Abstract
Fluorescent probes are powerful and cost-effective tools for the detection of metal ions in biological systems. Compared to non-redox-active metal ions, the design of fluorescent probes for biological copper is challenging. Within the reducing cellular environment, copper is predominantly present in its monovalent oxidation state; therefore, the design of fluorescent probes for biological copper must take into account the rich redox and coordination chemistry of Cu(I). Recent progress in understanding the underlying solution chemistry and photophysical pathways led to the development of new probes that offer high fluorescence contrast and excellent selectivity towards monovalent copper.
Introduction
Copper is an essential trace nutrient that is required for a broad range of biological processes, including mitochondrial respiration, bone metabolism, wound healing, connective tissue formation, and the mobilization and uptake of iron [1]. Compared to zinc and iron, the average concentration of cellular copper is approximately one order of magnitude lower. For example, a single mouse fibroblast cells contains only between 3–5 femtogram of total copper [2], an amount that falls below the detection limit of standard elemental speciation techniques, such as inductively-coupled plasma mass spectrometry (ICP-MS) or atomic emission spectroscopy (AES). In contrast, synthetic fluorescent probes offer sufficient sensitivity and have found widespread applications for the in situ detection of a broad range of metal ions in live cells [3]. Compared to non-redox active metal ions such as Mg(II), Ca(II), or Zn(II), the design of fluorescent probes for the detection of Cu(I) is challenging due to interfering quenching pathways. This review offers an overview of recent approaches for the fluorescence detection of Cu(I) with an emphasis on the underlying photophysical principles.
Chemical Speciation of Biological Copper
Under physiological conditions, only the mono- and divalent oxidation states of copper are relevant according to the corresponding standard reduction potentials. Given the reducing intracellular environment with an average potential of −0.25 V [4], the chemical speciation of biological copper is likely dominated by monovalent complexes. Prior to import across the plasma membrane, extracellular Cu(II) is reduced by membrane reductases [5]. Once inside the cell, a host of selective metallochaperones are responsible for escorting Cu(I) to various subcellular destinations [5–7]. Although these metallochaperones bind Cu(I) very tightly, typically with dissociation constants in the femto- to attomolar concentration range [8], the metal transfer to the downstream recipient occurs with rapid kinetics [9]. The metallochaperones achieve this by coordinating Cu(I) with a surface-exposed bidentate CXXC motif, which allows for an associative exchange mechanism without transient release of aqueous Cu(I).
Several compelling arguments suggest that the intracellular environment is devoid of free copper ions. O’Halloran et al. were first to conclude that metalloenzymes are not activated by a pool of free copper ions [10]. Based on the binding affinity of superoxide dismutase (SOD1), the concentration of cytosolic free copper was estimated to be lower than 10−18 M, corresponding to less than a single atom per cell. It could be argued that many biological processes do not operate at thermodynamic equilibrium, a condition that would likely also apply to a slow-exchanging metalloprotein such as SOD1. There are however additional reasons that speak against the presence of a sizable pool of free aqueous Cu(I) ions. For example, at micromolar concentrations, aqueous Cu(I) would either spontaneously disproportionate into Cu(II) and Cu(0) at acidic pH, or precipitate in the form of cuprous oxide (Cu2O) under neutral or basic conditions. As both decomposition pathways become thermodynamically unfavorable at nanomolar concentrations or below, it makes sense that cellular Cu(I) is buffered at a low concentration by coordination to endogenous ligands. Glutathione (GSH), a low molecular weight thiol present at millimolar concentrations in the cytosol, has been proposed to serve this purpose [11]; however, its affinity appears to be too weak to compete for Cu(I) binding with endogenous copper chaperone proteins [8]. Another important point of consideration is the instability of aqueous Cu(I) towards dioxygen. In air-saturated water at 25°C, free Cu(I) is rapidly oxidized to Cu(II) with a half-lifetime in the millisecond range [12]. At the same time, the reducing thiol-rich intracellular environment would convert any adventitiously formed Cu(II) back to Cu(I); therefore, free copper ions, regardless of the valence state, would result in depletion of glutathione or other cellular reductants via Cu(II/I) redox cycling. Finally, aqueous Cu(I) would engage in Fenton-type chemistry with H2O2, which itself could be generated by the reaction of Cu(I) with dioxygen. The resulting highly reactive free radical species would damage a broad range of biomolecules, including lipids, proteins, or DNA.
Based on these arguments, the intracellular environment is likely devoid of free copper ions of either valence state. Then, how useful are fluorescent probes, if there is no analyte present? Concluding from the rapid exchange of copper with the extracellular medium, not all copper ions are trapped within proteins. This labile pool is therefore the source of Cu(I) that can be probed with a synthetic fluorescent sensor. It is important to note that the probe does not report on actual cellular copper concentrations, but rather it senses the availability and distribution of this exchangeable copper pool.
Design of Cu(I)-Responsive Fluorescent Probes
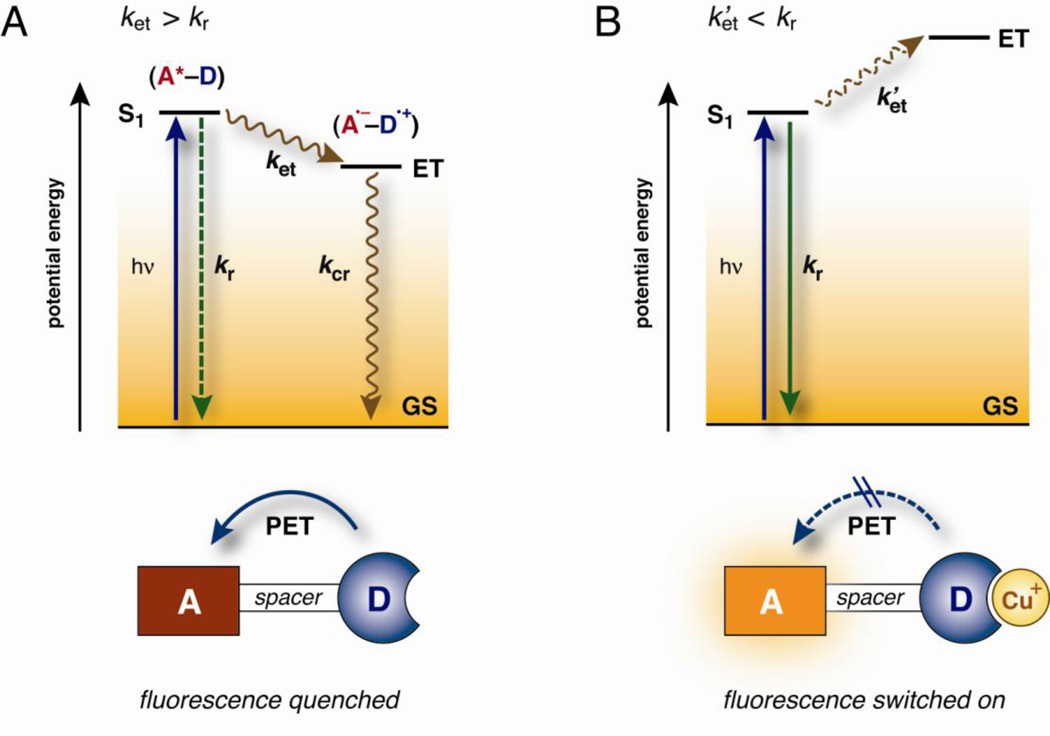
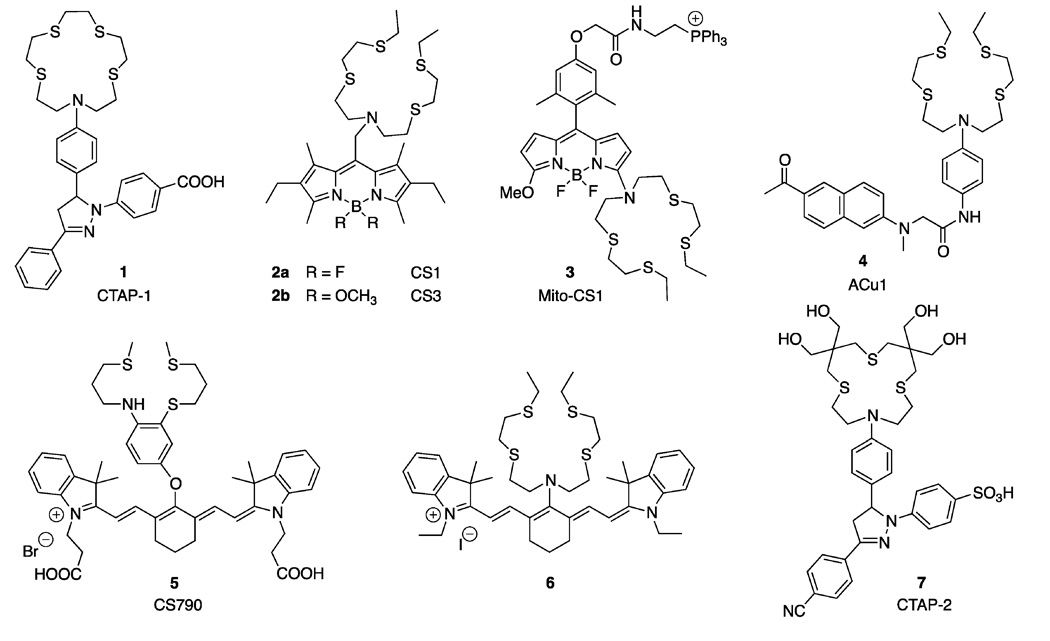
The majority of cation-selective fluorescent probes used in cellular biology are synthetic organic molecules composed of a chelator for ion recognition and a fluorophore for signal transduction upon binding of the analyte. Because Cu(I) is an effective fluorescence quencher, the design of Cu(I)-responsive fluorescent probes is challenging. The quenching pathways typically involve metal-to-ligand or ligand-to-metal charge transfer states, which undergo rapid intersystem crossing to non-fluorescent, long-lived triplet states. By spatially separating the chelator moiety from the fluorophore with a spacer, the formation of the quenching charge transfer states can be suppressed. Binding of Cu(I) is then signaled through a photoinduced electron transfer (PET) reaction between the chelator and the excited fluorophore, which act as the electron donor and acceptor in the PET process, respectively (Figure 1). Due to the spatial separation between the ion recognition site and the fluorophore, PET probes are inherently modular and allow for easy tuning of their photophysical and thermodynamic properties. At present, the number of fluorescence turn-on probes for aqueous Cu(I) is still small (Figure 2), though they already offer a distinct array of thermodynamic and photophysical properties (Table 1).
Figure 1.
Simplified Jablonski diagram illustrating the fluorescence switching mechanism of Cu(I)-responsive probes based on a photoinduced electron transfer (PET) process. a) In the absence of Cu+, electron transfer from the donor (D) to the excited fluorophore (A*) is thermodynamically favorable. The rate of electron transfer (ket) is faster than the rate for radiative deactivation (kr), resulting in emission quenching. Charge-recombination (kcr) of the formed radical ion-pair (A•−–D•+) is a non-radiative process b) Cu+-coordination to the donor (D) decreases the driving force for PET, and slows down the electron transfer process (ket’). Radiative deactivation favorably competes with PET, and the fluorescence is switched on (ket’ < kr). Legend: GS = ground state, ET = electron transfer state, S1 = lowest excited singlet state.
Figure 2.
Fluorescent probes for the detection of monovalent copper in aqueous buffer.
Table 1.
Photophysical Data of Fluorescent Probes for the Detection of Cu(I) in Aqueous Buffers
| compound | λabs max [nm] |
λem max [nm] |
ΦFa | feb | logKCu(I) | pH/buffer | mechanism c | ref |
|---|---|---|---|---|---|---|---|---|
| CTAP-1 (1) | 365 | 485 | 0.14 | 4.6 | 10.4 | 7.2 (PIPES) d | PET | [13] |
| CS1 (2a) | 540 | 561 | 0.13 | 10 | 11.4 | 7.0 (HEPES) d | PET | [14,15] |
| CS3 (2b) | 540 | 548 | 0.40 | 75 | 13.1 | 7.0 (HEPES) d | PET | [16•] |
| Mito-CS1 (3) | 550 | 558 | 0.05 | 10 | 11.1 | 7.4 (PBS) d | CT | [17] |
| ACu1 (4) | 363 (750)f | 482 | 0.13 | 4 | 10.7 | 7.0 (HEPES) d | PET | [18] |
| CS790 (5) | 760 | 790 | 0.072 | 15 | 10.5 | 7.0 (HEPES) d | PET | [19•] |
| 6 | 750 | 792 | n.d. | 9.6 | 11.2 | 7.0 (PBS, 10% EtOH)e | CT | [20] |
| CTAP-2 (7) | 396 | 508 | 0.083 | 65 | 11.4 | 7.2 (MOPS) | PET | [21•] |
| 10 | 394 | 506 | 0.074 | 57 | 9.7 | 7.2 (MOPS) | PET | [22] |
fluorescence quantum yield of the Cu(I)-saturated probe.
fluorescence enhancement factor (contrast) between the free and Cu(I)-saturated probe.
PET = photoinduced electron transfer switching; CT = charge transfer switching mechanism.
probe diluted into buffer from DMSO stock solution.
probe diluted from ethanol stock solution.
two-photon absorption maximum
The first synthetic fluorescence turn-on probe reported for the detection of aqueous Cu(I) was CTAP-1 (1). Its molecular architecture is composed of an NS4-thiazacrown ligand serving as the PET donor and analyte recognition site, and a 1,3-diaryl-2-pyrazoline fluorophore acting as the PET electron acceptor and fluorescence signal transducer [13]. The probe binds Cu(I) with a logK of 10.4 at pH 7.2 and exhibits a 4.6-fold emission increase with an excitation maximum at 365 nm. The cellular staining pattern of CTAP-1 revealed significant colocalization with the elemental copper topography as visualized by x-ray fluorescence microscopy (microXRF) [13].
The acyclic NS4 thiaza-ligand of coppersensor 1 (CS1, 2a) offers a 10-fold higher Cu(I)-affinity compared to CTAP-1. The attached BODIPY fluorophore can be excited with the 488 nm line of an argon laser and responds with a 10-fold fluorescence enhancement upon saturation with Cu(I) in HEPES buffer at pH 7.0 [14, 15]. Replacement of the BODIPY fluoro-substituents with a pair of methoxy groups yielded CS3 (2b) [16•], which exhibits a much improved fluorescence contrast over CS1. As demonstrated by Nagano and coworkers, this structural alteration increases the BODIPY reduction potential by approximately 190 mV, which in turn reduces the PET driving force and electron transfer kinetics (Figure 1) [23]. A comparison of the CS1 and CS3 quantum yields in absence of Cu(I) reveals, however, that apo-CS3 is more efficiently quenched, suggesting a different or competing quenching mechanism other than PET. It is unclear why the binding affinity of CS3 is 50-fold higher compared to CS1, as both probes utilize identical thiaza-ligands for copper-recognition. Imaging studies with CS3-stained live human embryonic kidney cells (HEK293T) revealed a markedly reduced fluorescence intensity upon treatment with the extracellular Cu(I)-selective chelator bathocuproine sulfonate (BCS). Furthermore, combined microXRF and fluorescence imaging studies with CS3 indicated a calcium-dependent copper redistribution in neuronal cells [16•].
To probe mitochondrial copper pools, the BODIPY core of Mito-CS1 (3) was conjugated to a lipophilic triphenylphosphonium cation [17], a proven strategy for targeting synthetic dyes to the mitochondrial matrix [24]. The Cu(I)-binding site of the probe is based on the NS4 motif of CS1 (2a) and thus exhibits a similar binding affinity. Because the nitrogen donor of the ligand is directly conjugated to the BODIPY π-system, the fluorescence switching mechanism of Mito-CS1 likely involves a pathway different from PET. Despite the proximity of the binding site to the fluorophore, the fluorescence is not quenched; rather it undergoes a 10-fold increase upon binding of Cu(I).
The fluorescent probe ACu1 (4) was developed to detect copper in live tissues by two-photon excitation microscopy (TPEM) [18]. Compared to conventional visible light fluorescence microscopy, TPEM offers increased depth penetration, lower background fluorescence, reduced photodamage, and intrinsic 3D-imaging capabilities [25, 26]. The probe features an acyclic NS4 ligand for selective recognition of Cu(I) and a donor-acceptor substituted naphthalene core as the two-photon excitable PET reporter. The utility of Acu1 (4) was assessed in live HeLa cells as well as in acute hippocampal slices from 2-day old rats [18].
Increased tissue penetration and reduced autofluorescence can be also achieved by conventional one-photon excitation in the near-infrared (NIR) region, an approach that has been widely employed for molecular imaging in whole animals [27]. Built upon a Cy7 NIR fluorophore platform, the copper sensor CS790 (5) offers an excitation maximum at 760 nm and exhibits a 15-fold emission increase upon saturation with Cu(I) in aqueous buffer [19•]. The NS3 thiaza-receptor used as a Cu(I)-selective chelator has been shown to maximize the switching potential through suppressing ternary complex formation with solvent molecules (vide infra) [28•]. The probe responded with a 1.9-fold fluorescence increase in HEK293T cells upon supplementation with 100 µM CuCl2 for 12 hours and was employed for imaging exchangeable copper pools in SKH-1 mice as well as Atp7b−/−, a murine model of Wilson disease [19•]. Combination of the acyclic NS4 thiaza chelator with a Cy7 fluorophore yielded the NIR probe 6 [20], which exhibits a similar binding affinity and fluorescence contrast compared to the parent probe CS1 (2a). Similar to Mito-CS1 (3), the NS4 receptor moiety is attached to the fluorophore π-system without a spacer, suggesting that the fluorescence switching mechanism does not involve PET.
Water-Solubility and Aggregation
In order to cross the plasma membrane and reach cellular targets, fluorescent probes must be somewhat lipophilic, which in turn often renders them poorly water-soluble. For this reason, the probes are routinely dissolved in an organic solvent such as DMSO, followed by dilution into the incubation buffer. Although the resulting solutions are usually optically clear, lipophilic compounds tend to form colloidal aggregates under these conditions, even at low micromolar concentrations [29, 30]. As the colloids are composed of nanoparticles with sizes below the diffraction limit, the final incubation solutions appear optically transparent, and the high-molecular weight aggregates can only be detected with more specialized analytical techniques such as dynamic light scattering or electron microscopy. Inside cells, the interaction of dye particles with hydrophobic proteins or lipid bilayers may not only impact the cellular physiology, but also result in subcellular localization of the dye into vesicles, lipid droplets, lysosomes, and other organelles, thus potentially producing staining artifacts. The latter possibility is especially a concern if the photophysical properties, notably the brightness and emission wavelength, of the fluorophore are substantially different between the monomeric and aggregated forms. For example, TM-BODIPY (4,4-difluoro-1,3,5,7-tetramethyl-4-bora-3a,4a-diaza-s-indacene) is able to form H and J dimer species that have vastly different absorption and emission properties compared to the monomeric species [31]. In ethanol, TM-BODIPY does not self-associate and is brightly fluorescent, whereas upon dilution into pure water, the dye forms non-fluorescent colloidal aggregates that slowly equilibrate into non-fluorescent H dimers, apparent by a blue-shifted absorption maximum [32]. Furthermore, in lipid bilayers, the monomeric form of the dye can be converted to J dimers with a red-shifted absorption and emission profile [31, 33]. Dynamic light scattering studies with aqueous solutions of a series of lipophilic copper probes, including the BODIPY-based coppersensors CS1 and CS3, revealed the presence of colloidal aggregates with hydrodynamic radii ranging between 60–100 nm [21•]. As the fluorescence response of such probes may not only be triggered by the analyte but also environmental changes, the interpretation of the cellular staining pattern and fluorescence response becomes challenging as recently demonstrated for the coppersensor CS1 [34•].
To suppress colloidal aggregation and improve the solubility in aqueous buffers, the Cu(I)-responsive probe CTAP-2 (7) was functionalized with hydrophilic hydroxy groups on the thiocrown binding site and a sulfonic acid substituent on the pyrazoline core (Figure 2) [21•]. In contrast to all previously published Cu(I)-responsive fluorescent probes, CTAP-2 (7) directly dissolves in water or aqueous buffer and does not require dilution from an organic stock solution. The probe underwent a 65-fold fluorescence enhancement upon saturation with Cu(I) and proved to be very selective for Cu(I) over all other biologically relevant metal ions. In addition, CTAP-2 was suitable for the direct in-gel detection of Cu(I) bound to a metallochaperone (Atox1) with an accessible metal-binding site [21•].
Contrast Optimization
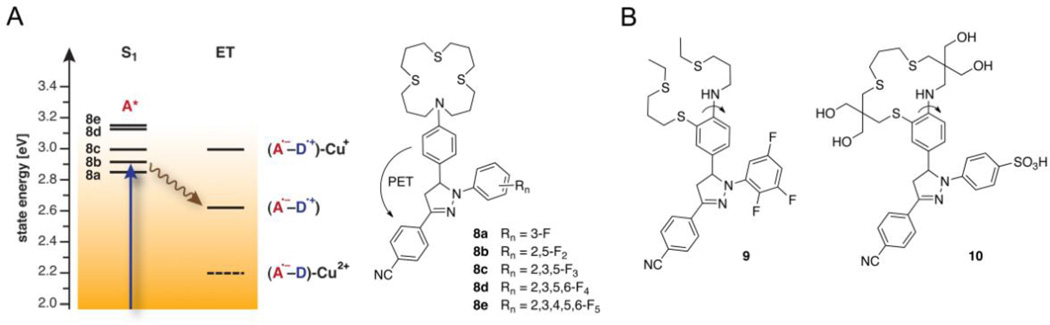
Within the complex environment of biological samples, the distribution of fluorescent probes is likely non-homogeneous. To avoid staining artifacts, it is critical to minimize the background fluorescence of the free probe and maximize the contrast upon saturation with the analyte. As evident from the Jablonski diagram in Figure 1, the background fluorescence is determined by the relative rates of ET quenching (ket) vs. radiative deactivation of the excited fluorophore (kr), while the fluorescence enhancement is related to the decrease of ket to k’et upon Cu(I)-binding. According to Marcus theory, the ET kinetics is governed by its thermodynamic driving force [35]; thus, the larger the energy gap between S1 and ET, the more effective the fluorescence quenching and the lower the background fluorescence. As illustrated with the probe series 8a–8e (Figure 3), the excited state energy of the pyrazoline fluorophore can be gradually increased by increasing the electron withdrawing character of the N-aryl substituents [36••]. Because the N-aryl substituents have little effect on the reduction potential of the fluorophore [37, 38], the energy of the ET state remains steady across the entire compound series. As a consequence, the ET driving force can be tuned in a step-wise fashion until the optimal balance is achieved between the quenching efficiency of the free probe and the fluorescence recovery upon Cu(I)-binding [39]. A comparison of the excited state energies (S1) and the ET levels in the presence and absence of Cu(I) reveals that the optimal balance is achieved with 8b, whereas the quenching efficiencies of the other probes are either too high (8c–d) or too low (8a). Consistent with this model, 8b exhibits the highest fluorescence contrast upon saturation with Cu(I) [36••]. Interestingly, protonation of the aniline nitrogen yields a more than 5-fold higher fluorescence contrast, suggesting that Cu(I) participates in adverse PET quenching, for example, by means of an energetically low-lying CT state (labeled (A•−–D)-Cu2+ in Figure 3A). However, femtosecond-resolved pump-probe experiments provided no evidence for the formation of a transient Cu(II) species; instead, variable temperature NMR studies indicated the presence of ternary complexes in which the interaction of the Cu(I) center with the aniline nitrogen is weakened through coordination with solvent molecules, presumably to alleviate steric strain between the aniline ring and the ligand backbone [36••].
Figure 3.
Contrast optimization of Cu(I)-responsive fluorescent probes. A) Simplified Jablonski diagram illustrating the tunability of the PET driving force in 1,3,5-triaryl-pyrazoline derivatives 8a–e: The excited state energy (S1) increases with increasing number of fluoro-substituents, whereas the energy of the electron transfer state (ET) remains largely unaffected. Although electron transfer from Cu(I) to the excited fluorophore is thermodynamically favorable, formation of the corresponding charge transfer state (A•−–D)-Cu2+ is too slow to compete with radiative deactivation from S1. B) Design of high-contrast probes based on synergistic conformational and electronic switching and suppression of ternary complex formation.
By integrating the aniline donor into the ligand backbone, steric crowding upon Cu(I)-coordination was reduced in probe 9, and the donor potential was maximized through synergistic conformational and electronic switching. The probe yielded a greater than 200-fold fluorescence enhancement with a quantum yield of 49% upon saturation with Cu(I) in methanol [28•]. Since the large recovery quantum yield contradicts any participation of Cu(I) in fluorescence quenching via formation of the thermodynamically favorable charge-transfer state (Figure 3A), the PET reaction must occur under kinetic control. Altogether, these data firmly established that high-contrast ratios can be achieved even for redox-active metal cations such as Cu(I) due to kinetic control of the PET switching mechanism.
To design a water-soluble derivative based on this approach, probe 10 was functionalized with hydrophilic groups similar to CTAP-2 [22]. Despite these structural changes, the fluorescence contrast and quantum yield of 10 did not improve over CTAP-2 (7). Concluding from solvent isotope effects and responses to acidification, the fluorescence contrast of 10 is compromised by two distinct excited state proton transfer quenching pathways operating under neutral and acidic conditions [22]. Furthermore, the low basicity of the arylamine with a pKa around 1.0 combined with a 50-fold reduced Cu(I) affinity compared to CTAP-2 suggest that Cu(I) interacts only weakly with the PET donor.
Conclusions
Although the development of Cu(I)-selective fluorescent probes has been riddled with many challenges, important progress has been made towards optimizing the fluorescence contrast, suppressing colloidal aggregation, improving the water-solubility, and understanding the photophysical pathways relevant to the probe design. In comparison, tuning of the Cu(I) affinity has so far received only minimal attention. Recent advances in characterizing proteins involved in copper homeostasis suggest that the cellular Cu(I) pool is buffered at femtomolar concentration or lower [8], a value that is not well matched by any of the synthetic probes listed in Table 1. At present, genetically encoded probes that are built upon Cu(I)-binding motifs of metalloregulatory proteins represent a promising alternative to synthetic probes [40••, 41–42]. Furthermore, the catalytic activity of Cu(I) offers intriguing opportunities to design ultrasensitive detection systems. For example, by harnessing the reactivity of a Cu(I) tris(2-pyridylmethyl)amine (TPA) complex to uncage a fluorescein derivative, Taki et al. were able to detect copper in live HeLa cells [43•]. Common to all fluorescent probes, a rigorous characterization of their response within the complex environment of biological systems is imperative, especially in case of probes that form colloidal aggregates or show a solvent dependent fluorescence response. While significant challenges remain to be addressed, there is no doubt that the ability of fluorescent probes to visualize analytes in live cells, tissues, and even whole organisms renders them an invaluable tool to advance our understanding of copper biology.
Highlights.
Fluorescent probes are powerful tools for the detection of copper in biological systems
Ternary complex formation with solvent molecules leads to reduced quantum yields
Electronic tuning of the fluorophore properties are key to maximize contrast
Lipophilic probes tend to form colloidal aggregates that may produce staining artifacts
Acknowledgements
Financial support from the National Institutes of Health (GM067169) is gratefully acknowledged.
Abbreviations
- GSH
Glutathione
- PET
Photoinduced electron transfer
- CT
Charge transfer
- SOD1
Superoxide dismutase 1
- BCS
Bathocuproine sulfonate
- microXRF
Synchrotron x-ray fluorescence microscopy
- TPEM
Two photon excitation microscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kim B-E, Nevitt T, Thiele DJ. Mechanisms for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008;4:176–185. doi: 10.1038/nchembio.72. [DOI] [PubMed] [Google Scholar]
- 2.McRae R, Lai B, Fahrni CJ. Copper redistribution in Atox1-deficient mouse fibroblast cells. J. Biol. Inorg. Chem. 2010;15:99–105. doi: 10.1007/s00775-009-0598-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Domaille DW, Que EL, Chang CJ. Synthetic fluorescent sensors for studying the cell biology of metals. Nat. Chem. Biol. 2008;4:168–175. doi: 10.1038/nchembio.69. [DOI] [PubMed] [Google Scholar]
- 4.Go YM, Jones DP. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta. 2008;1780:1271–1290. doi: 10.1016/j.bbagen.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lutsenko S. Human copper homeostasis: a network of interconnected pathways. Curr. Opin. Chem. Biol. 2010;14:211–217. doi: 10.1016/j.cbpa.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nevitt T, Ohrvik H, Thiele DJ. Charting the travels of copper in eukaryotes from yeast to mammals. Biochim. Biophys. Acta. 2012;1823:1580–1593. doi: 10.1016/j.bbamcr.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huffman DL, O'Halloran TV. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu. Rev. Biochem. 2001;70:677–701. doi: 10.1146/annurev.biochem.70.1.677. [DOI] [PubMed] [Google Scholar]
- 8.Xiao Z, Brose J, Schimo S, Ackland SM, La Fontaine S, Wedd AG. Unification of the copper(I) binding affinities of the metallo-chaperones Atx1, Atox1 and related proteins: detection probes and affinity standards. J. Biol. Chem. 2011;286:11047–11055. doi: 10.1074/jbc.M110.213074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benítez JJ, Keller AM, Huffman DL, Yatsunyk LA, Rosenzweig AC, Chen P. Relating dynamic protein interactions of metallochaperones with metal transfer at the single-molecule level. Faraday Discuss. 2011;148:71–82. doi: 10.1039/c004913a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rae TD, Schmidt PJ, Pufahl RA, Culotta VC, O'Halloran TV. Undetectable intracellular free copper: the requirement of a copper chaperone for superoxide dismutase. Science. 1999;284:805–808. doi: 10.1126/science.284.5415.805. [DOI] [PubMed] [Google Scholar]
- 11.Viguier RFH, Hulme AN. A sensitized europium complex generated by micromolar concentrations of copper(I): toward the detection of copper(I) in biology. J. Am. Chem. Soc. 2006;128:11370–11371. doi: 10.1021/ja064232v. [DOI] [PubMed] [Google Scholar]
- 12.Mi L, Zuberbühler AD. Cuprous complexes and dioxygen. Part 11. Concomitant one-and two-electron reduction of O2 by the Cuaq+ ion. Helv. Chim. Acta. 1991;74:1679–1688. [Google Scholar]
- 13.Yang L, McRae R, Henary MM, Patel R, Lai B, Vogt S, Fahrni CJ. Imaging of the intracellular topography of copper with a fluorescent sensor and by synchrotron x-ray fluorescence microscopy. Proc. Natl. Acad. SciU.SA. 2005;102:11179–11184. doi: 10.1073/pnas.0406547102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller EW, Zeng L, Domaille DW, Chang CJ. Preparation and use of Coppersensor-1, a synthetic fluorophore for live-cell copper imaging. Nat. Protoc. 2006;1:824–827. doi: 10.1038/nprot.2006.140. [DOI] [PubMed] [Google Scholar]
- 15.Zeng L, Miller EW, Pralle A, Isacoff EY, Chang CJ. A selective turn-on fluorescent sensor for imaging copper in living cells. J. Am. Chem. Soc. 2006;128:10–11. doi: 10.1021/ja055064u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dodani SC, Domaille DW, Nam CI, Miller EW, Finney LA, Vogt S, Chang CJ. Calcium-dependent copper redistributions in neuronal cells revealed by a fluorescent copper sensor and x-ray fluorescence microscopy. Proc. Natl. Acad. SciU.SA. 2011;108:5980–5985. doi: 10.1073/pnas.1009932108. • Live rat hippocampal neurons were depolarized with 50 mM KCl, and the redistribution of cellular copper was assessed by microXRF and with coppersensor 3, a synthetic fluorescent probe.
- 17.Dodani SC, Leary SC, Cobine PA, Winge DR, Chang CJ. A targetable fluorescent sensor reveals that copper-deficient SCO1 and SCO2 patient cells prioritize mitochondrial copper homeostasis. J. Am. Chem. Soc. 2011;133:8606–8616. doi: 10.1021/ja2004158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lim CS, Han JH, Kim CW, Kang MY, Kang DW, Cho BR. A copper(I)-ion selective two-photon fluorescent probe for in vivo imaging. Chem. Commun. 2011;47:7146–7148. doi: 10.1039/c1cc11568e. [DOI] [PubMed] [Google Scholar]
- 19. Hirayama T, Van de Bittner GC, Gray LW, Lutsenko S, Chang CJ. Near-infrared fluorescent sensor for in vivo copper imaging in a murine Wilson disease model. Proc. Natl. Acad. Sci. U.S.A. 2012;109:2228–2233. doi: 10.1073/pnas.1113729109. • The utility of coppersensor 790, a near-infrared Cu(I)-responsive fluorescent probe was explored in live HEK 293T cells as well as in a murine model of Wilson disease.
- 20.Cao X, Lin W, Wan W. Development of a near-infrared fluorescent probe for imaging of endogenous Cu(I) in live cells. Chem. Commun. 2012;48:6247–6249. doi: 10.1039/c2cc32114a. [DOI] [PubMed] [Google Scholar]
- 21. Morgan MT, Bagchi P, Fahrni CJ. Designed to dissolve: suppression of colloidal aggregation of Cu(I)-selective fluorescent probes in aqueous buffer and in-gel detection of a metallochaperone. J. Am Chem. Soc. 2011;133:15906–15909. doi: 10.1021/ja207004v. • To suppress colloidal aggregation observed with earlier Cu(I) probes, this Cu(I)-responsive probe was functionalized with hydrophilic groups and its fluorescence contrast optimized by electronic tuning. The probe directly dissolves in aqueous buffer without dilution from an organic stock solution.
- 22.Morgan MT, Bagchi P, Fahrni CJ. High-contrast fluorescence sensing of aqueous Cu(I) with triarylpyrazoline probes: dissecting the roles of ligand donor strength and excited state proton transfer. Dalton Trans. 2013;42:3240–3248. doi: 10.1039/c2dt31985c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gabe Y, Ueno T, Urano Y, Kojima H, Nagano T. Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Anal. Bioanal. Chem. 2006;386:621–626. doi: 10.1007/s00216-006-0587-y. [DOI] [PubMed] [Google Scholar]
- 24.Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. U.S.A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmchen F, Denk W. Deep tissue two-photon microscopy. Nat. Methods. 2005;2:932–940. doi: 10.1038/nmeth818. [DOI] [PubMed] [Google Scholar]
- 26.Sumalekshmy S, Fahrni CJ. Metal-ion-responsive fluorescent probes for two-photon excitation microscopy. Chem. Mater. 2011:823–830. doi: 10.1021/cm1021905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hilderbrand SA, Weissleder R. Near-infrared fluorescence: application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010;14:71–79. doi: 10.1016/j.cbpa.2009.09.029. [DOI] [PubMed] [Google Scholar]
- 28. Chaudhry AF, Mandal S, Hardcastle KI, Fahrni CJ. High-contrast Cu(I)-selective fluorescent probes based on synergistic electronic and conformational switching. Chem. Sci. 2011;2:1016–1024. doi: 10.1039/C1SC00024A. • A redesigned ligand architecture and systematic electronic tuning were key in the design of a high-contrast Cu(I)-probe that responded with a greater than 200-fold fluorescence enhancement and a quantum yield of 49%. The study demonstrates that high-contrast ratios can be achieved even for redox-active metal ions, providing the metal-initiated quenching pathways are kinetically unfavorable.
- 29.Doak AK, Wille H, Prusiner SB, Shoichet BK. Colloid formation by drugs in simulated intestinal fluid. J. Med. Chem. 2010;53:4259–4265. doi: 10.1021/jm100254w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng BY, Shelat A, Doman TN, Guy RK, Shoichet BK. High-throughput assays for promiscuous inhibitors. Nat. Chem. Biol. 2005;1:146–148. doi: 10.1038/nchembio718. [DOI] [PubMed] [Google Scholar]
- 31.Bergstrom F, Mikhalyov I, Hagglof P, Wortmann R, Ny T, Johansson LBA. Dimers of dipyrrometheneboron difluoride (BODIPY) with light spectroscopic applications in chemistry and biology. J. Am. Chem. Soc. 2002;124:196–204. doi: 10.1021/ja010983f. [DOI] [PubMed] [Google Scholar]
- 32.Tleugabulova D, Zhang Z, Brennan JD. Characterization of bodipy dimers formed in a molecularly confined environment. J. Phys. Chem. B. 2002;106:13133–13138. [Google Scholar]
- 33.Ohsaki Y, Shinohara Y, Suzuki M, Fujimoto T. A pitfall in using BODIPY dyes to label lipid droplets for fluorescence microscopy. Histochem. Cell Biol. 2010;133:477–480. doi: 10.1007/s00418-010-0678-x. [DOI] [PubMed] [Google Scholar]
- 34. Price KA, Hickey JL, Xiao ZG, Wedd AG, James SA, Liddell JR, Crouch PJ, White AR, Donnelly PS. The challenges of using a copper fluorescent sensor (CS1) to track intracellular distributions of copper in neuronal and glial cells. Chem. Sci. 2012;3:2748–2759. • A word of caution: Systematic studies with coppersensor 1 (CS1) showed that the source of fluorescence enhancement may be a consequence of altered pH, compromised vesicle maturation, increased probe uptake, or trapping in lysosomal compartments.
- 35.Marcus RA, Sutin N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta. 1985;811:265–322. [Google Scholar]
- 36. Chaudhry AF, Verma M, Morgan MT, Henary MM, Siegel N, Hales JM, Perry JW, Fahrni CJ. Kinetically controlled photoinduced electron transfer switching in Cu(I)-responsive fluorescent probes. J. Am. Chem. Soc. 2010;132:737–747. doi: 10.1021/ja908326z. •• A combination of femtosecond-resolved pump-probe experiments, time-resolved fluorescence decay data, and variable temperature NMR studies revealed that the incomplete recovery of Cu(I)-responsive probes is not due to reductive quenching from Cu(I) but rather a consequence of ternary complex formation with solvent molecules.
- 37.Cody J, Mandal S, Yang LC, Fahrni CJ. Differential tuning of the electron transfer parameters in 1,3,5-triarylpyrazolines: A rational design approach for optimizing the contrast ratio of fluorescent probes. J. Am. Chem. Soc. 2008;130:13023–13032. doi: 10.1021/ja803074y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma M, Chaudhry AF, Fahrni CJ. Predicting the photoinduced electron transfer thermodynamics in polyfluorinated 1,3,5-triarylpyrazolines based on multiple linear free energy relationships. Org. Biomol. Chem. 2009;7:1536–1546. doi: 10.1039/b821042j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma M, Chaudhry AF, Morgan MT, Fahrni CJ. Electronically tuned 1,3,5-triarylpyrazolines as Cu(I)-selective fluorescent probes. Org. Biomol. Chem. 2010;8:363–370. doi: 10.1039/b918311f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wegner SV, Arslan H, Sunbul M, Yin J, He C. Dynamic copper(I) imaging in mammalian cells with a genetically encoded fluorescent copper(I) sensor. J. Am. Chem. Soc. 2010;132:2567–2568. doi: 10.1021/ja9097324. •• Built upon the evolved Cu(I) binding site of an yeast transcription factor, this genetically encoded FRET-based probe was expressed in CHO-K1 cells and responded within 1 minute to supplementation of the extracellular medium with 100 nM Cu(II)SO4.
- 41.Yan X, Li X, Lv S-S, He D-C. A novel genetically encoded fluorescent protein as a Cu(I) indicator. Dalton Trans. 2012;41:727–729. doi: 10.1039/c1dt11002k. [DOI] [PubMed] [Google Scholar]
- 42.Liang J, Qin M, Xu R, Gao X, Shen Y, Xu Q, Cao Y, Wang W. A genetically encoded copper(I) sensor based on engineered structural distortion of EGFP. Chem. Commun. 2012;48:3890–3892. doi: 10.1039/c2cc30531c. [DOI] [PubMed] [Google Scholar]
- 43. Taki M, Iyoshi S, Ojida A, Hamachi I, Yamamoto Y. Development of highly sensitive fluorescent probes for detection of intracellular copper(I) in living systems. J. Am. Chem. Soc. 2010;132:5938–5939. doi: 10.1021/ja100714p. • A Cu(I)-catalyzed reaction led to uncaging of a fluorescein derivative and allowed for the in situ detection of Cu(I) in live HeLa cells.