SUMMARY
Since its first appearance in the US in 1999, West Nile virus (WNV) has emerged as the most common cause of epidemic meningoencephalitis in North America. In the 6 years following the 1999 outbreak, the geographic range and burden of the disease in birds, mosquitoes and humans has greatly expanded to include the 48 contiguous US and 7 Canadian provinces, as well as Mexico, the Caribbean islands and Colombia. WNV has shown an increasing propensity for neuroinvasive disease over the past decade, with varied presentations including meningitis, encephalitis and acute flaccid paralysis. Although neuroinvasive disease occurs in less than 1% of infected individuals, it is associated with high mortality. From 1999–2005, more than 8,000 cases of neuroinvasive WNV disease were reported in the US, resulting in over 780 deaths. In this review, we discuss epidemiology, risk factors, clinical features, diagnosis and prognosis of WNV meningoencephalitis, along with potential treatments.
Keywords: acute flaccid paralysis, encephalitis, meningitis, neuroinvasive disease, West Nile virus
INTRODUCTION
West Nile virus (WNV) is an arthropod-borne flavivirus that was first isolated from the blood of a febrile Ugandan woman in 1937. The virus was subsequently associated primarily with epidemics of flu-like febrile illness—and sporadically with encephalitis—throughout Africa, Asia and Europe. WNV had never been detected in North America before its first appearance in the US in 1999, but in the six ensuing years, it has emerged as the most common cause of epidemic meningoencephalitis in this region. In outbreaks occurring over the past decade, WNV has shown an increasing propensity to cause neuro invasive disease, including meningitis, encephalitis and acute flaccid paralysis (AFP). The largest epidemics of neuroinvasive WNV ever reported occurred in the US in 2002 and 2003.
In this review, we first summarize the epidemiology and transmission of WNV since its initial appearance in North America. We then review the salient clinical features of WNV neuroinvasive disease, including meningitis, encephalitis and AFP syndrome. Laboratory and radiographic findings are summarized, and risk factors, outcome and long-term prognosis are discussed. We end with a discussion of current treatment modalities and prospects for future therapies, including vaccination strategies.
EPIDEMIOLOGY AND TRANSMISSION
Epidemiology
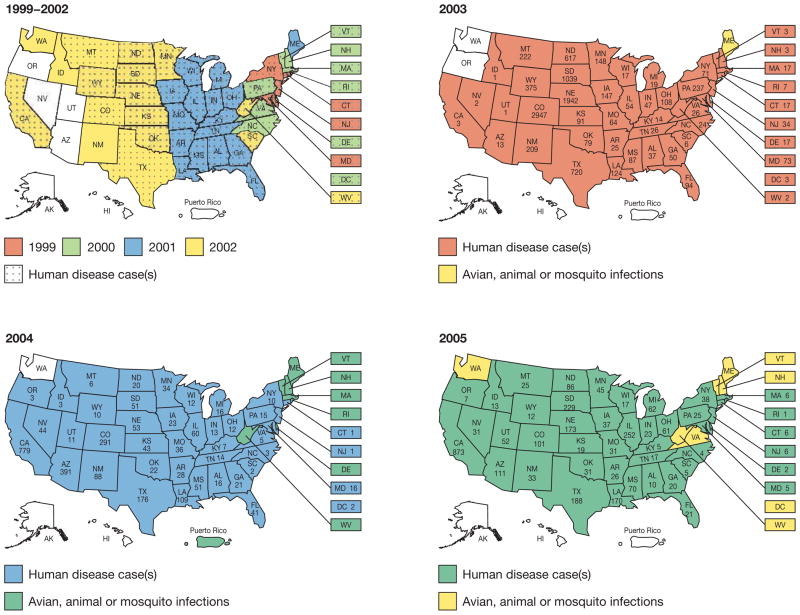
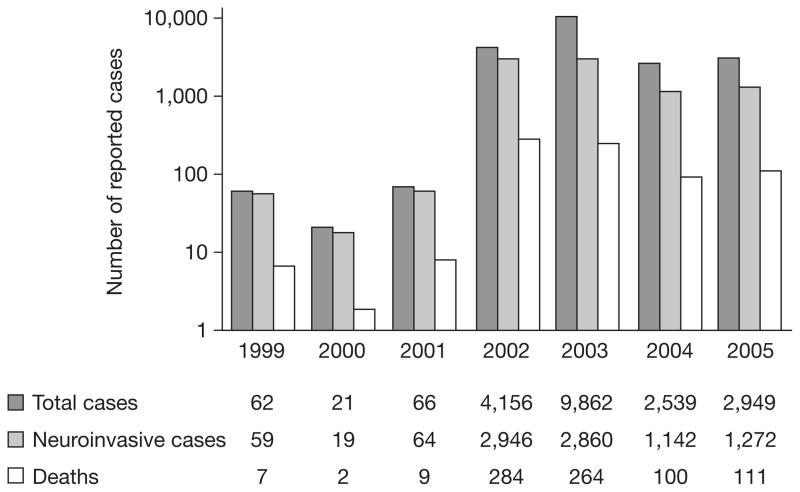
WNV disease first appeared in North America in the summer of 1999, with the simultaneous occurrence of an unusual number of deaths of exotic birds and crows in the New York City (NYC) Metropolitan Area. This first appearance was followed by an outbreak of 62 cases of encephalitis in humans, resulting in 7 deaths.1 Since this outbreak, the geographic range and burden of disease in birds, mosquitoes and humans has greatly expanded to include the 48 contiguous US States (Figure 1), as well as 7 Canadian provinces, Mexico, the Caribbean islands, and Colombia.2–6 From 1999–2005, 19,506 cases of human WNV disease were reported in the US—including 8,362 that were characterized as neuroinvasive—resulting in 782 deaths (Figure 2).3,7
Figure 1.
Geographical distribution of West Nile virus in the US 1999–2005 (source: Centers for Disease Control and Prevention).7
Figure 2.
Numbers of human West Nile virus cases in the US from 1999–2005 (source: Centers for Disease Control and Prevention).7
Analysis of sequences of genome fragments isolated from dead birds and mosquitoes by REVERSE TRANSCRIPTASE POLYMERASE CHAIN REACTION (RT-PCR) led to the identification of WNV as the causative agent of the 1999 NYC epidemic. The virus sequenced from bird and human cases was closely related to a virus isolated in 1998 from the brain of a goose in Israel.8 Genetic sequencing of WNV strains has identified two WNV lineages, the first of which is subdivided into four clades. All North American isolates have so far been classified as Lineage 1, Clade B, and are closely related to strains from Israel, with over 99.7% nucleotide sequence homology.9 Detailed phylogenic analysis of isolates was conducted across North America to determine whether a dominant genotype has arisen, and to better understand how the virus has evolved as its distribution has expanded. These studies provide evidence that a dominant variant has emerged across much of North America. Isolates with phenotypic differences from the dominant North American WNV variant, including apparent attenuation in animal models, have been reported.10 There is no evidence for strong selection pressure for any phenotypic differences,2 although one recent study indicated that the emergence of the dominant variant might be associated with a fitness advantage in mosquito infectivity and transmissibility.11
Since its introduction into North America, WNV has spread prolifically within birds (over 200 species have been infected), and has infected an unprecedented number of mosquito species (43 of 174 North American species).4 Corvids (crows, magpies and jays), house sparrows, house finches and grackles seem to be highly competent reservoirs for mosquito infection with WNV. By contrast, mammals (including humans) do not develop sufficiently high bloodstream titers of WNV to play a significant role in transmission. In mosquitoes, WNV can be transmitted vertically, and can overwinter in hibernating females,12 providing the mechanisms for viral persistence and re-emergence each spring.3
Transmission
Transmission of WNV to humans occurs predominantly following a bite from an infected mosquito, which acquires the virus after feeding on vertebrate amplifying hosts, primarily birds.3 Peak transmission occurs between July and October, but cases have occurred as early as April and as late as December. Despite high attack rates during earlier epidemics in Africa (serological studies indicated that more than 50% of the susceptible population exposed to the virus was infected), recent seroprevalence surveys indicate that less than 3% of affected US populations acquired the infection during epidemic transmission periods.13 These levels of seroprevalence are thought to be too low to decrease the frequency of epidemics or modulate their intensity through protective immunity.3
Although person-to-person transmission of WNV does not generally occur, cases have resulted from transfusion of blood products and organ transplantation, as well as following intrauterine, percutaneous (occupational) or breastfeeding exposure. Other isolated reports of possible routes of transmission include respira tory aerosol (in workers at a turkey farm) and dialysis.3
Twenty-three cases of WNV infection via transfusion of blood products (from asymptomatic, viremic donors) were reported in the US in 2002,14 leading to the mandate for nucleic-acid-amplification-based screening of donor minipools. After FDA approval and widespread implementation of screening, over 1,000 potentially WNV-viremic blood donations were intercepted during the 2003–2004 period.15 Nevertheless, seven transfusion-related cases of WNV were reported during this period, prompting investigations into more-sensitive screening methodologies, including single-donor screening, in areas of high WNV activity.16 A single donor (who acquired WNV by transfusion of blood products) transmitted WNV to four organ transplant recipients, all of whom went on to develop severe neuroinvasive disease;17 other cases have also been reported.18,19
Although the spectrum of disease following intrauterine transmission is not yet fully understood, in one case of proven intrauterine transmission the affected infant had chorioretinitis and severe cerebral abnormalities, including microcephaly and intracranial calcifications.20 The Centers for Disease Control (CDC) are gathering data on pregnancy outcomes for approximately 70 women with WNV illness during pregnancy, but unpublished data indicate that most of the women analyzed to date have delivered healthy infants.3 Thorough assessment of the fetus or infant is recommended if WNV infection is documented in a pregnant mother.21 Two laboratory-acquired cases of WNV infection have been reported,22 neither of which resulted in severe disease.
DIAGNOSIS
Clinical features
In animal models of WNV infection, following subcutaneous inoculation, the virus spreads to regional lymph nodes and spleen, and subsequently reaches the bloodstream before disseminating to the CNS.23 WNV-infected individuals are asymptomatic in 80% of cases. Symptomatic illness develops 2–14 days following inoculation; 20% of patients develop West Nile fever, a self-limited flu-like illness characterized by fever, myalgia, headache and gastrointestinal disturbance (25–30%), with an associated maculopapular rash in 25–50% of cases.24 Neuroinvasive disease occurs in less than 1% of cases (1 in 150 infected individuals), following viral penetration of the blood–brain barrier and direct invasion of neurons, particularly those in the brainstem, deep nuclei, and anterior horn of the spinal cord. In mouse models, WNV has been shown to increase blood–brain barrier permeability by inducing a Toll-like-receptor-3 (Tlr3)-dependent inflammatory response.25 The clinical and laboratory criteria for distinguishing neuroinvasive from non-neuroinvasive arthropod-borne viral diseases (including WNV) have recently been updated.7 Neuroinvasive presentations (Box 1) are varied and include aseptic meningitis, meningoencephalitis and AFP syndrome (a poliomyelitis-like illness).5,26–30 Brainstem encephalitis, cerebellitis, movement disorders, cranial neuropathies, polyneuropathy/radiculopathy, chorioretinitis and optic neuritis are also recognized WNV neurological presentations. The proportion of neuroinvasive disease manifesting as meningitis, as opposed to encephalitis or myelitis, has varied greatly within a given epidemic season and locale.
Box 1. Diagnostic criteria for neuroinvasive West Nile virus disease.
West Nile meningitis
Clinical signs of meningeal inflammation (collectively known as meningismus), including NUCHAL RIGIDITY, KERNIG SIGN, BRUDZINSKI’S SIGN, photophobia or phonophobia
Additional evidence of acute infection, including one or more of the following: fever (temperature >38 °C) or hypothermia (temperature <35 °C); cerebrospinal fluid pleocytosis (>5 leukocytes/mm3); peripheral leukocyte count >10,000/mm3; or neuroimaging findings consistent with acute meningeal inflammation
West Nile encephalitis
Encephalopathy (depressed or altered level of consciousness, lethargy, or personality change lasting 24 hours)
Additional evidence of CNS inflammation, including two or more of the following: fever (temperature >38 °C) or hypothermia (temperature <35 °C); cerebrospinal fluid pleocytosis (>5 leukocytes/mm3); peripheral leukocyte count >10,000/mm3; neuroimaging findings consistent with acute inflammation (with or without involvement of the meninges) or acute demyelination; presence of focal neurological deficit; meningismus; electroencephalography findings consistent with encephalitis; or seizures (either new-onset or exacerbation of previously controlled)
Acute flaccid paralysis
Acute onset of limb weakness with marked progression over 48 hours
At least two of the following: asymmetry of weakness; areflexia/hyporeflexia of affected limb(s); absence of pain, paresthesia, or numbness in affected limb(s); cerebrospinal fluid pleocytosis (>5 leukocytes/mm3) and elevated protein levels (>450 mg/l); electrodiagnostic studies consistent with an anterior horn cell process; or spinal cord MRI documenting abnormal increased signal in the anterior gray matter
Modified with permission from reference 26 © (2003) American Medical Association.
Several clinical features might provide a clue to the diagnosis of CNS infection with WNV.5 As in other forms of encephalitis, nonspecific symptoms such as fever (in 70–100% of patients), headache (50–100%) and altered mental status (50–100%) are common. Vomiting (30–75%), diarrhea (15–35%) and rash (5–50%) are seen in a significant proportion of patients.5,28–30 In contrast to other encephalitides, muscle weakness is often a prominent finding in patients with WNV meningoencephalitis (in 30–50%), and is often of a lower-motor-neuron pattern with flaccid paralysis and hyporeflexia. The presence of this pattern of weakness in the absence of sensory abnormalities is typical of WNV-associated AFP.27 Other distinctive findings include cranial neuropathies (in 20% of patients with WNV meningoencephalitis)—most commonly unilateral or bilateral peripheral facial palsy—which might have a delayed onset in the second or third week following onset of illness.5,27–30 Movement disorders (dyskinesias) are common in patients with WNV meningoencephalitis, and can include postural or kinetic tremor (in up to 90% of patients), parkinsonism (including cogwheel rigidity, bradykinesia and postural instability [70%]), and myoclonus (20–40%).26
AFP can occur in isolation, or in combination with meningitis or meningoencephalitis.27 The relative frequency of pure AFP compared with AFP in combination with meningoencephalitis is uncertain, but might be as high as 50%.5 Unlike meningoencephalitis, the WNV AFP syndrome does not predominantly affect the elderly, but has been reported in all age groups. Patients with WNV AFP syndrome have acute onset and rapid progression (within 24–48 hours) of asymmetric flaccid weakness, with associated hyporeflexia or areflexia in involved limbs. Motor weakness is usually the result of a poliomyelitis-like process (pure motor deficit), rather than a Guillain–Barré-like syndrome (motor and sensory deficit), although cases of WNV-associated Guillain–Barré syndrome have also been reported. In a recent prospective, population-based study of 32 patients with WNV-associated paralysis, poliomyelitis-like syndrome was demonstrated in 84% of patients (equating to an incidence of 3.7/100,000 in the general population) and a Guillain–Barré-like syndrome in 13%.27 Weakness can occur in a single limb, or in any combination of the four extremities. Respiratory insufficiency might also occur, including respiratory failure requiring endotracheal intubation, as seen in 38% of affected patients in a recent series.27 Bowel and bladder dysfunction occurs in a third of patients. Electromyography demonstrates reduced amplitudes of compound muscle action potentials, with normal amplitudes of sensory nerve action potentials. Follow-up studies 3 or more weeks later can show denervation changes. Pathologic studies in cases of fatal AFP have shown that the associated signs and symptoms result from an acute anterior poliomyelitis.
Risk factors
The most important risk factor for acquiring WNV infection is exposure to infected mosquitoes.28 People over 50 years of age (particularly those of 60–89 years of age) are at highest risk (20-fold increase) for developing meningo encephalitis, with a slightly higher incidence among males (Figure 3).3,29,31 Immunosuppressed recipients of transplanted organs have an increased risk of developing neuroinvasive disease (estimated at up to 40-fold increase in some studies),18,32 and when disease develops it is often more severe than in immunocompetent individuals.33 Other immunosuppressed individuals might be at higher risk, but this has not been shown conclusively. Diabetes, hypertension and cerebrovascular disease have also been considered as possible risk factors, but generalization from current studies is difficult owing to small sample sizes.4 Despite equal susceptibility to WNV infection, neuro-invasive disease is less common in children than in adults. Cases have, however, been confirmed (including poliomyelitis-like disease) in patients from all age groups, including infants, young children and adolescents.34–36 During the 2002–2004 US epidemic of WNV, the CDC confirmed 317 cases of neuroinvasive disease in children under 19 years of age; 106 of these (34%) were under 10 years of age.36
Figure 3.
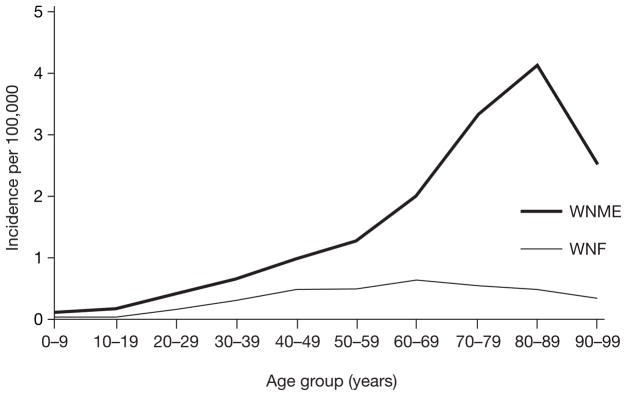
Incidence of West Nile virus neuroinvasive disease by age.81
WNF, West Nile fever; WNME, West Nile meningoencephalitis.
Genetic determinants of WNV resistance, including the 1B isoform of 2′-5′ oligoadenylate synthetase (Oas), have been identified in mice.37,38 In the presence of interferon (IFN) and viral double-stranded RNA, expression of enzymes belonging to the 2′-5′-Oas family is induced, and these enzymes are converted into their active form. This process results in synthesis of oligoadenylates, which bind to and activate RNase L. Active RNase L in turn degrades viral RNAs. Mice that are resistant to WNV infection have normal Oas genes, whereas mouse strains that are susceptible to WNV have mutations that truncate 2′-5′-Oas1b (also referred to as Oasl1). 2′-5′-Oas1b is also a potent inhibitor of WNV replication in infected cells.39 The role of the Oas system in human susceptibility to WNV infection is unknown. In one recent study, however, differences were observed in the frequency distribution of at least one polymorphism in OAS genes in hospital ized patients with WNV infection compared with controls, raising the possibility that the Oas system also has a role in human susceptibility to WNV infection.40
Mouse models of WNV infection have proved valuable for studying the importance of various components of the host immune system in control of WNV infection. In mice, IFN-α/β, B cells and antibody, complement, and CD8+ T cells, all have crucial roles in the control of WNV infection.23,41–44 In addition, secretion by WNV-infected neurons of the chemokine CXCL10 facilitates recruitment of CD8+ T cells to the CNS.45,46 WNV infection leads to activation of Tlr3, which recognizes viral double-stranded RNA. Tlr3-dependent inflammatory responses include enhanced production of various cytokines, including IFN, tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which help to control WNV replication at extraneural sites. TNF-α, however, also facilitates WNV entry into the CNS by compromising the integrity of the blood–brain barrier. Tlr3-deficient mice have increased WNV load at peripheral sites, but lower viral loads, less inflammation, and less injury in the CNS, compared with normal mice.25
Laboratory findings
Serology
Serological testing of serum and cerebrospinal fluid (CSF) remains the gold standard for the diagnosis of human WNV disease.5,28,47 IgM antibody to WNV develops in 75% of WNV-infected patients, and is usually measurable by ENZYME IMMUNOASSAY by the fourth day of symptom onset (detectable in CSF earlier than serum); 95% of infected patients develop IgM antibody by 7 days of symptom onset. Detection of WNV IgM in CSF is diagnostic of neuroinvasive disease; IgM antibodies do not readily cross the blood–brain barrier, so their presence in CSF is indicative of intrathecal synthesis. As infection by related flaviviruses might elicit cross-reactive test results by enzyme immunoassay, a positive WNV IgM should be confirmed by WNV PLAQUE REDUCTION NEUTRALIZATION ASSAY. Unlike most IgM responses, WNV IgM antibody persists for at least 6 months, and sometimes for 12–16 months, in the serum of previously infected patients (20–36% in one study).47 IgM antibody is also detectable in the CSF up to 7 months after illness. Confirmation of acute infection (rather than past infection) might, therefore, require evaluation of acute and convalescent (after 2–3 weeks) sera to identify a fourfold rise in antibody titer. IgG avidity testing is an additional diagnostic modality that might aid the differentiation between recently acquired and previous infections with WNV.48
RT-PCR of serum or CSF is not generally useful—or indeed recommended—for the diagnosis of WNV infection, as peak viremia occurs 3–4 days before symptom onset, resulting in poor sensitivity. The sensitivity of RT-PCR among 28 patients with serologically confirmed neuroinvasive WNV disease was 57% in CSF and 14% in serum.49 Sensitivity might be better in the case of immunocompromised hosts, who might not mount an adequate antibody response and could have prolonged viremia. The sensitivity of CSF viral culture is low, and it is not routinely employed for diagnosis.
Cerebrospinal fluid
Typical CSF findings in WNV neuroinvasive disease include PLEOCYTOSIS (polymorpho-nuclear or lymphocytic predominance), with elevated protein but normal glucose levels.1,50 Features of the pleocytosis that are indicative of WNV include a prolonged predominance of polymorphonuclear cells, and the presence of abnormal-appearing reactive lymphocytes or monocytes, including PLASMA CELL-like and MOLLARET-LIKE CELL. In a recent study of 250 hospitalized patients with WNV CNS disease,50 patients with encephalitis and meningitis had similar mean cell counts (227 cells/mm3 and 226 cells/mm3, respectively). Less than 5% of patients with either meningitis or encephalitis had fewer than 5 cells/mm3, and 8% had more than 500 cells/mm3. In patients with meningitis, the mean percentage of neutrophils was 41%, and in those with encephalitis the corresponding figure was 45%. In approximately 37% of patients with encephalitis and 50% of patients with meningitis, more than 50% of the cells in their initial CSF specimen were neutrophils. Nearly half of the patients with encephalitis, but only 16% of those with meningitis, had CSF protein levels of more than 1 g/l. CSF parameters were only a modest predictor of disease outcome.
Peripheral blood counts can be normal in patients with WNV invasive disease, or can demonstrate anemia, thrombocytopenia, leukocytosis or leukopenia. Elevated creatinine kinase and hyponatremia, and elevated serum ferritin late in the course of illness, have also been reported.
Radiological findings
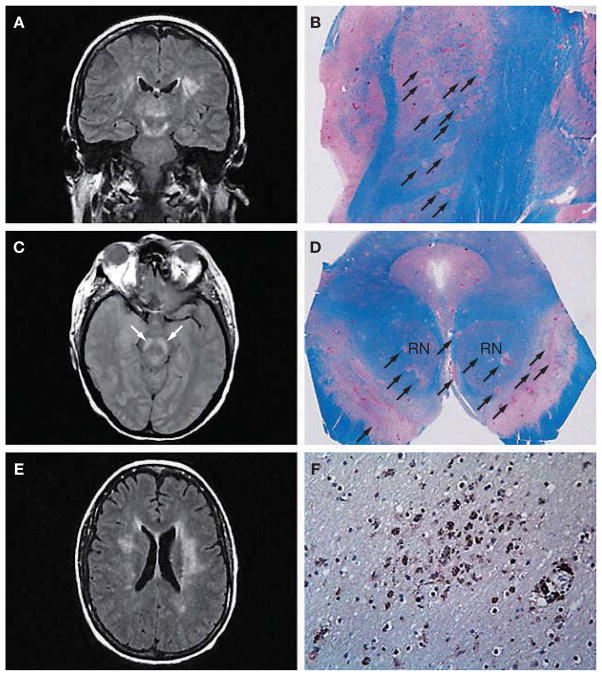
The incidence of acute MRI abnormalities in patients with WNV neuroinvasive disease has been extremely variable (Figures 4 and 5).30,51–53 In a recent series of 39 consecutive cases of WNV neuroinvasive disease (including both meningitis and meningoencephalitis), MRI was unremarkable in all except one patient.30 In each of two more recent series,51,52 however, around 70% of patients with WNV CNS disease had abnormal MRI findings. In the first study, 12/17 patients had abnormal findings on MRI: 4/17 had abnormalities only on diffusion-weighted (DW) imaging (involving the corona radiata and internal capsule), 3/17 had abnormal signal intensity on fluid-attenuated inversion recovery (FLAIR) or T2-weighted sequences (involving the cortical gray and white matter, cerebellum, basal ganglia, thalamus, internal capsule, pons and midbrain), 2/17 had meningeal enhancement, and 3/17 had abnormalities involving the spinal cord, cauda equina or nerve roots. Patients with normal MRI or only DW-image abnormalities had the best prognosis, whereas those with T2 and FLAIR abnormalities had the worst outcomes. A WNV MRI registry has been established by the CDC in the hope that, as imaging data are accumulated and consolidated, a more comprehensive picture of the imaging characteristics of WNV infection will emerge.53
Figure 4.
Radiographic and neuropathologic findings in West Nile virus encephalitis. (A) Coronal fluid-attenuated inversion recovery (FLAIR) magnetic resonance image shows an area of abnormally increased signal in the thalami, substantia nigra (extending superiorly toward the subthalamic nuclei) and white matter. (B) Corresponding tissue section from the same patient at autopsy 15 days later, stained with Luxol fast blue–periodic acid Schiff for myelin, shows numerous ovoid foci of necrosis and pallor throughout the thalamus and subthalamic nucleus (arrows). (C) Axial proton density image at the level of the midbrain shows a bilaterally increased signal in the substantia nigra (arrows). (D) Corresponding tissue section at autopsy, stained with Luxol fast blue–periodic acid Schiff, illustrates multifocal involvement of the substantia nigra (arrows), with nearly 50% of the area destroyed; the red nuclei are clearly affected. (E) Axial FLAIR image at the level of the lateral ventricle bodies shows a bilaterally increased signal within the white matter. A scan performed approximately 5 months earlier demonstrated an abnormal signal in the left periventricular white matter. This signal increased once West Nile virus encephalitis developed, and the lesions in the right cerebral white matter (left side of photograph) were new. (F) Photomicrograph taken from the right periventricular white matter, immunostained with the HAM56 antibody, shows numerous macrophages, both in perivascular areas (lower right) and diffusely throughout the white matter (center). Figures reproduced with permission from reference 33 © 2004 American Medical Association. RN, red nucleus.
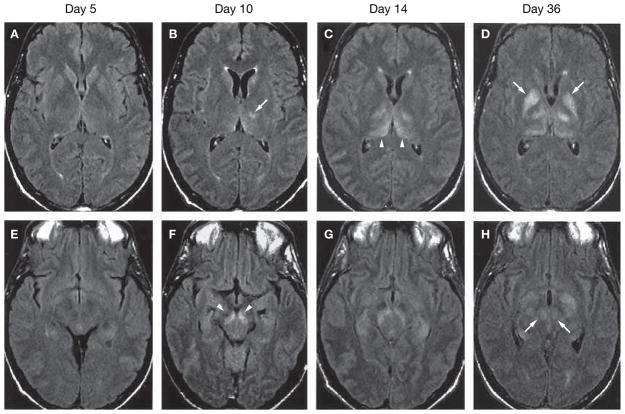
Figure 5.
Radiographic findings during disease progression in West Nile virus encephalitis. Serial fluid-attenuated inversion recovery (FLAIR) magnetic resonance images at the level of the basal ganglia (A–D) and midbrain (E–H). At day 5 after the onset of neurological symptoms, the scan is normal. The day 10 scan shows lesion in the left thalamus (B) and substantia nigra (F). The day 18 scan shows progression of thalamic lesions (C). On day 36, lesions are visible in the globus pallidus (D) and red nuclei (H). All of the lesion sites are indicated by arrows Figures reproduced with permission from reference 67 © 2004 American College of Physicians.
Mortality and prognosis
The overall case-fatality rate for WNV infection is 2–7%, based on US cases reported to the CDC from 2002–2005. Almost all mortality is confined to patients with neuroinvasive disease, in whom the death rate is approximately 9%. In patients with WNV encephalitis, the overall mortality is approximately 12–15%, although it could be as high as 35% in elderly patients.7
Prognostic data on WNV neuroinvasive disease are currently rather limited.54–57 Most patients with WNV meningitis and no associated focal neurological deficits make a complete recovery. Long-term outcomes and sequelae from WNV meningoencephalitis are highly variable, but severe neurological deficits have been reported to persist for months or even years and, in some cases, are likely to be lifelong. Among the prolonged symptoms reported are fatigue, myalgia, residual tremor and parkinsonism.26
The initial severity of encephalitic signs and symptoms is not necessarily predictive of outcome. In one study, five of eight patients with severe WNV encephalitis had excellent outcomes, achieving premorbid levels of functioning without residual disability within 4 months of illness.26 In a 1-year follow-up study of patients affected in the 1999 NYC WNV encephalitis outbreak,54 the prevalence of physical, functional and cognitive symptoms, including muscle weakness, loss of concentration, confusion and lightheadedness, remained significantly higher than at baseline, and only 37% of the patients had recovered fully to their premorbid status. Younger age at infection was the only significant predictor of recovery. The extent to which radiographic findings correlate with prognosis is unknown, but a recent study indicated that patients with normal MRI, or with abnormalities detected only on DW images, had better outcomes that those with abnormalities on FLAIR and T2-weighted images.51
Patients with AFP have the worst overall prognosis, and often have significant residual weakness.5,27,55 Improvements in limb strength, however, can occur over time. In a prospective study of 32 patients with WNV-associated paralysis, 25 showed varying degrees of improvement at 4 months after the onset of symptoms, and the remaining 7 patients showed no improvement.27 Clinical recovery was variable in another study of 11 patients over a 21-month follow-up period, and was attributed to differing degrees or combinations of motor-neuron loss and motor-nerve-terminal changes, as assessed by electrophysiology and muscle biopsy.56 In a retrospective case series of five patients with severe WNV disease, all patients demonstrated some degree of functional improvement, with the lowest functional outcomes noted in patients with severe muscle weakness and axonal neuropathy.57
CURRENT TREATMENT AND PROSPECTS FOR FUTURE THERAPIES
Therapeutics
At present, there is no specific therapy of proven efficacy for the treatment of WNV infection. Current treatment of WNV disease is largely supportive, including pain control for headaches, antiemetic therapy and rehydration for associated nausea and vomiting, monitoring for development of elevated intracranial pressure, and control of seizures if present. Acute respiratory failure can develop rapidly, particularly in patients with prominent bulbar signs.26 In transplant patients with presumed meningo-encephalitis, early withdrawal of immuno-suppression is recommended.18,33 The efficacy of corticosteroids for the treatment of WNV has not been adequately studied.
Ribavirin appears to have limited clinical efficacy (and seems to produce detrimental effects in rodent models),58,59 despite demonstrated efficacy against WNV in vitro. This drug was administered to some patients during a WNV outbreak in Israel in 2000, but no clinical benefit was observed when treated patients were compared with untreated patients.59 Inhibitors of nucleoside triphosphatase (NTPase)/helicase activities of Flaviviridae have not shown promising results against WNV in vitro.60
IFN-α2b inhibits growth of WNV in vitro and can protect BALB/c mice and golden hamsters from WNV-induced disease, although its efficacy is greatly diminished when treatments are delayed beyond 4–6 hours before viral challenge.41,44,58 IFN-α/β-receptor knockout mice have increased mortality following WNV challenge, whereas treatment of primary mouse neuronal cultures with IFN-β before or after infection increased neuronal survival in dependently of the effect on WNV replication.44 The efficacy of IFN treatment in humans is still unclear, as it has only been studied in a non-blinded, non-placebo-controlled clinical trial. A randomized, placebo-controlled clinical trial is, however, currently in progress.61
Passive transfer of WNV-specific antibodies or immunoglobulin can protect mice and hamsters against WNV infection in experimental models of disease.62,63 Antibody transfer can offer some protection to mice even after the virus has reached the CNS, although efficacy declines markedly with time after WNV infection, indicating that rapid diagnosis and timely administration are vital. Uncontrolled clinical reports describe an apparent beneficial effect in two patients given the Israeli human intravenous immunoglobulin preparation Omr-IgG-am, which contains high titers of neutralizing antibody to WNV, although a third treated patient died.64–66 A multicenter randomized, placebo-controlled trial sponsored by the NIH to test the effectiveness of Omr-IgG-am in humans with WNV disease is currently in progress.67,68 The humanized monoclonal antibody E16, which targets the WNV envelope protein, recently showed therapeutic efficacy in mice, conferring reduced mortality even when administered as a single dose 5 days after WNV infection.69 E16 inhibits infection after the viral attachment stage, possibly by blocking envelope glycoprotein conformational changes.70
A proprietary antisense oligomer construct (AVI-4020; AVI BioPharma, Corvallis, OR, USA), which inhibits viral replication,71 was found to be safe in a small pilot phase I human clinical trial, and is currently undergoing more-extensive testing in a phase II placebo-controlled trial to assess tolerability, pharmacokinetics and potential efficacy.72 RNA INTERFERENCE constructs have also been studied in human cells in vitro, but have not shown efficacy.73 A screening program sponsored by the National Institute of Allergy and Infectious Diseases (NIAID) to identify agents with potent in vitro activity against WNV is ongoing, and has identified several effective compounds that are inhibitors of cellular enzymes involved in nucleotide synthesis. These compounds will now be studied in animal models of WNV infection.67
Vaccination
An effective formalin-inactivated equine vaccine, and a recombinant vaccine based on expression of WNV antigens by the canary pox virus, are currently licensed for use in horses.62,74 In some animal models, vaccination with Japanese encephalitis virus,75 St Louis encephalitis virus or Kunjin virus (a subtype of WNV) confers relative protection from severe WNV infection, but two studies have shown that prior Japanese encephalitis, yellow fever or dengue virus vaccination does not induce protective neutralizing antibody responses to WNV.76,77 Potential strategies for human WNV vaccine development include use of inactivated or attenuated WNV strains, chimeric live virus vaccines (in which WNV genes are inserted into the genetic background of another flavivirus), and recombinant-subunit-based vaccines. A chimeric vaccine that incorporates WNV into a genomic backbone of attenuated serotype-4 dengue virus induced protective immunity against WNV challenge in monkeys,78 and is currently undergoing Phase I safety and immunogenicity studies in humans. Another chimeric vaccine that incorporates WNV precursor transmembrane and envelope genes into the genome backbone of an attenuated yellow fever virus 17D has been shown to be effective in mice and monkeys,79 and is currently undergoing phase II safety and immunogenicity trials in humans.80 A Phase I human study, using a recombinant plasmid-based DNA vaccine (encoding WNV precursor transmembrane and envelope genes), is also underway.
CONCLUSIONS
In the 6 years since its first detection in the US, WNV has become the most frequent cause of epidemic meningoencephalitis in humans in North America. Although less than 1% of infected individuals develop neuroinvasive disease manifesting as meningitis, meningo-encephalitis or AFP, mortality is high, and neurological sequelae among survivors are severe and often permanent. No specific therapy has yet been shown to be effective for treatment of WNV encephalitis. Potential treatments, including antiviral compounds, immunomodulatory therapies and vaccines, are all areas of active research in animals and humans. Human clinical trials are essential for assessing the efficacy of potential treatments, as WNV is likely to continue to circulate in the Northern Hemisphere for many years to come.
REVIEW CRITERIA.
PubMed was searched using Entrez for articles published up to December 30, 2005, including electronic early release publications. Search terms included “West Nile Virus” and “West Nile Virus Encephalitis”, as well as “WNV Acute Flaccid Paralysis, “WNV Radiographic” and “WNV Vaccine”. The abstracts of retrieved citations were reviewed and prioritized by relative content. Full articles were obtained and references were checked for additional material when appropriate.
KEY POINTS.
Since its first appearance in the US in 1999, West Nile virus (WNV), an arthropod-borne flavivirus, has emerged as the most common cause of epidemic meningoencephalitis in North America
Transmission of WNV to humans occurs predominantly following a bite from an infected mosquito, which acquires virus after feeding on vertebrate amplifying hosts, primarily birds
Neuroinvasive disease occurs in less than 1% of cases; neuroinvasive presentations are varied and include aseptic meningitis, meningoencephalitis and acute flaccid paralysis
Serological testing of serum and cerebrospinal fluid remains the gold standard for the diagnosis of human WNV disease, and some patients with WNV neuroinvasive disease show abnormalities on brain MRI
Almost all mortality from WNV is confined to patients with neuroinvasive disease
Current treatment of WNV disease is largely supportive, including pain control, antiemetic therapy and rehydration, monitoring for development of elevated intracranial pressure, and control of seizures
A vaccine that was shown to induce protective immunity against WNV challenge in monkeys is currently undergoing Phase I safety and immunogenicity studies in humans
Acknowledgments
RLD receives research support from the NIH’s National Institute of Allergy and Infectious Diseases (5K08AI052261). KLT receives research support from the Department of Veterans Affairs, the NIH’s National Institute of Neurological Disease and Stroke (R01NS050138, R01NS051403), and the Reuler-Lewin Family Professorship of Neurology.
GLOSSARY
- REVERSE TRANSCRIPTASE POLYMERASE CHAIN REACTION (RT-PCR)
A molecular diagnostic tool that uses amplification to detect specific RNA; West Nile virus and other flaviviruses have a positive-sense, single-stranded RNA genome that is detectable by this methodology
- CLADE
A branch of biological taxa or species whose members share homologous features inherited from a common ancestor
- NUCHAL RIGIDITY
A physical sign of meningitis in which rigidity of neck muscles limits movement of the neck, including preventing its flexion
- KERNIG SIGN
A physical sign of meningitis in which the supine patient whose hip is flexed to 90° is unable to completely extend the leg at the knee joint
- BRUDZINSKI’S SIGN
A physical sign of meningitis in which flexion of the supine patient’s neck causes flexion of the patient’s hip and knees
- ENZYME IMMUNOASSAY
An assay that uses an enzyme-conjugated antibody to detect antigen (or serum antibody in the case of IgG enzyme immunoassay); the enzyme catalyzes a color reaction when exposed to substrate
- PLAQUE REDUCTION NEUTRALIZATION ASSAY
A viral plaque is a visible zone of cell destruction formed when virus propagates within cell culture; the plaque reduction neutralization assay is used to measure reductions in virus infectivity caused by specific antibody present in serum samples
- PLEOCYTOSIS
The presence of more cells than normal in the cerebrospinal fluid
- PLASMA CELL
A type of white blood cell of B-cell lineage that produces antibodies
- MOLLARET-LIKE CELL
A cell within the cerebrospinal fluid that appears large, friable, and ‘endothelial’ in appearance; considered to be large activated cells of the monocyte/macrophage lineage
- RNA INTERFERENCE
Post-transcriptional gene silencing in which double-stranded RNA mediates the destruction of messenger RNAs in a sequence-specific fashion
Footnotes
Competing interests
KL Tyler declared competing interests; go to the article online for details.
RL DeBiasi declared she has no competing interests.
Contributor Information
Roberta L. DeBiasi, Associate professor with co-appointments in the Departments of Pediatrics and Neurology
Kenneth L. Tyler, Reuler-Lewin Family Professor of Neurology and Professor of Medicine, Microbiology and Immunology, at the University of Colorado Health Sciences Center, Denver, CO, USA.
References
- 1.Nash D, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344:1807–1814. doi: 10.1056/NEJM200106143442401. [DOI] [PubMed] [Google Scholar]
- 2.Davis CT, et al. Phylogenetic analysis of North American West Nile virus isolates, 2001–2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252–265. doi: 10.1016/j.virol.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 3.Hayes EB, et al. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167–1173. doi: 10.3201/eid1108.050289a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Granwehr BP, et al. West Nile virus: where are we now? Lancet Infect Dis. 2004;4:547–556. doi: 10.1016/S1473-3099(04)01128-4. [DOI] [PubMed] [Google Scholar]
- 5.Tyler KL. West Nile virus infection in the United States. Arch Neurol. 2004;61:1190–1195. doi: 10.1001/archneur.61.8.1190. [DOI] [PubMed] [Google Scholar]
- 6.Public Health Agency of Canada: West Nile Virus Surveillance Information. [ http://www.phac-aspc.gc.ca/wnv-vwn]
- 7.Centers for Disease Control and Prevention. [ http://www.cdc.gov]
- 8.Lanciotti RS, et al. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333–2337. doi: 10.1126/science.286.5448.2333. [DOI] [PubMed] [Google Scholar]
- 9.Lanciotti RS, et al. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- 10.Davis CT, et al. Emergence of attenuated West Nile virus variants in Texas, 2003. Virology. 2004;330:342–350. doi: 10.1016/j.virol.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Ebel GD, et al. Genetic and phenotypic variation of West Nile virus in New York, 2000–2003. Am J Trop Med Hyg. 2004;71:493–500. [PubMed] [Google Scholar]
- 12.Nasci RS, et al. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742–744. doi: 10.3201/eid0704.010426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mostashari F, et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention . Update: Detection of West Nile virus in blood donations—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:916–919. [PubMed] [Google Scholar]
- 15.Petersen LR, Epstein JS. Problem solved? West Nile virus and transfusion safety. N Engl J Med. 2005;353:516–517. doi: 10.1056/NEJMe058144. [DOI] [PubMed] [Google Scholar]
- 16.Custer B, et al. The cost-effectiveness of screening the US blood supply for West Nile virus. Ann Intern Med. 2005;143:486–492. doi: 10.7326/0003-4819-143-7-200510040-00007. [DOI] [PubMed] [Google Scholar]
- 17.Iwamoto M, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196–2203. doi: 10.1056/NEJMoa022987. [DOI] [PubMed] [Google Scholar]
- 18.DeSalvo D, et al. West Nile virus encephalitis in organ transplant recipients: another high-risk group for meningoencephalitis and death. Transplantation. 2004;77:466–469. doi: 10.1097/01.TP.0000101434.98873.CB. [DOI] [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention . West Nile virus infections in organ transplant recipients—New York and Pennsylvania, August–September, 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1021–1023. [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention . Intrauterine West Nile virus infection—New York, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1135–1136. [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention . Interim guidelines for the evaluation of infants born to mothers infected with West Nile virus during pregnancy. MMWR Morb Mortal Wkly Rep. 2004;53:154–157. [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention . Laboratory-acquired West Nile virus infections—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:1133–1135. [PubMed] [Google Scholar]
- 23.Diamond MS, et al. B cells and antibody play critical roles in the immediate defense of disseminated infection by West Nile encephalitis virus. J Virol. 2003;77:2578–2586. doi: 10.1128/JVI.77.4.2578-2586.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Watson JT, et al. Clinical characteristics and functional outcomes of West Nile virus fever. Ann Intern Med. 2004;141:360–365. doi: 10.7326/0003-4819-141-5-200409070-00010. [DOI] [PubMed] [Google Scholar]
- 25.Wang T, et al. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- 26.Sejvar JJ, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290:511–515. doi: 10.1001/jama.290.4.511. [DOI] [PubMed] [Google Scholar]
- 27.Sejvar JJ, et al. West Nile virus-associated flaccid paralysis. Emerg Infect Dis. 2005;11:1021–1027. doi: 10.3201/eid1107.040991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes EB, et al. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174–1179. doi: 10.3201/eid1108.050289b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen LR, et al. West Nile virus encephalitis. N Engl J Med. 2002;347:1225–1226. doi: 10.1056/NEJMo020128. [DOI] [PubMed] [Google Scholar]
- 30.Brilla R, et al. Clinical and neuroradiologic features of 39 consecutive cases of West Nile Virus meningoencephalitis. J Neurol Sci. 2004;220:37–40. doi: 10.1016/j.jns.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 31.O’Leary DR, et al. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004;4:61–70. doi: 10.1089/153036604773083004. [DOI] [PubMed] [Google Scholar]
- 32.Kumar D, et al. A seroprevalence study of West Nile virus infection in solid organ transplant recipients. Am J Transplant. 2004;4:1883–1888. doi: 10.1111/j.1600-6143.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 33.Kleinschmidt-DeMasters BK, et al. Naturally-acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic and neuropathological features. Arch Neurol. 2004;61:1210–1220. doi: 10.1001/archneur.61.8.1210. [DOI] [PubMed] [Google Scholar]
- 34.DeBiasi RL, et al. West Nile virus meningoencephalitis in an immunocompetent adolescent. Pediatr Neurol. 2005;33:217–219. doi: 10.1016/j.pediatrneurol.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Yim R, et al. Spectrum of clinical manifestations of West Nile virus infection in children. Pediatrics. 2004;114:1673–1675. doi: 10.1542/peds.2004-0491. [DOI] [PubMed] [Google Scholar]
- 36.Hayes EB, O’Leary DR. West Nile virus infection: a pediatric perspective. Pediatrics. 2004;113:1375–1381. doi: 10.1542/peds.113.5.1375. [DOI] [PubMed] [Google Scholar]
- 37.Mashimo T, et al. A nonsense mutation in the gene encoding 2′-5′-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc Natl Acad Sci USA. 2002;99:11311–11316. doi: 10.1073/pnas.172195399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perelygin AA, et al. Positional cloning of the murine flavivirus resistance gene. Proc Natl Acad Sci USA. 2002;99:9322–9327. doi: 10.1073/pnas.142287799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kajaste-Rudnitski A, et al. The 2′5′ oligoadenylate synthetase 1b is a potent inhibitor of West Nile virus replication inside infected cells. J Biol Chem. 2006;281:4624–4637. doi: 10.1074/jbc.M508649200. [DOI] [PubMed] [Google Scholar]
- 40.Yakub I, et al. Single nucleotide polymorphisms in genes for 2′5′-oligoadenylate synthetase and RNase L in patients hospitalized with West Nile virus infection. J Infect Dis. 2005;192:1741–1748. doi: 10.1086/497340. [DOI] [PubMed] [Google Scholar]
- 41.Diamond MS, et al. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259–278. doi: 10.1089/088282403322396082. [DOI] [PubMed] [Google Scholar]
- 42.Shrestha B, Diamond MS. Role of CD8+ T cells in control of West Nile virus infection. J Virol. 2004;78:8312–8321. doi: 10.1128/JVI.78.15.8312-8321.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehlhop E, et al. Complement activation is required for induction of a protective antibody response against West Nile virus infection. J Virol. 2005;79:7466–7477. doi: 10.1128/JVI.79.12.7466-7477.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79:13350–13361. doi: 10.1128/JVI.79.21.13350-13361.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Glass WG, et al. Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection. J Exp Med. 2005;202:1087–1098. doi: 10.1084/jem.20042530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein RS, et al. Neuronal CXCL10 directs CD8+ T-cell recruitment and control of West Nile virus encephalitis. J Virol. 2005;79:11457–11466. doi: 10.1128/JVI.79.17.11457-11466.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roehrig JT, et al. Persistence of virus-reactive serum immunoglobulin M antibody in confirmed West Nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376–379. doi: 10.3201/eid0903.020531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levett PN, et al. Use of immunoglobulin G avidity assays for differentiation of primary from previous infections with West Nile virus. J Clin Microbiol. 2005;43:5873–5875. doi: 10.1128/JCM.43.12.5873-5875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanciotti RS, Kerst AJ. Nucleic acid sequence-based amplification assays for rapid detection of West Nile and St. Louis encephalitis viruses. J Clin Microbiol. 2001;39:4506–4513. doi: 10.1128/JCM.39.12.4506-4513.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tyler KL, et al. CSF findings in 250 patients with serologically confirmed West Nile Virus meningitis and encephalitis. Neurology. 2006;14:361–365. doi: 10.1212/01.wnl.0000195890.70898.1f. [DOI] [PubMed] [Google Scholar]
- 51.Ali M, et al. West Nile virus infection: MR imaging findings in the nervous system. AJNR Am J Neuroradiol. 2005;26:289–297. [PMC free article] [PubMed] [Google Scholar]
- 52.Petropoulou KA, et al. West Nile virus meningoencephalitis: MR imaging findings. AJNR Am J Neuroradiol. 2005;26:1986–1995. [PMC free article] [PubMed] [Google Scholar]
- 53.Robertson HJ, Sejvar JJ. The need for a West Nile virus MRI registry. AJNR Am J Neuroradiol. 2003;24:1741–1742. [PMC free article] [PubMed] [Google Scholar]
- 54.Klee AL, et al. Long-term prognosis for clinical West Nile virus infection. Emerg Infect Dis. 2004;10:1405–1411. doi: 10.3201/eid1008.030879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marciniak C, et al. Acute flaccid paralysis associated with West Nile virus: motor and functional improvement in 4 patients. Arch Phys Med Rehabil. 2004;85:1933–1938. doi: 10.1016/j.apmr.2004.04.038. [DOI] [PubMed] [Google Scholar]
- 56.Cao NJ, et al. Recovery and prognosticators of paralysis in West Nile virus infection. J Neurol Sci. 2005;236:73–80. doi: 10.1016/j.jns.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 57.Rao N, et al. Rehabilitation outcomes of 5 patients with severe West Nile virus infection: a case series. Arch Phys Med Rehabil. 2005;86:449–452. doi: 10.1016/j.apmr.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 58.Morrey JD, et al. Effect of interferon-alpha and interferon-inducers on West Nile virus in mouse and hamster animal models. Antivir Chem Chemother. 2004;15:101–109. doi: 10.1177/095632020401500202. [DOI] [PubMed] [Google Scholar]
- 59.Chowers MY, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675–678. doi: 10.3201/eid0704.010414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bretner M, et al. Synthesis and evaluation of ATP-binding site directed potential inhibitors of nucleoside triphosphatases/helicases and polymerases of hepatitis C and other selected Flaviviridae viruses. Antivir Chem Chemother. 2004;15:35–42. doi: 10.1177/095632020401500104. [DOI] [PubMed] [Google Scholar]
- 61.Rahal JJ, Wehbeh WA. Double-blind placebo controlled trial of alpha-interferon for West Nile virus meningoencephalitis. [accessed 9 March 2006];Protocol WIN-102. 2004 revised 7/26/2004 [ http://www.nyhq.org/posting/rahal.html]
- 62.Ben Nathan D, et al. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188:5–12. doi: 10.1086/376870. [DOI] [PubMed] [Google Scholar]
- 63.Engle MJ, Diamond MS. Antibody prophylaxis and therapy against West Nile virus infection in wild-type and immunodeficient mice. J Virol. 2003;77:12941–12949. doi: 10.1128/JVI.77.24.12941-12949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shimoni Z, et al. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7:759. doi: 10.3201/eid0704.010432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamdan A, et al. Possible benefit of intravenous immunoglobulin therapy in a lung transplant recipient with West Nile virus encephalitis. Transpl Infect Dis. 2002;4:160–162. doi: 10.1034/j.1399-3062.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- 66.Haley M, et al. The role for intravenous immunoglobulin in the treatment of West Nile virus encephalitis. Clin Infect Dis. 2003;37:e88–e90. doi: 10.1086/377172. [DOI] [PubMed] [Google Scholar]
- 67.Gea-Banacloche J, et al. West Nile virus: pathogenesis and therapeutic options. Ann Intern Med. 2004;140:545–553. doi: 10.7326/0003-4819-140-7-200404060-00015. [DOI] [PubMed] [Google Scholar]
- 68.Agrawal AG, Petersen LR. Human immunoglobulin as a treatment for West Nile virus infection. J Infect Dis. 2003;188:1–4. doi: 10.1086/376871. [DOI] [PubMed] [Google Scholar]
- 69.Oliphant T, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nybakken GE, et al. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Deas TS, et al. Inhibition of flavivirus infections by antisense oligomers specifically suppressing viral translation and RNA replication. J Virol. 2005;79:4599–4609. doi: 10.1128/JVI.79.8.4599-4609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.An exploratory study of AVI-4020 in patients with possible acute neuroinvasive West Nile virus (WNV) disease. [ http://www.clinicaltrials.gov/ct/show/NCT00091845]
- 73.Geiss BJ, et al. Actively replicating West Nile virus is resistant to cytoplasmic delivery of siRNA. Virol J. 2005;2:53. doi: 10.1186/1743-422X-2-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Minke JM, et al. Recombinant canarypoxvirus vaccine carrying the prM/E genes of West Nile virus protects horses against a West Nile virus-mosquito challenge. Arch Virol Suppl. 2004;18:221–230. doi: 10.1007/978-3-7091-0572-6_20. [DOI] [PubMed] [Google Scholar]
- 75.Siger L, et al. Assessment of the efficacy of a single dose of a recombinant vaccine against West Nile virus in response to natural challenge with West Nile virus-infected mosquitoes in horses. Am J Vet Res. 2004;65:1459–1462. doi: 10.2460/ajvr.2004.65.1459. [DOI] [PubMed] [Google Scholar]
- 76.Kanesa-Thasan N, et al. Short report: absence of protective neutralizing antibodies to West Nile virus in subjects following vaccination with Japanese encephalitis or dengue vaccines. Am J Trop Med Hyg. 2002;66:115–116. doi: 10.4269/ajtmh.2002.66.115. [DOI] [PubMed] [Google Scholar]
- 77.Johnson BW, et al. West Nile virus infection and serologic response among persons previously vaccinated against yellow fever and Japanese encephalitis viruses. Vector Borne Zoonotic Dis. 2005;5:137–145. doi: 10.1089/vbz.2005.5.137. [DOI] [PubMed] [Google Scholar]
- 78.Pletnev AG, et al. Molecularly engineered live-attenuated chimeric West Nile/dengue virus vaccines protect rhesus monkeys from West Nile virus. Virology. 2003;314:190–195. doi: 10.1016/s0042-6822(03)00450-1. [DOI] [PubMed] [Google Scholar]
- 79.Arroyo J, et al. ChimeriVax–West Nile virus live-attenuated vaccine: preclinical evaluation of safety, immunogenicity, and efficacy. J Virol. 2004;78:12497–12507. doi: 10.1128/JVI.78.22.12497-12507.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Acambis. [accessed 9 March 2006];Acambis becomes first company to enter Phase II testing of vaccine against West Nile virus. (online 20 December 2005) [ http://www.acambis.com/default.asp?id=1446]
- 81.Hayes EB, Gubler DJ. Epidemiology and clinical features of an emerging epidemic in the United States. Ann Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]