SUMMARY
Objective
To determine whether quadriceps weakness is associated with elevated risk of worsening knee pain over 5 years.
Methods
The Multicenter Osteoarthritis Study (MOST) is a longitudinal study of 50–79-year-old adults with knee osteoarthritis (OA) or known risk factors for knee OA. The predictor variable was baseline isokinetic quadriceps strength. Covariates included baseline body mass index (BMI), physical activity level, and history of knee surgery. The outcome was worsening pain reported on the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index pain subscale or knee replacement surgery between baseline and 5-year follow-up. Analyses were knee-based and used generalized estimating equations, stratified by sex to assess whether the lowest compared with the highest tertile of baseline quadriceps strength was associated with an increased risk of worsening knee pain at 5-year follow-up, controlling for age, BMI, history of knee surgery, and physical activity level as well as correlation between knees within participants.
Results
Analyses of worsening knee pain included 4,648 knees from 2,404 participants (61% female). Men with lower quadriceps strength did not have a higher risk of worsening knee pain (RR {95% CI} = 1.01 {0.78–1.32}, P = 0.9183). However, women in the lowest compared with the highest strength tertile had a 28% increased risk of worsening knee pain (RR {95% CI} = 1.28 {1.08–1.52}, P = 0.0052).
Conclusion
Quadriceps weakness was associated with an increased risk of worsening of knee pain over 5 years in women, but not in men.
Keywords: Quadriceps weakness, Knee pain, Knee osteoarthritis
Introduction
Knee pain is the hallmark symptom of knee osteoarthritis (OA) and the main reason patients seek medical care1–3. According to a recent report, the prevalence of knee pain among older adults in the United States has increased by as much as 65% over a span of 20 years in analyses adjusted for age and body mass index (BMI)4. Despite this considerable increase, causes of knee pain are still poorly understood.
It is believed that knee pain results from factors that may be biological and/or psycho-social in origin. Among biological factors, data suggest that quadriceps muscle weakness may contribute to worsening of knee pain. Recent investigations have provided evidence that higher quadriceps strength may inhibit worsening of knee joint structure5,6. However, because knee pain does not always correlate with radiographic changes, these data do not provide direct evidence that quadriceps strength protects against worsening of knee pain. Interventional studies have shown that exercise may improve knee pain and function7,8, providing indirect evidence for a relationship between quadriceps muscle strength and knee pain. One randomized trial of the effects of weight-bearing and non-weight-bearing exercise on knee pain and function revealed that both exercise programs led to statistically significant improvements in WOMAC scores compared with the control condition9. However, exercise-related improvements in pain may be due to factors such as improvements in joint range of motion, or hormonal effects, such as endogenous opioid secretion both reducing pain and improving mood, even without affecting strength. This is illustrated by the fact that quadriceps weakness does not predict incident knee symptoms10, despite strengthening exercises alleviating knee symptoms7. Moreover, cross-sectional and longitudinal investigations of the relationship between quadriceps strength and knee pain have had mixed results.
In cross-sectional studies, quadriceps weakness has been consistently associated with knee pain11–13, while longitudinal studies of individuals with10,14 or at increased risk of14 knee OA have not supported an association between quadriceps strength and a reduced risk of developing knee pain10,14 or a change in knee pain14 approximately 30 months later. Likewise, a study of similar duration also found no association between quadriceps strength and a longitudinal change in knee pain in individuals with symptomatic knee OA15. However, those with greater baseline quadriceps strength had less pain at both the baseline and 30-month follow-up visits compared with those with less strength15. Because this cohort was made up of individuals with symptomatic knee OA, it is possible that the ability to observe a longitudinal change over a 30-month duration in individuals with knee symptoms was limited.
As the number of older adults is substantially increasing, identification of modifiable factors, such as quadriceps strength, that could mitigate the severity or pace of worsening of knee pain in older adults could potentially help to reduce the significant burden of subsequent disability. Thus, this study evaluated whether quadriceps weakness at baseline was longitudinally associated with an increased risk of worsening of knee pain over 5 years in older adults with or at increased risk of knee OA.
Methods
Study population
The Multicenter Osteoarthritis Study (MOST) is an National Institutes of Health (NIH)-funded longitudinal observational study of 3,026 community-dwelling men and women age 50–79 with knee OA or known risk factors for knee OA including age, female sex, overweight status, and history of knee symptoms, knee injury and/or surgery. Participant enrollment has been previously described16. Briefly, potential participants were identified by mass mailings or advertisements and screened by telephone for eligibility. Potential participants were excluded for a history of (or planned) bilateral knee replacement, cancer (with the exception of non-melanoma skin cancer or breast, cervical, colon, prostate, rectal, or uterine cancer successfully treated with surgery), history of chemotherapy or radiation therapy, history of rheumatologic disease, or plan to move out of the area within 3 years of enrollment. In addition, knees with pain too severe to undergo the strength measurements (n = 702 knees from 414 participants) or knees with a history of total knee replacement at baseline (n = 78 knees from 78 participants) were excluded from the present study. All participants provided written informed consent using forms approved by the investigators' institutional review boards. Participating institutions' review boards approved this study.
Baseline assessments
Anthropometric measures
BMI(kg/m2) was calculated from baseline weight in kilograms divided by the square of the height in meters (Stadiometer, Holtain, Wales, UK), as measured by trained and certified staff17.
Physical activity
The Physical Activity Scale for the Elderly questionnaire (PASE: New England Research Institute, Watertown, MA) was used for estimating baseline activity level18–20. The PASE is a validated questionnaire that evaluates frequency and duration of specific leisure time, household and work activities in the past 7 days.
Strength
Isokinetic quadriceps and hamstrings strength for each lower limb was measured using a Cybex 350 computerized dynamometer (HUMAC software version 4.3.2/Cybex 300 for Windows 98, Avocent, Huntsville, AL)16. Certified examiners completed testing at 60° per second with a chair back angle of 85°, using a standardized protocol to assure uniformity among test sites. Participants completed three repetitions at 50% effort to become familiar with the test. After practice trials, four repetitions were completed at maximum effort and the peak torque (N•m) out of the four repetitions was recorded. To reduce potential for pain exacerbation or injury associated with a maximal eccentric contraction, peak torque was measured concentrically. The equipment was calibrated monthly according to manufacturer's guidelines.
Baseline and follow-up assessments
Knee-specific pain
The Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index, a validated instrument widely used in knee and hip osteoarthritis clinical trials, was completed at baseline and 60 months later. The knee pain subscale of the WOMAC was utilized for the present study. This subscale is comprised of five items with responses that range from no (0) to extreme (4) pain with a total possible score of 20. Participants provided pain scores for each knee (knee-based score). Higher scores on the WOMAC indicate greater pain. This study utilized a modified version of the WOMAC Likert format 5.0. The modification included the addition of a “don't do” response for participants unable to rate their pain severity because they either avoided or were unable to do specific activities. A “don't do” response was considered missing data. If two or more questions were missing from the WOMAC pain subscale, then the pain score was considered unusable. In addition, the WOMAC question about climbing up and down stairs was separated into two questions, with the worst score of the two used for calculating the WOMAC pain score. If one of the questions about climbing stairs was missing or not answered, then the non-missing answer was used for calculating the pain score.
Definition of worsening
This study utilized the Minimal Detectable Change (MDC, 90% confidence level), a modification of the standard error of the measurement (SEM) formula utilized by Wyrwich et al21. The MDC90 is a distribution-based approach for determining change that exceeded the error of the WOMAC instrument at a 90% confidence level, through using the SEM.
For this study, the MDC90 was calculated using the following formula:
where .
SEM, the standard error of the measurement, was defined as the baseline standard deviation (σ) of the WOMAC pain subscale in the study population times the square root of one minus the 14-day test–retest reliability coefficient for the WOMAC pain subscale in MOST (r = 0.79). Based on this formula, an increase of greater than three points was the cut-off for worsening for the pain subscale. Because pain is the main reason patients undergo knee replacement, the definition for worsening was further expanded to include knee replacement surgery between baseline and 60-month follow-up. This also enabled inclusion of participants who would otherwise have missing WOMAC follow-up data and those with baseline pain scores too high to meet the cut-off for worsening.
Statistical methods
Analyses were performed using SAS software version 9.2 (SAS Institute Inc., Cary, NC). All analyses were stratified by sex, due to differences in both strength and knee symptoms in men and women. Participant characteristics were summarized with frequencies and means. Participants were grouped into sex-specific tertiles of baseline quadriceps strength to define cut-offs for higher and lower strength in this cohort with the highest tertile used to define higher strength and the lowest tertile used to define lower strength. The potential relationships between baseline quadriceps strength and worsening of knee pain were assessed using a logistic regression model adjusted for age, BMI, history of knee surgery, and physical activity level (PASE) at baseline. Analyses were knee-based and utilized generalized estimating equations to adjust for correlations between knees within participants. To avoid selection bias, analyses were repeated using imputed data for participants missing baseline and follow-up WOMAC pain scores not due to total knee replacement. These data were estimated with a multiple imputation model using the Markov Chain Monte Carlo (MCMC) method. This included 620 knees from 320 participants. Missing data were assumed to be missing at random.
Results
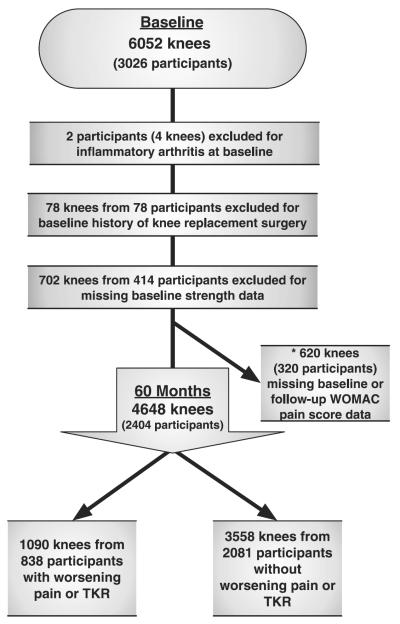
Out of 3,026 participants (6,052 knees) at baseline, two participants (four knees) were excluded due to inflammatory arthritis, 78 knees from 78 participants were excluded due to history of knee replacement surgery, one knee was excluded for missing WOMAC pain score data and 702 knees from 414 participants had missing strength data due to pain too severe to participate in the strength measurement (Fig. 1). At follow-up, there were 619 knees with missing WOMAC pain score data excluded from the analyses. A total of 4,648 knees from 2,404 participants (61% female) met eligibility criteria, had complete baseline and follow-up data and were included in the study. Baseline characteristics are shown in Table I. At baseline, men and women did not significantly differ in BMI. However, women were slightly older (P = 0.0181) had significantly higher pain scores (P < 0.0001) and significantly lower physical activity levels (P < 0.0001) and strength compared with men (P < 0.0001). A greater percentage of men had a history of knee surgery (P < 0.0001). A total of 35.1% of male knees and 37.0% of female knees had a KL grade ≥2 at baseline.
Fig. 1.
Participant inclusion diagram. Analyses were knee-based and a participant's right and left knees may have differed in eligibility as well as outcome. *Analyses repeated using imputed WOMAC pain score data for the 620 knees missing baseline or follow-up WOMAC pain score data.
Table I.
Baseline characteristics
| Participants | Age (years) | BMI (kg/m2) | PASE | WOMAC pain | Knee surgery (%) | Knee extensor strength (Nm) |
|---|---|---|---|---|---|---|
| Men | 61.7 ± 8.1 | 30.5 ± 5.2 | 207.5 ± 97.2 | 2.6 ± 3.3 | 15.2 | 122.5 ± 42.2 |
| n = 918 participants | n = 1,798 knees | |||||
| Women | 62.5 ± 7.8 | 30.6 ± 6.2 | 159.1 ± 76.7 | 3.4 ± 3.7 | 8.4 | 67.5 ± 25.1 |
| n = 1,486 participants | n = 2,850 knees |
Knee surgery = unilateral or bilateral WOMAC pain, knee surgery and knee extensor strength measured by knee.
There were a total of 1,090 knees from 838 participants (67% female) that experienced worsening of knee pain at 60-month follow-up. This included the 6.3% of male and 8.9% of female knees that underwent total knee replacement between baseline and 60-month follow-up. In men, lower compared with higher strength was not associated with an increased risk of knee pain worsening (RR {95% CI} = 1.01 {0.78–1.32}, P = 0.9183) (Table II). However, lower compared with higher baseline quadriceps strength was significantly associated with an increased risk of knee pain worsening in women (RR {95% CI} = 1.28 {1.08–1.52}, P = 0.0052).
Table II.
The relationship between baseline quadriceps strength and knee pain worsening at 60-month follow-up adjusted for baseline age, BMI, PASE score, and history of knee surgery
| Sex | Number of knees (% with progression) | Strength tertile (Range) | RR | (95% CI) | P-value |
|---|---|---|---|---|---|
| Male | 604 (18.0) | High (referent) 137.00–270.00 | 1.00 | ||
| 595 (20.5) | Middle 102.00–136.00 | 1.05 | 0.82–1.34 | 0.7082 | |
| 599 (21.4) | Low 20.00–101.00 | 1.01 | 0.78–1.32 | 0.9183 | |
| Female | 941 (20.5) | High (referent) 77.00–206.00 | 1.00 | ||
| 979 (25.4) | Middle 55.00–76.00 | 1.12 | 0.96–1.32 | 0.1545 | |
| 930 (31.1) | Low 20.00–54.00 | 1.28 | 1.08–1.52 | 0.0052 |
Data analyses using imputed data
There were 620 knees with either missing baseline or 60-month follow-up WOMAC pain scores. This included one knee with missing baseline data and 619 knees with missing follow-up data from participants who either attended the 60-month follow-up visit (n = 315 knees from 172 participants) or were lost to follow-up (304 knees from 159 participants). Because those missing follow-up data significantly differed from those with complete data, missing WOMAC pain scores were imputed. Those with missing 60-month follow-up WOMAC pain scores were similar in age (P = 0.2348) and knee extensor strength (P = 0.6305), but had greater baseline knee pain (P < 0.0001), higher BMI's (P = 0.0010) and lower physical activity scores (P = 0.0063) compared with those with complete data. In addition, there were more women with missing data (P = 0.0010). This brought the total number of knees included in the analyses from 4,648 knees (2,404 participants) up to 5,268 knees (2,736 participants). However, results were similar to those from analyses using only complete data.
There were a total of 444 knees from 339 men and 824 knees from 631 women that experienced worsening of knee pain at 60-month follow-up. Results are shown in Table III. In men, lower compared with higher strength was not associated with an increased risk of knee pain worsening (RR {95% CI} = 0.99 {0.75–1.30}, P = 0.9396). Lower compared with higher baseline quadriceps strength was still significantly associated with an increased risk of knee pain worsening in women (RR {95% CI} = 1.23 {1.01–1.49}, P = 0.0394).
Table III.
The relationship between baseline quadriceps strength and knee pain worsening at 60-month follow-up adjusted for baseline age, BMI, PASE score, and history of knee surgery including imputed data from 620 knees
| Sex | Number of knees (% with progression) | Strength tertile (Range) | RR | (95% CI) | P-value |
|---|---|---|---|---|---|
| Male | 709 (19.8) | High (referent) 137.00–270.00 | 1.00 | ||
| 689 (22.1) | Middle 102.00–136.00 | 1.06 | 0.82–1.37 | 0.6386 | |
| 703 (21.6) | Low 20.00–101.00 | 0.99 | 0.75–1.30 | 0.9396 | |
| Female | 1,061 (21.3) | High (referent) 77.00–206.00 | 1.00 | ||
| 1,046 (25.2) | Middle 55.00–76.00 | 1.09 | 0.90–1.31 | 0.4012 | |
| 1,060 (31.5) | Low 20.00–54.00 | 1.23 | 1.01–1.49 | 0.0394 |
Discussion
This study examined the longitudinal relationship between quadriceps weakness and worsening knee pain in men and women with or at increased risk for knee OA. We found that baseline quadriceps weakness was associated with worsening knee pain at 60-month follow-up in women but not men in the MOST cohort. To our knowledge, this is the largest longitudinal, observational study to evaluate this relationship.
The effects of quadriceps strength on progression of knee pain in individuals with knee OA has previously been investigated in longitudinal studies with different results14,15. Brandt and colleagues evaluated both incidence and progression of knee pain in 79 individuals with knee OA over 2.5 years, using the WOMAC pain subscale and defined progressive pain as an increase in WOMAC pain score of ≥15% compared with the baseline score14. With this different definition of knee pain progression, quadriceps strength was not found to be associated with progression of knee pain in this smaller cohort. Similarly, Amin et al found that baseline quadriceps strength was not associated with change in VAS knee pain ratings over 2.5 years in 265 men and women with symptomatic knee OA15. However, they reported that in comparison with those in the lowest tertile of strength, those in the highest tertile of strength had less pain at baseline and that this association with less pain persisted at follow-up.
Differences between the findings of these studies and those of our study could be due to the larger sample size in MOST. Our study also had a longer follow-up duration of 5 rather than 2.5 years, which may have increased the likelihood that worsening could be observed. In addition, our cohort was composed of men and women with or at risk of knee OA as opposed to only those with knee OA14 or symptomatic knee OA15. The fact that, in the study by Amin et al., subjects with greater baseline strength had less pain at both baseline and 30-month follow-up compared with those with lower baseline strength may support a relationship between quadriceps strength and knee pain. It is possible that a relationship with the change in pain was not observed in that study due to the shorter study duration, the use of VAS rather than WOMAC for assessment of pain, or the more advanced symptomatic knee OA in that cohort in comparison with the MOST cohort.
We evaluated whether quadriceps weakness predicts worsening knee pain because the quadriceps muscle is a primary dynamic contributor to knee joint stability. Weakness could reduce shock absorption and neuromuscular control and impair structural integrity of the knee joint. This may lead to abnormal loading and subsequent structural pathology associated with knee symptoms, such as bone marrow lesions or bone attrition22–24. In this study, we found quadriceps weakness may be a modifiable risk factor of worsening knee pain in women but not in men. Reasons for the apparent differences between men and women in the role of quadriceps strength may be related to a certain amount of strength being necessary to protect the knee from adverse loading. Women have less strength than men and therefore may start closer to that threshold, and cross it sooner than men25,26.
Our findings also may suggest sex-specific differences in risk factors for knee pain. Data from other studies have shown greater prevalence of pain conditions as well as pain sensitivity in women than men27. This was confirmed in a recent study that evaluated 11,000 patient medical records and found higher pain scores in women with musculoskeletal disorders compared with men28. Thus, it is possible that our finding that quadriceps muscle weakness predicts worsening knee pain in women but not in men may be due to underlying sex-specific differences in risk factors for worsening knee pain.
Several strengths of the current study contributed to advancing understanding of the relationship between muscle function and worsening of knee pain. First, to evaluate worsening of knee-related pain, our study evaluated whether participants met specific criteria for worsening, the MDC, rather than mean change in WOMAC scores from baseline which may have resulted in statistically significant findings simply due to the large sample size21. The Minimal Clinically Important Difference (MCID), an indicator of the clinical significance of an outcome, could not be utilized because the requisite external criterion for defining an important change was not administered in MOST. Though an MCID for worsening WOMAC pain has been reported in the literature, it was not utilized because MCID's may vary depending on the population under study as well as the method used for determining it29–33. However, the MDC90 cut-off for worsening in this study represented at least a 15% change from the maximal possible score of the baseline pain WOMAC subscale. Participants who met this cut-off were likely to have experienced worsening because this cut-off was similar to or exceeded the only reported MCID's for worsening pain on the WOMAC instrument in patients with hip or knee OA30,31,34. Further, inclusion of total knee replacement in our definition for worsening pain allowed for inclusion of participants who would have had missing data at 60-month follow-up due to surgery and those who had baseline scores too high to worsen. Because worsening of knee pain may progress slowly35, our study duration of 60 months and the inclusion of older adults with or at high risk for knee OA provided the opportunity to observe worsening over a longer duration and in a wider sample than prior studies. The longitudinal study design allowed for determination of the temporal relationship between quadriceps weakness and worsening of knee pain. In addition, the MOST cohort is made up of individuals with or at increased risk of knee OA, so the study findings are generalizable to this clinically relevant group, who is most likely to have concerns about their knees.
Despite these strengths, our findings should be interpreted in lightof the following. First, there are potential limitations in the use of the WOMAC to assess pain. The WOMAC only covers certain tasks, and each question on the WOMAC has the same potential total score despite representing tasks that may not result in equivalent pain, such as going down stairs compared with walking on a flat surface. In addition, if certain activities covered by the WOMAC questionnaire were either not done oravoided by participants, then these responses were considered missing information in our study. These limitations of the WOMAC index may hinder the ability to observe a change in individuals at the severe end of the spectrum, who may have avoided activities associated with pain or that they were no longer able to perform. However, missing data not due to total knee replacement were estimated (imputed) for those participants and the results when including these data (Table III) were similar to the results obtained when those participants were not included (Table II). It is also possible that our definition for worsening may have limited the ability to detect a change in those with greater pain at baseline. There were three participants with baseline WOMAC pain scores too high to meet the definition for worsening (three points). These participants also did not meet the definition for worsening pain through under-going total knee replacement surgery by 60-month follow-up. While the inability to detect worsening pain in these participants could bias results toward the null, the small number who met this criterion suggests that this is unlikely to have biased the study findings. Finally, there may be limitations to the way strength was measured. Iso-kinetic strength is not necessarily representative of functional strength. In addition, participants with pain at baseline may have had lower strength due to pain inhibition interfering with their ability to provide full effort for the strength test. It is possible these individuals were more likely to experience worsening pain.
Conclusion
In MOST, quadriceps weakness was associated with an increased risk of worsening of knee pain over 5 years in women. These findings expand on those of smaller studies of shorter duration on the relationship between quadriceps strength and knee pain and suggest the possibility of underlying sex-specific differences in risk factors for worsening of knee pain.
Acknowledgments
Role of the funding source
This study was supported by NIH grants to: Boston University (David Felson, MD – AG18820); University of Iowa (James Torner, PhD – AG18832); University of Alabama (Cora E. Lewis, MD MSPH – AG18947); University of California San Francisco (Michael Nevitt, PhD – AG19069). The study sponsor had no involvement in the study design, collection, analysis and interpretation of data, writing of the manuscript and decision to submit the manuscript for publication.
Footnotes
Author contributions
Responsibility for the integrity of the work as a whole: N. Glass, N. A. Segal.
Study concept and design: N. Glass, N. A. Segal.
Acquisition of data: J. C. Torner, M. Nevitt, D. T. Felson, C. E. Lewis. Analysis and interpretation of data: N. Glass, N. Segal, K. Wang, T. Yang.
Drafting of the manuscript: N. Glass, N. A. Segal.
Critical revision of the manuscript for important intellectual content: all authors.
Statistical expertise: K. Wang, T. Yang.
Obtained funding: J. C. Torner, M. D. Nevitt, D. T. Felson, C. E. Lewis.
Administrative, technical, or material support: J. C. Torner, M. Nevitt, D. T. Felson, C. E. Lewis.
Conflict of interest
The authors have declared no professional relationships with companies or manufacturers who will benefit from the results of the present study.
References
- 1.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 2.Dieppe PA, Cushnaghan J, Shepstone L. The Bristol 'OA500' study: progression of osteoarthritis (OA) over 3 years and the relationship between clinical and radiographic changes at the knee joint. Osteoarthritis Cartilage. 1997;5:87–97. doi: 10.1016/s1063-4584(97)80002-7. [DOI] [PubMed] [Google Scholar]
- 3.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;39:490–6. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Segal NA, Anderson DD, Iyer KS, Baker J, Torner JC, Lynch JA, et al. Baseline articular contact stress levels predict incident symptomatic knee osteoarthritis development in the MOST cohort. J Orthopaedic Res. 2009;27:1562–8. doi: 10.1002/jor.20936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segal NA, Glass NA, Torner J, Yang M, Felson DT, Sharma L, et al. Quadriceps weakness predicts risk for knee joint space narrowing in women in the MOST cohort. Osteoarthritis Cartilage. 2010;18:769–75. doi: 10.1016/j.joca.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker K, McAlindon T. Exercise for knee osteoarthritis. Curr Opin Rheumatol. 2000;12:456–63. doi: 10.1097/00002281-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 8.Thomas KS, Muir KR, Doherty M, Jones AC, O'Reilly SC, Bassey EJ. Home based exercise programme for knee pain and knee osteoarthritis: randomised controlled trial. BMJ. 2002;325:752. doi: 10.1136/bmj.325.7367.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jan MH, Lin CH, Lin YF, Lin JJ, Lin DH. Effects of weight-bearing versus nonweight-bearing exercise on function, walking speed, and position sense in participants with knee osteoarthritis: a randomized controlled trial. Arch Phys Med Rehabil. 2009;90:897–904. doi: 10.1016/j.apmr.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Segal NA, Torner JC, Felson DT, Niu J, Sharma L, Lewis CE, et al. Knee extensor strength does not protect against incident knee symptoms at 30 months in the multicenter knee osteoarthritis (MOST) cohort. PMR. 2009;1:459–65. doi: 10.1016/j.pmrj.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Reilly SC, Jones A, Muir KR, Doherty M. Quadriceps weakness in knee osteoarthritis: the effect on pain and disability. Ann Rheum Dis. 1998;57:588–94. doi: 10.1136/ard.57.10.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall MC, Mockett SP, Doherty M. Relative impact of radiographic osteoarthritis and pain on quadriceps strength, proprioception, static postural sway and lower limb function. Ann Rheum Dis. 2006;65:865–70. doi: 10.1136/ard.2005.043653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Madsen OR, Bliddal H, Egsmose C, Sylvest J. Isometric and isokinetic quadriceps strength in gonarthrosis; inter-relations between quadriceps strength, walking ability, radiology, subchondral bone density and pain. Clin Rheumatol. 1995;14:308–14. doi: 10.1007/BF02208344. [DOI] [PubMed] [Google Scholar]
- 14.Brandt KD, Heilman DK, Slemenda C, Katz BP, Mazzuca SA, Braunstein EM, et al. Quadriceps strength in women with radiographically progressive osteoarthritis of the knee and those with stable radiographic changes. J Rheumatol. 1999;26:2431–7. [PubMed] [Google Scholar]
- 15.Amin S, Baker K, Niu J, Clancy M, Goggins J, Guermazi A, et al. Quadriceps strength and the risk of cartilage loss and symptom progression in knee osteoarthritis. Arthritis Rheum. 2009;60:189–98. doi: 10.1002/art.24182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis Rheum. 2009;61:1210–7. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Arch Phys Med Rehabil. 2007;88:988–92. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinger MK, Oman RF, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the Physical Activity Scale for the Elderly (PASE) J Sports Med Phys Fitness. 2004;44:186–92. [PubMed] [Google Scholar]
- 19.Martin KA, Rejeski WJ, Miller ME, James MK, Ettinger WH, Jr, Messier SP. Validation of the PASE in older adults with knee pain and physical disability. Med Sci Sports Exerc. 1999;31:627–33. doi: 10.1097/00005768-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–62. doi: 10.1016/0895-4356(93)90053-4. [DOI] [PubMed] [Google Scholar]
- 21.Wyrwich KW, Nienaber NA, Tierney WM, Wolinsky FD. Linking clinical relevance and statistical significance in evaluating intra-individual changes in health-related quality of life. Med Care. 1999;37:469–78. doi: 10.1097/00005650-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Felson DT, Chaisson CE, Hill CL, Totterman SM, Gale ME, Skinner KM, et al. The association of bone marrow lesions with pain in knee osteoarthritis. Ann Intern Med. 2001;134:541–9. doi: 10.7326/0003-4819-134-7-200104030-00007. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Niu J, Guermazi A, Roemer F, Aliabadi P, Clancy M, et al. Correlation of the development of knee pain with enlarging bone marrow lesions on magnetic resonance imaging. Arthritis Rheum. 2007;56:2986–92. doi: 10.1002/art.22851. [DOI] [PubMed] [Google Scholar]
- 24.Hernandez-Molina G, Neogi T, Hunter DJ, Niu J, Guermazi A, Reichenbach S, et al. The association of bone attrition with knee pain and other MRI features of osteoarthritis. Ann Rheum Dis. 2008;67:43–7. doi: 10.1136/ard.2007.070565. [DOI] [PubMed] [Google Scholar]
- 25.Buchner D, de Lateur BJ. The importance of skeletal muscle strength to physical function in older adults. Ann Behav Med. 1991;13:91–8. [Google Scholar]
- 26.Ploutz-Snyder LL, Manini T, Ploutz-Snyder RJ, Wolf DA. Functionally relevant thresholds of quadriceps femoris strength. J Gerontol A Biol Sci Med Sci. 2002;57:B144–52. doi: 10.1093/gerona/57.4.b144. [DOI] [PubMed] [Google Scholar]
- 27.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. J Pain. 2009;10:447–85. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ruau D, Liu LY, Clark JD, Angst MS, Butte AJ. Sex differences in reported pain across 11,000 patients captured in electronic medical records. J Pain. 2012;13:228–34. doi: 10.1016/j.jpain.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Terwee CB, Roorda LD, Dekker J, Bierma-Zeinstra SM, Peat G, Jordan KP, et al. Mind the MIC: large variation among populations and methods. J Clin Epidemiol. 2010;63:524–34. doi: 10.1016/j.jclinepi.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Quintana JM, Escobar A, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after hip joint replacement. Osteoarthritis Cartilage. 2005;13:1076–83. doi: 10.1016/j.joca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 31.Escobar A, Quintana JM, Bilbao A, Arostegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthritis Cartilage. 2007;15:273–80. doi: 10.1016/j.joca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45:384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 33.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–8. [PubMed] [Google Scholar]
- 35.van Dijk GM, Dekker J, Veenhof C, van den Ende CH. Course of functional status and pain in osteoarthritis of the hip or knee: a systematic review of the literature. Arthritis Rheum. 2006;55:779–85. doi: 10.1002/art.22244. [DOI] [PubMed] [Google Scholar]