Abstract
A new strategy for iminium ion isomerization was applied to the direct, redox-neutral α-alkynylation of amines. Cu(II) 2-ethylhexanoate was identified as the optimal catalyst for this three-component coupling reaction of secondary amines, aldehydes and alkynes.
Keywords: C–H activation, redox-neutral, azomethine ylides, heterocycles, propargylic amines
Propargylic amines represent important building blocks,[1] and compounds of this class (e.g., 1) are readily assembled by three-component reactions of amines, aldehydes and alkynes, frequently referred to as A3 reactions (eq 1).[2–4] Methods that enable direct access to ring-substituted isomers 2 are much more limited. As part of a program to develop redox-neutral[5] reactions of broad utility,[6] we recently reported an α-amino acid based decarboxylative three-component coupling strategy to access ring-substituted propargylic amines such as 2 (eq 2).[6e] A nearly identical approach was also reported by Li et al.[7–9] Replacement of the α-amino acid with a simple amine would represent a significant advance (eq 3). Here we report the first examples of this elusive transformation.
 |
(1) |
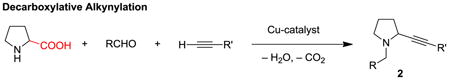 |
(2) |
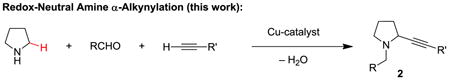 |
(3) |
Direct α-alkynylation of tertiary amines has previously been accomplished by means of oxidative C–H functionalization,[10,11] including photoredox catalysis.[12] These methods require stoichiometric amounts of oxidant and are often limited to N-aryl tetrahydroisoquinolines and N,N-dialkylanilines.[13,14] As an attractive alternative, we envisioned a direct, redox-neutral three-component coupling with concurrent amine α-alkynylation (Figure 1). To realize such a process, conditions must be identified that prevent the regular course of events in an A3 reaction, namely addition of the metal acetylide to the initially formed iminium ion 3 to give undesired isomer 1. Access to 2 requires the isomerization of iminium ion 3 to 5, which must proceed in the presence of the metal acetylide. This could in principle be accomplished by iminium deprotonation/reprotonation via azomethine ylide intermediate 4. In fact, α-deprotonation of iminium ions as a means to form azomethine ylides has been reported in the context of pericyclic azomethine ylide chemistry.[15,16] We have previously developed powerful reactions of azomethine ylides that lead to intramolecular C–N and C–C bond formation via non-pericyclic pathways.[6,17] Upon considering potential solutions to the added challenge of performing amine α-functionalizations in an intermolecular setting, we reasoned that an electron withdrawing group R on 3 would serve to accelerate iminium isomerization through acidification of the amine α-proton. In addition, increasing the steric demand of R would be expected to slow down the rate of formation of 1.
Figure 1.
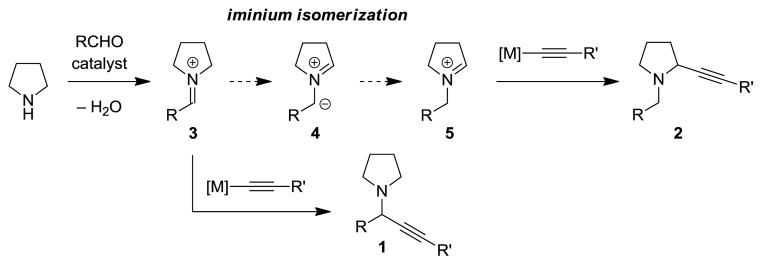
Competing reaction pathways in the formation of isomeric propargylic amines.
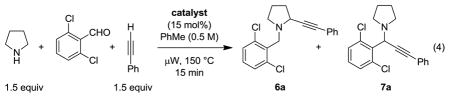
With the above considerations in mind and encouraged by our recent success in developing a redox-neutral amine α-cyanation,[6m] we selected 2,6-dichlorobenzaldehyde as the reaction partner in the proposed three-component reaction with pyrrolidine and phenylacetylene (eq 4). Different catalysts were evaluated as part of this survey that was conducted under microwave conditions (Table 1). Using CuBr and TMEDA, the catalyst combination that proved optimal in the decarboxylative alkynylation (eq 2),[6e] a 1:3 mixture of 6a/7a was obtained in 65% yield (entry 1). CuBr or CuBr2 provided higher overall yields, but less favorable product ratios (entries 2 and 3). Interestingly, Cu(II) triflate provided a favorable 5:1 ratio of regioisomers, albeit in moderate yield (entry 4). Gratifyingly, Cu(II) acetate gave a 7:1 ratio of regioisomers but without improvement in yield (entry 5). Other Cu(II) carboxylate salts such as Cu(II) benzoate also yielded 6a as the major product. Cu(II) carboxylates with enhanced solubilities provided favorable product ratios and good product yields (entries 8–10). Readily available Cu(II) 2-ethylhexanoate (Cu(2-EH)2) was identified as an excellent catalyst, allowing for the isolation of 6a/7a in a 20:1 ratio and 82% yield. Notably, Cu(acac)2 provided product ratios vastly different from that of the corresponding perfluorinated catalyst (entries 11, 12). No desired products were obtained with 2-ethylhexanoic acid (2-EHA) or in the absence of a catalyst. In all Cu(II) catalyzed reactions, 1,4-diphenylbuta-1,3-diyne, the corresponding Glaser coupling product[18] of phenylacetylene was isolated as a byproduct.
Table 1.
Evaluation of Catalysts for the Direct Three-Component α-Alkynylation.[a]
| |||
|---|---|---|---|
| entry | catalyst | ratio 6a/7a | yield 6a+7a (%) |
| 1 | CuBr + TMEDA (30 mol%) | 1:3 | 65 |
| 2 | CuBr | 1:5 | 94 |
| 3 | CuBr2 | 1:4 | 82 |
| 4 | Cu(OTf)2 | 5:1 | 45 |
| 5 | Cu(OAc)2•H2O | 7:1 | 47 |
| 6 | Cu(HCOO)2•H2O | 1:1 | 82 |
| 7 | Cu(OBz)2•H2O | 7:1 | 74 |
| 8 | Cu(II) 2-ethylhexanoate | 20:1 | 82 |
| 9 | Cu(II) cyclohexylbutanoate | 14:1 | 76 |
| 10 | Cu(II) pivalate | 15:1 | 82 |
| 11 | Cu(acac)2 | 1:4 | 67 |
| 12 | Cu(hfacac)2•H2O | 3:1 | 76 |
| 13 | Cu(NO2)2•H2O | 1:1 | 67 |
| 14 | 2-ethylhexanoic acid | N/A | 0 |
| 15 | none | N/A | 0 |
Reactions were performed on a 0.5 mmol scale with anhydrous toluene. Yields are isolated yields of chromatographically purified compounds.
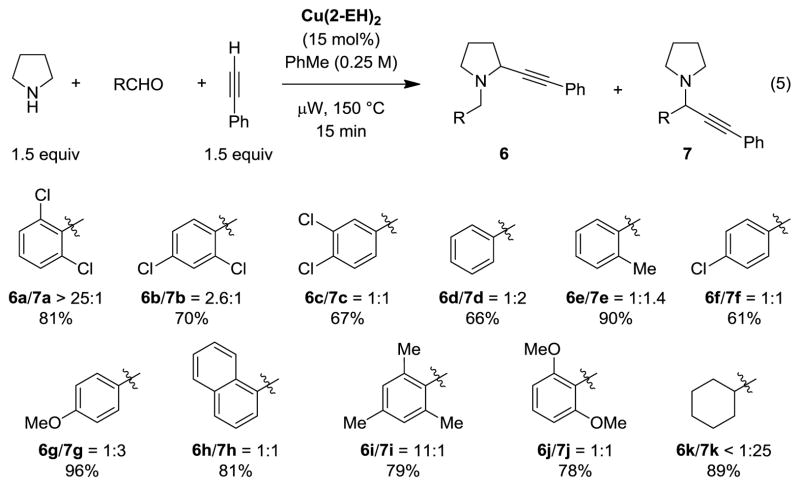
Upon further examination of parameters, we found that replacement of anhydrous toluene for HPLC grade toluene had no deleterious effect on the outcome of the reaction. Lowering the concentration from 0.5 M to 0.25 M was found to be beneficial. Under these conditions, the reaction of 2,6-dichlorobenzaldehyde, pyrrolidine, and phenylacetylene gave products 6a/7a in a >25:1 ratio and 81% yield (Chart 1).
Chart 1.
Dependence of Product Ratios on the Aldehyde.
To evaluate the impact of the aldehyde on the selectivity of the reaction, we tested a collection of electronically diverse aldehydes with varying steric demands (Chart 1). Interestingly, replacement of 2,6-dichlorobenzaldehyde with electronically similar 2,4-dichlorobenzaldehyde resulted in a dramatic reduction of the product ratio from >25:1 to 2.6:1. Further reduction in ratio to 1:1 was seen for 3,4-dichlorobenzaldehyde. Unsubstituted benzaldehyde gave rise to 6d/7d in a 1:2 ratio, favoring the undesired regioisomer. 2-Methylbenzaldehyde performed slightly better, providing a 1:1.4 ratio of products 6e/7e. A comparison of the results obtained with benzaldehyde, 4-Cl-benzaldehyde, and 4-MeO-benzaldehyde (products 6d, 6f, 6g) clearly established the impact of electronic factors on the regioselectivity, with more electron-poor aldehydes providing more favorable product ratios. However, upon inspection of all results, it can be concluded that steric factors outweigh electronics. The case in point is mesitaldehyde which provided an excellent product ratio of 11:1. Even the electron-rich 2,6-di-MeO-benzaldehyde provided a more favorable product ratio than benzaldehyde. In contrast, cyclohexane-carbaldehyde gave rise to almost none of the desired product but rather underwent the standard A3 reaction. As a side note, the reaction of 2,6-dichlorobenzaldehyde, pyrrolidine, and phenylacetylene can be performed under reflux but otherwise identical conditions. In this instance, products 6a/7a were isolated in a 19:1 ratio (86% yield) following a reaction time of just 30 min.
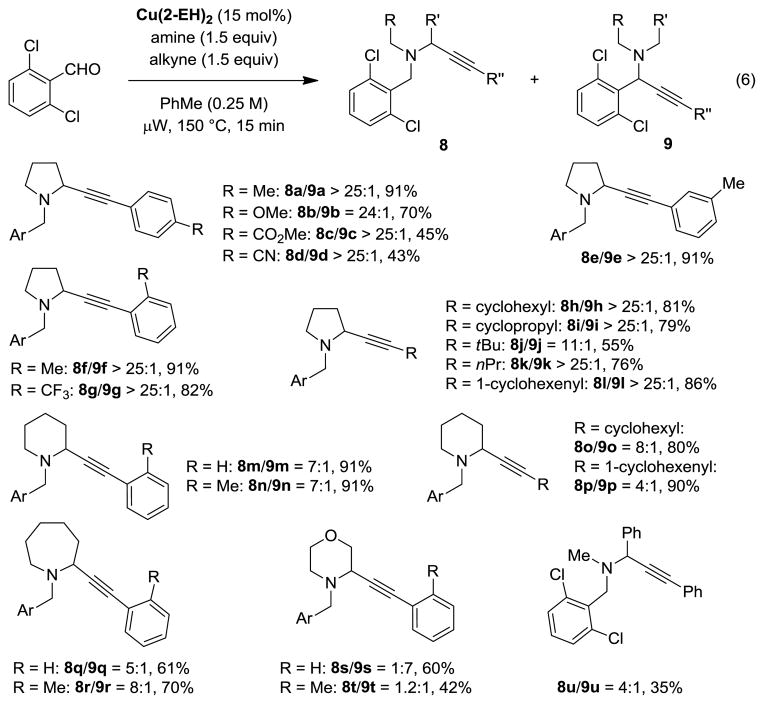
The scope of the three-component coupling reaction was explored under the optimized microwave conditions (Chart 2). Reactions of 2,6-dichlorobenzaldehyde, pyrrolidine, and various terminal alkynes resulted in the formation of the desired products in generally good to excellent yields. Aromatic, alkenyl, and aliphatic substituents on the alkyne were readily accommodated. In the majority of cases, products were obtained with regioselectivities exceeding 25:1. Importantly, the α-alkynylation is not limited to pyrrolidine. Piperidine and azepane also underwent Cu(2-EH)2-catalyzed couplings with various terminal alkynes to provide propargylic amines in good to excellent yields. While the observed regioselectivities for these more challenging substrates are lower than for pyrrolidine, they are still in a synthetically useful range. Morpholine provided only small amounts of desired regioisomer in a reaction with phenylacetylene. Interestingly, the introduction of an ortho-methyl substituent in phenylacetylene allowed for the isolation of 8t/9t in a 1.2:1 ratio. Finally, although alkynylation products from acyclic amines are available via traditional A3 chemistry, we decided to test N-methylbenzylamine under the standard reaction conditions. In the event, products 8u/9u were isolated in a 4:1 ratio, albeit in only 35% yield.
Chart 2.
Scope of the Direct α-Alkynylation.
 |
(7) |
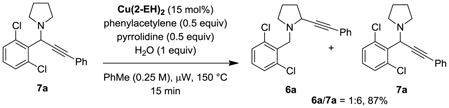
In order to establish whether the regioselectivity of the alkynylation could be affected by product isomerization, we exposed 7a to the reaction conditions.[19] To best mimic the original conditions, phenylacetylene, pyrrolidine and water were added (eq 7). Very little isomerization was observed in addition to the formation of 1,4-diphenylbuta-1,3-diyne, and 6a/7a were recovered in a 1:6 ratio (87% yield). While this study establishes the potential reversibility of the reaction, the degree of isomerization is insufficient to account for the observed regioselectivity of the parent reaction. Therefore, the product ratios likely reflect the intrinsic reactivities of the reaction intermediates. This is in stark contrast to our previous study on α-cyanation in which product isomerization is an important contributor to the overall selectivity.[6m]
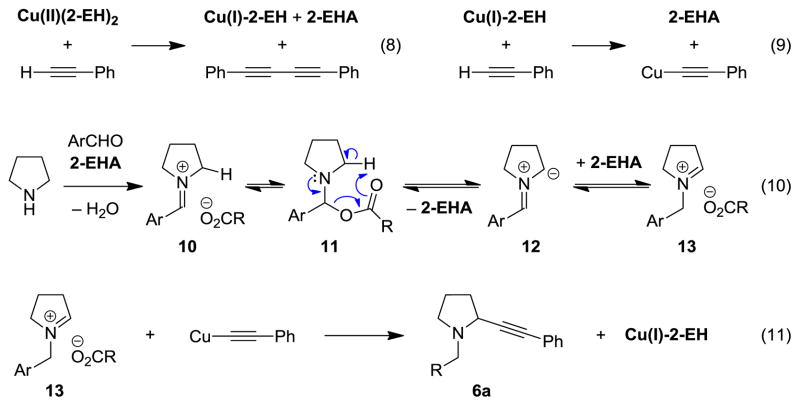
Outlined in Figure 2 is a potential mechanism for the redox-neutral α-alkynylation. Reaction of Cu(2-EH)2 with phenylacetylene results in the formation of Cu(I)-2-EH, the Glaser product, and one equivalent of 2-EHA (eq 8). The active Cu-acetylide is formed upon reaction of Cu(I)-2-EH with phenylacetylene, with concurrent release of another equivalent of 2-EHA (eq 9). 2-EHA is believed to play a crucial role in the overall reaction (eq 10). Firstly, it is expected to facilitate the formation of iminium ion 10. As indicated in Figure 1, the carboxylate anion of 10 could bring about iminium isomerization via deprotonation/reprotonation. Alternatively, N,O-acetal 11, which should exist in equilibrium with 10, could eliminate 2-EHA via a concerted pathway to form azomethine ylide 12.[16,20] Regioselective protonation of azomethine ylide 12 by 2-EHA results in iminium ion 13, which may exist in equilibrium with the corresponding N,O-acetal (not shown). In the final step, iminium ion 13 engages the copper acetylide to form propargylic amine 6a, concomitant with the regeneration of Cu(I)-2-EH. Although Cu(2-EH)2 is the only additive, the two active catalysts Cu(I)-2-EH and 2-EHA are formed in situ and activate both reaction partners separately in what may be considered an example of synergistic catalysis.[21]
Figure 2.
Proposed Mechanism for α-Alkynylation.
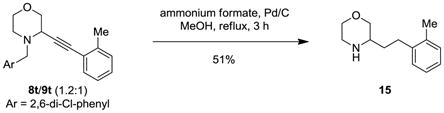
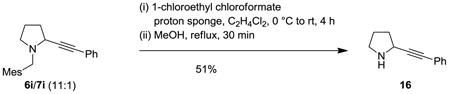
To demonstrate the utility of the propargylic amines derived from the redox-neutral α-alkynylation, a number of products were selectively transformed. Debenzylation of 8a with simultaneous reduction of the triple bond was accomplished by transfer hydrogenation to provide product 14 (eq 12). This approach can also be employed to remove the undesired regioisomer in cases where the alkynylation is less regioselective. For instance, exposure of a 1.2:1 mixture of 8t/9t to the transfer hydrogenation conditions led to clean formation of morpholine 15 (eq 13). An alternative two-step debenzylation strategy designed to preserve the alkyne functionality allowed for the synthesis of propargylamine 16 (eq 14).
 |
(12) |
 |
(13) |
 |
(14) |
In summary, we have developed an unprecedented approach for the one-step synthesis of ring-substituted propargylic amines. The combination of a reductive amine N-alkylation with an oxidative α-functionalization effectively renders this process redox-neutral. We anticipate wide adoption of this concept as a means to rapidly access α-functionalized amines from simple precursors.
Experimental Section
General procedure
A 10 mL microwave reaction tube was charged with a 10 × 8 mm SiC passive heating element, Cu(II) 2-ethylhexanoate (0.038 mmol, 0.15 equiv.), toluene (1 mL), alkyne (0.375 mmol, 1.5 equiv.), amine (0.375 mmol, 1.5 equiv.) and aldehyde (0.25 mmol). The reaction tube was sealed with a Teflon-lined snap cap, and heated in a microwave reactor at 150 °C (200 W, 30–80 psi) for 15 minutes (Note: SiC passive heating elements must not be used in conjunction with stir bars for they may score glass and cause vessel failure). After cooling with compressed air flow, the reaction mixture was directly loaded onto a column and purified by silica gel chromatography.
Footnotes
Financial support from the NIH–NIGMS (R01GM101389-01) is gratefully acknowledged. Partial support (microwave purchase) was provided by the National Science Foundation (Grant CHE-0911192). A.X.S. acknowledges support from the Aresty Research Center for Undergraduates and Rutgers University School of Arts and Sciences. D.S. is a fellow of the Alfred P. Sloan Foundation and the recipient of an Amgen Young Investigator Award.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.See:. de Armas P, Tejedor D, Garcia-Tellado F. Angew Chem Int Ed. 2010;49:1013. doi: 10.1002/anie.200906018. and references cited therein.
- 2.A3 reactions, selected key contributions: Dyatkin AB, Rivero RA. Tetrahedron Lett. 1998;39:3647.Kabalka GW, Wang L, Pagni RM. Synlett. 2001:676.Li CJ, Wei CM. Chem Commun. 2002:268. doi: 10.1039/b108851n.Wei CM, Li CJ. J Am Chem Soc. 2003;125:9584. doi: 10.1021/ja0359299.Gommermann N, Koradin C, Polborn K, Knochel P. Angew Chem Int Ed. 2003;42:5763. doi: 10.1002/anie.200352578.
- 3.A3 reactions, selected reviews: Wei C, Li Z, Li CJ. Synlett. 2004:1472.Zani L, Bolm C. Chem Commun. 2006:4263. doi: 10.1039/b607986p.Kouznetsov VV, Mendez LYV. Synthesis. 2008:491.Yoo WJ, Zhao L, Li CJ. Aldrichimica Acta. 2011;44:43.Peshkov VA, Pereshivko OP, Van der Eycken EV. Chem Soc Rev. 2012;41:3790. doi: 10.1039/c2cs15356d.
- 4.A3 reactions, selected recent contributions: Graf TA, Anderson TK, Bowden NB. Adv Synth Catal. 2011;353:1033.Cheng M, Zhang Q, Hu XY, Li BG, Ji JX, Chan ASC. Adv Synth Catal. 2011;353:1274.Patil SS, Patil SV, Bobade VD. Synlett. 2011:1157.Saifuddin M, Agarwal PK, Kundu B. J Org Chem. 2011;76:10122. doi: 10.1021/jo201942r.Chen MT, Landers B, Navarro O. Org Biomol Chem. 2012;10:2206. doi: 10.1039/c2ob06900h.Meyet CE, Pierce CJ, Larsen CH. Org Lett. 2012;14:964. doi: 10.1021/ol2029492.Buckley BR, Khan AN, Heaney H. Chem Eur J. 2012;18:3855. doi: 10.1002/chem.201103987.Pierce CJ, Nguyen M, Larsen CH. Angew Chem Int Ed. 2012;51:12289. doi: 10.1002/anie.201206674.
- 5.a) Burns NZ, Baran PS, Hoffmann RW. Angew Chem Int Ed. 2009;48:2854. doi: 10.1002/anie.200806086. [DOI] [PubMed] [Google Scholar]; b) Newhouse T, Baran PS, Hoffmann RW. Chem Soc Rev. 2009;38:3010. doi: 10.1039/b821200g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Zhang C, De CK, Mal R, Seidel D. J Am Chem Soc. 2008;130:416. doi: 10.1021/ja077473r. [DOI] [PubMed] [Google Scholar]; b) Murarka S, Zhang C, Konieczynska MD, Seidel D. Org Lett. 2009;11:129. doi: 10.1021/ol802519r. [DOI] [PubMed] [Google Scholar]; c) Zhang C, Murarka S, Seidel D. J Org Chem. 2009;74:419. doi: 10.1021/jo802325x. [DOI] [PubMed] [Google Scholar]; d) Murarka S, Deb I, Zhang C, Seidel D. J Am Chem Soc. 2009;131:13226. doi: 10.1021/ja905213f. [DOI] [PubMed] [Google Scholar]; e) Zhang C, Seidel D. J Am Chem Soc. 2010;132:1798. doi: 10.1021/ja910719x. [DOI] [PubMed] [Google Scholar]; f) Deb I, Seidel D. Tetrahedron Lett. 2010;51:2945. [Google Scholar]; g) Zhang C, Das D, Seidel D. Chem Sci. 2011;2:233. [Google Scholar]; h) Deb I, Das D, Seidel D. Org Lett. 2011;13:812. doi: 10.1021/ol1031359. [DOI] [PubMed] [Google Scholar]; i) Haibach MC, Deb I, De CK, Seidel D. J Am Chem Soc. 2011;133:2100. doi: 10.1021/ja110713k. [DOI] [PubMed] [Google Scholar]; j) Deb I, Coiro D, Seidel D. Chem Commun. 2011;47:6473. doi: 10.1039/c1cc11560j. [DOI] [PubMed] [Google Scholar]; k) Vecchione MK, Sun AX, Seidel D. Chem Sci. 2011;2:2178. [Google Scholar]; l) Das D, Richers MT, Ma L, Seidel D. Org Lett. 2011;13:6584. doi: 10.1021/ol202957d. [DOI] [PubMed] [Google Scholar]; m) Ma L, Chen W, Seidel D. J Am Chem Soc. 2012;134:15305. doi: 10.1021/ja308009g. [DOI] [PubMed] [Google Scholar]
- 7.Bi HP, Teng Q, Guan M, Chen WW, Liang YM, Yao X, Li CJ. J Org Chem. 2010;75:783. doi: 10.1021/jo902319h. [DOI] [PubMed] [Google Scholar]
- 8.For an oxidative variant, see: Bi HP, Zhao L, Liang YM, Li CJ. Angew Chem Int Ed. 2009;48:792. doi: 10.1002/anie.200805122.
- 9.Decarboxylative A3 reactions: Feng H, Ermolat’ev DS, Song G, Van der Eycken EV. J Org Chem. 2011;76:7608. doi: 10.1021/jo2013725.Feng H, Ermolat’ev DS, Song G, Van der Eycken EV. Org Lett. 2012;14:1942. doi: 10.1021/ol3006612.Feng H, Ermolat’ev DS, Song G, Van der Eycken EV. J Org Chem. 2012;77:5149. doi: 10.1021/jo300562j.
- 10.Selected reviews on amine α-functionalization: Murahashi SI. Angew Chem Int Ed Engl. 1995;34:2443.Doye S. Angew Chem Int Ed. 2001;40:3351. doi: 10.1002/1521-3773(20010917)40:18<3351::aid-anie3351>3.0.co;2-b.Campos KR. Chem Soc Rev. 2007;36:1069. doi: 10.1039/b607547a.Murahashi SI, Zhang D. Chem Soc Rev. 2008;37:1490. doi: 10.1039/b706709g.Li CJ. Acc Chem Res. 2009;42:335. doi: 10.1021/ar800164n.Yoo WJ, Li CJ. Top Curr Chem. 2010;292:281. doi: 10.1007/128_2009_17.Jazzar R, Hitce J, Renaudat A, Sofack-Kreutzer J, Baudoin O. Chem Eur J. 2010;16:2654. doi: 10.1002/chem.200902374.Yeung CS, Dong VM. Chem Rev. 2011;111:1215. doi: 10.1021/cr100280d.Sun CL, Li BJ, Shi ZJ. Chem Rev. 2011;111:1293. doi: 10.1021/cr100198w.Liu C, Zhang H, Shi W, Lei AW. Chem Rev. 2011;111:1780. doi: 10.1021/cr100379j.Wendlandt AE, Suess AM, Stahl SS. Angew Chem Int Ed. 2011;50:11062. doi: 10.1002/anie.201103945.Jones KM, Klussmann M. Synlett. 2012;23:159.Zhang C, Tang CH, Jiao N. Chem Soc Rev. 2012;41:3464. doi: 10.1039/c2cs15323h.Mitchell EA, Peschiulli A, Lefevre N, Meerpoel L, Maes BUW. Chem Eur J. 2012;18:10092. doi: 10.1002/chem.201201539.Shi L, Xia W. Chem Soc Rev. 2012;41:7687. doi: 10.1039/c2cs35203f.
- 11.Selected articles on oxidative amine α-alkynylation: Li Z, Li C-J. J Am Chem Soc. 2004;126:11810. doi: 10.1021/ja0460763.Li Z, Li CJ. Org Lett. 2004;6:4997. doi: 10.1021/ol047814v.Li Z, MacLeod PD, Li CJ. Tetrahedron: Asymmetry. 2006;17:590.Turcaud S, Sierecki E, Martens T, Royer J. J Org Chem. 2007;72:4882. doi: 10.1021/jo070631c.Niu M, Yin Z, Fu H, Jiang Y, Zhao Y. J Org Chem. 2008;73:3961. doi: 10.1021/jo800279j.Zhao L, Li CJ. Angew Chem Int Ed. 2008;47:7075. doi: 10.1002/anie.200801367.Xu XL, Li XN. Org Lett. 2009;11:1027. doi: 10.1021/ol802974b.Volla CMR, Vogel P. Org Lett. 2009;11:1701. doi: 10.1021/ol9002509.Liu P, Zhou CY, Xiang S, Che CM. Chem Commun. 2010;46:2739. doi: 10.1039/c001209b.Su WK, Yu JB, Li ZH, Jiang ZJ. J Org Chem. 2011;76:9144. doi: 10.1021/jo2015533.Boess E, Schmitz C, Klussmann M. J Am Chem Soc. 2012;134:5317. doi: 10.1021/ja211697s.Singh KN, Singh P, Kaur A. Synlett. 2012:760.
- 12.Photoredox approaches to amine α-alkynylation: Freeman DB, Furst L, Condie AG, Stephenson CR. Org Lett. 2012;14:94. doi: 10.1021/ol202883v.Rueping M, Koenigs RM, Poscharny K, Fabry DC, Leonori D, Vila C. Chem Eur J. 2012;18:5170. doi: 10.1002/chem.201200050.
- 13.For conceptually different α-alkynylations of tertiary amines, see: Sugiishi T, Nakamura H. J Am Chem Soc. 2012;134:2504. doi: 10.1021/ja211092q.
- 14.For Ir-catalyzed α-alkenylations of imines with alkynes, see: Sakaguchi S, Kubo T, Ishii Y. Angew Chem Int Ed. 2001;40:2534. doi: 10.1002/1521-3773(20010702)40:13<2534::aid-anie2534>3.0.co;2-2.
- 15.Huisgen R, Grashey R, Steingruber E. Tetrahedron Lett. 1963:1441. [Google Scholar]
- 16.Selected reviews on azomethine ylides: Padwa A, Pearson WH. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products. Vol. 59 Wiley; Chichester, U. K: 2002. Najera C, Sansano JM. Curr Org Chem. 2003;7:1105.Coldham I, Hufton R. Chem Rev. 2005;105:2765. doi: 10.1021/cr040004c.Pandey G, Banerjee P, Gadre SR. Chem Rev. 2006;106:4484. doi: 10.1021/cr050011g.Pinho e Melo TMVD. Eur J Org Chem. 2006:2873.Najera C, Sansano JM. Top Heterocycl Chem. 2008;12:117.Nyerges M, Toth J, Groundwater PW. Synlett. 2008:1269.Adrio J, Carretero JC. Chem Commun. 2011;47:6784. doi: 10.1039/c1cc10779h.
- 17.Selected recent articles on redox-neutral amine functionalization: Pastine SJ, McQuaid KM, Sames D. J Am Chem Soc. 2005;127:12180. doi: 10.1021/ja053337f.Tobisu M, Chatani N. Angew Chem Int Ed. 2006;45:1683. doi: 10.1002/anie.200503866.Matyus P, Elias O, Tapolcsanyi P, Polonka-Balint A, Halasz-Dajka B. Synthesis. 2006:2625.Oda M, Fukuchi Y, Ito S, Thanh NC, Kuroda S. Tetrahedron Lett. 2007;48:9159.Zheng L, Yang F, Dang Q, Bai X. Org Lett. 2008;10:889. doi: 10.1021/ol703049j.Barluenga J, Fananas-Mastral M, Aznar F, Valdes C. Angew Chem Int Ed. 2008;47:6594. doi: 10.1002/anie.200802268.Mori K, Ohshima Y, Ehara K, Akiyama T. Chem Lett. 2009;38:524.Cui L, Peng Y, Zhang L. J Am Chem Soc. 2009;131:8394. doi: 10.1021/ja903531g.Vadola PA, Sames D. J Am Chem Soc. 2009;131:16525. doi: 10.1021/ja906480w.Pahadi NK, Paley M, Jana R, Waetzig SR, Tunge JA. J Am Chem Soc. 2009;131:16626. doi: 10.1021/ja907357g.Lo VKY, Zhou CY, Wong MK, Che CM. Chem Commun. 2010;46:213. doi: 10.1039/b914516h.Kuang J, Ma S. J Am Chem Soc. 2010;132:1786. doi: 10.1021/ja910503k.Kang YK, Kim SM, Kim DY. J Am Chem Soc. 2010;132:11847. doi: 10.1021/ja103786c.Dunkel P, Turos G, Benyei A, Ludanyi K, Matyus P. Tetrahedron. 2010;66:2331.Zhou G, Zhang J. Chem Commun. 2010;46:6593. doi: 10.1039/c0cc01946a.Mao H, Xu R, Wan J, Jiang Z, Sun C, Pan Y. Chem Eur J. 2010;16:13352. doi: 10.1002/chem.201001896.Cao WD, Liu XH, Wang WT, Lin LL, Feng XM. Org Lett. 2011;13:600. doi: 10.1021/ol1028282.Zhou GH, Liu F, Zhang JL. Chem Eur J. 2011;17:3101. doi: 10.1002/chem.201100019.Ghavtadze N, Narayan R, Wibbeling B, Wuerthwein EU. J Org Chem. 2011;76:5185. doi: 10.1021/jo200896y.Mori K, Ehara K, Kurihara K, Akiyama T. J Am Chem Soc. 2011;133:6166. doi: 10.1021/ja2014955.He YP, Du YL, Luo SW, Gong LZ. Tetrahedron Lett. 2011;52:7064.Mahoney SJ, Fillion E. Chem Eur J. 2012;18:68. doi: 10.1002/chem.201103155.Jurberg ID, Peng B, Woestefeld E, Wasserloos M, Maulide N. Angew Chem Int Ed. 2012;51:1950. doi: 10.1002/anie.201108639.Han YY, Han WY, Hou X, Zhang XM, Yuan WC. Org Lett. 2012;14:4054. doi: 10.1021/ol301559k.Chen LJ, Zhang L, Lv J, Cheng JP, Luo SZ. Chem Eur J. 2012;18:8891. doi: 10.1002/chem.201201532.
- 18.a) Glaser C. Ber Dtsch Chem Ges. 1869;2:422. [Google Scholar]; b) Siemsen P, Livingston RC, Diederich F. Angew Chem Int Ed. 2000;39:2633. [PubMed] [Google Scholar]
- 19.Reversibility in an iminium alkynylation has previously been demonstrated: Sugiishi T, Kimura A, Nakamura H. J Am Chem Soc. 2010;132:5332. doi: 10.1021/ja9109055.
- 20.This mode of azomethine ylide formation from a carboxylic acid derived N,O-acetal was proposed by Yu et al. as part of a computational investigation of Tunge’s benzoic acid catalyzed synthesis of N-alkyl pyrroles from 3-pyrroline: Xue XS, Yu A, Cai Y, Cheng JP. Org Lett. 2011;13:6054. doi: 10.1021/ol2025247. See also reference 17j.
- 21.Allen AE, MacMillan DWC. Chem Sci. 2012;3:633. doi: 10.1039/C2SC00907B. [DOI] [PMC free article] [PubMed] [Google Scholar]