Abstract
Background
Case reports suggest that duloxetine hepatotoxicity may arise but risk factors, presenting features, and clinical course are not well-described.
Aim
To describe the presenting features and outcomes of 7 well-characterized patients with suspected duloxetine hepatotoxicity.
Methods
Patients enrolled in the DILIN Prospective Study undergo an extensive laboratory and clinical evaluation to exclude competing etiologies of liver injury as well as a standardized assessment for causality and disease severity.
Results
Between 1/06 and 9/09, 6 of the 7 cases of DILI attributed to duloxetine were assessed as definite or very likely. Median patient age was 49 years, 6 (86%) were women and the median latency from drug initiation to DILI onset was 50 days. Six patients developed jaundice and the median peak ALT in the 5 patients with acute hepatocellular injury was 1633 IU/L. Ascites developed in 1 patient and acute renal dysfunction in 2 others (29%). All patients recovered without liver transplantation even though 3 had pre-existing chronic liver disease. Liver histology in 4 cases demonstrated varying patterns of liver injury.
Conclusions
Duloxetine hepatotoxicity developed within 2 months of drug intake and led to clinically significant liver injury. A spectrum of laboratory, histological, and extra-hepatic features were noted at presentation.
Keywords: DILI, Duloxetine, renal failure, causality assessment, RUCAM
Introduction
Mood disorders such as depression and anxiety disorder are highly prevalent in the general population1 and are associated with substantial morbidity and resource utilization.2 Specifically, depression has an estimated life-time prevalence of 16% and a 12-month prevalence of 6.6% in the United States.3 Therefore, it is not surprising that antidepressants are one of the most commonly used medications in adults. Although antidepressants have been classified into several classes based on the mechanism of action, they are typically categorized as “first generation” (monoamine oxidase inhibitors and tricyclic antidepressants) or “second generation” (selective serotonin reuptake inhibitors [SSRIs], inhibitors of the reuptake of both serotonin and norepinephrine [SNRIs], and atypicals that have unique modes of action). Duloxetine (Cymbalta®) is one of the newer SNRIs approved in 2004 with indications for use in the treatment of major depressive disorder, diabetic neuropathy, fibromyalgia and generalized anxiety disorder.4 Duloxetine is currently the second most commonly prescribed antidepressant with 14.4 million prescriptions written in 2008 alone.
Drug induced liver injury (DILI) from antidepressants has been reported with several agents, but data regarding duloxetine induced liver injury are scant. In a pooled analysis of 17,615 subjects, the incidence of serum alanine aminotransferase (ALT) levels > 3 times the upper limit of normal (ULN), > 5 ULN, and > 10 ULN were 1%, 0.5%, and 0.2%, respectively.5 Almost all subjects maintained normal values of alkaline phosphatase and total bilirubin with no case of jaundice and hepatocellular injury (“Hy’s law”) reported.5 However, post-marketing surveillance identified 406 cases with potential hepatotoxicity from duloxetine between 8/2004 and 8/2006.6 Of these, 58 cases were considered clinically significant. A careful review of these cases led to the following observations: (a) there was no dose-dependent increase in the incidence of hepatic injury, (b) a large number of cases occurred between 2 and 8 weeks of therapy and 74% with onset within 16 weeks, (c) 31% had either pre-existing liver disease or clinical risk factors for liver disease, and (d) there were two fatal cases possibly related to duloxetine.6 These cases prompted the manufacturer, to issue a “Dear Health Care Professional” letter and the product label was revised to include a warning that duloxetine “should ordinarily not be prescribed to a patient with substantial alcohol use or evidence of chronic liver disease.”
In this paper, we report 7 well-characterized consecutive cases of suspected DILI due to duloxetine enrolled in the DILIN Prospective Study. Our aim is to describe their presenting clinical and laboratory features, potential risk factors, and short-term outcomes.
Methods
Seven subjects enrolled into the DILIN Prospective Study with suspected liver injury attributed to duloxetine were identified between 1/2006 and 9/2009. The DILIN Prospective Study was approved by the IRB at all participating sites. All eligible subjects provided written informed consent and were required to meet predefined eligibility criteria including being seen within 6 months of DILI onset.9 At enrollment, a thorough evaluation was performed to assess for any competing or co-existing cause of liver injury. All clinical and laboratory data was reconciled with the source documents prior to the causality assessment as previously described.7 Briefly, all cases were scored by expert consensus opinion on a scale of 1 (Definite) to 5 (Unlikely). For the current analysis, a single reviewer (JHH) independently calculated Roussel Uclaf Causality Assessment Method (RUCAM) and DILIN severity scores that ranged from 1 (Asymptomatic) to 5 (Life-threatening).
Case series
Patient 1
A 37-year-old Caucasian man presented with a two day history of nausea, vomiting, upper abdominal pain, myalgia, and flu-like symptoms six weeks after starting duloxetine [60 mg once daily] for depression. Medical history included chronic alcoholism, hypothyroidism, obesity, and bipolar disorder. Concomitant medications included levothyroxine, fluoxetine and aripiprazole. He denied use of acetaminophen, over the counter (OTC) medications or herbal and dietary supplements (HDS). He had no history of liver disease but he reported his average alcohol consumption as 6 to 7 drinks per day 3 to 4 times per week for many years. Physical examination revealed jaundice and mild right upper quadrant abdominal tenderness. There was no rash, fever or signs of chronic liver disease. Initial laboratory tests showed a total bilirubin of 5.7 mg/dL, alkaline phosphatase (Alk P) 175 U/L, aspartate aminotransferase (AST) 7,500 U/L and alanine aminotransferase (ALT) 10,000 U/L. The white blood cell count was normal with 4% eosinophils and INR was elevated at 1.7 but serum albumin was normal (4.1 g/dL). Tests for viral hepatitis, serum auto-antibodies and abdominal imaging showed no evidence for a competing diagnosis (Table 1). Serum acetaminophen levels at admission were undetectable, and acetaminophen-cysteine protein adducts were not detected from a sample obtained within 12 hours after presentation.8 A transjugular liver biopsy showed extensive, predominantly zone-3 liver necrosis and moderate to marked steatosis associated with polymorphonuclear cells with a relatively small number of mononuclear cells (Figure 1). Mallory bodies were not seen.
Table 1.
Select clinical characteristics of cases with liver injury after exposure to duloxetine
| Clinical Characteristics | Total (N=7) |
|---|---|
| Demographics: | |
| Median Age (25th, 75th) Years | 49 (43, 54) |
| Female gender | 6 (86%) |
| Self-reported Race | |
| White or Caucasian | 6 (86%) |
| Black or African American | 1 (14%) |
| Median BMI (25th, 75th) (kg/m2) | 32.3 (28.4, 33.1) |
| Pre-Existing Medical Conditions: | |
| Underlying liver disease | 2 |
| Prior Drug Allergies | 3 (43%) |
| Alcohol Use | 1 (14%) |
| Diabetes/endocrine disorder | 1 (14%) |
| Neurological disease | 3 (43%) |
| Congestive heart failure | 0 |
| Chronic renal disease | 0 |
| Dosage, mg/day | |
| 60 | 5 (71%) |
| 30 | 2 (29%) |
| Median Duration of exposure (25th,75th), days | 46 (32, 57) |
| Latency period, Median (25th,75th), days | |
| Onset of symptoms | 50 (34,59) |
| Onset of lab abnormalities | 50 (35,56) |
| Onset of jaundice | 50 (40,57) |
| Reported symptoms profile | |
| Itching | 3 (43%) |
| Nausea | 6 (86%) |
| Pain | 5 (71%) |
| Rash | 1 (14%) |
| Fever | 3 (43%) |
| Median absolute eosinophils per mm3(25th,75th) | 128 (120–354) |
| Positive auto-antibodies | 3 (43%) |
| Other organ dysfunction | |
| Acute renal dysfunction | 2 (29%) |
| Altered mental status | 1 (14%) |
| Thrombocytopenia | 1 (14%) |
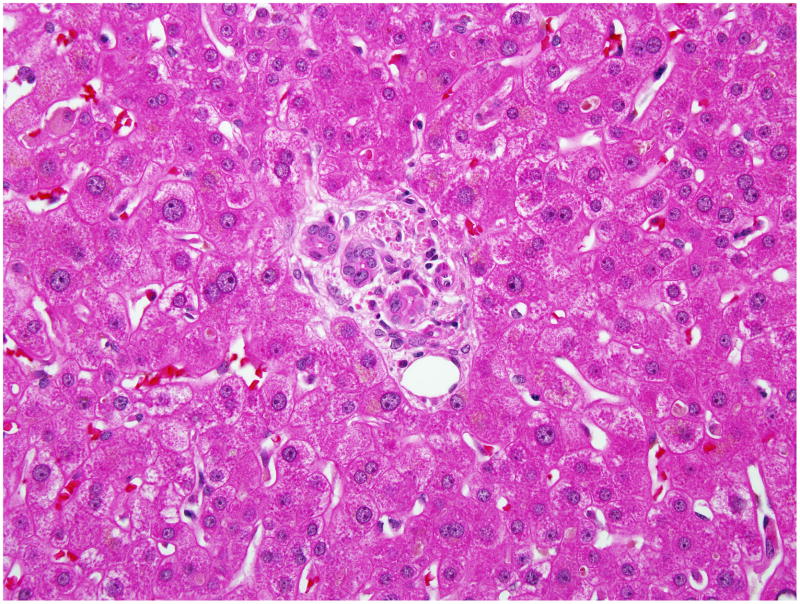
Figure 1. Liver biopsy of subject # 1.
Sections of the liver with H&E stain viewed under light microscopy and high power field showed centrilobular coagulative necrosis and marked fatty change. Inflammatory infiltrate is predominantly polymorphonuclear and relatively small number of mononuclear cells. The interlobular bile ducts were unremarkable.
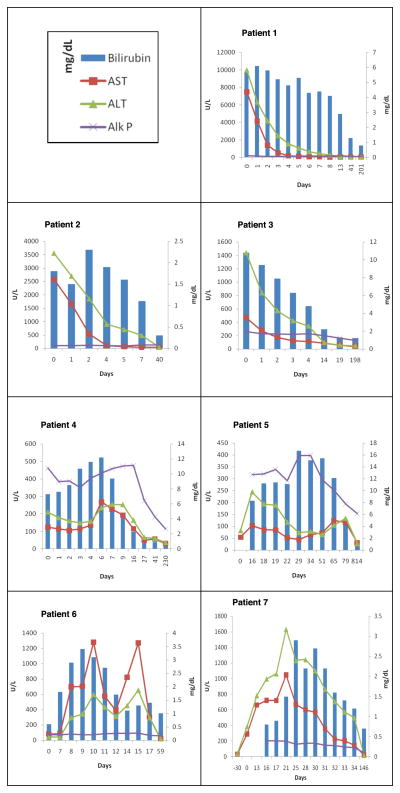
Duloxetine was discontinued and symptoms and laboratory abnormalities improved rapidly over the next two weeks (Figure 2). However, serum creatinine was 7.3 mg/dL on admission and rose to 12.3 mg/dL, eventually requiring temporary hemodialysis. He was discharged after more than a week in hospital and when seen in follow up, 6 weeks after onset, he was asymptomatic, had resumed therapy with levothyroxine, fluoxetine and aripiprazole and all liver and kidney tests were normal. Comment: This patient presented with severe hepatic necrosis and renal failure resembling toxic liver injury such as occurs with acetaminophen overdose. However, he denied use of analgesics and acetaminophen levels and adducts were not detected. The history of excessive alcohol use may have contributed to the severity of illness and affected the liver histology (accounting for steatosis and polymorphonuclear cell infiltrates), but the clinical course and liver biopsy were not compatible with acute alcoholic hepatitis alone. The DILIN causality score was “definite” and the RUCAM score was 9 (“highly probable”). The DILIN severity score was “severe” (4) (Table 3).
Figure 2. Biochemical profile and clinical course of liver injury of all 7 subjects.
Liver biochemistries include total bilirubin (T.Bili), aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (Alk P).
Table 3.
Causality and severity of outcomes of patients with duloxetine hepatotoxicity
| Patient | DILIN Causality Score | Expert RUCAM score | DILIN severity scale |
|---|---|---|---|
| 1 | 1 | 9 | 4 |
| 2 | 1 | 8 | 3 |
| 3 | 2 | 8 | 4 |
| 4 | 2 | 5 | 3 |
| 5 | 2 | 7 | 3 |
| 6 | 4*(possible) | 8 | 1 |
| 7 | 2 | 6 | 2 |
The DILIN causality score ranges from 1–5 [3- probable; 5- unlikely]. The DILIN severity of the DILI episode is categorized on a scale of 1(mild) to5 (fatal resulting in death or liver transplantation).
Please refer to the narrative for Case 6 for reasons resulting in assignment of causality score of 4.
Patient 2
A 29 year-old Caucasian woman with chronic leg and back pain on long-term oxycodone and ibuprofen was started on duloxetine [60 mg per day] as adjuvant therapy for pain. One month later, she stopped duloxetine because of symptoms of fatigue which she attributed to the medication. Two weeks later she continued to have fatigue and had developed nausea, abdominal pain and dark urine. At an emergency room, she was treated with fluids and intravenous ciprofloxacin and was sent home on oral ciprofloxacin; Two days later she was hospitalized. She had been taking oxycodone, ibuprofen, and birth control pills for several years. She did not drink alcohol and denied a history of liver disease or recent use of acetaminophen-containing products, OTC or herbal products. Blood tests showed total bilirubin of 1.8 mg/dL, Alk P 109 U/L, AST 2,586 U/L, and ALT 3,557 with INR of 2.0 and serum creatinine of 2.3 mg/dL. Serum acetaminophen levels were negative. The serum antinuclear antibody titer was 1:640, but smooth muscle antibody, immunoglobulin levels were normal, tests for hepatitis A, B and C were negative, and imaging showed no evidence for biliary obstruction (Table 1). With supportive care she experienced rapid clinical and laboratory improvement within a month of initial testing except for the ANA which was still positive (Figure 2). She restarted oxycodone and ibuprofen therapy. Comment: This patient presented with severe acute hepatic necrosis with renal injury approximately 3 weeks after stopping a one-month course of duloxetine. The recent exposure to ciprofloxacin provided another possible cause of liver injury, but her symptoms pre-dated the administration of this agent. The DILIN causality score was 2 “very likely”. The RUCAM score was 5 (probable) and the DILIN severity score was 4 (Table 3).
Patient 3
A 49-year-old Caucasian woman with presumed nonalcoholic fatty liver disease developed nausea, vomiting, abdominal pain, and anorexia 4 weeks after starting duloxetine [30 mg daily]. She consumed clonazepam long-term and also took low doses of acetaminophen (<1 gm daily) for several days before admission. She denied a previous history of liver disease, alcohol use, OTC, or HDS. She had undergone gastric bypass surgery for obesity 14 years previously, and was initially thought to have small bowel obstruction. On examination, she was jaundiced, dehydrated, and had marked abdominal tenderness. Laboratory tests showed a total bilirubin of 10.8 mg/dL, Alk P 259 U/L, AST 467 U/L and ALT 1,437 U/L. The INR was 2.0 and blood counts showed thrombocytopenia (33,000/mm3). Acetaminophen levels were 7.2 μg/mL (low normal range). Tests for hepatitis A, B and C were negative as were smooth muscle and antinuclear antibodies. A small bowel series showed no evidence of obstruction. Duloxetine was discontinued on admission and liver test abnormalities began to improve rapidly (Figure 2). She was discharged within four days due to improvement in liver tests, but was readmitted to another hospital 10 days later because of abdominal distension. Abdominal ultrasound demonstrated an enlarged liver with increased echogenicity and ascites, but no evidence of biliary obstruction and a normal spleen size. Serum bilirubin had fallen to 2.2 mg/dL and INR to 1.2 and the platelet count had risen to 149,000/mm3. A liver biopsy showed steatohepatitis and bridging hepatic fibrosis but not frank cirrhosis. An MRCP showed no evidence of biliary obstruction. She was treated with diuretics and her ascites ultimately improved. When seen in follow up two years later, she no longer required diuretics and liver tests were normal. Comment: This patient appeared to have an episode of acute hepatic necrosis superimposed upon presumed nonalcoholic steatohepatitis which led to hepatic decompensation. The DILIN causality score was 2 “very likely”, RUCAM score was 8 and DILIN severity score was 4 (jaundice and hepatic decompensation) (Table 3).
Patient 4
A 56-year old Caucasian woman with depression and sciatica was started on duloxetine [30 mg/day] for pain control. Nine days later, the dose was increased to 60 mg/day. Approximately 9 weeks later, she presented with chest pain, non-specific abdominal discomfort and nausea. She had taken multiple medications for low back pain in the past, but for the previous several weeks was only taking oxycodone, prednisone and low doses of acetaminophen. She denied use of alcohol, OTC, or HDS. Her physical examination was largely unremarkable except for jaundice. A comprehensive inpatient cardiac evaluation identified no evidence of myocardial ischemia or dysfunction. Initial blood tests showed total bilirubin of 7.6 mg/dL, Alk P 384 U/L, AST 114 U/L, and ALT 179 U/L. Tests for viral hepatitis and auto-antibodies were negative, and abdominal imaging identified no evidence for biliary obstruction (Table 1). The prothrombin time was normal (INR 0.9) and serum albumin 3.4 g/dL. A liver biopsy showed marked portal infiltrates composed of activated lymphocytes, which breached the limiting plates. There was also lymphocytic lobular infiltrates in a sinusoidal pattern, with a “pseudomononucleosis” pattern but stains for EBV antigens were negative. Kupffer cells appeared activated and rare foci of necrosis were seen as well as mild macro- and medium droplet fat. Endothelitis was present in central and portal venules. (Figure 3A and 3B). Trichrome stain failed to reveal significant fibrosis. Duloxetine was stopped upon admission and symptoms and laboratory tests began to improve after 4 or 5 days, normalizing over the next five weeks (Figure 2). Comment: This patient presented with a cholestatic hepatitis slightly more than two months after starting duloxetine. No other cause of acute liver injury was identified and the subsequent course was typical for a drug-induced cholestatic injury. DILIN causality score was 1 as “definite”, RUCAM score was 8 and severity grade 3+ (jaundice and hospitalization without hepatic failure) (Table 3).
Figure 3.
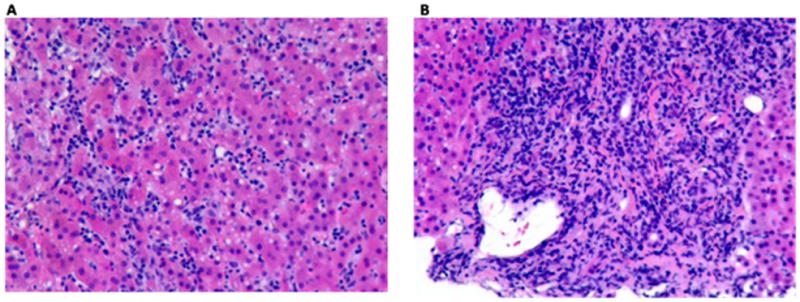
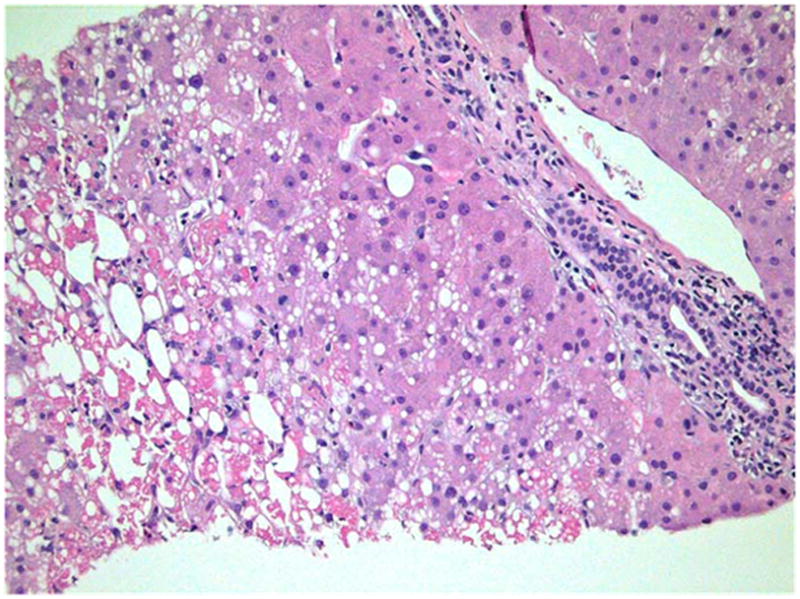
Figure 3A and 3B: Liver Biopsy of subject # 4
Sections of the liver with H&E stain viewed under light microscopy and high power field.
Figure 3A: The lobules showed marked inflammatory infiltrate which consisted of necroinflammatory foci as well as prominent infiltration of sinusoids by lymphocytes.
Figure 3B: A heavy lymphocytic inflammatory infiltrate was present in portal tracts and this infiltrate breached the limiting plate. Endothelitis was seen and the bile ducts were preserved.
Patient 5
A 49 year old Caucasian woman developed nausea, fatigue, dark urine, and itching followed by jaundice and weight loss, 5 weeks after starting duloxetine (60 mg daily) for chronic pain and depression. She had no history of liver disease and denied use of alcohol, acetaminophen, OTC, or HDS. Physical examination showed jaundice, without fever, rash or signs of chronic liver disease. Laboratory testing showed a total bilirubin of 8.3 mg/dL, Alk P 317 U/L, AST 103 U/L and ALT 245 U/L. Serum acetaminophen levels were undetectable. Tests for viral hepatitis were negative as was antinuclear antibody, but low levels of anti-smooth muscle antibody were detected (32 units; normal <30 units). Serum immunoglobulin levels were normal. Duloxetine was stopped on admission but she remained jaundiced, and serum bilirubin rose to 16.7 mg/dL with a cholestatic pattern of serum enzyme elevations (Figure 2). Repeat imaging studies showed no evidence of biliary obstruction and a liver biopsy showed intrahepatic cholestasis without bile duct injury or loss (Figure 4A and 4B). There was no fibrosis and scant portal inflammation or evidence of chronic hepatitis. Jaundice persisted for more than two months but ultimately resolved. During long-term follow up, however, she continued to have mild elevations in serum AP (153 U/L, 2 years after onset). Comment: This patient developed a prolonged and symptomatic cholestatic hepatitis which progressively worsened for several weeks even after stopping her one-month course of duloxetine. DILIN causality score was 2 “very likely” RUCAM score was 7 (“probable”) and severity score was 3 (jaundice and hospitalization) (Table 3).
Figure 4.
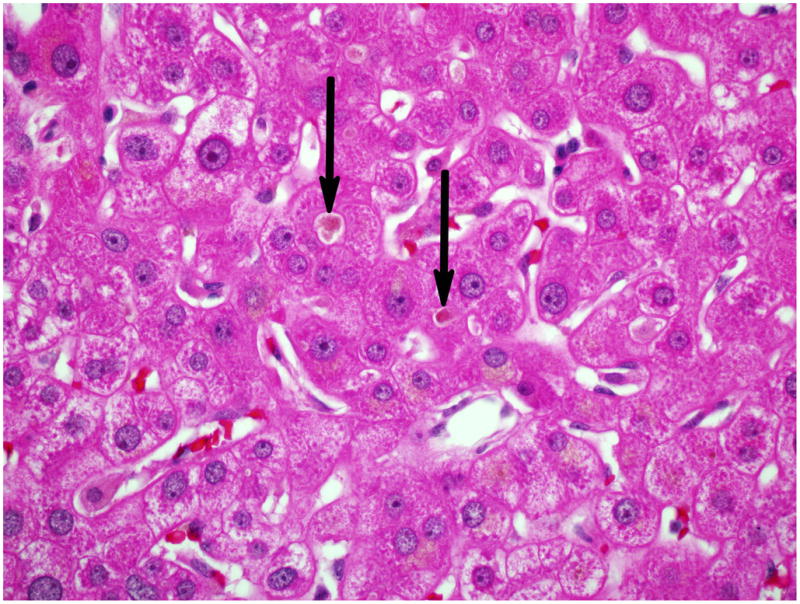
Figure 4A and 4B: Liver Biopsy of subject # 5
Figure 4A: Only minimal portal and lobular inflammation was seen. Most portal areas showed only a few inflammatory cells, mainly neutrophils and eosinophils (400x, H&E).
Figure 4B: Canalicular and hepatocellular cholestasis was easily identified (arrows) (600x, H&E).
Patient 6
A 58-year-old, African-American woman with chronic hepatitis C, diabetes, obesity, gout, chronic anxiety, and chronic low back pain had duloxetine [30 mg daily] added to a chronic regimen of amitriptyline, clonazepam, oxycodone, propranolol, allopurinol, colchicine, and acetaminophen. Two weeks later she was brought to the emergency room after being found unresponsive at home. She was stuporous but vital signs were stable. She was admitted to the intensive care unit and started on antibiotics for suspected meningitis and levetiracetam for persistent obtundation. A toxicology screen showed the presence of benzodiazepines and opiates but no alcohol. Initial laboratory tests showed minimal liver test abnormalities with total bilirubin 0.6 mg/dL, Alk P 71 U/L, AST 81 U/L and ALT 42 U/L. Duloxetine was given intermittently, but was stopped when serum enzymes rose (ALT 125, AST 289 U/L, Alk P 78 U/L). Serum aminotransferase levels peaked several days later and then began to fall (Figure 2). Duloxetine was restarted, but held again when ALT levels rose from 310 to 654 U/L and AST from 390 to 1269 U/L, thereafter falling to normal. Tests for hepatitis A and B were negative. She was positive for both antibody to HCV and HCV RNA, which she was known to have had for several years. Imaging of the liver by ultrasound and CT scan showed a normal appearing liver and spleen and no evidence of biliary obstruction. After 3 weeks in the hospital her sensorium had cleared and serum enzymes had fallen to near normal levels (ALT 61 U/L, AST 39 U/L) (Figure 2) and she was discharged. Comment: This patient had an unexplained episode of confusion and obtundation that occurred two weeks after starting duloxetine. During her hospitalization, restarting duloxetine was followed by prompt rises in serum ALT and AST levels, without jaundice. While the timing of serum enzyme elevations were supportive of the role of duloxetine in their etiology, her underlying chronic hepatitis C and the multiple medications and procedures that she underwent during the hospitalization made it difficult to assign causality to duloxetine alone. DILIN causality score was 4 “possible”, RUCAM score was 8 and the severity score 1 (serum enzyme elevations without jaundice) (Table 3).
Patient 7
A 52-year-old Caucasian woman with rheumatoid arthritis and coronary artery disease developed nausea, vomiting and abdominal pain 8 weeks after starting duloxetine [60 mg daily]. She was also taking methotrexate (started 3 years previously) and monthly infliximab infusions (for the previous 4 months). She had no history of liver disease, had normal liver tests on methotrexate and did not drink alcohol or take OTC or HDS. Physical examination was unremarkable; she was anicteric and afebrile. Laboratory testing showed total bilirubin of 0.8 mg/dL, Alk P 203 U/L, AST 719 U/L and ALT 996 U/L. Tests for hepatitis A, B, and C were negative and auto-antibodies were not present. An ultrasound showed no evidence of biliary obstruction. A liver biopsy demonstrated a mild hepatitis with lobular and portal inflammation and mild fibrosis. The dose of duloxetine was decreased and she was started on prednisone [40 mg daily]. Because of a lack of improvement in liver tests (Figure 2), duloxetine was discontinued 22 days after initial presentation, whereupon liver tests rapidly improved. Comment: This patient presented with an acute hepatitis like syndrome without jaundice which was initially not attributed to duloxetine. After a lack of improvement and development of mild jaundice, however, duloxetine was stopped, and liver tests subsequently improved and were normal during followed up (total bilirubin 0.6 mg/dL, Alk P 48 U/L, AST 22 U/L and ALT 22 U/L approximately 100 days after the initial event was recognized). The DILIN causality score was 2 or “very likely”, RUCAM score was 6 and severity score was 2 (jaundiced but not hospitalized) (Table 3).
Results
A total of 7 subjects were identified in the DILIN database of approximately 600 sequential cases (~1% of total) with suspected hepatotoxicity from exposure to duloxetine (Table 1). In six cases, duloxetine was the only implicated agent and causality rated either definite (n=2) or very likely (n=4). The median age of patients was 49 years and all except one (86%) were women (Table 1). The median time to onset of symptoms or laboratory abnormalities was 50 days. Five patients had a hepatocellular pattern of serum enzyme elevations and two a cholestatic pattern. There were wide variations in the peak values of liver tests (Table 2). Skin rash, fever and eosinophilia, and features of immunoallergic or autoimmune hepatitis were not common. All patients showed improvement in their liver test abnormalities during the follow up period. Three patients had underlying chronic liver disease from hepatitis C, alcoholic liver disease, and nonalcoholic steatohepatitis. In one patient with underlying chronic liver disease, new onset ascites was observed. Acute renal insufficiency occurred in two patients and bone marrow suppression (thrombocytopenia and mild leucopenia) in one. Four of the seven cases were considered severe, demonstrating evidence of hepatic failure or accompanying bone marrow or renal failure, but all patients recovered without liver transplantation. No deaths were reported during the study follow up.
Table 2.
Pattern of liver injury, biochemistries and time-course for improvement
| Patient | Pattern of liver injury | Peak T. Bili (mg/dL) | Peak ALT (U/L) | Peak Alk P (U/L) | Time to improve by ≥ 50% from peak (days) | ||
|---|---|---|---|---|---|---|---|
| ALT | T.Bili | Alk P | |||||
| 1 | Hepatocellular | 6.1 | 10,000 | 175 | 3 | 13 | 41 |
| 2 | Hepatocellular | 2.3 | 3,557 | 109 | 4 | 5 | - |
| 3 | Hepatocellular | 10.8 | 467 | 259 | 2 | 4 | 15 |
| 4 | Cholestatic | 12.2 | 265 | 468 | 21 | 4 | 18 |
| 5 | Cholestatic | 16.7 | 245 | 397 | 6 | 50 | 50 |
| 6 | Hepatocellular | 3.4 | 654 | 92 | 3 | 5 | - |
| 7 | Hepatocellular | 2.9 | 1,633 | 203 | 11 | 8 | - |
Discussion
Spontaneous reports of hepatic injury from duloxetine use to the manufacturer and FDA have raised concerns about the safety of this widely used drug. However, inherent limitations associated with such a reporting system makes causality assessment difficult and inconclusive due to incomplete information. An identifiable clinical signature and pattern of liver injury often cannot be recognized from such scant data. To date, there is only one published case report of duloxetine hepatotoxicity.9 In this report, the investigators described various tests including serology, imaging and liver biopsy to make an argument for duloxetine hepatotoxicity. Unfortunately, this patient also received concomitant mirtazapine making the case less compelling. The current series of seven patients with liver injury after exposure to duloxetine provide a more detailed description of presentations and outcomes of liver injury associated with this agent. These 7 cases were enrolled between 2006 (two years after FDA approval) and 2008 at three sites participating in the DILIN prospective study. The prospective nature of the study design and use of standardized case report forms for data capture insured a uniformly high data capture rate and exclusion of competing causes of liver injury. The use of ‘expert consensus’ opinion for causality assessment not only insured a thorough evaluation to conclusively exclude competing etiologies but also addressed the issue of wide spread variability in RUCAM scores assigned by different reviewers.10 In the 6 cases attributed to duloxetine alone in our series, all of them were scored as definite or highly likely while the 1 case with multiple suspect drugs had a score of ”possible” duloxetine hepatotoxicity.
Several observations from this case series merit attention. Hepatotoxicity from duloxetine appears to be more common at a daily dose of 60 mg compared to 30 mg (5 vs. 2 respectively). It is noteworthy that 6 of the affected patients were women, raising the possibility that female sex might be a risk factor for developing severe duloxetine hepatotoxicity. The onset of symptoms of liver injury averaged 6 to 7 weeks after staring the drug. Features of hypersensitivity (rash, fever, and eosinophilia) and autoimmunity were not prominent. Three patterns of hepatic injury occurred; three patients had severe acute hepatic necrosis with a precipitous rise in serum aminotransferase levels, damage to other organs (bone marrow or kidney) and rapid recovery; two patients had a cholestatic hepatitis; and two had serum ALT and AST elevations with no or minimal jaundice. Of particular significance, two patients with acute hepatic necrosis developed concurrent evidence of acute renal injury and one required hemodialysis. Therefore, duloxetine hepatotoxicity may be severe at presentation.
Based on post marketing adverse event reports, alcohol abuse and pre-existing chronic liver disease have been cited as potential risk factors for duloxetine hepatotoxicity. Underlying liver disease has also been implicated in patients with isoniazid and antiretroviral hepatotoxicity.11–14 In this case series one patient admitted to significant alcohol consumption and two others appeared to have underlying chronic liver disease (one with nonalcoholic steatohepatitis (NASH) and with chronic hepatitis C). The patient with NASH developed signs of acute liver decompensation with new onset ascites and jaundice. All patients showed improvement in their liver test abnormalities during the follow up period and none required liver transplantation. No deaths were reported during the study follow up.
Patients with underlying liver disease may be at increased risk of developing DILI due to increased intrahepatic immune activity, altered drug toxification/detoxification pathways, etc. Since patients with mood disorders frequently have underlying liver disease, a careful consideration of the risk versus benefit of duloxetine in patients with liver disease is recommended. As a minimum, assessing baseline liver biochemistries would be advisable as well as repeat laboratory monitoring during the first 12 to 16 weeks of duloxetine therapy. In addition, a careful and slow titration of the duloxetine dose would be advisable particularly in patients with pre-existing liver disease. The lack of hypersensitivity features noted in our series does not entirely exclude an immunological mechanism in duloxetine hepatotoxicity as was recently shown with flucloxacillin hepatoxicity. Therefore, collection of DNA samples from well-characterized cases like these enrolled in the DILIN study and other registries around the world may allow for future mechanistic investigation of the potential role of host genetic factors in these rare adverse events.
In summary, duloxetine-induced liver injury appears to be idiosyncratic in nature and its onset is within 2 months after its initiation. Several patterns of hepatic injury (signatures) occur, including hepatocellular and cholestatic features. The available liver histology is highly variable. Acute kidney failure or other organ involvement may occur. The hepatic injury can be severe although full recovery is the usual outcome. This report raises the possibility that duloxetine administered at 60 mg per day is more hepatotoxic than 30 mg daily dose. The relationship between duloxetine hepatotoxicity and underlying liver disease and female sex requires further investigation. Physicians using this drug should be aware of the spectrum of liver injury and consider duloxetine as another agent capable of causing life-threatening liver injury. In patients with underlying liver disease, slow and deliberate dosage titration with careful monitoring for adverse hepatic effects in the first few months of duloxetine therapy is suggested.
Acknowledgments
We are grateful for Laura P. James, MD (University of Arkansas for Medical Sciences) for measuring acetaminophen adducts from the serum of patient 1. We thank research participants for their enrollment in the DILIN studies and research coordinators for their dedication and contributions to the DILIN.
Source of Funding: The DILIN network is supported by the National Institute of Diabetes and Digestive and Kidney Diseases under the following cooperative agreements: 1U01DK065021, 1U01DK065193, 1U01DK065201, 1U01DK065193, 1U01DK065184, 1U01DK065211, 1U01DK065238, and 1U01DK065176.
Abbreviations
- DILIN
Drug-induced Liver Injury Network
- AP
alkaline phosphatase
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- ANA
antinuclear antibody
- INR
international normalized ratio
Footnotes
Conflicts of Interest: Dr. Chalasani has received consulting fees regarding drug-induced liver injury in the past 12 months from the following companies: Teva pharmaceuticals, KaroBio, J&J, Abbott, Salix Pharmaceuticals, and Gilead. He has received research support from Eli Lilly for research on drug-induced liver disease. In the preceding 12 months, Dr. Bonkovsky served as a paid advisor to Clinuvel, Inc., Novartis Pharmaceuticals and Lundbeck SA. He is on the speakers’ bureau of Lundbeck. He receives support for research studies from The National Institutes of Health, The American Porphyria Foundation, Clinuvel, Novartis, and Vertex. During the past 12 months, Dr. Bonkovsky has served as an expert witness for plaintiffs in litigation regarding suspected drug-induced liver injury. Dr. Fontana served as a paid consultant to Glaxo-Smith-Kine, Hoffman-La Roche, Bristol-Myer-Squibbs, and Novartis. Dr. Vuppalanchi has served on the Roche Speaker’s bureau. Drs. Hoofnagle, Kleiner, Hayashi, and Saxena have no conflicts to declare.
Contributor Information
Raj Vuppalanchi, Email: rvuppala@iupui.edu.
Paul Hayashi, Email: paul_hayashi@med.unc.edu.
Robert J Fontana, Email: rfontana@med.umich.edu.
Herbert Bonkovsky, Email: Herbert.bonkovsky@carolinashealthcare.org.
Romil Saxena, Email: rsaxena@iupui.edu.
David Kleiner, Email: Kleinerd@mail.nih.gov.
Jay H. Hoofnagle, Email: Hoofnaglej@extra.niddk.nih.gov.
References
- 1.Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–75. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- 2.Williams N, Simpson AN, Simpson K, Nahas Z. Relapse rates with long-term antidepressant drug therapy: a meta-analysis. Hum Psychopharmacol. 2009;24:401–8. doi: 10.1002/hup.1033. [DOI] [PubMed] [Google Scholar]
- 3.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 4.Hunziker ME, Suehs BT, Bettinger TL, Crismon ML. Duloxetine hydrochloride: a new dual-acting medication for the treatment of major depressive disorder. Clin Ther. 2005;27:1126–43. doi: 10.1016/j.clinthera.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Gahimer J, Wernicke J, Yalcin I, Ossanna MJ, Wulster-Radcliffe M, Viktrup L. A retrospective pooled analysis of duloxetine safety in 23,983 subjects. Curr Med Res Opin. 2007;23:175–84. doi: 10.1185/030079906X162719. [DOI] [PubMed] [Google Scholar]
- 6.Wernicke J, Acharya N, Strombom I, Gahimer JL, D’Souza DN, DiPietro N, Uetrecht JP. Hepatic effects of duloxetine-II: spontaneous reports and epidemiology of hepatic events. Curr Drug Saf. 2008;3:143–53. doi: 10.2174/157488608784529198. [DOI] [PubMed] [Google Scholar]
- 7.Fontana RJ, Watkins PB, Bonkovsky HL, Chalasani N, Davern T, Serrano J, Rochon J. Drug-Induced Liver Injury Network (DILIN) prospective study: rationale, design and conduct. Drug Saf. 2009;32:55–68. doi: 10.2165/00002018-200932010-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.James LP, Wilson JT, Simar R, Farrar HC, Kearns GL, Simpson PM, Hinson JA, Pumford NR. Evaluation of occult acetaminophen hepatotoxicity in hospitalized children receiving acetaminophen. Pediatric Pharmacology Research Unit Network. Clin Pediatr (Phila) 2001;40:243–8. doi: 10.1177/000992280104000501. [DOI] [PubMed] [Google Scholar]
- 9.Hanje AJ, Pell LJ, Votolato NA, Frankel WL, Kirkpatrick RB. Case report: fulminant hepatic failure involving duloxetine hydrochloride. Clin Gastroenterol Hepatol. 2006;4:912–7. doi: 10.1016/j.cgh.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Rockey DC, Seeff LB, Rochon J, Freston J, Chalasani N, Bonacini M, Fontana RJ, Hayashi PH. Causality assessment in drug-induced liver injury using a structured expert opinion process: comparison to the Roussel-Uclaf causality assessment method. Hepatology. 51:2117–26. doi: 10.1002/hep.23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopanoff DE, Snider DE, Jr, Caras GJ. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis. 1978;117:991–1001. doi: 10.1164/arrd.1978.117.6.991. [DOI] [PubMed] [Google Scholar]
- 12.McGlynn KA, Lustbader ED, Sharrar RG, Murphy EC, London WT. Isoniazid prophylaxis in hepatitis B carriers. Am Rev Respir Dis. 1986;134:666–8. doi: 10.1164/arrd.1986.134.4.666. [DOI] [PubMed] [Google Scholar]
- 13.Gronhagen-Riska C, Hellstrom PE, Froseth B. Predisposing factors in hepatitis induced by isoniazid-rifampin treatment of tuberculosis. Am Rev Respir Dis. 1978;118:461–6. doi: 10.1164/arrd.1978.118.3.461. [DOI] [PubMed] [Google Scholar]
- 14.Dieterich D. Managing antiretroviral-associated liver disease. J Acquir Immune Defic Syndr. 2003;34 (Suppl 1):S34–9. doi: 10.1097/00126334-200309011-00006. [DOI] [PubMed] [Google Scholar]