and identify immunological correlates of protection will be critical. Certainly, the public health ramifications of continued explosion of DENV in the tropics and semi-tropics provide an enormous incentive to address these issues.
Key Issues.
Dengue virus is the most significant human arboviral pathogen and poses a global public health threat which will continue without intervention.
The phenomenon of immune-mediated enhancement and unpredictable circulation of DENV serotypes necessitate a tetravalent dengue virus vaccine that will provide long-lasting protection against all four dengue virus serotypes.
Complex and unpredictable epidemiology as well as regional epidemiological differences will require testing of vaccine candidates in multiple geographic areas.
Assessment of genetic differences between circulating DENV strains and vaccine strains may provide insight into vaccine efficacy and should be incorporated into clinical trials.
The live-attenuated tetravalent vaccine candidate based on the backbone of yellow fever 17D vaccine strain is currently the most advanced having recently completed a phase 2b clinical trial demonstrating only 30% overall efficacy. Phase 3 trials are underway with an estimated completion in 2016.
Extensive post-licensure surveillance for adverse events will be necessary to detect delayed occurrence of more frequent or severe vaccine-associated dengue, including immune-mediated enhancement.
Identification of immune correlates of protection requires continued field trials to associate immune responses with protection against disease and will facilitate the development of second-generation vaccines.
Development of second-generation dengue vaccine candidates should continue in pursuit of affordable, immunogenic vaccines that can be administered in one or two doses over a short period of time and provide >80% protective efficacy.
Considerable political and financial support will be required for wide-spread adoption of a dengue vaccine, once licensed.
Acknowledgements
This work was supported, in part, by NIAID, NIH, DHHS federal research grant U19 AI082655 (CCHI) to MBS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
List of abbreviations
- ADE
antibody dependent enhancement
- AdV5
adenovirus type 5
- Ae.
Aedes
- C
capsid
- CMI
cell mediated immunity
- CYD
chimeric YF17D-DENV
- DIII
domain III
- DENV
dengue virus
- DF
dengue fever
- DHF
dengue hemorrhagic fever
- DSS
dengue shock syndrome
- E
envelope
- EBV
Epstein Barr Virus
- EPI
Expanded Program on Immunization
- FRhL
fetal rhesus lung
- GSK
GlaxoSmithKline
- HBV
hepatitis B virus
- HPV
human papilloma virus
- IFN
interferon
- JEV
Japanese encephalitis virus
- M
membrane
- NHP
non-human primate
- NIAID
National Institute of Allergy and Infectious Diseases
- NS
non-structural
- PDK
primary dog kidney
- prM
pre-membrane
- PRNT
plaque reduction neutralization test
- TBEV
tick-borne encephalitis virus
- TDV
tetravalent dengue vaccine
- TNF
tumor necrosis factor
- UTR
untranslated region
- VEE
Venezuelan equine encephalitis
- VLP
virus-like particle
- WHO
World Health Organization
- WNV
West Nile virus
- WRAIR
Walter Reed Army Institute of Research
- YFV
yellow fever virus
Footnotes
Financial Disclosure:
The authors have no financial conflicts to disclose.
Contributor Information
Monica A. McArthur, Email: mmcarthu@medicine.umaryland.edu.
Marcelo B. Sztein, Email: msztein@medicine.umaryland.edu.
References
- 1. Beatty ME, Beutels P, Meltzer MI, et al. Health economics of dengue: a systematic literature review and expert panel's assessment. Am J Trop Med Hyg. 2011;84(3):473–488. doi: 10.4269/ajtmh.2011.10-0521. This literature review of the economic burden of dengue provides an expert panel opinion of research needs pertaining to the health economice of dengue.
- 2.Gubler DJ. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res. 2002;33(4):330–342. doi: 10.1016/s0188-4409(02)00378-8. [DOI] [PubMed] [Google Scholar]
- 3.Scientific Working Group on Dengue. Report of the Scientific Working Group on Dengue, 1–5 October 2006. Report of the Scientific Working Group on Dengue, 1–5 October 2006. 2007;31:183–186. [Google Scholar]
- 4.Who. Sustaining the drive to overcome the global impact of neglected tropical diseases. Second WHO report on neglected tropical diseases. 2013 [Google Scholar]
- 5.World Health Organization Dengue and severe dengue. World Health Organization Dengue and severe dengue. 2012 2012(October 3, 2012) [Google Scholar]
- 6.Bhatt S, Gething PW, Brady OJ, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gubler DJ. The economic burden of dengue. Am J Trop Med Hyg. 2012;86(5):743–744. doi: 10.4269/ajtmh.2012.12-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Halasa YA, Shepard DS, Zeng W. Economic cost of dengue in Puerto Rico. Am J Trop Med Hyg. 2012;86(5):745–752. doi: 10.4269/ajtmh.2012.11-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halstead SB. Dengue. Lancet. 2007;370(9599):1644–1652. doi: 10.1016/S0140-6736(07)61687-0. [DOI] [PubMed] [Google Scholar]
- 10.Whitehorn J, Farrar J. Dengue. Br Med Bull. 2010;95:161–173. doi: 10.1093/bmb/ldq019. [DOI] [PubMed] [Google Scholar]
- 11.Lindenbach BD, Rice CM. Molecular biology of flaviviruses. Adv Virus Res. 2003;59:23–61. doi: 10.1016/s0065-3527(03)59002-9. [DOI] [PubMed] [Google Scholar]
- 12.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30(29):4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 13.Kanai R, Kar K, Anthony K, et al. Crystal structure of west nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80(22):11000–11008. doi: 10.1128/JVI.01735-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modis Y, Ogata S, Clements D, Harrison SC. A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A. 2003;100(12):6986–6991. doi: 10.1073/pnas.0832193100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Modis Y, Ogata S, Clements D, Harrison SC. Variable surface epitopes in the crystal structure of dengue virus type 3 envelope glycoprotein. J Virol. 2005;79(2):1223–1231. doi: 10.1128/JVI.79.2.1223-1231.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80(23):11467–11474. doi: 10.1128/JVI.01125-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rey FA, Heinz FX, Mandl C, Kunz C, Harrison SC. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature. 1995;375(6529):291–298. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Zhang W, Ogata S, et al. Conformational changes of the flavivirus E glycoprotein. Structure. 2004;12(9):1607–1618. doi: 10.1016/j.str.2004.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heinz FX, Allison SL. Flavivirus structure and membrane fusion. Adv Virus Res. 2003;59:63–97. doi: 10.1016/s0065-3527(03)59003-0. [DOI] [PubMed] [Google Scholar]
- 20.Wahala WM, Silva AM. The human antibody response to dengue virus infection. Viruses. 2011;3(12):2374–2395. doi: 10.3390/v3122374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Who. Dengue guidelines for diagnosis, treatment, prevention, and control. Geneva: World Helath Organization; 2009. [PubMed] [Google Scholar]
- 22.Who. Dengue Hemorrhagic Fever: Diagnosis, treatment, prevention, and control. Dengue Hemorrhagic Fever: Diagnosis, treatment, prevention, and control. 1997 [Google Scholar]
- 23.Narvaez F, Gutierrez G, Perez MA, et al. Evaluation of the traditional and revised WHO classifications of Dengue disease severity. PLoS Negl Trop Dis. 2011;5(11):e1397. doi: 10.1371/journal.pntd.0001397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Were F. The dengue situation in Africa. Paediatr Int Child Health. 2012;32(Suppl 1):18–21. doi: 10.1179/2046904712Z.00000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Who. Working to Overcome the Global Impact of Neglected Tropical Diseases. First WHO Report on Neglected Tropical Diseases. Geneva, Switzerland. Working to Overcome the Global Impact of Neglected Tropical Diseases. First WHO Report on Neglected Tropical Diseases. Geneva, Switzerland. 2010 [Google Scholar]
- 26.Lambrechts L, Scott TW, Gubler DJ. Consequences of the expanding global distribution of Aedes albopictus for dengue virus transmission. PLoS Negl Trop Dis. 2010;4(5):e646. doi: 10.1371/journal.pntd.0000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gubler DJ. Dengue, Urbanization and Globalization: The Unholy Trinity of the 21(st) Century. Trop Med Health. 2011;39(4 Suppl):3–11. doi: 10.2149/tmh.2011-S05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tantawichien T. Dengue fever and dengue haemorrhagic fever in adolescents and adults. Paediatr Int Child Health. 2012;32(Suppl 1):22–27. doi: 10.1179/2046904712Z.00000000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cardoso IM, Cabidelle Ade S, Borges Pde C, et al. Dengue: clinical forms and risk groups in a high incidence city in the southeastern region of Brazil. Rev Soc Bras Med Trop. 2011;44(4):430–435. doi: 10.1590/s0037-86822011005000044. [DOI] [PubMed] [Google Scholar]
- 30.Gubler DJ. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–103. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 31.Tapia-Conyer R, Betancourt-Cravioto M, Mendez-Galvan J. Dengue: an escalating public health problem in Latin America. Paediatr Int Child Health. 2012;32(Suppl 1):14–17. doi: 10.1179/2046904712Z.00000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blanton RE, Silva LK, Morato VG, et al. Genetic ancestry and income are associated with dengue hemorrhagic fever in a highly admixed population. Eur J Hum Genet. 2008;16(6):762–765. doi: 10.1038/ejhg.2008.4. [DOI] [PubMed] [Google Scholar]
- 33.Halstead SB, Streit TG, Lafontant JG, et al. Haiti: absence of dengue hemorrhagic fever despite hyperendemic dengue virus transmission. Am J Trop Med Hyg. 2001;65(3):180–183. doi: 10.4269/ajtmh.2001.65.180. [DOI] [PubMed] [Google Scholar]
- 34.Racloz V, Ramsey R, Tong S, Hu W. Surveillance of dengue fever virus: a review of epidemiological models and early warning systems. PLoS Negl Trop Dis. 2012;6(5):e1648. doi: 10.1371/journal.pntd.0001648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banu S, Hu W, Hurst C, Tong S. Dengue transmission in the Asia-Pacific region: impact of climate change and socio-environmental factors. Trop Med Int Health. 2011;16(5):598–607. doi: 10.1111/j.1365-3156.2011.02734.x. [DOI] [PubMed] [Google Scholar]
- 36.Alvarez M, Pavon-Oro A, Rodriguez-Roche R, et al. Neutralizing antibody response variation against dengue 3 strains. J Med Virol. 2008;80(10):1783–1789. doi: 10.1002/jmv.21234. [DOI] [PubMed] [Google Scholar]
- 37. Sabin AB. Research on dengue during World War II. Am J Trop Med Hyg. 1952;1(1):30–50. doi: 10.4269/ajtmh.1952.1.30. A classic paper. Human challenge studies directly demonstrated serotype cross-protection of limited duration.
- 38.Halstead SB. Pathogenesis of dengue: challenges to molecular biology. Science. 1988;239(4839):476–481. doi: 10.1126/science.3277268. [DOI] [PubMed] [Google Scholar]
- 39.Thein S, Aung MM, Shwe TN, et al. Risk factors in dengue shock syndrome. Am J Trop Med Hyg. 1997;56(5):566–572. doi: 10.4269/ajtmh.1997.56.566. [DOI] [PubMed] [Google Scholar]
- 40.Halstead SB. Antibodies determine virulence in dengue. Ann N Y Acad Sci. 2009;1171(Suppl 1):E48–E56. doi: 10.1111/j.1749-6632.2009.05052.x. [DOI] [PubMed] [Google Scholar]
- 41.Vaughn DW, Green S, Kalayanarooj S, et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis. 2000;181(1):2–9. doi: 10.1086/315215. [DOI] [PubMed] [Google Scholar]
- 42.Pang T, Cardosa MJ, Guzman MG. Of cascades and perfect storms: the immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS) Immunol Cell Biol. 2007;85(1):43–45. doi: 10.1038/sj.icb.7100008. [DOI] [PubMed] [Google Scholar]
- 43.Rothman AL. T lymphocyte responses to heterologous secondary dengue virus infections. Ann N Y Acad Sci. 2009;1171(Suppl 1):E36–E41. doi: 10.1111/j.1749-6632.2009.05055.x. [DOI] [PubMed] [Google Scholar]
- 44.Halstead SB. Neutralization and antibody-dependent enhancement of dengue viruses. Adv Virus Res. 2003;60:421–467. doi: 10.1016/s0065-3527(03)60011-4. [DOI] [PubMed] [Google Scholar]
- 45.Pierson TC, Fremont DH, Kuhn RJ, Diamond MS. Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe. 2008;4(3):229–238. doi: 10.1016/j.chom.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Avirutnan P, Punyadee N, Noisakran S, et al. Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis. 2006;193(8):1078–1088. doi: 10.1086/500949. [DOI] [PubMed] [Google Scholar]
- 47.Malasit P. Complement and dengue haemorrhagic fever/shock syndrome. Southeast Asian J Trop Med Public Health. 1987;18(3):316–320. [PubMed] [Google Scholar]
- 48. Dowd KA, Pierson TC. Antibody-mediated neutralization of flaviviruses: a reductionist view. Virology. 2011;411(2):306–315. doi: 10.1016/j.virol.2010.12.020. A thorough review of the current understanding of the mechanisms of neutralizing antibody in flaviviral infections.
- 49.Chau TN, Quyen NT, Thuy TT, et al. Dengue in Vietnamese infants-results of infection-enhancement assays correlate with age-related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis. 2008;198(4):516–524. doi: 10.1086/590117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kliks SC, Nisalak A, Brandt WE, Wahl L, Burke DS. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg. 1989;40(4):444–451. doi: 10.4269/ajtmh.1989.40.444. This is a prospective study in which the enhancing ability of serum specimens collected from naturally-infected school-aged children in Bangkok were shown to enhance dengue virus 2 infection in vitro.
- 51.Moi ML, Lim CK, Takasaki T, Kurane I. Involvement of the Fc gamma receptor IIA cytoplasmic domain in antibody-dependent enhancement of dengue virus infection. J Gen Virol. 2010;91(Pt 1):103–111. doi: 10.1099/vir.0.014829-0. [DOI] [PubMed] [Google Scholar]
- 52.Laoprasopwattana K, Libraty DH, Endy TP, et al. Dengue Virus (DV) enhancing antibody activity in preillness plasma does not predict subsequent disease severity or viremia in secondary DV infection. J Infect Dis. 2005;192(3):510–519. doi: 10.1086/431520. [DOI] [PubMed] [Google Scholar]
- 53.Libraty DH, Acosta LP, Tallo V, et al. A prospective nested case-control study of Dengue in infants: rethinking and refining the antibody-dependent enhancement dengue hemorrhagic fever model. PLoS Med. 2009;6(10):e1000171. doi: 10.1371/journal.pmed.1000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rothman AL. Cellular immunology of sequential dengue virus infection and its role in disease pathogenesis. Curr Top Microbiol Immunol. 2010;338:83–98. doi: 10.1007/978-3-642-02215-9_7. [DOI] [PubMed] [Google Scholar]
- 55.Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of Cytokines and Chemokines during Febrile Episodes Associated with Endothelial Cell Damage and Plasma Leakage in Dengue Hemorrhagic Fever. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e31826fd456. [DOI] [PubMed] [Google Scholar]
- 56.Gunther VJ, Putnak R, Eckels KH, et al. A human challenge model for dengue infection reveals a possible protective role for sustained interferon gamma levels during the acute phase of illness. Vaccine. 2011;29(22):3895–3904. doi: 10.1016/j.vaccine.2011.03.038. [DOI] [PubMed] [Google Scholar]
- 57.Mangada MM, Endy TP, Nisalak A, et al. Dengue-specific T cell responses in peripheral blood mononuclear cells obtained prior to secondary dengue virus infections in Thai schoolchildren. J Infect Dis. 2002;185(12):1697–1703. doi: 10.1086/340822. [DOI] [PubMed] [Google Scholar]
- 58.Mongkolsapaya J, Dejnirattisai W, Xu XN, et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003;9(7):921–927. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 59.Bossi F, Fischetti F, Pellis V, et al. Platelet-activating factor and kinin-dependent vascular leakage as a novel functional activity of the soluble terminal complement complex. J Immunol. 2004;173(11):6921–6927. doi: 10.4049/jimmunol.173.11.6921. [DOI] [PubMed] [Google Scholar]
- 60.De Alwis R, Smith SA, Olivarez NP, et al. Identification of human neutralizing antibodies that bind to complex epitopes on dengue virions. Proc Natl Acad Sci U S A. 2012;109(19):7439–7444. doi: 10.1073/pnas.1200566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Teoh EP, Kukkaro P, Teo EW, et al. The structural basis for serotype-specific neutralization of dengue virus by a human antibody. Sci Transl Med. 2012;4(139):139ra183. doi: 10.1126/scitranslmed.3003888. [DOI] [PubMed] [Google Scholar]
- 62.Endy TP, Nisalak A, Chunsuttitwat S, et al. Relationship of preexisting dengue virus (DV) neutralizing antibody levels to viremia and severity of disease in a prospective cohort study of DV infection in Thailand. J Infect Dis. 2004;189(6):990–1000. doi: 10.1086/382280. [DOI] [PubMed] [Google Scholar]
- 63.Roehrig JT, Hombach J, Barrett AD. Guidelines for Plaque-Reduction Neutralization Testing of Human Antibodies to Dengue Viruses. Viral Immunol. 2008;21(2):123–132. doi: 10.1089/vim.2008.0007. [DOI] [PubMed] [Google Scholar]
- 64.Rodrigo WW, Alcena DC, Rose RC, Jin X, Schlesinger JJ. An automated Dengue virus microneutralization plaque assay performed in human Fc{gamma} receptor-expressing CV-1 cells. Am J Trop Med Hyg. 2009;80(1):61–65. [PubMed] [Google Scholar]
- 65.Moi ML, Lim CK, Chua KB, Takasaki T, Kurane I. Dengue virus infection-enhancing activity in serum samples with neutralizing activity as determined by using FcgammaR-expressing cells. PLoS Negl Trop Dis. 2012;6(2):e1536. doi: 10.1371/journal.pntd.0001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sun W, Eckels KH, Putnak JR, et al. Experimental Dengue Virus Challenge of Human Subjects Previously Vaccinated With Live Attenuated Tetravalent Dengue Vaccines. J Infect Dis. 2013 doi: 10.1093/infdis/jis744. A live-attenuated tetravalent dengue virus vaccine showed variable protection in a human challenge study. This study highlights the safety and potential utility of a human challenge model of dengue virus infection.
- 67. Sabchareon A, Wallace D, Sirivichayakul C, et al. Protective efficacy of the recombinant, live-attenuated, CYD tetravalent dengue vaccine in Thai schoolchildren: a randomised, controlled phase 2b trial. Lancet. 2012;380(9853):1559–1567. doi: 10.1016/S0140-6736(12)61428-7. In this first, published efficacy field trial of any live-attenuated tetravalent dengue virus vaccine, there was an overall efficacy of only 30.2%; however, type-specific protection was 55.6%, 9.2%, 75.3%, and 100% for dengue virus 1, 2, 3, and 4 respectively.
- 68.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis. 2012;54(11):1615–1617. doi: 10.1093/cid/cis238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Raviprakash K, Luke TC, Doukas J, et al. A dengue DNA vaccine formulated with Vaxfectin ((R)) is well tolerated, and elicits strong neutralizing antibody responses to all four dengue serotypes in New Zealand white rabbits. Hum Vaccin Immunother. 2012;8(12) doi: 10.4161/hv.21806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zompi S, Harris E. Animal models of dengue virus infection. Viruses. 2012;4(1):62–82. doi: 10.3390/v4010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cassetti MC, Durbin A, Harris E, et al. Report of an NIAID workshop on dengue animal models. Vaccine. 2010;28(26):4229–4234. doi: 10.1016/j.vaccine.2010.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Atrasheuskaya A, Petzelbauer P, Fredeking TM, Ignatyev G. Anti-TNF antibody treatment reduces mortality in experimental dengue virus infection. FEMS Immunol Med Microbiol. 2003;35(1):33–42. doi: 10.1111/j.1574-695X.2003.tb00646.x. [DOI] [PubMed] [Google Scholar]
- 73.Shresta S, Kyle JL, Robert Beatty P, Harris E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology. 2004;319(2):262–273. doi: 10.1016/j.virol.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 74.Solomon T, Dung NM, Vaughn DW, et al. Neurological manifestations of dengue infection. Lancet. 2000;355(9209):1053–1059. doi: 10.1016/S0140-6736(00)02036-5. [DOI] [PubMed] [Google Scholar]
- 75.Chen HC, Hofman FM, Kung JT, Lin YD, Wu-Hsieh BA. Both virus and tumor necrosis factor alpha are critical for endothelium damage in a mouse model of dengue virus-induced hemorrhage. J Virol. 2007;81(11):5518–5526. doi: 10.1128/JVI.02575-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Paes MV, Pinhao AT, Barreto DF, et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology. 2005;338(2):236–246. doi: 10.1016/j.virol.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 77.Souza DG, Fagundes CT, Sousa LP, et al. Essential role of platelet-activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci U S A. 2009;106(33):14138–14143. doi: 10.1073/pnas.0906467106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beaumier CM, Rothman AL. Cross-reactive memory CD4+ T cells alter the CD8+ T-cell response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. Viral Immunol. 2009;22(3):215–219. doi: 10.1089/vim.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.White LJ, Parsons MM, Whitmore AC, Williams BM, De Silva A, Johnston RE. An immunogenic and protective alphavirus replicon particle-based dengue vaccine overcomes maternal antibody interference in weanling mice. J Virol. 2007;81(19):10329–10339. doi: 10.1128/JVI.00512-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.An J, Zhou DS, Zhang JL, Morida H, Wang JL, Yasui K. Dengue-specific CD8+ T cells have both protective and pathogenic roles in dengue virus infection. Immunol Lett. 2004;95(2):167–174. doi: 10.1016/j.imlet.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 81.Blaney JE, Jr, Johnson DH, Manipon GG, et al. Genetic basis of attenuation of dengue virus type 4 small plaque mutants with restricted replication in suckling mice and in SCID mice transplanted with human liver cells. Virology. 2002;300(1):125–139. doi: 10.1006/viro.2002.1528. [DOI] [PubMed] [Google Scholar]
- 82.Lin YL, Liao CL, Chen LK, et al. Study of Dengue virus infection in SCID mice engrafted with human K562 cells. J Virol. 1998;72(12):9729–9737. doi: 10.1128/jvi.72.12.9729-9737.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Shresta S, Sharar KL, Prigozhin DM, Beatty PR, Harris E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J Virol. 2006;80(20):10208–10217. doi: 10.1128/JVI.00062-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Williams KL, Zompi S, Beatty PR, Harris E. A mouse model for studying dengue virus pathogenesis and immune response. Ann N Y Acad Sci. 2009;1171(Suppl 1):E12–E23. doi: 10.1111/j.1749-6632.2009.05057.x. [DOI] [PubMed] [Google Scholar]
- 85.Akkina R. Human immune responses and potential for vaccine assessment in humanized mice. Curr Opin Immunol. 2013 doi: 10.1016/j.coi.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jaiswal S, Pazoles P, Woda M, et al. Enhanced humoral and HLA-A2-restricted dengue virus-specific T-cell responses in humanized BLT NSG mice. Immunology. 2012;136(3):334–343. doi: 10.1111/j.1365-2567.2012.03585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halstead SB, Shotwell H, Casals J. Studies on the pathogenesis of dengue infection in monkeys. I. Clinical laboratory responses to primary infection. J Infect Dis. 1973;128(1):7–14. doi: 10.1093/infdis/128.1.7. [DOI] [PubMed] [Google Scholar]
- 88.Whitehead RH, Chaicumpa V, Olson LC, Russell PK. Sequential dengue virus infections in the white-handed gibbon (Hylobates lar) Am J Trop Med Hyg. 1970;19(1):94–102. doi: 10.4269/ajtmh.1970.19.94. [DOI] [PubMed] [Google Scholar]
- 89.Goncalvez AP, Engle RE, St Claire M, Purcell RH, Lai CJ. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc Natl Acad Sci U S A. 2007;104(22):9422–9427. doi: 10.1073/pnas.0703498104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Durbin AP, Karron RA, Sun W, et al. Attenuation and immunogenicity in humans of a live dengue virus type-4 vaccine candidate with a 30 nucleotide deletion in its 3'-untranslated region. Am J Trop Med Hyg. 2001;65(5):405–413. doi: 10.4269/ajtmh.2001.65.405. [DOI] [PubMed] [Google Scholar]
- 91.Durbin AP, Kirkpatrick BD, Pierce KK, Schmidt AC, Whitehead SS. Development and clinical evaluation of multiple investigational monovalent DENV vaccines to identify components for inclusion in a live attenuated tetravalent DENV vaccine. Vaccine. 2011;29(42):7242–7250. doi: 10.1016/j.vaccine.2011.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Durbin AP, Mcarthur J, Marron JA, et al. The live attenuated dengue serotype 1 vaccine rDEN1Delta30 is safe and highly immunogenic in healthy adult volunteers. Hum Vaccin. 2006;2(4):167–173. doi: 10.4161/hv.2.4.2944. [DOI] [PubMed] [Google Scholar]
- 93.Durbin AP, Whitehead SS, Mcarthur J, et al. rDEN4delta30, a live attenuated dengue virus type 4 vaccine candidate, is safe, immunogenic, and highly infectious in healthy adult volunteers. J Infect Dis. 2005;191(5):710–718. doi: 10.1086/427780. [DOI] [PubMed] [Google Scholar]
- 94.Mcarthur JH, Durbin AP, Marron JA, et al. Phase I clinical evaluation of rDEN4Delta30–200,201: a live attenuated dengue 4 vaccine candidate designed for decreased hepatotoxicity. Am J Trop Med Hyg. 2008;79(5):678–684. [PMC free article] [PubMed] [Google Scholar]
- 95.Guy B, Barrere B, Malinowski C, Saville M, Teyssou R, Lang J. From research to phase III: preclinical, industrial and clinical development of the Sanofi Pasteur tetravalent dengue vaccine. Vaccine. 2011;29(42):7229–7241. doi: 10.1016/j.vaccine.2011.06.094. [DOI] [PubMed] [Google Scholar]
- 96.Osorio JE, Brewoo JN, Silengo SJ, et al. Efficacy of a tetravalent chimeric dengue vaccine (DENVax) in Cynomolgus macaques. Am J Trop Med Hyg. 2011;84(6):978–987. doi: 10.4269/ajtmh.2011.10-0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Osorio JE, Huang CY, Kinney RM, Stinchcomb DT. Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever. Vaccine. 2011;29(42):7251–7260. doi: 10.1016/j.vaccine.2011.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Blanc G, Caminopetros J. Contribution a l'etude de la vaccination contre la dengue. Bulletin de l'Académie nationale de médecine. 1929;102(24):1–4. [Google Scholar]
- 99.Simmons J, St John J, Reynolds F. Experimental studies of dengue. The Philippine Journal of Science. 1931;44:1–252. [Google Scholar]
- 100.Mcarthur MA, Holbrook MR. Japanese Encephalitis Vaccines. J Bioterror Biodef. 2011;S1:2. doi: 10.4172/2157-2526.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Monath TP. Yellow fever: an update. Lancet Infect Dis. 2001;1(1):11–20. doi: 10.1016/S1473-3099(01)00016-0. [DOI] [PubMed] [Google Scholar]
- 102.Capeding RZ, Luna IA, Bomasang E, et al. Live-attenuated, tetravalent dengue vaccine in children, adolescents and adults in a dengue endemic country: randomized controlled phase I trial in the Philippines. Vaccine. 2011;29(22):3863–3872. doi: 10.1016/j.vaccine.2011.03.057. [DOI] [PubMed] [Google Scholar]
- 103.Durbin AP, Whitehead SS, Shaffer D, et al. A single dose of the DENV-1 candidate vaccine rDEN1Delta30 is strongly immunogenic and induces resistance to a second dose in a randomized trial. PLoS Negl Trop Dis. 2011;5(8):e1267. doi: 10.1371/journal.pntd.0001267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lanata CF, Andrade T, Gil AI, et al. Immunogenicity and safety of tetravalent dengue vaccine in 2–11 year-olds previously vaccinated against yellow fever: randomized, controlled, phase II study in Piura, Peru. Vaccine. 2012;30(41):5935–5941. doi: 10.1016/j.vaccine.2012.07.043. [DOI] [PubMed] [Google Scholar]
- 105.Simasathien S, Thomas SJ, Watanaveeradej V, et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus naive children. Am J Trop Med Hyg. 2008;78(3):426–433. [PubMed] [Google Scholar]
- 106.Sun W, Cunningham D, Wasserman SS, et al. Phase 2 clinical trial of three formulations of tetravalent live-attenuated dengue vaccine in flavivirus-naive adults. Hum Vaccin. 2009;5(1):33–40. doi: 10.4161/hv.5.1.6348. [DOI] [PubMed] [Google Scholar]
- 107.Watanaveeradej V, Simasathien S, Nisalak A, et al. Safety and immunogenicity of a tetravalent live-attenuated dengue vaccine in flavivirus-naive infants. Am J Trop Med Hyg. 2011;85(2):341–351. doi: 10.4269/ajtmh.2011.10-0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wright PF, Durbin AP, Whitehead SS, et al. Phase 1 trial of the dengue virus type 4 vaccine candidate rDEN4{Delta}30–4995 in healthy adult volunteers. Am J Trop Med Hyg. 2009;81(5):834–841. doi: 10.4269/ajtmh.2009.09-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Guirakhoo F, Arroyo J, Pugachev KV, et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol. 2001;75(16):7290–7304. doi: 10.1128/JVI.75.16.7290-7304.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Guirakhoo F, Weltzin R, Chambers TJ, et al. Recombinant chimeric yellow fever-dengue type 2 virus is immunogenic and protective in nonhuman primates. J Virol. 2000;74(12):5477–5485. doi: 10.1128/jvi.74.12.5477-5485.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiao M, Shaw D, Forrat R, Wartel-Tram A, Lang J. Priming effect of dengue and yellow fever vaccination on the immunogenicity, infectivity, and safety of a tetravalent dengue vaccine in humans. Am J Trop Med Hyg. 2011;85(4):724–731. doi: 10.4269/ajtmh.2011.10-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sin Leo Y, Wilder-Smith A, Archuleta S, et al. Immunogenicity and safety of recombinant tetravalent dengue vaccine (CYD-TDV) in individuals aged 2–45 y: Phase II randomized controlled trial in Singapore. Hum Vaccin Immunother. 2012;8(9):1259–1271. doi: 10.4161/hv.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morrison D, Legg TJ, Billings CW, Forrat R, Yoksan S, Lang J. A novel tetravalent dengue vaccine is well tolerated and immunogenic against all 4 serotypes in flavivirus-naive adults. J Infect Dis. 2010;201(3):370–377. doi: 10.1086/649916. [DOI] [PubMed] [Google Scholar]
- 114.Poo J, Galan F, Forrat R, Zambrano B, Lang J, Dayan GH. Live-attenuated Tetravalent Dengue Vaccine in Dengue-naive Children, Adolescents, and Adults in Mexico City: Randomized Controlled Phase 1 Trial of Safety and Immunogenicity. Pediatr Infect Dis J. 2010 doi: 10.1097/INF.0b013e3181fe05af. [DOI] [PubMed] [Google Scholar]
- 115.Edelman R, Wasserman SS, Bodison SA, et al. Phase I trial of 16 formulations of a tetravalent live-attenuated dengue vaccine. Am J Trop Med Hyg. 2003;69(6 Suppl):48–60. doi: 10.4269/ajtmh.2003.69.48. [DOI] [PubMed] [Google Scholar]
- 116.Thomas SJ, Eckels KH, Carletti I, et al. A Phase II, Randomized, Safety and Immunogenicity Study of a Re-Derived, Live-Attenuated Dengue Virus Vaccine in Healthy Adults. Am J Trop Med Hyg. 2013;88(1):73–88. doi: 10.4269/ajtmh.2012.12-0361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huang CY, Butrapet S, Tsuchiya KR, Bhamarapravati N, Gubler DJ, Kinney RM. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. J Virol. 2003;77(21):11436–11447. doi: 10.1128/JVI.77.21.11436-11447.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brewoo JN, Kinney RM, Powell TD, et al. Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice. Vaccine. 2012;30(8):1513–1520. doi: 10.1016/j.vaccine.2011.11.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Murphy BR, Whitehead SS. Immune response to dengue virus and prospects for a vaccine. Annu Rev Immunol. 2011;29:587–619. doi: 10.1146/annurev-immunol-031210-101315. [DOI] [PubMed] [Google Scholar]
- 120.Blaney JE, Jr, Hanson CT, Firestone CY, Hanley KA, Murphy BR, Whitehead SS. Genetically modified, live attenuated dengue virus type 3 vaccine candidates. Am J Trop Med Hyg. 2004;71(6):811–821. [PubMed] [Google Scholar]
- 121.Blaney JE, Jr, Hanson CT, Hanley KA, Murphy BR, Whitehead SS. Vaccine candidates derived from a novel infectious cDNA clone of an American genotype dengue virus type 2. BMC Infect Dis. 2004;4:39. doi: 10.1186/1471-2334-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Durbin AP, Mcarthur JH, Marron JA, et al. rDEN2/4Delta30(ME), a live attenuated chimeric dengue serotype 2 vaccine is safe and highly immunogenic in healthy dengue-naive adults. Hum Vaccin. 2006;2(6):255–260. doi: 10.4161/hv.2.6.3494. [DOI] [PubMed] [Google Scholar]
- 123. Durbin AP, Kirkpatrick BD, Pierce KK, et al. A single dose of any of four different live attenuated tetravalent dengue vaccines is safe and immunogenic in flavivirus-naive adults: a randomized, double blind clinical trial. J Infect Dis. 2013 doi: 10.1093/infdis/jis936. Multiple admixtures of a live-attenuated tetravalent dengue virus vaccine candidate were tested in a phase 1 trial. The vaccine was found to be safe and induced tri- or tetravalent neutralizing antibody responses in 75 – 90% of volunteers after a single dose.
- 124.Putnak R, Barvir DA, Burrous JM, et al. Development of a purified, inactivated, dengue-2 virus vaccine prototype in Vero cells: immunogenicity and protection in mice and rhesus monkeys. J Infect Dis. 1996;174(6):1176–1184. doi: 10.1093/infdis/174.6.1176. [DOI] [PubMed] [Google Scholar]
- 125.Putnak R, Cassidy K, Conforti N, et al. Immunogenic and protective response in mice immunized with a purified, inactivated, Dengue-2 virus vaccine prototype made in fetal rhesus lung cells. Am J Trop Med Hyg. 1996;55(5):504–510. doi: 10.4269/ajtmh.1996.55.504. [DOI] [PubMed] [Google Scholar]
- 126.Coller BA, Clements DE, Bett AJ, Sagar SL, Ter Meulen JH. The development of recombinant subunit envelope-based vaccines to protect against dengue virus induced disease. Vaccine. 2011;29(42):7267–7275. doi: 10.1016/j.vaccine.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Clements DE, Coller BA, Lieberman MM, et al. Development of a recombinant tetravalent dengue virus vaccine: immunogenicity and efficacy studies in mice and monkeys. Vaccine. 2010;28(15):2705–2715. doi: 10.1016/j.vaccine.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Robert Putnak J, Coller BA, Voss G, et al. An evaluation of dengue type-2 inactivated, recombinant subunit, and live-attenuated vaccine candidates in the rhesus macaque model. Vaccine. 2005;23(35):4442–4452. doi: 10.1016/j.vaccine.2005.03.042. [DOI] [PubMed] [Google Scholar]
- 129.Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine. 2012;30(Suppl 5):F123–F138. doi: 10.1016/j.vaccine.2012.04.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44(2–3):98–114. doi: 10.1159/000050037. [DOI] [PubMed] [Google Scholar]
- 131.Arora U, Tyagi P, Swaminathan S, Khanna N. Virus-like particles displaying envelope domain III of dengue virus type 2 induce virus-specific antibody response in mice. Vaccine. 2012 doi: 10.1016/j.vaccine.2012.12.016. [DOI] [PubMed] [Google Scholar]
- 132.Arora U, Tyagi P, Swaminathan S, Khanna N. Chimeric Hepatitis B core antigen virus-like particles displaying the envelope domain III of dengue virus type 2. J Nanobiotechnology. 2012;10:30. doi: 10.1186/1477-3155-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23(3):377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 134.Khanam S, Pilankatta R, Khanna N, Swaminathan S. An adenovirus type 5 (AdV5) vector encoding an envelope domain III-based tetravalent antigen elicits immune responses against all four dengue viruses in the presence of prior AdV5 immunity. Vaccine. 2009;27(43):6011–6021. doi: 10.1016/j.vaccine.2009.07.073. [DOI] [PubMed] [Google Scholar]
- 135.Raviprakash K, Wang D, Ewing D, et al. A tetravalent dengue vaccine based on a complex adenovirus vector provides significant protection in rhesus monkeys against all four serotypes of dengue virus. J Virol. 2008;82(14):6927–6934. doi: 10.1128/JVI.02724-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.White LJ, Sariol CA, Mattocks MD, et al. An alphavirus vector based tetravalent dengue vaccine induces a rapid and protective immune response in macaques that differs qualitatively from immunity induced by live virus infection. J Virol. 2013 doi: 10.1128/JVI.02298-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Danko JR, Beckett CG, Porter KR. Development of dengue DNA vaccines. Vaccine. 2011;29(42):7261–7266. doi: 10.1016/j.vaccine.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 138.Lu H, Xu XF, Gao N, Fan DY, Wang J, An J. Preliminary evaluation of DNA vaccine candidates encoding dengue-2 prM/E and NS1: Their immunity and protective efficacy in mice. Mol Immunol. 2012;54(2):109–114. doi: 10.1016/j.molimm.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 139.Beckett CG, Tjaden J, Burgess T, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine. 2011;29(5):960–968. doi: 10.1016/j.vaccine.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 140.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21(3):346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.George JA, Eo SK. Distinct Humoral and Cellular Immunity Induced by Alternating Prime-boost Vaccination Using Plasmid DNA and Live Viral Vector Vaccines Expressing the E Protein of Dengue Virus Type 2. Immune Netw. 2011;11(5):268–280. doi: 10.4110/in.2011.11.5.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Simmons M, Burgess T, Lynch J, Putnak R. Protection against dengue virus by non-replicating and live attenuated vaccines used together in a prime boost vaccination strategy. Virology. 2010;396(2):280–288. doi: 10.1016/j.virol.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 143.Crill WD, Hughes HR, Trainor NB, Davis BS, Whitney MT, Chang GJ. Sculpting humoral immunity through dengue vaccination to enhance protective immunity. Front Immunol. 2012;3:334. doi: 10.3389/fimmu.2012.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Deroeck D, Deen J, Clemens JD. Policymakers' views on dengue fever/dengue haemorrhagic fever and the need for dengue vaccines in four southeast Asian countries. Vaccine. 2003;22(1):121–129. doi: 10.1016/s0264-410x(03)00533-4. [DOI] [PubMed] [Google Scholar]
- 145.Who. Guidelines for the clinical evaluation of dengue vaccines in endemic areas. 2008 doi: 10.1016/j.vaccine.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 146.Mahoney RT, Francis DP, Frazatti-Gallina NM, et al. Cost of production of live attenuated dengue vaccines: a case study of the Instituto Butantan, Sao Paulo, Brazil. Vaccine. 2012;30(32):4892–4896. doi: 10.1016/j.vaccine.2012.02.064. [DOI] [PubMed] [Google Scholar]
- 147.Wilder-Smith A, Deen JL. Dengue vaccines for travelers. Expert Rev Vaccines. 2008;7(5):569–578. doi: 10.1586/14760584.7.5.569. [DOI] [PubMed] [Google Scholar]
- 148.Who. Vaccine introduction guidelines. WHO/IVB/05.18. 2005 [Google Scholar]
- 149.Durbin AP, Whitehead SS. The Dengue Human Challenge Model: Has the Time Come to Accept This Challenge? J Infect Dis. 2013 doi: 10.1093/infdis/jis749. [DOI] [PubMed] [Google Scholar]