Abstract
Background
As statin therapy increases risks of diabetes, the balance of benefit and risk in primary prevention for these agents has become controversial. We undertook an analysis of participants from the JUPITER trial to address the balance of vascular benefits and diabetes hazard of statin use.
Methods
In the randomized, double-blind JUPITER trial, 17,603 men and women without prior cardiovascular disease or diabetes were randomly allocated to rosuvastatin 20 mg or placebo and followed for up to 5 years for the trial primary endpoint (myocardial infarction, stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death) and the protocol pre-specified secondary endpoints of venous thromboembolism (VTE), all-cause mortality, and incident diabetes. To address balance of vascular benefits and diabetes hazard, participants were stratified on the basis of having none or at least one of the following major risk factors for developing diabetes: metabolic syndrome, impaired fasting glucose, body mass index >30 kg/m2, or HbA1c > 6 percent.
Findings
Trial participants with one or more major diabetes risk factor (N=11,508) were at higher risk of developing diabetes; for such individuals, statin allocation was associated with a 39 percent reduction in the primary endpoint (P=0.0001), a 36 percent reduction in VTE (P=0.08), a 17 percent reduction in total mortality (P=0.15) and a 28 percent increase in diabetes (P=0.01). Thus, for those with diabetes risk factors, 93 vascular events or deaths were avoided for every 54 new cases of diabetes diagnosed. For trial participants with no major diabetes risk factor (N=6,095), statin allocation was associated with a 52 percent reduction in the primary endpoint (P=0.0001), a 53 percent reduction in VTE (P=0.05), a 22 percent reduction in total mortality (P=0.08) and no increase in diabetes (HR 0.99, P= 0.99). For such individuals, a total of 86 vascular events or deaths were avoided with no new cases of diabetes diagnosed. In analysis limited to the 486 participants who developed diabetes during follow-up (270 on rosuvastatin vs. 216 on placebo group, P=0.01), the point estimate of cardiovascular risk reduction associated with statin therapy (hazard ratio 0.63) was consistent with that observed for the trial as a whole (hazard ratio 0.56). As compared to placebo, statin allocation accelerated the average time to diagnosis of diabetes by 5.4 weeks.
Interpretation
In the JUPITER primary prevention trial, the cardiovascular and mortality benefits of statin therapy exceed the diabetes hazard, including among those at higher risk for developing diabetes
Introduction
Statin therapy is effective for reducing cardiovascular events. Yet, trial data1 and meta-analyses2–4 indicate that statins also confer an increased risk of developing diabetes. In particular, recent overviews indicate that all statin agents are associated with a modest increase in the risk of incident type 2 diabetes (hazard ratio 1.09, 95%CI 1.02–1.17)3, and that intensive-dose therapy may be associated with somewhat higher risk than moderate dose therapy (hazard ratio 1.12, 95%CI 1.04–1.22)4. For these reasons, on March 1, 2012, the United States Food and Drug Administration added a warning regarding diabetes risk to the labeling of all statin agents5 and similar concern has been raised by European drug authorities. These regulatory changes have engendered controversy in the lay and medical press as to whether the cardiovascular benefit of treatment with statins exceeds the diabetes risk, particularly in primary prevention, a setting in which these agents have seen increasing use. The Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER)1 trial provides a contemporary opportunity to address this issue directly.
Methods
JUPITER was a randomized, double-blind, placebo controlled trial designed to investigate whether rosuvastatin 20 mg daily compared to placebo would decrease the rate of first-ever cardiovascular events among 17,802 apparently healthy men and women with LDLC<130 mg/dL and high sensitivity C-reactive protein (hsCRP) ≥2 mg/L1,6. An important pre-specified secondary aim of the trial was to address the effects of rousvastatin on incident type 2 diabetes; as such, a prior history of diabetes was an exclusion criterion for the trial. However, large numbers of participants in the JUPITER trial had major risk factors for diabetes at study entry including metabolic syndrome, impaired fasting glucose, body mass index ≥ 30 kg/m2, or entry HbA1c > 6 percent; these diabetes risk factors were selected post hoc on the basis of literature review and to be consistent with prior publications. For all JUPITER analyses, metabolic syndrome is defined according to American Heart Association/National Heart Lung and Blood Institute 2005 consensus criteria7 while impaired fasting glucose is defined as a fasting glucose level greater than 100 but less than 126 mg/dL. For this analysis, trial participants were divided into those with none or at least one of these major diabetes risk factors.
During a follow-up period of up to 5 years, all trial participants underwent prospective follow-up for incident vascular events, incident diabetes, and other adverse events. The pre-specified JUPITER trial endpoint included first events of myocardial infarction, stroke, hospitalization for unstable angina, arterial revascularization, or cardiovascular death, while protocol pre-specified secondary endpoints designed to be used in analyses of net clinical benefit included venous thromboembolism, physician reported diabetes, and all-cause mortality.
Consistent with prior reports from the JUPITER trial and as specified in the protocol, cardiovascular events included those occurring at any time between randomization and March 30, 2008, the date of unblinding of the JUPITER trial. As physician reported diabetes was considered as an adverse event, these are included if they occurred any time between randomization and the last study visit for each individual participant, a process that continued until August 2008.
All components of the JUPITER primary endpoint were adjudicated by an endpoints committee unaware of randomization status using pre-specified endpoint criterion. Incident events of diabetes were physician reported and total mortality was based upon filed reports.
To address the net cardiovascular and mortality benefit and diabetes hazard associated with rosuvastatin, we used Cox proportional hazard regression models to calculate hazard ratios and 95% confidence intervals for first major cardiovascular events or death and for incident diabetes comparing those on active therapy to those on placebo. Absolute numbers of vascular events or deaths prevented and diabetes cases diagnosed were also calculated for each study group. In addition to the total number of vascular events or deaths prevented, we performed an additional analysis to be as conservative as possible in assessing net clinical benefit in which we allowed only the first vascular event to be counted for any given trial participant; thus, in this latter analysis, a trial participant who suffered non-fatal myocardial infarction, VTE, non-fatal stroke, and death was counted as having 1 rather than 4 events. So as not to underestimate potential hazards, we conservatively elected to include all physician reported cases of diabetes regardless of whether there was formal biochemical confirmation. Individual participants were allowed to contribute both to the cardiovascular and diabetes endpoints if each of these occurred for that participant during the trial follow-up period. All P values reported are two-sided and all confidence intervals computed at the 95% level.
Role of the Funding Source
The JUPITER trial protocol was designed and written by the study chair (PMR) and approved by the local institutional review board at each participating center. The trial data were analyzed by the study chair, the academic study statistician (RJG), and the academic programmer (JM) who vouch for the accuracy and completeness of the data and the analyses. The trial was financially supported by Astra-Zeneca. The sponsor collected the trial data and monitored the study sites but played no role in the conduct of these analyses, in the drafting of this manuscript, or in the decision to submit these analyses for publication. The corresponding author (PMR) had full access to all data in the study and had final responsibility to submit for publication.
Results
Of 17,802 JUPITER trial participants, 121 (0.7 percent) were missing data on at least one risk factor for diabetes and 78 (0.4 percent) were found at randomization to have fasting glucose ≥126 mg/dL or clinical diabetes. The remaining 17,603 trial participants (98.9 percent) had complete data and were included in the current analysis.
Compared to trial participants with no major diabetes risk factors (N=6095), those with one or more major diabetes risk factor (N=11508) were more likely to be female, have lower baseline levels of HDL cholesterol, and higher baseline levels of blood pressure, HbA1c, glucose, and triglycerides. By contrast, smoking was more prevalent among those with no major diabetes risk factor. In gender specific analyses, levels of hsCRP were higher among those with one or more major diabetes risk factor (Table 1). As anticipated, trial participants with one or more major diabetes risk factor had higher risk of developing diabetes during trial follow-up (incidence rate 1.88 vs 0.18 per 100 person years, HR = 10.5, 95%CI 6.98–15.8, P=0.001). Tabular data stratifying these groups by randomized treatment assignment are shown in the appendix.
Table 1.
Baseline characteristics of participants in the JUPITER trial among those with none or at least one major risk factor for diabetes (metabolic syndrome, impaired fasting glucose, HbA1c > 6 percent, or body mass index ≥ 30 kg/m2).
| Baseline Characteristic | Major Risk Factors for Diabetes | P | |
|---|---|---|---|
| None (N=6095) | One or More (N=11508) | ||
| Age, years | 66 (60–72) | 66 (60–71) | 0.37 |
| Female, N (%) | 1963 (32.2) | 4771 (41.5) | <0.0001 |
| Race/Ethnicity, N (%) | |||
| Caucasian | 4544 (74.6) | 8010 (69.6) | <0.0001 |
| Black | 772 (12.7) | 1439 (12.5) | |
| Hispanic | 549 (9.0) | 1663 (14.5) | |
| Other/unknown | 230 (3.8) | 396 (3.4) | |
| Body Mass Index, kg/m2 | 25.4 (23.2–27.5) | 30.7 (27.5–34.0) | <0.0001 |
| Hypertension, N (%) | 2757 (45.2) | 7338 (63.8) | <0.0001 |
| Current Smoking, N (%) | 1318 (21.6) | 1475 (12.8) | <0.00001 |
| hsCRP, mg/L | |||
| men | 3.9 (2.6–6.7) | 4.2 (2.8–6.7) | <0.0001 |
| women | 3.8 (2.7–6.2) | 5.0 (3.3–8.3) | <0.0001 |
| LDL-C, mg/dL | 108 (93–119) | 109 (95–119) | 0.001 |
| HDL-C, mg/dL | 54 (45–66) | 46 (38–56) | <0.0001 |
| Triglycerides, mg/dL | 97 (73–128) | 134 (96–192) | <0.0001 |
| Total Cholesterol, mg/dL | 184 (167–199) | 186 (169–200) | 0.001 |
| Apo A, mg/dL | 170 (151–193) | 158 (141–179) | <0.0001 |
| Apo B, mg/dL | 104 (91–116) | 112 (98–125) | <0.0001 |
| Glucose, mg/dL | 89 (84–94) | 99 (91–106) | <0.0001 |
| HbA1c, % | 5.6 (5.4–5.7) | 5.8 (5.5–6.1) | 0.001 |
All values are median (interquartile range) or N (%). For hsCRP, values are based on the average of the screening and randomization visits
Overall, incident diabetes occurred more frequently in the rosuvastatin group (270 reports of diabetes, vs. 216 in the placebo group, HR=1.25, 95%CI 1.05–1.49, P=0.01). The average time from randomization to diagnosis of diabetes was 84.3 weeks in the rosuvastatin group and 89.7 weeks in the placebo group, an acceleration of 5.4 weeks.
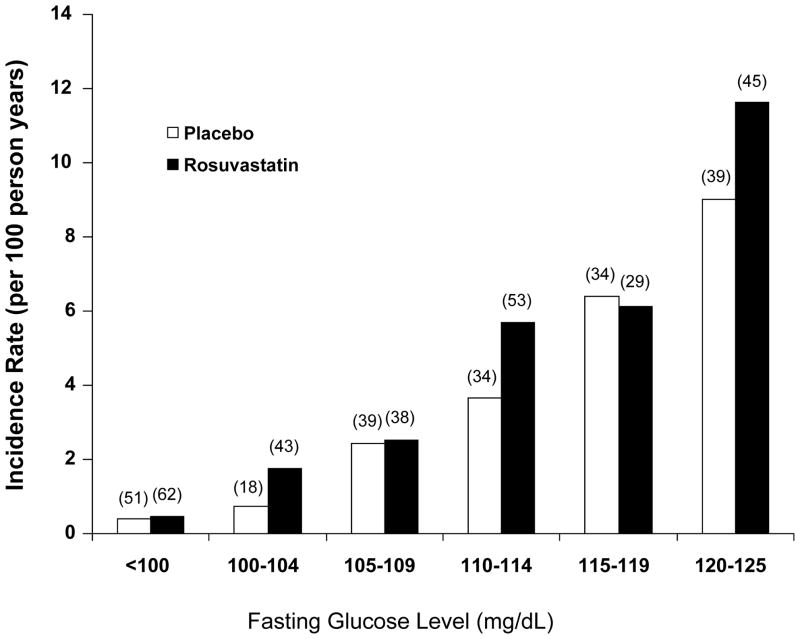
As shown in Figure 1, virtually all of the excess risk of diabetes associated with rosuvastatin occurred among those with baseline evidence of impaired fasting glucose.
Figure 1.
Incidence rates (per 100 person years) of physician diagnosed diabetes in the JUPITER trial according to baseline fasting glucose levels. Data are shown separately for those allocated to placebo (white bars) and those allocated to rosuvastatin (black bars). Numbers in parentheses indicate the absolute number of individuals who developed diabetes in each group.
Table 2 presents incidence rates for cardiovascular events, total mortality, and diabetes among those with and without at least one major diabetes risk factor, according to statin or placebo allocation. For trial participants with at least one major diabetes risk factor, random allocation to rosuvastatin was associated with a 39 percent reduction in the primary endpoint (HR 0.61,95%CI 0.47–0.79, P=0.0001), a 36 percent reduction in venous thromboembolism (HR 0.64,95%CI 0.39–1.06, P=0.08), a 17 percent reduction in total mortality (HR 0.83,95%CI 0.64–1.07, P=0.15) and a 28 percent increase in diabetes (HR 1.28,95%CI 1.07–1.54, P=0.01). In absolute terms for those with diabetes risk factors, 134 total cardiovascular events or deaths were avoided for every 54 new cases of diabetes diagnosed. In analyses limited to first events only, the number of major cardiovascular events or deaths avoided among those with one or more diabetes risk factors was 93.
Table 2.
Absolute number of events, incidence rates (per 100 person years), and hazard ratios (HR) for cardiovascular endpoints, death, and diabetes in the JUPITER trial among those with or without major diabetes risk factors, according to random allocation to rosuvastatin or placebo.
| Endpoint | No Major Diabetes Risk Factor (N=6095) | One or More Major Diabetes Risk Factors (N=11508) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rosuvastatin | Placebo | Delta | HR (95%CI) | P | Rosuvastatin | Placebo | Delta | HR (95%CI) | P | |
| Primary Endpoint* | 44 (0.69) | 91 (1.45) | −47 | 0.48 (0.33–0.68) | 0.0001 | 96 (0.80) | 157 (1.31) | −61 | 0.61 (0.47–0.79) | 0.0001 |
| Primary, Any Death | 118 (1.85) | 174 (2.77) | −56 | 0.67 (0.53–0.85) | 0.0007 | 175 (1.46) | 262 (2.18) | −87 | 0.67 (0.55–0.81) | 0.0001 |
| Primary, VTE, Any Death | 122 (1.92) | 187 (2.99) | −65 | 0.64 (0.51–0.81) | 0.0001 | 196 (1.64) | 289 (2.41) | −93 | 0.68 (0.57–0.81) | 0.0001 |
| MI, Stroke, Any Death | 99 (1.55) | 147 (2.33) | −48 | 0.67 (0.52–0.86) | 0.002 | 139 (1.15) | 202 (1.67) | −63 | 0.69 (0.56–0.86) | 0.0006 |
| Any Death | 89 (1.32) | 113 (1.69) | −24 | 0.78 (0.59–1.03) | 0.08 | 109 (0.85) | 132 (1.02) | −23 | 0.83 (0.64–1.07) | 0.15 |
| Diabetes | 12 (0.18) | 12 (0.18) | + 0 | 0.99 (0.45–2.21) | 0.99 | 258 (2.12) | 204 (1.65) | + 54 | 1.28 (1.07–1.54) | 0.01 |
Primary endpoint = nonfatal myocardial infarction, nonfatal stroke, unstable angina/revascularization, cardiovascular death; VTE = venous thromboembolism; MI = myocardial infarction; Delta = absolute difference in events between rosuvastatin and placebo
For trial participants with no major diabetes risk factor, random allocation to rosuvastatin yielded a 52 percent reduction in the primary endpoint (HR 0.48,95%CI 0.33–0.68, P=0.0001), a 53 percent reduction in VTE (HR 0.47,95CI 0.21–1.03, P=0.05), a 22 percent reduction in total mortality (HR 0.78,95%CI 0.59–1.03, P=0.08) and no increase in diabetes (HR 0.99, 95%CI 0.45–2.21, P= 0.99). In absolute terms for those without a major diabetes risk factor, 86 total cardiovascular events or deaths were avoided with no excess new cases of diabetes diagnosed. In analyses limited to first events only, the number of major cardiovascular events or deaths avoided among those with no major diabetes risk factor was 65.
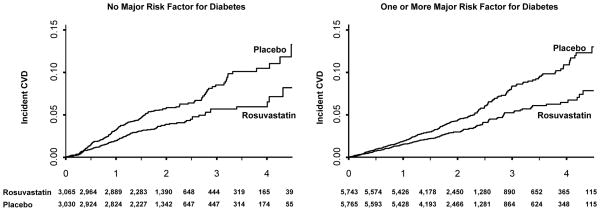
The cumulative incidence of cardiovascular events or death for those with and without major diabetes risk factors is shown in Figure 2, while the cumulative incidence of diabetes for those with and without major diabetes risk factors is shown in Figure 3. There were no significant violations of the proportional hazards assumptions for the data contained in these Figures. As anticipated with respect to the primary cardiovascular endpoint, the relative treatment benefits attributable to rosuvastatin were similar among those with and without diabetes risk factors (P- for interaction = 0.28).
Figure 2.
Cumulative incidence of cardiovascular events and total mortality among those with and without major risk factors for diabetes.
Figure 3.
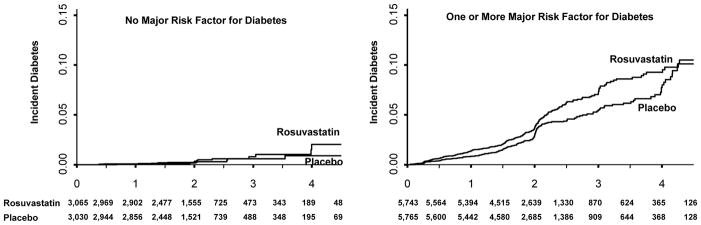
Cumulative incidence of diabetes among those with and without major risk factors for diabetes.
Risks of diabetes associated with rosuvastatin allocation did not change substantially as the number of major diabetes risk factors increased. For those with 1, 2, 3, or 4 major risk factors for diabetes, the observed hazard ratios for physician diagnosed diabetes associated with rosuvastatin were 1.2, 1.2, 1.4, and 1.4, respectively; none of these hazard ratios differed significantly from that observed for the study as a whole.
As shown in Figure 4, the relative benefits and risks of rosuvastatin were generally consistent for all components of the JUPITER primary and secondary endpoints and in all subgroups evaluated, including among those with or without metabolic syndrome, with or without impaired fasting glucose, with or without body mass index ≥ 30 kg/m2, or with or without HbA1c > 6 percent. In no instance were tests for interaction significantly different from that observed in the main analyses of those with none or at least one of these major diabetes risk factors.
Figure 4.
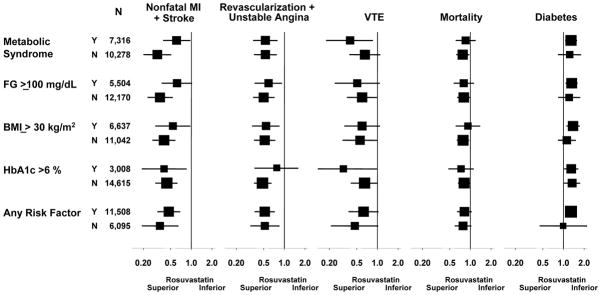
Hazard ratios and 95% confidence intervals for specific vascular events, total mortality, and diabetes in subgroup analyses among those with or without metabolic syndrome; among those with fasting glucose ≥ or < 100 mg/dL; among those with body mass index ≥ or < 30 kg/m2; and among those with HbA1c > or ≤ 6 percent.
Table 3 provides data concerning rates of adverse events (other than incident diabetes) and measured laboratory values in the JUPITER trial comparing rosuvastatin to placebo among those with and without one or more major diabetes risk factor. As shown, those with and without diabetes risk factors had similar non-diabetes adverse event rates attributable to rosuvastatin. As also shown, among those with and without diabetes risk factors, measured HbA1c at 24 months increased by 0.1 percent among those allocated to rosuvastatin (both p-values 0.001). Of interest, measured fasting glucose levels were not significantly different in the rosuvastatin and placebo groups; thus, as anticipated, had we relied on biochemical determination of diabetes rather than physician diagnosis, we could have systematically underestimated true effects.
Table 3.
Adverse events and measured laboratory values for fasting glucose and HbA1c during follow-up among those with and without major diabetes risk factors.
| No Major Diabetes Risk Factor | One or More Major Diabetes Risk Factors | |||||
|---|---|---|---|---|---|---|
| Randomized Rosuvastatin | Randomized Placebo | P | Randomized Rosuvastatin | Randomized Placebo | P | |
| Adverse Events, rate | ||||||
| Muscular weakness, stiffness, or pain | 8.72 | 8.53 | 0.76 | 8.20 | 7.75 | 0.28 |
| Myopathy | 0.06 | 0.06 | 0.98 | 0.05 | 0.04 | 0.75 |
| Rhabdomyolysis | 0.02* | 0.0 | -- | 0.0 | 0.0 | -- |
| Cancer | 1.98 | 1.70 | 0.24 | 1.38 | 1.61 | 0.14 |
| Renal disorders | 3.10 | 2.65 | 0.14 | 2.77 | 2.52 | 0.24 |
| Bleeding | 1.32 | 1.52 | 0.34 | 1.41 | 1.42 | 0.90 |
| Hepatic disorders | 1.12 | 1.08 | 0.84 | 1.14 | 0.93 | 0.10 |
| Hemorrhagic stroke | 0.03 | 0.03 | 1.00 | 0.02 | 0.05 | 0.32 |
| Laboratory values** | ||||||
| HbA1c (% at 24 months) | 5.8 (5.6–6.0) | 5.7 (5.5–5.9) | 0.001 | 6.0 (5.7–6.2) | 5.9 (5.6–6.2) | 0.001 |
| Fasting glucose (mg/dL at 24 months) | 94 (87–100) | 93 (87–99) | 0.20 | 101 (94–110) | 101 (93–110) | 0.19 |
Rates are per 100 person years
Occurred after trial completion.
Hemoglobin A1c (HbA1c, %) and fasting glucose (mg/dL) are reported as median (IQR) values.
In an analysis limited to those who developed diabetes during the JUPITER trial (N = 270 on rosuvastatin, N = 216 on placebo), 18 primary cardiovascular endpoints occurred. Of these, 8 were on rosuvastatin (incidence rate 1.10 per 100 person years) and 10 were on placebo (incidence rate 1.73 per 100 person years). Thus, among the 486 JUPITER trial participants who developed diabetes during follow-up, the cardiovascular risk reduction associated with rosuvastatin (hazard ratio 0.63, 95% confidence interval 0.25–1.60) was consistent with that observed for the trial as a whole (0.56, 95% confidence interval 0.46–0.69).
In sensitivity analyses, we found no substantive change for any of these findings when alternative definitions of metabolic syndrome or alternative cut-points for body mass index or HbA1c were used.
In analyses stratified by age, the hazard ratio (95%CI) for incident diabetes associated with rosuvastatin as compared to control was 1.26 (1.02–1.56) for those aged 50 to 69 years and 1.25 (0.90–1.74) for those aged 70 and over8.
Discussion
Although JUPITER was the first placebo-controlled statin trial to formally report an increased risk of developing diabetes1, post-hoc evaluations of previously completed trials demonstrate that this modest increase in risk is present for all statins and may relate to drug potency2–4. In higher risk secondary prevention patients with established coronary artery disease, the diabetes risk associated with statin therapy is low in absolute terms when compared with the reduction in cardiovascular events. However, in lower risk primary prevention patients where statin therapy is increasingly being utilized for vascular prevention, there has been controversy in the lay and medical press as to whether the absolute benefit of treatment outweighs the diabetes risk.
The current analyses from a contemporary primary prevention trial suggests that the risk of developing diabetes on statin therapy appears limited to those with baseline evidence of impaired fasting glucose, metabolic syndrome, severe obesity, or elevated HbA1c, a group of patients already at high risk for developing diabetes9,10. Of equal importance, within the JUPITER trial, the cardiovascular and mortality benefits of statin therapy exceeded the diabetes hazard in the trial population as a whole as well as among those at higher risk for developing diabetes. Further, in analyses limited to the 486 participants who developed diabetes, the point estimate for the relative risk reduction for cardiovascular events (hazard ratio 0.63) conformed with that observed for the trial as a whole (hazard ratio 0.56). These cardiovascular benefits, however, came with the hazard of diagnosing new onset diabetes 5 to 6 weeks earlier among those allocated to rosuvastatin as compared to placebo. Whether this latter observation has clinical relevance is uncertain as most diabetic patients are treated with statin therapy. In these data, we observed no effect modification by age.
Strengths of our analysis include its sample size, random allocation of statin therapy, and blinded ascertainment of incident events. Our analysis plan is also highly conservative in several respects, an approach we took on an a priori basis so as not to underestimate potential hazards of treatment. For example, we used the observed hazard ratio for diabetes within JUPITER of 1.25 rather than the smaller hazard ratios of 1.18 and 1.09 described for rosuvastatin and all statins, respectively, in the most comprehensive recent meta-analysis3. We also elected to conservatively include all incident cases of physician reported diabetes that occurred during the trial (including those reported between the time of study completion and the last patient closeout visit) as well as those cases that lacked biochemical confirmation; both of these approaches further reduce the risk of systematically under-reporting incident diabetes. Further, although we believe most physicians and patients would consider myocardial infarction, stroke, and death to be more severe outcomes than new-onset diabetes (which in some cases was simply a biochemical change in glucose levels from below to above 126 mg/dL), we elected not to introduce subjective bias into our analysis by weighting these events differently in our analysis plan. Finally, in addition to our primary analysis of total vascular events prevented, we conducted an additional analysis in which we limited each individual participant to a maximum of one vascular event. Even in this highly constrained analysis we found that statin therapy was associated with 65 fewer vascular events or deaths at no risk of diabetes among those with no major diabetes risk factors, and with 93 fewer vascular events or deaths at a cost of 54 new diagnoses of diabetes among those with major diabetes risk factors.
Limitations of our analysis include the fact that all study participants had elevated levels of CRP, an independent risk marker for both incident type 2 diabetes and incident cardiovascular events11,12. Thus, care should be used when considering these primary prevention data for those with CRP levels less than 2 mg/L. Further, although all statins increase diabetes risk2–4, our data are limited to rosuvastatin at a single dose (20 mg daily). Last, although we had more than 1,000 participants followed for 4 to 5 years, median follow-up within JUPITER was 2 years and thus long term data on benefits and risks cannot be gleaned from this study. This may be particularly relevant if an increased risk of diabetes results in microvascular as well as macrovascular disease that may not manifest for several years. On the other hand, as demonstrated here, almost all individuals who experienced an increased risk of diabetes while taking statin therapy already had underlying evidence of impaired fasting glucose (Figure 1). These are the very individuals most likely to develop diabetes in the near future and who thus would typically be treated with a statin as part of their routine care.
We believe the current data have clinical relevance for several reasons. First, we hope the benefit and risk data presented here in a primary prevention setting will better inform physician debate about the net utility of statin therapy, an issue that has recently become controversial particularly in the lay press.
Second, as the increase in risk of diabetes associated with statin therapy appears limited to those with major risk factors for diabetes, monitoring of glucose levels when initiating statin therapy may not be needed among those who have normal pretreatment glucose levels or lack multiple characteristics of metabolic syndrome.
Third, we anticipate that these and related data will spur research into the as yet unknown mechanisms by which statin therapy increases diabetes risk. Our observations that statins modestly accelerate the time to diabetes diagnosis and that risk is largely limited to those with impaired fasting glucose suggest directions for such mechanistic work. To this end, ongoing work will evaluate change in biochemical markers reflecting beta-cell function, insulin resistance and endothelial injury, adipokines, and other metabolic markers upon statin initiation and as predictors of incident diabetes in the trial.
Finally, for our patients, we hope these data ease concern about risks associated with statin therapy when they are appropriately prescribed for cardiovascular risk reduction as an adjunct to dietary discretion, increased exercise, and smoking cessation.
Supplementary Material
Panel: Research in context.
Systematic review
Three meta-analyses published between 2009 and 20112–4 indicate that all statin agents are associated with a modest increase in the risk of incident type 2 diabetes (hazard ratio 1.09, 95%CI 1.02–1.17)3, and that intensive-dose statin therapy is associated with somewhat higher risk than moderate dose therapy (hazard ratio 1.12, 95%CI 1.04–1.22)4. In absolute terms, however, these risks are low when compared to the absolute benefit of statin therapy in the setting of secondary prevention where most data derive. We were unable to find any data in the literature directly addressing the cardiovascular benefits and diabetes risks in the setting of primary prevention, an issue that has had considerable controversy in both the medical and lay press. Further, we were unable to find any data addressing whether the risks and benefits of statin therapy in primary prevention differ among those with and without risk factors for diabetes.
Interpretation
In the randomized, placebo controlled JUPITER trial of rosuvastatin 20 mg conducted in the setting of primary prevention, we observed that the modest risk of developing diabetes on statin therapy was limited to those who had biochemical evidence of impaired fasting glucose or multiple components of metabolic syndrome, groups already at high risk for developing diabetes. Further, both among those with and without diabetes risk factors, the absolute benefit of statin therapy on vascular events was greater than the hazard for developing new onset diabetes. These data should provide reassurance for patients and physicians regarding the use of lipid lowering as an adjunct to diet, exercise, and smoking cessation in the primary prevention of myocardial infarction, stroke, and cardiovascular death.
Acknowledgments
We thank the 17,802 JUPITER study participants and their individual physicians worldwide for their personal time and commitment to this project.
Funding. AstraZeneca (ClinicalTrials.gov number, NCT00239681)
Footnotes
ClinicalTrials.gov number, NCT00239681
Author’s Contributions: PMR designed the study and its analysis plan, contributed to the collection of data and its analysis and interpretation, provided study logistics, wrote the manuscript, and gave final approval of the report; AP and PL contributed to the collection of data and its interpretation and reviewed the report; JGM performed the primary data analyses, constructed tables and figures, and reviewed the report; RJG contributed to the study design and analysis plan, oversaw all statistical analyses, contributed to data interpretation and data analysis, and gave approval of the report.
Conflicts of Interest: The JUPITER trial was an investigator-initiated project funded by AstraZeneca. Dr. Ridker is the Principle Investigator of the trial and received grant support from AstraZeneca for its conduct. Dr. Ridker has served as a consultant to Merck, ISIS, Vascular Biogenics, Boerhinger, Abbott, and Genzyme; receives additional research grant support from Novartis; and is listed as a co-inventor on patents held by the Brigham and Women’s Hospital related to inflammatory biomarkers in cardiovascular disease and diabetes that have been licensed to Seimens and AstraZeneca. Dr. Glynn is the independent academic trial statistician for JUPITER and has received grant support from AstraZeneca for its conduct. Dr. Libby is an unpaid consultant or involved in clinical trials for AstraZeneca, GlaxoSmithKline, Merck, Novartis, Pfizer, ProNova, and Sigma-Tau, and is a member of the scientific advisory boards for Athera Biotechnolgies, Carolus Therapeutics, Interleukin Genetics, and BIND Biosciences. Dr. Pradhan, and Ms. MacFadyen report no conflicts of interest with regard to this manuscript.
References
- 1.Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–207. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 2.Rajpathak SN, Kumbhani DJ, Crandall J, et al. Statin therapy and risk of developing type 2 diabetes: a meta-analysis. Diabetes Care. 2009;32:1924–9. doi: 10.2337/dc09-0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735–42. doi: 10.1016/S0140-6736(09)61965-6. [DOI] [PubMed] [Google Scholar]
- 4.Preiss D, Seshasai SRK, Wels P, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy. JAMA. 2011;305:2556–64. doi: 10.1001/jama.2011.860. [DOI] [PubMed] [Google Scholar]
- 5.United States Food and Drug Administration. [accessed March 4, 2012];FDA Drug Safety Communication: Important safety label changes to cholesterol lowering statin drugs. http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm.
- 6.Glynn RJ, Danielson E, Fonseca FA, et al. A randomized trial of rosuvastatin in the prevention of venous thromboembolism. N Engl J Med. 2009;360:1851–61. doi: 10.1056/NEJMoa0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement: executive summary. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 8.Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomized trial. Ann Intern Med. 2010;152:488–96. doi: 10.1059/0003-4819-152-8-201004200-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Waters DW, Ho JE, DeMicco DA, et al. Predictors of new-onset diabetes in patients treated with atorvastatin. J Am Coll Cardiol. 2011;57:1535–45. doi: 10.1016/j.jacc.2010.10.047. [DOI] [PubMed] [Google Scholar]
- 10.Wilson PW, D’Agostino RB, Parise H, Sullivan L, Meigs JB. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112:3066–72. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 11.Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and the risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non HDL-cholesterol, apolipoproteins A-1 and B100, standard lipid measures, lipid ratios and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.