Abstract
Cajal-Retzius (CR) cells are a transient cell population of the CNS that is critical for brain development. In the neocortex, CR cells secrete reelin to instruct the radial migration of projection neurons. It has remained unexplored, however, whether CR cells provide additional molecular cues important for brain development. Here, we show that CR cells express the immunoglobulin-like adhesion molecule nectin1, whereas neocortical projection neurons express its preferred binding partner, nectin3. We demonstrate that nectin1/3-mediated interactions between CR cells and the leading processes of migrating neurons are critical for radial migration. Furthermore, reelin signaling to Rap1 promotes Cdh2 function in neurons via nectin3 and afadin, thus directing the broadly expressed homophilic cell adhesion molecule Cdh2 towards mediating heterotypic cell-cell interactions between neurons and CR cells. Our findings identify nectins/afadin as components of the reelin signaling pathway and demonstrate that coincidence signaling between CR cell-derived secreted and short-range guidance-cues direct neuronal migration.
INTRODUCTION
Developmental processes frequently depend on transient cell populations to guide migrating cells. One such population in the CNS are the Cajal-Retzius (CR) cells, which have crucial functions in the developing neocortex and hippocampus (Soriano and Del Río, 2005). In the neocortex, CR cells reside in the marginal zone (MZ) and secrete reelin, which signals to projection neurons to control their radial migration (Franco et al., 2011; Gupta et al., 2003; Jossin and Cooper, 2011; Olson et al., 2006; Sekine et al., 2011). At early stages of neocortical development, radially migrating neurons enter the cortical plate (CP) using a migration mode called glia-independent somal translocation, which is characterized by the movement of neuronal cell bodies along their leading processes that are located in the marginal zone (MZ) (Nadarajah et al., 2001; Tabata and Nakajima, 2003). Later-born neurons must migrate further and thus use several modes of migration (Noctor et al., 2004; Tabata and Nakajima, 2003), but ultimately complete their migration by switching to glia-independent somal translocation once their leading processes enter the MZ (Nadarajah et al., 2001). Reelin specifically regulates glia-independent somal translocation in early- and late-born neurons (Franco et al., 2011), but is dispensable for other modes of motility (Franco et al., 2011; Jossin and Cooper, 2011). During glia-independent somal translocation, reelin regulates the activity of cadherin 2 (Cdh2) to maintain neuronal leading processes in the MZ (Franco et al., 2011), possibly through their interaction with CR cells.
Cdh2 is widely expressed in radial glial cells (RGCs) and neurons of the developing neocortex and is critical for a variety of cellular processes. In migrating neurons, Cdh2 is not only required for forming stable attachments to cell in the MZ (Franco et al., 2011), but also for establishing dynamic adhesions with RGCs during glia-dependent migration (Kawauchi et al., 2010). In contrast, Cdh2 forms stable adherens junctions between RGCs at the ventricular surface (Kadowaki et al., 2007; Rasin et al., 2007). We therefore hypothesized that migrating neurons and other neocortical cell types, such as RGCs and CR cells, might express additional cell surface receptors that direct the specificity of the homophilic cell adhesion molecule Cdh2 towards the establishment of heterotypic cell-cell contacts with distinct functional properties. Candidate molecules for such interactions are the nectins, a branch of the immunoglobulin superfamily that consists of four members (Takai et al., 2008). Outside the nervous system, nectins cooperate with cadherins in the assembly of adherens junctions (Takahashi et al., 1999; Takai et al., 2008). Within the nervous system, nectins have important functions at synaptic sites (Rikitake et al., 2012). Importantly, some nectins, such as nectin1 and nectin3, preferentially engage in heterophilic interactions that play critical roles during development (Honda et al., 2006; Inagaki et al., 2005; Okabe et al., 2004; Rikitake et al., 2012; Togashi et al., 2011; 2006). However, the functions of nectins in the developing neocortex are not known.
Here we show that nectin1 and nectin3 are expressed in complementary patterns in the neocortex, in which radially migrating neurons express nectin3 and CR cells express nectin1. We demonstrate that nectin1 in CR cells mediates heterotypic interactions with nectin3 in the leading processes of migrating projection neurons. These nectin-based adhesions control radial migration by acting in concert with reelin and Cdh2 to promote interactions between migrating neurons and CR cells. Overall, our findings reveal that CR cells instruct the directional migration of neocortical projection neurons by coincident presentation of secreted molecules, such as reelin, and cell-surface bound guidance cues, such as cadherins and nectins. Our results also clarify how the homophilic cell adhesion molecule Cdh2, which is expressed in many neocortical cell types, mediates specific interactions between two defined cell types by combinatorial signaling with other cell adhesion molecules.
RESULTS
Nectin expression in the developing neocortex
Previous studies have shown that nectins cooperate with cadherins in adherens junction assembly (Takahashi et al., 1999; Takai et al., 2008). Since Cdh2 regulates radial neuronal migration (Franco et al., 2011; Jossin and Cooper, 2011; Kawauchi et al., 2010), we hypothesized that nectins might regulate Cdh2 function during migration. We therefore analyzed by in situ hybridization the expression patterns of all four nectin family members in the developing neocortex. At E13.5, nectin2 and nectin4 showed weak, if any, expression in the neocortex (not shown). In contrast, nectin1 was prominently expressed in the cortical hem and MZ (Fig. 1A; Fig. S1A,B), whereas nectin3 was expressed in the neocortical VZ, subventricular zone (SVZ) and intermediate zone (IZ) (Fig. 1H). The adaptor protein afadin, which binds to the cytoplasmic domains of all nectins (Miyahara et al., 2000; Takahashi et al., 1999), was expressed throughout the neocortical wall (Fig. 1K).
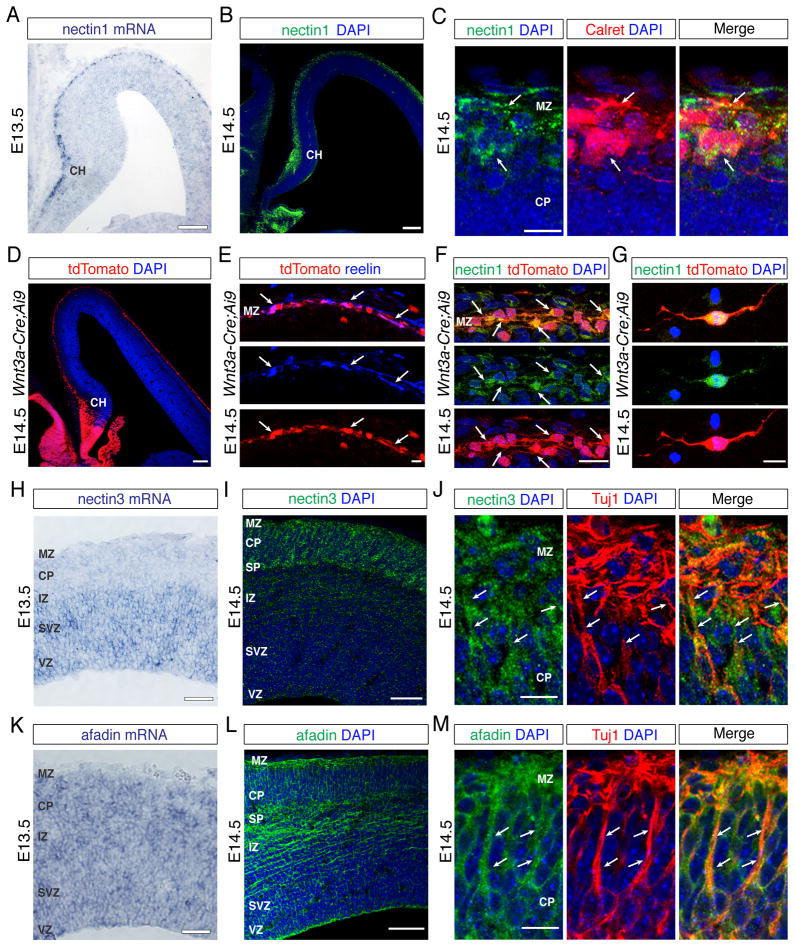
Fig. 1. Expression patterns of nectins and afadin in the developing neocortex.
(A) Nectin1 mRNA and (B) protein are expressed in the cortical hem (CH) and marginal zone (MZ). Nuclei are stained in these and subsequent panels with DAPI (blue). (C) High magnification views show co-expression (arrows) of nectin1 with calretinin (Calret) in CR cells. (D–G) Analysis of nectin1 expression in Wnt3a-Cre;Ai9 mice. (D) Low magnification view of tdTomato expression in the CH and MZ. (E) tdTomato+ cells in the MZ (arrows) are reelin+ CR cells. (F) tdTomato+ CR cells in the MZ express nectin1. (G) Nectin1 expression is maintained in cultured tdTomato+ CR cells. (H–M) Nectin3 and afadin are expressed in the leading processes of projection neurons. (H) Nectin3 mRNA expression is high in the ventricular zone (VZ), subventricular zone (SVZ), and intermediate zone (IZ). (I) Nectin3 protein is prominently expressed in the subplate (SP), cortical plate (CP), and MZ. (J) High magnification views show that nectin3 is expressed in the leading processes (arrows) of cultured Tuj1+ neurons. (K–L) Afadin mRNA (K) and protein (L) are widely expressed in the neocortical primordium. (M) Afadin is expressed in the leading processes (arrows) of cultured Tuj1+ neurons. Scale bars (A, B, D) 100 μm, (H, I, K, L) 50 μm, (E–G) 10 μm, (C, J, M) 5 μm. See also Figure S1.
We next determined by immunohistochemistry the cell types that express nectins and afadin. At E14.5, Nectin1 was confined to the cortical hem and MZ (Fig. 1B; Fig. S1B), the source and destination of CR cells, respectively (Meyer et al., 2002; Yoshida et al., 2006; Zhao et al., 2006). Co-staining with calretinin, a marker for CR cells (Weisenhorn et al., 1994) and interneurons (Gonchar and Burkhalter, 1997), revealed nectin1 expression in calretinin+ cells (Fig. 1C). Even though interneurons are rare in the MZ at E14.5 (Xu et al., 2004), we wanted to confirm that the nectin1+ cells were CR cells. We therefore generated a Wnt3a-Cre mouse line (Fig. S1C) that expresses Cre in CR cells (Louvi et al., 2007; Yoshida et al., 2006) and crossed them with Ai9 mice (Fig. 1D), which carry a Cre-inducible tdTomato allele (Madisen et al., 2010). tdTomato+ cells in the MZ expressed reelin, confirming their identity as CR cells (Fig. 1E). These cells also expressed nectin1 in vivo (Fig. 1F) and in vitro (Fig. 1G).
Next, we determined the expression pattern of nectin3, the preferred binding partner for nectin1 (Satoh-Horikawa et al., 2000; Togashi et al., 2006; 2011). In contrast to nectin1, nectin3 was present throughout the neocortical wall, including the sublate (SP), CP and MZ (Fig. 1I). In the CP and MZ, nectin3 was enriched in Tuj1+ leading processes of radially migrating neurons (Fig. 1J). Similarly, nectin3 was prominently localized to the processes of cultured neocortical neurons (Fig. S1D). Co-staining for nectin3 and nestin revealed additional staining in the endfeet of RGCs (Fig. S1F). A similar expression pattern in neurons (Fig. 1L,M; Fig. S1E) and RGCs (Fig. S1G) was observed for afadin.
Early-born projection neurons require nectin3 and afadin for glia-independent somal translocation
To define the functions of nectin1, nectin3, and their effector afadin during radial neuronal migration, we used small hairpin (sh) RNAs to knock down their expression. We first focused on nectin3 and afadin, which are expressed in radially migrating neurons. shRNAs for nectin3, afadin or a non-silencing control were introduced by in utero electroporation into the neocortical primordium of E12.5 embryos (Fig. 2A). We chose E12.5 because neurons generated at this time point largely migrate by glia-independent somal translocation (Nadarajah et al., 2001), thus allowing us to specifically study the roles of these molecules in this mode of migration. Our shRNA vectors co-expresses EGFP for detection of electroporated cells. Four days after electroporation, control cells had migrated into the CP and populated emerging layers V and VI (Fig. 2C). In contrast, knockdown of nectin3 or afadin with two different shRNA constructs each blocked migration and caused neurons to accumulate in the IZ (Fig. 2B,C; Fig. S2A,B). The knockdown efficiency of each shRNA was confirmed by western blotting (Fig. S2C) and the migratory defects were rescued by co-electroporation with nectin3 or afadin cDNAs that are not targeted by the shRNAs (Fig. 2B,C), thus confirming the shRNA on-target specificity.
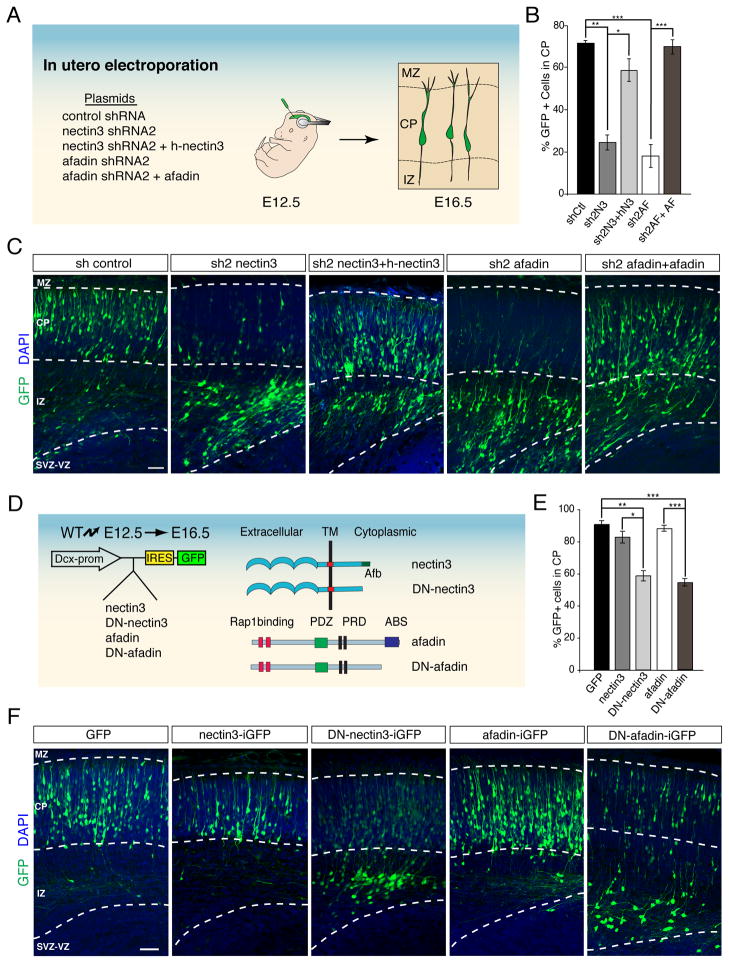
Fig. 2. Nectin3 and afadin regulate glia-independent somal translocation of early-born projection neurons.
(A) Strategy to perturb the functions of nectin3 and afadin. Embryos were electroporated in utero at E12.5 with control, nectin3 or afadin shRNAs to target early-born neurons that migrate by glia-independent somal translocation. Positions of the electroporated cells were analyzed at E16.5. (B) Quantification (mean ± SEM) of the percentage of electroporated neurons from (C) that migrated into the CP. *p < 0.001, **p < 0.0001, ***p < 0.00001 by Student’s t-test. (C) Representative images showing the positions of electroporated neurons (green). Nuclei were stained with DAPI (blue). Knockdown of nectin3 (sh2 nectin3) or afadin (sh2 afadin) blocks migration into the CP compared to a non-silencing control (sh control). Migration defects are rescued by co-expression of shRNAs and their respective refractory cDNAs (sh2 nectin3 + h-nectin3; sh2 afadin + afadin*). (D) Strategy to perturb nectin3 and afadin specifically in neurons. Wild-type or dominant-negative versions of nectin3 or afadin (depicted at right) were expressed with GFP from the neuron-specific Dcx promoter. In utero electroporations were performed as in (A). (E) Quantification (mean ± SEM) of the percentage of electroporated neurons from (F) that enter the CP plate. *p < 0.005, **p < 0.0002, ***p < 0.00005 by Student’s t-test. (F) Representative examples of neuron-specific perturbations. Overexpression of wild-type nectin3 (nectin3-iGFP) or afadin (afadin-iGFP) does not affect neuronal migration compared to control (GFP). In contrast, expression of dominant-negative versions of nectin3 (DN-nectin3-iGFP) or afadin (DN-afadin-iGFP) impairs migration.Ctl, control; N3, nectin3; AF, afadin; TM, transmembrane domain; Afb, afadin binding site; PRD, proline rich domain; ABS, actin binding site. Scale bar 50 μm. See also Figure S2.
Nectin3 acts in migrating neurons
The vector used in the knockdown experiments expresses shRNAs in RGCs and neurons. To define the cell type that is affected and determine the mechanisms responsible for the migration defects, we first evaluated neuronal differentiation using molecular markers. Knockdown of nectin3 or afadin did not affect the normal differentiation process. Cells that failed to migrate were positive for the neuronal marker Tuj1 (Fig. S2D,E), but not for the RGC marker Pax6 (Fig. S2F,G) the intermediate progenitor marker Tbr2 (Fig. S2H,I), or the proliferation marker Ki67 (Fig. S2J,K).
Next, we analyzed the extent to which nectin3 and afadin act cell-autonomously in migrating neurons using a doublecortin (Dcx) promoter construct that is expressed in migrating neurons, but not in RGCs (Fig. 2D) (Franco et al., 2011; Wang et al., 2007). The Dcx vector contains an internal ribosome entry site (IRES) for simultaneous expression of cDNAs and EGFP. We cloned into the Dcx vector: (i) full-length control cDNAs for nectin3 and afadin (Dcx-nectin3-iGFP, Dcx-afadin-iGFP); (ii) truncated nectin3 (Dcx-DN-nectin3-iGFP) that lacks the afadin-binding site and acts as a dominant negative (Brakeman et al., 2009; Takahashi et al., 1999); (iii) truncated afadin (Dcx-DN-afadin-iGFP) lacking the F-actin binding site that is critical for stabilizing adherens junctions (Mandai et al., 1997; Ozaki-Kuroda et al., 2002). As an additional control, we used the Dcx-iGFP vector lacking a cDNA insert. When embryos were electroporated at E12.5 and analyzed at E16.5, the positions of neurons expressing full-length nectin3 or afadin were identical to those expressing the Dcx-iGFP control (Fig. 2E,F), indicating that over-expression of the wild-type proteins does not disrupt migration. In contrast, expression of DN-nectin3 or DN-afadin caused electroporated cells to accumulate near the IZ (Fig. 2E,F) indicating that nectin3 and afadin act at least in part in neurons to regulate glia-independent somal translocation.
Nectin3 and afadin stabilize leading neuronal processes in the cortical MZ
We next determined the mechanism by which nectin3 and afadin regulate radial migration. We reasoned that the two proteins might help to anchor the leading processes of neurons in the MZ. We therefore evaluated neuronal morphology following perturbation of nectin3 or afadin function by knockdown and dominant-negative approaches, which gave similar results. Although neurons largely failed to migrate into the CP following perturbation of nectin3 or afadin, they still properly polarized the Golgi apparatus ahead of the nucleus (Fig. 3A) and also developed stereotypical polarized morphologies characterized by leading processes (Fig. 3B–D). Two to three days after electroporation, leading processes that extended towards or even into the MZ were observed both in control neurons and in neurons expressing shRNAs against nectin3 or afadin (Fig. 3B,C). A small decrease in the number of branches was observed after three days in the case of afadin shRNA electroporation suggesting onset of leading process retraction. However, only control neurons had their cell bodies located close to the MZ, indicative of somal translocation. Cell bodies in the knockdown experiments failed to translocate toward the MZ (Fig. 3B,C) and remained near the IZ and lower CP as non-electroporated cells bypassed them to expand the CP. This CP expansion initially caused the leading processes of affected neurons to appear longer than those of controls neurons two to three days after electroporation (Fig. 3B,C), but many of these processes were subsequently retracted by four days after electroporation (Fig. 2C,F). Additionally, whereas the leading processes of control neurons extensively branched in the MZ, no such branching was observed after nectin3 or afadin knockdown (Fig. 3D). Together these data indicate that nectin3 and afadin are not required for neuronal polarization or initial process extension, but are important for leading process anchorage and arborization in the MZ and subsequent somal translocation.
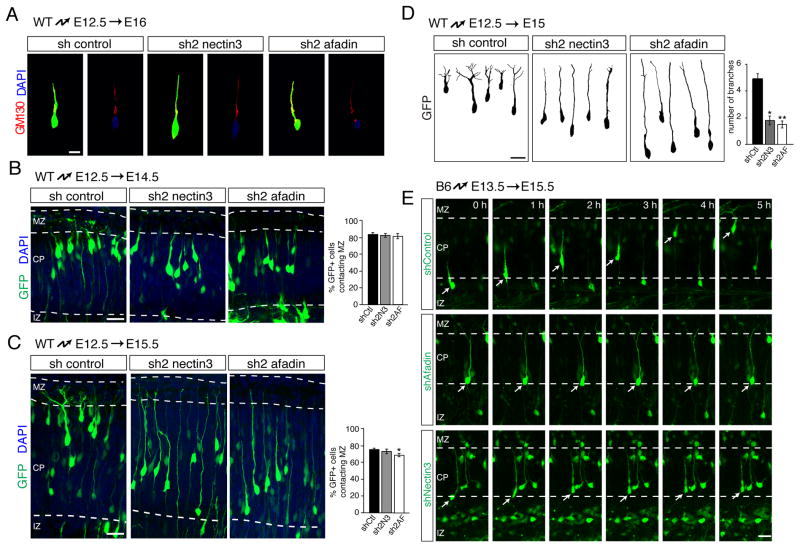
Fig. 3. Nectin3 and afadin are dispensible for cell polarization and process extension, but are required for leading process branching and somal translocation.
(A–D) Perturbation of nectin3 or afadin does not affect neuronal polarization or leading process extension. E12.5 brains were electroporated in utero with non-silencing control shRNA or shRNAs targeting nectin3 or afadin. (A) Staining of E16.5 brains with GM130 (red) and DAPI (blue) reveals normal polar localization of the Golgi apparatus ahead of the nucleus. (B, C) Analysis at E14.5 (B) and E15.5 (C) demonstrates similar numbers of leading processes contacting the marginal zone in the three conditions at these time points. Quantification is mean ± SEM. A small reduction in the number of processes contacting MZ is observed 3 days after electroporation. *p < 0.05 by Student’s t-test. (D) Morphological analysis of migrating neurons. shRNAs were electroporated into E12.5 embryos, followed by 3D reconstruction of GFP-positive neurons at E15.5. Leading processes of neurons electroporated with control shRNA profusely arborize in the MZ. Processes are poorly arborized in cells expressing shRNAs targeting nectin3 or afadin. Quantification is mean ± SEM of the number of branches in the MZ 3 days after electroporation. *p < 5x10−5, **p < 5x10−6 by Student’s t-test. (E) Time-lapse imaging of neurons migrating at E15.5, after electroporation of shRNAs at E13.5. Neurons in the IZ were imaged for 5 hours while undergoing glia-independent somal translocation into the CP. Control cells migrate out of the IZ to the top of the CP; cells expressing nectin3 or afadin shRNA fail to undergo somal translocation. Abbreviations as in Fig. 1 Scale bars (A–D) 10 μm, (E) 20 μm.
To directly determine whether nectin3 and afadin are required for somal translocation, we carried out time-lapse imaging experiments. Neurons from E13.5 animals were electroporated with control, nectin3 or afadin shRNAs, and neocortical slice cultures were prepared at E15.5. As reported (Franco et al., 2011), control neurons translocated their cell bodies along their leading processes towards the MZ (Fig. 3E). In contrast, neurons expressing shRNAs for afadin or nectin3 extended leading processes, but failed to undergo somal translocation (Fig. 3E).
Interactions between the leading processes of migrating neurons and CR cells
Since nectin3 mediates cell-cell adhesion, we reasoned that it might mediate interactions of the leading neuronal processes with CR cells in the cortical MZ. To test this model, we again took advantage of Wnt3a-Cre:Ai9 mice to study interactions between migrating neurons and tdTomato+ CR cells in vitro and in vivo. We electroporated E13.5 embryos with Dcx-GFP to label migrating neurons, then isolated these primary neurons at E15.5. In parallel, we isolated primary tdTomato+ CR cells from Wnt3a-Cre:Ai9 embryos by magnetic activated cell sorting (Fig. 4A). Next, we combined the GFP+ neurons with tdTomato+ CR cells for in vitro co-cultures. Using this paradigm, we consistently observed pairs of cells in which a GFP+ neuron interacted with a tdTomato+ CR cell. Some of the cells were in tight apposition and aligned their membranes (Fig. 4B, arrowheads), while some neurons sent out processes to CR cells (Fig. 4B, arrows). These data indicated that neurons and CR cells can engage in cell-cell interactions with one another, at least in vitro.
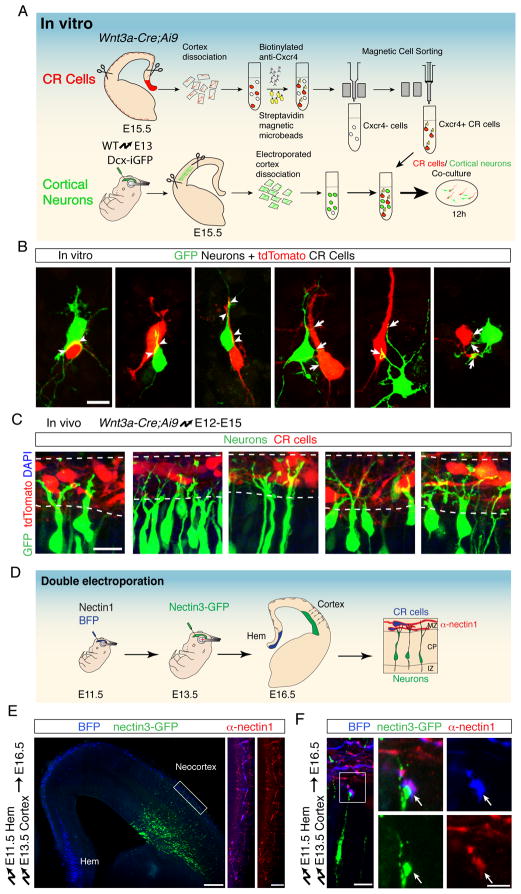
Fig. 4. Interactions between neurons and CR cells.
(A) Schematic of the co-culture experiment to visualize in vitro interactions between migrating neurons and CR cells. tdTomato+ CR cells (red) were isolated from E15.5 Wnt3a-Cre;Ai9 brains using anti-Cxcr4 magnetic bead purification. Cortical neurons (green) were microdissected from E15.5 brains electroporated at E13.5 with Dcx-GFP. Equal numbers of each cell type were mixed and co-cultured for 12 h. (B) Representative examples of cortical neurons (green) contacting CR cells (red). Arrowheads point to closely aligned membranes between neurons and CR cells. Arrows point to neuronal branches that contact CR cells. (C) In vivo interactions between the leading processes of migrating neurons and CR cells. Wnt3a-Cre;Ai9 embryos were electroporated at E12.5 to express GFP in cortical neurons. Coronal sections showing the MZ at E15.5 demonstrate branched leading processes of projection neurons (green) in contact with CR cells (red). (D) Double-electroporation protocol to visualize nectin1 and nectin3 at contact sites between CR cells and cortical neurons. CR cells in the cortical hem were electroporated at E11.5 with vectors expressing BFP and nectin1. Migrating neurons in the same embryos were then electroporated at E13.5 with nectin3-GFP. Double-electroporated brains were analyzed at E16.5. To obtain cellular resolution in dense tissue, overexpressed nectin1 was detected using an antibody dilution 10-fold higher than normally used to detect endogenous protein. (E) Coronal section of an E16.5 double-electroporated brain to visualize interactions between CR cells and migrating neurons. Hem-electroporated cells express BFP (blue) and projection neurons express GFP (green). Magnification on the right shows the MZ after nectin1 immunohistrochemistry (red). (F) High magnification showing the leading process of an individual nectin3-GFP+ projection neuron contacting a branch of a nectin1+ CR cell. Panels on the right show the co-localization of nectin1 and nectin3 at the contact point (arrows) between cells. Scale bars (B, C) 10 μm, (E) 200 μm, (F) 2.5 μm. See also Figure S3.
To extend these findings in vivo and to determine whether nectin3 and afadin are required in neurons to mediate interactions with CR cells, we electroporated Wnt3a-Cre;Ai9 embryos with shRNA vectors at E12.5 to obtain differentially labelled neurons and CR cells within the same brain. Neuronal processes were visualized at E15.5 in single confocal sections in relation to tdTomato+ CR cells. Similar to the in vitro experiments, the branched leading processes of control neurons overlapped with the cell bodies and projections of CR cells (Fig. 4C). Moreover, these interactions and the branching of the leading processes were disrupted upon knockdown of nectin3 or afadin (Fig. S3A), providing evidence that nectin3 expression in neurons is important for the formation of these contacts in vivo.
Nectin1 and Nectin3 colocalize at contact sites between the leading processes of migrating neurons and CR cells
To directly assess whether nectin1 and nectin3 are recruited in vivo to interaction sites between the leading processes of migrating neurons and CR cells, we established a protocol with sufficient resolution to visualize individual cell-cell contacts. For this purpose, we electroporated the cortical hem at E11.5, during peak times of CR cell generation (Yoshida et al., 2006), to label CR cells with a blue fluorescence (BFP) (Fig. 4D). The same embryos were re-electroporated at E13.5, but the neocortical VZ was targeted to introduce GFP-tagged nectin3 into migrating neurons. Three days after the second electroporation, BFP+ CR cells had migrated tangentially from the hem into the cortical MZ, while nectin3-GFP+ neurons had migrated radially to populate the emerging CP (Fig. 4E). The BFP+ cells expressed calretinin (Fig. S3B) and reelin (not shown), confirming their identity as CR cells. Staining of the electroporated brains with antibodies to nectin1 revealed a punctate staining in CR cells in the cortical MZ (Fig. 4E). At higher resolution, nectin1 could be seen concentrated around the tips of the leading processes of migrating neurons, which also contained high levels of nectin3-GFP (Fig. 4F). Co-localization between nectin1 and nectin3 was observed at multiple locations within the cell bodies and distal processes of CR cells (Fig. S3C). Together, these data demonstrate that nectin1 and nectin3 are appropriately localized to mediate interactions between CR cells and migrating neurons.
Nectin1 in CR cells non-autonomously regulates glia-independent somal-translocation of neurons
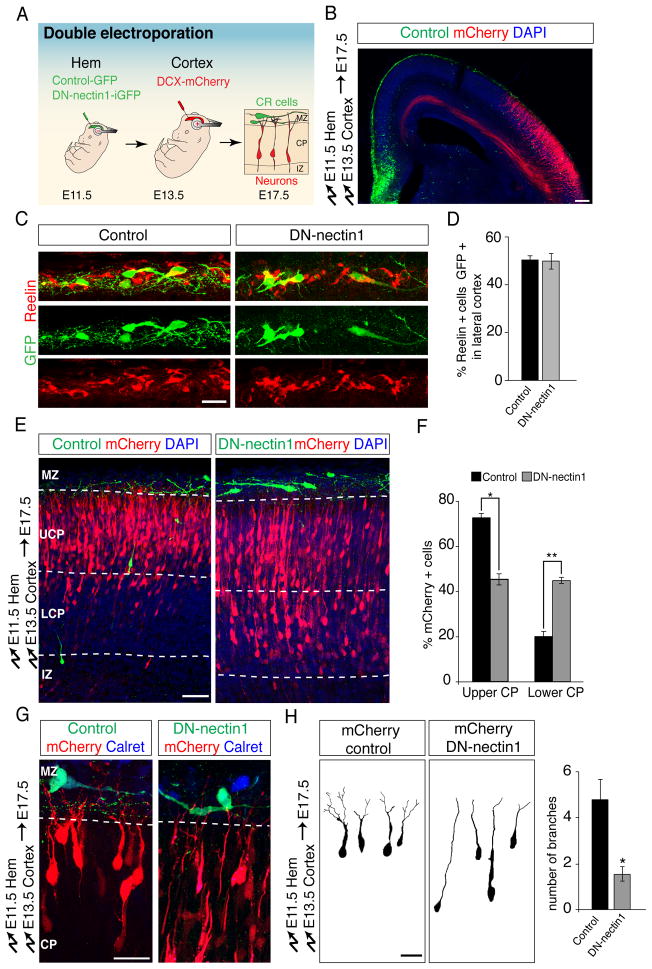
Because nectin3 preferentially forms heterotypic adhesions with nectin1 (Satoh-Horikawa et al., 2000), we next determined whether nectin1 expression in CR cells is required for the radial migration of nectin3-expressing neurons. For this purpose, we took advantage of our double electroporation strategy (Fig. 5A). We first electroporated hem-derived CR cells at E11.5 with a DN-nectin1 that lacks the afadin binding site (Brakeman et al., 2009; Takahashi et al., 1999). The same embryos were re-electropated at E13.5 with a Dcx-mCherry expression vector to label migrating neurons, then analyzed at E17.5.
Fig. 5. Nectin1 in CR cells is required non-autonomously for glia-independent somal translocation of radially migrating projection neurons.
(A) Double-electroporation to target CR cells and then label radially migrating neurons in the same embryo. Control-iGFP or DN-nectin1-iGFP was electroporated into the cortical hem at E11.5, then migrating neurons were electroporated with Dcx-mCherry at E13.5. Neuronal position was analyzed at E17.5. (B) Example of a double-electroporated brain showing GFP-expressing cells (green) in the hem and MZ and mCherry-expressing projection neurons (red). DAPI-stained nuclei are in blue. (C) High magnification view of the lateral cortex showing that hem-electroporated cells in the MZ (green) have typical CR cell morphologies and express reelin (red). Note that half of all reelin+ CR cells in the lateral cortex are electroporated, as quantified in (D). (D) Quantification (mean ± SEM) of the percentage of CR cells in the lateral cortex that express control GFP or DN-Nectin1 after hem electroporation. (E) Perturbation of nectin1 function in CR cells causes a non-cell-autonomous migration defect of early-born projection neurons. Neurons in controls reach the upper cortical plate (UCP) by E17.5, whereas expression of DN-nectin1 in CR cells (green) causes radially migrating neurons (red) to accumulate in the lower CP (LCP). (F) Quantification (mean ± SEM) of the data in (D). *p < 0.00005, **p < 0.000005, by Student’s t-test. (G) Leading processes of migrating neurons (red) show reduced arborization compared to controls when they encounter CR cells expressing DN-nectin1 (green). The CR cell marker caretinin is shown in blue. (H) 3D reconstructions of mCherry+ projection neurons in contact with the MZ after double electroporation. Leading processes that contact control CR cells arborize in the MZ, whereas neurons contacting CR cells expressing DN-nectin1 display minimal arborization. Quantification is mean ± SEM of the number of branches in the MZ 4 days after electroporation. *p <0.001 by Student’s t-test. Scale bars (B) 100 μm, (C,G,H) 10 μm and (E) 50 μm. See also Figure S4.
CR cells expressing DN-nectin1 still migrated along their normal route within the cortical MZ (Fig. S4A–D). Quantitative evaluation confirmed that ~50% of all reelin+ CR cells expressed DN-nectin1, even in the lateral cortex at a substantial distance from the cortical hem (Fig. 5C–D). These findings show that our electroporation method targets half of all CR cells and that DN-nectin1 does not significantly affect their tangential migration. However, the positions of radially migrating neurons were strikingly altered after nectin1 perturbation in CR cells. Neurons in controls had migrated into the upper part of the CP, whereas large numbers of neurons remained in the lower part of the CP following expression of DN-nectin1 in CR cells (Fig. 5E,F). Neurons in controls had normal bipolar morphologies with leading processes that branched in the MZ, whereas branch density was drastically decreased following expression of DN-nectin1 in CR cells (Fig. 5G,H). Similar defects in migration and leading process arborization were found when nectin1 function in CR cells was perturbed using shRNAs (Fig. S4E–I). Finally, nectin1 perturbation in CR cells did not produce obvious changes in the morphologies of RGC processes or the localization of RGC endfeet (Fig. S4J). We conclude that perturbation of nectin1 function in CR cells affects interactions between neuronal leading processes and CR cells, thereby non-autonomously perturbing somal translocation of radially migrating neurons into the CP.
Cdh2 acts in neurons in concert with nectin3
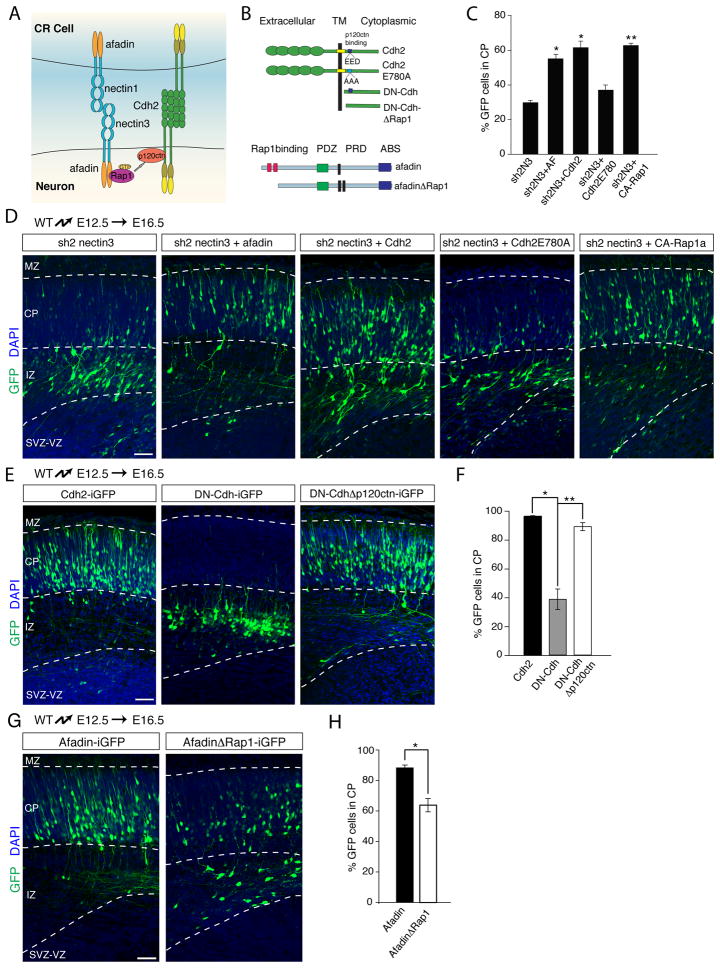
We have previously shown that Cdh2 is required for glia-independent somal translocation (Franco et al., 2011) and now show that nectin3 and afadin are also required for this process. In epithelial cells, nectins form nascent cell-cell adhesion sites, to which afadin is recruited by binding to the cytoplasmic tails of nectins. Afadin then stabilizes cadherin molecules at the cell surface to promote the establishment of stable adherens junctions containing nectins and cadherins (Fig. 6A) (Hoshino et al., 2005). We hypothesized that nectin3 and afadin in migrating neurons may also cooperate with Cdh2 to regulate the attachment of neuronal leading processes in the MZ.
Fig. 6. Nectins act upstream of Afadin, Rap1, p120ctn and Cadherins during glia-independent somal translocation.
(A) Illustration of the signaling pathway connecting nectins and cadherins. (B) Schematic of constructs to study the involvement of p120ctn binding to Cdh2 (upper part) and afadin binding to Rap1 (lower part) in glia-independent somal translocation. (C) Quantification (mean ± SEM) of the percentage of electroporated neurons from (D) that migrated into the CP. *p < 0.001, **p < 0.00005 by Student’s t-test, comparing to nectin3 knockdown (sh2N3). (D, E, G) Constructs were electroporated at E12.5 and neuronal position was determined at E16.5. Electroporated neurons are in green and nuclei in blue. (D) Overexpression of afadin, Cdh2 or constitutively-active Rap1 in neurons rescues the migration defects caused by nectin3 shRNA expression. Expression of a Cdh2 cDNA mutated in the p120ctn binding site (Cdh2-E780A) does not rescue the migratory phenotype caused by nectin3 knockdown. (E) Deletion of part of the p120ctn binding site in DN-Cdh relieves its inhibitory effect on radial migration. (F) Quantification (mean ± SEM) of the percentage of electroporated neurons from (E) that enter the CP. *p < 0.0005, **p < 0.00005 by Student’s t test. (G) Binding of Rap1 to afadin is required to regulate somal translocation. Electroporation of a truncated form of afadin lacking the Rap1-binding domain perturbs migration into the CP. (H) Quantification (mean ± SEM) of the percentage of electoporated neurons from (G) that enter the CP. *p < 0.003 by Student’s t test. Abbreviations as in Figs. 1 and 6. Scale bars 50 μm. See also Fig. S5.
We first determined the extent to which nectin3 and afadin act in a common pathway in migrating neurons. The similarity in the migration defects caused by knockdown of nectin3 or afadin suggested a functional link between the two. Further supporting this conclusion, nectin3 lacking the afadin-binding site (Fig. 2D) acts as a dominant negative (Brakeman et al., 2009; Takahashi et al., 1999) and affects radial migration (Fig. 2E,F), likely by preventing nectin-mediated recruitment of afadin to the cell membrane. We therefore reasoned that overexpression of afadin might rescue the defects caused by nectin3 inactivation, presumably by targeting sufficient amounts of afadin to the cell surface to regulate Cdh2 function. We co-expressed nectin3 shRNA with a full-length afadin cDNA in neurons at E12.5 and analyzed their positions at E16.5. Overexpression of afadin partially rescued the migration defect caused by knockdown of nectin3 (Fig. 6C,D). Similarly, expressing full-length Cdh2 also rescued the migration defect caused by expression of nectin3 shRNA (Fig. 6C,D) or afadin shRNA (Fig. S5A,B). Taken together, these findings suggest that Cdh2 acts in migrating neurons in concert with nectin3 and afadin to regulate glia-independent somal translocation. In support of this model, Cdh2 also co-localized with nectin1 and nectin3 at contact sites between the leading processes of migration neurons and CR cells in vivo (Fig. S5C,D) and in vitro (Fig. S5E). In addition, nectin1-coated latex beads attached to cultured cortical neurons and recruited nectin3, Cdh2, and the Cdh2-binding protein p120catenin (p120ctn) to the bead/neuron interface (Fig. S5F).
p120ctn function in glia-independent somal translocation
Stabilization of cadherins at adherens junctions by the nectin/afadin complex depends on p120ctn, which binds to the cytoplasmic domain of Cdh2 and regulates its endocytosis (Fig. 6A) (Davis et al., 2003; Hoshino et al., 2005; Sato et al., 2006). We therefore determined weather p120ctn is required in neurons for nectin3/afadin function during migration. We first evaluated the extent to which a mutated form of Cdh2 (E780A) (Fig. 6B) that does not bind p120ctn (Thoreson et al., 2000) can rescue the migratory defects caused by knockdown of nectin3 or afadin. In contrast to wild-type Cdh2 (Fig. 6C,D), Cdh2(E780A) was unable to rescue the migratory defect caused by nectin3 and afadin knockdown (Fig. 6C,D; Fig. S5A,B)
To independently confirm that binding of p120ctn to Cdh2 is important for Cdh2 function during migration, we took advantage of a dominant-negative cadherin construct (DN-Cdh) that consists of the cytoplasmic domain common to classical cadherins (Fig. 6B). This construct blocks neuronal migration (Franco et al., 2011; Jossin and Cooper, 2011), most likely by sequestering cytoplasmic binding partners of endogenous cadherins. Deletion of the binding site for p120ctn within DN-Cdh (Fig. 6B) released the dominant negative effect (Fig. 6E,F), likely because p120ctn was no longer sequestered, indicating that p120ctn binding to Cdh2 is important for glia-independent somal translocation.
Rap1 acts in concert with afadin and p120ctn to link nectin3 and Cdh2 functions
The nectin/afadin complex does not bind p120ctn directly, but does so via the small GTPase Rap1, which binds to both afadin and p120ctn (Fig. 6A) (Hoshino et al., 2005; Sato et al., 2006). We hypothesized that Rap1 might be the crucial link between nectin3/afadin and Cdh2/p120ctn. Several lines of evidence support this model. First, Rap1 is required for glia-independent somal translocation and overexpression of Cdh2 can rescue the migration defect caused by Rap1 loss-of-function, demonstrating that Cdh2 acts downstream of Rap1 in this process (Franco et al., 2011). In addition, we now show that a constitutively active form of Rap1 rescued the migration defect caused by nectin3 knockdown (Fig. 6C,D). Finally, an afadin construct lacking the Rap1 binding-site (Fig. 6B) acted as a dominant negative and disrupted radial migration (Fig. 6G,H). Taken together, our data suggest that nectin3 in migrating neurons recruits an afadin/Rap1 complex that regulates Cdh2 function via p120ctn, thereby promoting leading process attachment in the MZ and glia-independent somal translocation.
Cdh2 in CR cells is required for glia-independent somal translocation
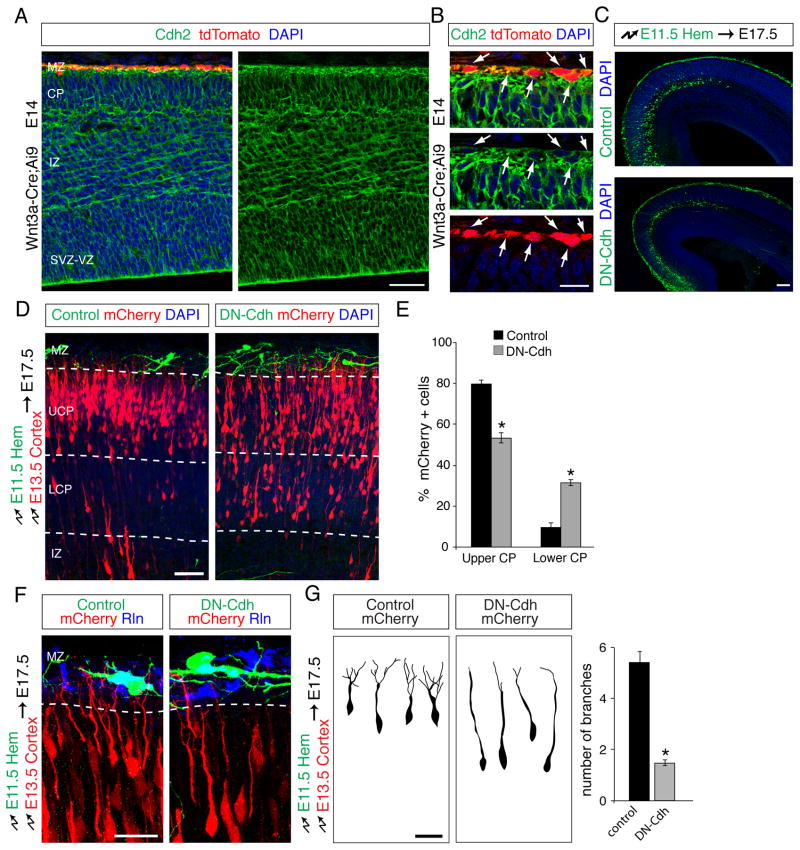
At adherens junctions, cadherins are recruited between neighboring cells through nectin/afadin to form stable adhesions. We therefore reasoned that CR cells might also express Cdh2 that acts in concert with nectin1 to mediate interactions with neurons. Indeed, Cdh2 was expressed in CR cells (Fig. 7A,B). For functional tests, we electroporated the cortical hem at E11.5 with Dcx-iGFP or Dcx-DN-Cdh-iGFP, then electroporated the neocortical VZ of the same embryos at E13.5 with Dcx-mCherry to label migrating neurons. By E17.5, GFP+ CR cells had migrated into the neocortical MZ (Fig. 7C), while mCherry+ radially migrating neurons populated the emerging CP (Fig. 7D). Expression of DN-Cdh did not inhibit the migration of CR cells within the MZ (Fig. 7C), but the positions of radially migrating neurons were significantly altered (Fig. 7D,E). Neurons in controls migrated into the upper CP, whereas large numbers of neurons remained in the lower CP following expression of DN-Cdh in CR cells (Fig. 7D,E). In addition, neurons in controls had leading processes that branched extensively in the MZ, but branch density was decreased following expression of DN-Cdh in CR cells (Fig. 7F,G). The defects in cell morphology and migration resembled those observed after perturbation of nectin1 in CR cells (Fig. 5E–H and Fig. S4E–G), indicating that nectin1 and Cdh2 in CR cells cooperate to establish stable interactions between neuronal leading processes and CR cells.
Fig. 7. Cdh2 in CR cells is required non-autonomously for glia-independent somal translocation of radially migrating projection neurons.
(A) Coronal section of an E14.5 brain from a Wnt3a-Cre;Ai9 embryo showing expression of Cdh2 protein in the developing neocortex. tdTomato+ CR cells in the MZ express Cdh2. Nuclei are in blue. (B) Higher magnification view of (A) showing the MZ. Arrows point to CR cells co-expressing Cdh2 and tdTomato. (C) CR cells targeted with DN-Cdh migrate normally to populate the MZ. Coronal sections of brains electroporated with control or DN-Cdh at E11.5 in the cortical hem and analyzed at E17.5. Electroporated CR cells are shown in green and DAPI stained nuclei in blue. (D–F) Double electroporation experiments as described in Fig. 5 were carried out to perturb Cdh2 function in CR cells and determine effects on radially migrating neurons. (D) Coronal sections of double-electroporated brains in which CR cells (green) express either GFP alone (control) or together with dominant-negative cadherin (DN-Cdh). Migrating projection neurons express mCherry (red). Nuclei are in blue. Radially migrating neurons accumulate in the lower CP (LCP) upon expression of DN-Cdh in CR cells, whereas neurons in controls populate the upper CP (UCP). (E) Quantification (mean ± SEM) of the data in (D). *p < 0.0002, by Student’s t-test. (F) Leading process arborization of migrating neurons (red) is reduced when they contact CR cells (green) expressing DN-Cdh compared to when they contact control GFP-expressing CR cells. Reelin staining is shown in blue. (G) 3D reconstructions of mCherry+ projection neurons in contact with the MZ after double electroporation. Leading processes that contact control CR cells arborize in the MZ, whereas neurons contacting CR cells expressing DN-Cdh display reduced arborization. Quantification is mean ± SEM of the number of branches in the MZ 4 days after electroporation. *p <0.0001 by Student’s t-test. Abbreviations as in Fig. 5. Scale bar (A, D) 50 μm, (B, F,G) 10 μm (C) 100 μm.
Reelin regulates recruitment of Cdh2 to the cell surface of neurons engaged in nectin-mediated adhesive interactions
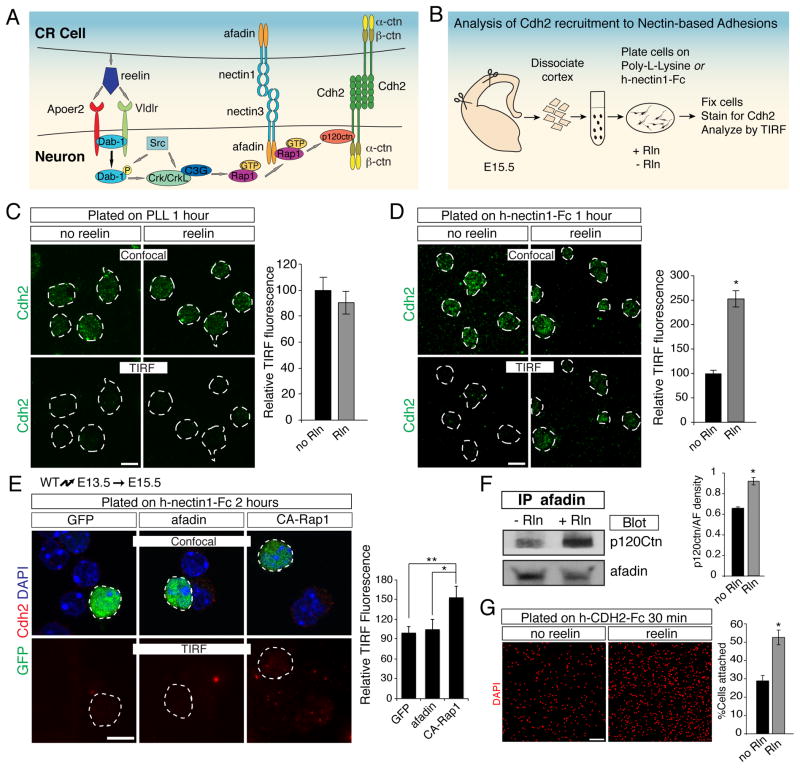
Reelin regulates glia-independent somal translocation by activating Cdh2 function via the adaptor protein Dab1 and the small GTPase Rap1 (Franco et al., 2011). However, the mechanism that links Dab1/Rap1 to Cdh2 function is unclear. Since Rap1 binds to afadin and p120ctn, we reasoned that afadin might provide the critical link between reelin signaling, nectins and Cdh2 (Fig 8A). We hypothesized that nectins initially mediate heterophilic interactions between migrating neurons and CR cells, leading to the subsequent recruitment of Cdh2 in a reelin-dependent manner to stabilize these nascent adhesion sites (Fig. 8A).
Fig. 8. Reelin signaling promotes recruitment of p120ctn/Cdh2 to nectin/afadin complexes and enhances Cdh2-mediated adhesion.
(A) Signaling pathways proposed to act during neuronal migration. (B) Experimental strategy to study the effects of reelin signaling on recruitment of Cdh2 to nectin-based adhesions. Dissociated neocortical neurons were plated on glass coated with poly-L-lysine or nectin1-Fc, in the presence or the absence of reelin. Recruitment of Cdh2 to the cell-substrate interface was analyzed by TIRF microscopy. (C–D) Reelin promotes recruitment of Cdh2 to nectin-based adhesions. Neocortical cells from E15.5 reeler brains were plated onto (C) poly-L-lysine-coated or (D) nectin1-Fc-coated glass ± reelin and immunostained for Cdh2 (green). Cells (dotted outlines) were identified by confocal microscopy (upper panels) and Cdh2 at the cell-substrate interface by TIRF microscopy (lower panels). Quantification (mean ± SEM) is Cdh2 mean pixel intensity per cell in TIRF images, normalized to control (no reelin). *p < 5x10−13 by Student’s t-test. (E) Cortical neurons were electroporated in utero with GFP alone or together with afadin or constitutively-active Rap1a (CA-Rap1) at E13.5, dissociated at E15.5 and plated on nectin1-Fc-coated glass for 2 hours before staining for Cdh2 (red). Electroporated cells (green) are outlined. Quantification (normalized mean ± SEM) of the Cdh2 TIRF signal (red) demonstrates that overexpression of afadin does not affect Cdh2 recruitment compared to control, whereas CA-Rap1 promotes Cdh2 recruitment to the nectin1 surface. *p<0.04, **p<0.01, by Student’s t-test. (F) Reelin treatment promotes co-immunoprecipitation of p120ctn with afadin in neurons. Quantification (mean ± SEM) is optical density of western-blot bands for p120ctn, normalized to afadin levels. *p < 0.005 by Student’s t-test. (G) Reelin enhances cadherin-mediated cell adhesion. E15.5 primary neocortical cells were plated on CDH2-Fc-coated coverslips in the presence or absence of recombinant reelin and allowed to adhere for 30 min prior to washing and analysis. For quantification, the percentage of cells adhering to poly-L-lysine was considered maximum adhesion, and cell attachment to CDH2-Fc is shown as percent maximum adhesion (mean ± SEM). *p < 0.002 by Student’s t-test. Rln, Reelin. Scale bars (C–E) 10 μm, (G) 100μm. See also Fig. S6.
To test this hypothesis in vitro, we modeled in vivo interactions between nectin3+ neurons and nectin1+ CR cells by coating glass-bottom wells with recombinant nectin1 and plating dissociated neurons on the coated surface (Fig. 8B). We then used TIRF microscopy to study the recruitment of Cdh2 to the adhesive interface between neurons and the nectin1-coated surface. When neurons were cultured overnight, neuronal Cdh2 was recruited to the cell-substrate interface in nectin1-coated wells, but not on control poly-L-lysine-coated glass (Fig. S6). As predicted by our model (Fig. 8A), this recruitment of Cdh2 was inhibited upon afadin knockdown in neurons (Fig S6), demonstrating that Cdh2 recruitment was dependent on afadin.
Next, we modified our TIRF assay to allow us to quantitatively evaluate effects of reelin on Cdh2 recruitment. Using primary neurons from reeler embryos to maximize response to reelin, we allowed dissociated neurons to make initial contacts with different substrates by plating them for only 1–2 hours (Fig. 8B). We then measured the effects of recombinant reelin on Cdh2 recruitment to the interface between neurons and the substrate. We also evaluated Cdh2 adhesive function. Recruitment of Cdh2 to nectin1 substrates was enhanced by treatment of neurons with recombinant reelin (Fig. 8D), whereas reelin had no effect on Cdh2 recruitment to poly-L-lysine (Fig. 8C). A similar increase in Cdh2 recruitment was observed by overexpression of constitutively active Rap1, but not by overexpression of afadin alone (Fig. 8E), suggesting that reelin/Rap1 signaling does not simply act by increasing afadin levels within the cell. Furthermore, interactions of afadin with p120ctn were enhanced by reelin treatment (Fig. 8F), suggesting that the reelin/Rap1 pathway facilitates complex formation between the two proteins. Finally, adhesion of dissociated primary neurons to Cdh2-coated cover slips was substantially increased following reelin treatment (Fig. 8G), confirming that cell-surface expressed Cdh2 was functionally active in mediating homophilic interactions. In conclusion, since p120ctn binding to cadherins stabilizes their expression at the cell surface (Hoshino et al., 2005), our findings suggest that reelin stabilizes Cdh2 at the surface by facilitating afadin/Rap1-mediated recruitment of p120ctn to nascent nectin-based cell-cell adhesions.
DISCUSSION
We provide here insights into the mechanisms by which CR cells instruct neocortical development and identify nectins as components of the reelin signaling pathway. Previous studies have shown that CR cell-derived reelin regulates the Cdh2-dependent anchorage of the leading processes of radially migrating neurons with yet to be defined cells in the cortical MZ (Franco et al., 2011). We now identify CR cells as the adhesion partners for migrating neurons and demonstrate that heterotypic binding specificity between the two cell types is achieved by a combinatorial adhesion code consisting of the homophilic cell adhesion molecule Cdh2 and the heterophilic cell adhesion molecules nectin1 and nectin3. Unlike ubiquitously expressed Cdh2, nectin1 and nectin3 are expressed specifically in CR cells and migrating neurons, respectively. Using functional perturbations, we show that nectin1 and nectin3 mediate heterotypic interactions between CR cells and the leading processes of migrating neurons. Cdh2 is then likely required to consolidate these initial interactions into stable contacts to facilitate translocation of the neuronal cell bodies along the leading processes.
Our findings also define components of the signaling pathway that couples reelin to nectins and cadherins. Reelin regulates Cdh2 function during glia-independent somal translocation via the adaptor protein Dab1 and the small GTPase Rap1 (Franco et al., 2011). We now show that nectin3 and afadin provide a critical link between reelin/Dab1/Rap1 and Cdh2. Accordingly, perturbation of nectin3 or afadin disrupts glia-independent somal translocation, and overexpression of Cdh2 in neurons rescues these migratory defects. Reelin signaling facilitates Cdh2 recruitment to nectin1/3-based adhesions, indicating that reelin promotes the assembly of adhesion sites consisting of nectins and cadherins. Afadin apparently serves a critical function in connecting reelin signaling to adhesion by binding to nectins and Rap1. In addition, afadin binds p120ctn in a Rap1-dependent manner, reelin signaling enhances recruitment of p120ctn to afadin, and p120ctn binding to Cdh2 is critical for glia-independent somal translocation. These results reveal a resemblance to the mechanism of adherens junction assembly in epithelial cells, in which nectins establish weak nascent adhesion sites that are then consolidated into stable adherens junctions by the nectin-dependent stabilization of cadherin function via afadin/Rap1/p120ctn (Hoshino et al., 2005; Sato et al., 2006). Since p120ctn inhibits cadherin endocytosis (Davis et al., 2003; Hoshino et al., 2005), this model is consistent with the observation that reelin increases Cdh2 cell surface levels (Jossin and Cooper, 2011).
Our findings also provide insights into the mechanisms by which heterotypic cell-cell interaction specificity is achieved by combinatorial interactions between homophilic cell adhesion molecules (Cdh2) and receptors that preferentially engage in heterophilic interactions, such as nectin1 and nectin3 (Satoh-Horikawa et al., 2000). This combinatorial signaling is likely critical in several developmental contexts. For example, previous studies have shown that nectin1 is expressed in hippocampal mossy fibers, whereas nectin3 is expressed in CA3 pyramidal neurons. These nectins are localized at synaptic contacts formed between the two cell types and perturbation of their function leads to synaptic defects (Honda et al., 2006). Cdh2 is also recruited to the synaptic sites and is required for their function (Brigidi and Bamji, 2011), suggesting that cooperation between nectins and cadherins determines synaptic specificity. However, in some instances nectins appear to function independently of cadherins. For example, nectin1 and nectin3 regulate pathfinding of commissural axons at the spinal cord midline independently of cadherins (Okabe et al., 2004). Instead, axonal pathfinding depends on the secreted signaling molecule netrin1 (Serafini et al., 1994). Nevertheless, there is a striking similarity between axonal pathfinding and radial neuronal migration, in that directional motility in both cases is regulated by combinations of secreted signaling molecules, such as reelin and netrin1, together with cell adhesion molecules, such as nectins and cadherins. The combinatorial code of these molecular cues likely varies depending on the cell type and developmental context, resulting in different functional outputs. In this regard, it will be interesting to analyze nectin and cadherin functions during other stages of neocortical development, for example during the formation of axonal processes or dendrites within neocortical cell layers.
Mice with mutations in nectin1 and nectin3 show defects in hippocampal synapse formation, but no neocortical defects have been reported in these mice when analyzed by general histology (Honda et al. 2006). In light of the current findings, it will be important to analyze the formation of neocortical cell layers in these mice further, for example by using molecular markers that define the identity and position of subtypes of projection neurons. In addition, it is feasible that in these knockout mice, which lack nectin1 or nectin3 throughout development, other cell adhesion molecules might be upregulated to functionally compensate for the loss of nectin1 and nectin3. This compensation may not be triggered by acute perturbations. Similar observations have been made in other instances, for example when the function of doublecortin was disrupted genetically or by RNAi. Only in the latter case were functional defects observed (Bai et al., 2003) whereas defects following genetic perturbation were compensated for at least in part by expression of doublecortin kinase (Deuel et al., 2006; Koizumi et al., 2006).
Our findings extend the notion that the neocortical MZ is an important signaling center for brain development. The MZ contains extracellular matrix (ECM) molecules and various cell types, including interneurons, meningeal fibroblasts and CR cells. Although CR cells are best-known for controlling neocortical lamination via reelin secretion, they are thought to regulate several other important developmental events and thus might provide additional molecular cues besides reelin. For example, CR cell subpopulations that have distinct extracortical origins populate different regions of the neocortical surface, suggesting that they might be involved in patterning the neocortex (Griveau et al., 2010). Projection neurons and CR cells also interact after projection neurons have settled into neocortical cell layers, raising the possibility that CR cells regulate the maturation of dendrites and synapses (Marín-Padilla, 1998; Radnikow et al., 2002). Finally, CR cells and GABAergic interneurons in the cortical MZ show synchronized neuronal activity (Aguiló et al., 1999; Radnikow et al., 2002; Schwartz et al., 1998; Soda et al., 2003), and CR cells receive synaptic inputs from the thalamus, entorhinal cortex and brainstem (Janusonis et al., 2004; Supèr et al., 1998). The functions of these developmental circuits are not known. Intriguingly, recent molecular profiling studies have identified secreted molecules and transmembrane proteins that are expressed in CR cells (Yamazaki et al., 2004), some of which likely instruct the formation of neocortical circuits by mechanisms that have yet to be explored.
EXPERIMENTAL PROCEDURES
Procedures are described in detail in Supplemental Experimental Procedures.
Mice
Wnt3a-Cre mice were generated by targeting an IRES-Cre cassette into the 3′ UTR of the Wnt3a gene. Ai9 mice have been described (Madisen et al., 2010). Reeler mice were purchased from JAX (Stock 000235). C57BL/6J mice were used for in utero electroporations.
Expression constructs
shRNAs for nectin3 and afadin were expressed from the U6 promoter in vectors also containing a CMV-GFP cassette. cDNAs were expressed in RGCs and neurons using the CAG-iGFP vector containing the chicken-β-actin promoter (CAG) and an IRES-EGFP (Hand et al., 2005). Neuron-specific expression was achieved using Dcx-iGFP, which contains the doublecortin promoter and an IRES-EGFP (Franco et al., 2011).
In utero electroporation and time-lapse imaging
Electroporations and time-lapse imaging were carried out as described (Franco et al., 2012). Static images were taken using a Nikon C2 laser-scanning confocal microscope. For quantification, the mean percentage of GFP+ or mCherry+ cells located in the CP or MZ was determined, ± S.E.M. At least 4 animals from 3 separate experiments were analyzed for each condition. Statistical significance was evaluated by Student’s t-test.
In situ hybridizations, immunohistochemistry, immunoprecipitations and western blots
In situ hybridizations, immunostainings, immunoprecipitations and western blots were carried out as described (Belvindrah et al., 2007; Franco et al., 2011; 2012; Tiveron et al., 1996). Probes and antibodies are summarized in Supplemental Experimental Procedures. Images were captured using a Nikon C2 laser-scanning confocal microscope or an Olympus AX70 microscope for bright field images.
Cdh2 adhesion assays
Coverglass was coated with 0.01% poly-L-Lysine (Sigma) or recombinant human CDH2-Fc (0.5 μg/ml; R&D) as detailed in Supplemental Experimental Procedures. Cortical neurons were plated in the presence or the absence of recombinant reelin (0.5 μg/ml; R&D). Cells were washed, fixed and stained with DAPI (Molecular Probes). The number of attached cells was counted in nine fields (10x magnification) for each cover slip using Image-J software. 5 independent experiments were performed. The number of cells attached was normalized as a percentage of cells attached to poly-L-lysine. Values are mean ± S.E.M. Statistical significance was evaluated by Student’s t-test.
Coculture of cortical neurons with CR cells
Embryos were electroporated with Dcx-GFP at E13.5, brains were dissected at E15.5 and primary neocortical cells were prepared as described (Belvindrah et al., 2007). E15.5 Wnt3a-Cre;Ai9 cortices were dissociated into single-cell suspensions and enriched for CR cells by magnetic cell sorting with biotinylated anti-CD184 (Cxcr4) (BD Biosciences) and anti-biotin MicroBeads (Miltenyi Biotec). Equal numbers of GFP+ neurons and tdTomato+ CR cells were mixed and plated on poly-L-lysine (Sigma) coated coverslips 12 hours at 37°C. Coverslips were processed for immunocytochemistry and imaged on a confocal microscope. 3 independent experiments were performed.
TIRF microscopy
Coverglass was coated with 0.01% poly-L-Lysine (Sigma) or recombinant human nectin1-Fc (38 μg/ml; Sino Biological) as detailed in Supplemental Experimental Procedures. cDNAs and shRNAs were introduced into neurons by in utero electroporation at E13.5. Neurons were dissociated at E15.5, plated and cultured on substrates with or without recombinant reelin (0.5 μg/ml; R&D), washed, fixed and immunostained. Cdh2 was detected by immunocytochemistry on a Nikon Ti Eclipse fluorescence TIRF microscope. Excitation was carried out with a 488-nm Coherent laser (Coherent, Inc.). Images were collected with an Andor iXon DU-897 EMCCD camera. Pixel intensity of the TIRF signal was quantified using Nikon Elements software. 3 independent experiments were performed. Values are mean ± S.E.M. Statistical significance was evaluated by Student’s t-test.
Supplementary Material
HIGHLIGHTS.
Cajal Retzius cells steer radial migration by secreted and short-range guidance cues
Nectins and cadherins cooperate to establish heterotypic cell contacts in the neocortex
Nectins and the adaptor protein afadin are components of the reelin signaling pathway
Acknowledgments
We thank K. Spencer for help with microscopy; C. Ramos, G. Martin and S. Kupriyanov for assistance in generating mice. This work was supported by funding from the NIH (SJF, NS060355; UM, NS046456, MH078833, HD070494), the Dorris Neurscience Center (UM), and the Skaggs Institute for Chemical Biology (UM), CIRM (IM-G; AE), Ministerio de Educacion (CG-S., EX2009-0416; IM-G., FU-2006-1238), Generalitat Valenciana (CG-S, APOSTD/2010/064).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguiló A, Schwartz TH, Kumar VS, Peterlin ZA, Tsiola A, Soriano E, Yuste R. Involvement of cajal-retzius neurons in spontaneous correlated activity of embryonic and postnatal layer 1 from wild-type and reeler mice. J Neurosci. 1999;19:10856–10868. doi: 10.1523/JNEUROSCI.19-24-10856.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–1283. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Belvindrah R, Graus-Porta D, Goebbels S, Nave KA, Müller U. Beta1 integrins in radial glia but not in migrating neurons are essential for the formation of cell layers in the cerebral cortex. J Neurosci. 2007;27:13854–13865. doi: 10.1523/JNEUROSCI.4494-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Liu KD, Shimizu K, Takai Y, Mostov KE. Nectin proteins are expressed at early stages of nephrogenesis and play a role in renal epithelial cell morphogenesis. Am J Physiol Renal Physiol. 2009;296:F564–F574. doi: 10.1152/ajprenal.90328.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigidi GS, Bamji SX. Cadherin-catenin adhesion complexes at the synapse. Curr Opin Neurobiol. 2011;21:208–214. doi: 10.1016/j.conb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–534. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuel TAS, Liu JS, Corbo JC, Yoo SY, Rorke-Adams LB, Walsh CA. Genetic interactions between doublecortin and doublecortin-like kinase in neuronal migration and axon outgrowth. Neuron. 2006;49:41–53. doi: 10.1016/j.neuron.2005.10.038. [DOI] [PubMed] [Google Scholar]
- Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Müller U. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco SJ, Martinez-Garay I, Gil-Sanz C, Harkins-Perry SR, Müller U. Reelin regulates cadherin function via Dab1/Rap1 to control neuronal migration and lamination in the neocortex. Neuron. 2011;69:482–497. doi: 10.1016/j.neuron.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonchar Y, Burkhalter A. Three distinct families of GABAergic neurons in rat visual cortex. Cereb Cortex. 1997;7:347–358. doi: 10.1093/cercor/7.4.347. [DOI] [PubMed] [Google Scholar]
- Griveau A, Borello U, Causeret F, Tissir F, Boggetto N, Karaz S, Pierani A. A novel role for Dbx1-derived Cajal-Retzius cells in early regionalization of the cerebral cortical neuroepithelium. PLoS Biol. 2010;8:e1000440. doi: 10.1371/journal.pbio.1000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Sanada K, Miyamoto DT, Rovelstad S, Nadarajah B, Pearlman AL, Brunstrom J, Tsai LH. Layering defect in p35 deficiency is linked to improper neuronal-glial interaction in radial migration. Nat Neurosci. 2003;6:1284–1291. doi: 10.1038/nn1151. [DOI] [PubMed] [Google Scholar]
- Hand R, Bortone D, Mattar P, Nguyen L, Heng JIT, Guerrier S, Boutt E, Peters E, Barnes AP, Parras C, et al. Phosphorylation of Neurogenin2 specifies the migration properties and the dendritic morphology of pyramidal neurons in the neocortex. Neuron. 2005;48:45–62. doi: 10.1016/j.neuron.2005.08.032. [DOI] [PubMed] [Google Scholar]
- Honda T, Sakisaka T, Yamada T, Kumazawa N, Hoshino T, Kajita M, Kayahara T, Ishizaki H, Tanaka-Okamoto M, Mizoguchi A, et al. Involvement of nectins in the formation of puncta adherentia junctions and the mossy fiber trajectory in the mouse hippocampus. Mol Cell Neurosci. 2006;31:315–325. doi: 10.1016/j.mcn.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–24103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- Inagaki M, Irie K, Ishizaki H, Tanaka-Okamoto M, Morimoto K, Inoue E, Ohtsuka T, Miyoshi J, Takai Y. Roles of cell-adhesion molecules nectin 1 and nectin 3 in ciliary body development. Development. 2005;132:1525–1537. doi: 10.1242/dev.01697. [DOI] [PubMed] [Google Scholar]
- Janusonis S, Gluncic V, Rakic P. Early serotonergic projections to Cajal-Retzius cells: relevance for cortical development. J Neurosci. 2004;24:1652–1659. doi: 10.1523/JNEUROSCI.4651-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jossin Y, Cooper JA. Reelin, Rap1 and N-cadherin orient the migration of multipolar neurons in the developing neocortex. Nat Neurosci. 2011;14:697–703. doi: 10.1038/nn.2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki M, Nakamura S, Machon O, Krauss S, Radice GL, Takeichi M. N-cadherin mediates cortical organization in the mouse brain. Dev Biol. 2007;304:22–33. doi: 10.1016/j.ydbio.2006.12.014. [DOI] [PubMed] [Google Scholar]
- Kawauchi T, Sekine K, Shikanai M, Chihama K, Tomita K, Kubo KI, Nakajima K, Nabeshima YI, Hoshino M. Rab GTPases-dependent endocytic pathways regulate neuronal migration and maturation through N-cadherin trafficking. Neuron. 2010;67:588–602. doi: 10.1016/j.neuron.2010.07.007. [DOI] [PubMed] [Google Scholar]
- Koizumi H, Tanaka T, Gleeson JG. Doublecortin-like kinase functions with doublecortin to mediate fiber tract decussation and neuronal migration. Neuron. 2006;49:55–66. doi: 10.1016/j.neuron.2005.10.040. [DOI] [PubMed] [Google Scholar]
- Louvi A, Yoshida M, Grove EA. The derivatives of the Wnt3a lineage in the central nervous system. J Comp Neurol. 2007;504:550–569. doi: 10.1002/cne.21461. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai K, Nakanishi H, Satoh A, Obaishi H, Wada M, Nishioka H, Itoh M, Mizoguchi A, Aoki T, Fujimoto T, et al. Afadin: A novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J Cell Biol. 1997;139:517–528. doi: 10.1083/jcb.139.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- Meyer G, Perez-Garcia CG, Abraham H, Caput D. Expression of p73 and Reelin in the developing human cortex. J Neurosci. 2002;22:4973–4986. doi: 10.1523/JNEUROSCI.22-12-04973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara M, Nakanishi H, Takahashi K, Satoh-Horikawa K, Tachibana K, Takai Y. Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J Biol Chem. 2000;275:613–618. doi: 10.1074/jbc.275.1.613. [DOI] [PubMed] [Google Scholar]
- Nadarajah B, Brunstrom JE, Grutzendler J, Wong RO, Pearlman AL. Two modes of radial migration in early development of the cerebral cortex. Nat Neurosci. 2001;4:143–150. doi: 10.1038/83967. [DOI] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Okabe N, Shimizu K, Ozaki-Kuroda K, Nakanishi H, Morimoto K, Takeuchi M, Katsumaru H, Murakami F, Takai Y. Contacts between the commissural axons and the floor plate cells are mediated by nectins. Dev Biol. 2004;273:244–256. doi: 10.1016/j.ydbio.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Olson EC, Kim S, Walsh CA. Impaired neuronal positioning and dendritogenesis in the neocortex after cell-autonomous Dab1 suppression. J Neurosci. 2006;26:1767–1775. doi: 10.1523/JNEUROSCI.3000-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki-Kuroda K, Nakanishi H, Ohta H, Tanaka H, Kurihara H, Mueller S, Irie K, Ikeda W, Sakai T, Wimmer E, et al. Nectin couples cell-cell adhesion and the actin scaffold at heterotypic testicular junctions. Curr Biol. 2002;12:1145–1150. doi: 10.1016/s0960-9822(02)00922-3. [DOI] [PubMed] [Google Scholar]
- Radnikow G, Feldmeyer D, Lübke J. Axonal projection, input and output synapses, and synaptic physiology of Cajal-Retzius cells in the developing rat neocortex. J Neurosci. 2002;22:6908–6919. doi: 10.1523/JNEUROSCI.22-16-06908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasin MR, Gazula VR, Breunig JJ, Kwan KY, Johnson MB, Liu-Chen S, Li HS, Jan LY, Jan YN, Rakic P, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10:819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Mandai K, Takai Y. The role of nectins in different types of cell-cell adhesion. J Cell Sci. 2012;125:3713–3722. doi: 10.1242/jcs.099572. [DOI] [PubMed] [Google Scholar]
- Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281:5288–5299. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- Satoh-Horikawa K, Nakanishi H, Takahashi K, Miyahara M, Nishimura M, Tachibana K, Mizoguchi A, Takai Y. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J Biol Chem. 2000;275:10291–10299. doi: 10.1074/jbc.275.14.10291. [DOI] [PubMed] [Google Scholar]
- Schwartz TH, Rabinowitz D, Unni V, Kumar VS, Smetters DK, Tsiola A, Yuste R. Networks of coactive neurons in developing layer 1. Neuron. 1998;20:541–552. doi: 10.1016/s0896-6273(00)80993-9. [DOI] [PubMed] [Google Scholar]
- Sekine K, Honda T, Kawauchi T, Kubo KI, Nakajima K. The outermost region of the developing cortical plate is crucial for both the switch of the radial migration mode and the Dab1-dependent “inside-out” lamination in the neocortex. J Neurosci. 2011;31:9426–9439. doi: 10.1523/JNEUROSCI.0650-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424. doi: 10.1016/0092-8674(94)90420-0. [DOI] [PubMed] [Google Scholar]
- Soda T, Nakashima R, Watanabe D, Nakajima K, Pastan I, Nakanishi S. Segregation and coactivation of developing neocortical layer 1 neurons. J Neurosci. 2003;23:6272–6279. doi: 10.1523/JNEUROSCI.23-15-06272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano E, Del Río JA. The cells of cajal-retzius: still a mystery one century after. Neuron. 2005;46:389–394. doi: 10.1016/j.neuron.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Supèr H, Martínez A, Del Río JA, Soriano E. Involvement of distinct pioneer neurons in the formation of layer-specific connections in the hippocampus. J Neurosci. 1998;18:4616–4626. doi: 10.1523/JNEUROSCI.18-12-04616.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Nakajima K. Multipolar migration: the third mode of radial neuronal migration in the developing cerebral cortex. J Neurosci. 2003;23:9996–10001. doi: 10.1523/JNEUROSCI.23-31-09996.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Nakanishi H, Miyahara M, Mandai K, Satoh K, Satoh A, Nishioka H, Aoki J, Nomoto A, Mizoguchi A, et al. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with Afadin, a PDZ domain-containing protein. J Cell Biol. 1999;145:539–549. doi: 10.1083/jcb.145.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Ikeda W, Ogita H, Rikitake Y. The Immunoglobulin-Like Cell Adhesion Molecule Nectin and Its Associated Protein Afadin. 2008;24:309–342. doi: 10.1146/annurev.cellbio.24.110707.175339. Http://Dx.Doi.org/10.1146/Annurev.Cellbio.24.110707.175339. [DOI] [PubMed] [Google Scholar]
- Thoreson MA, Anastasiadis PZ, Daniel JM, Ireton RC, Wheelock MJ, Johnson KR, Hummingbird DK, Reynolds AB. Selective uncoupling of p120(ctn) from E-cadherin disrupts strong adhesion. J Cell Biol. 2000;148:189–202. doi: 10.1083/jcb.148.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron MC, Hirsch MR, Brunet JF. The expression pattern of the transcription factor Phox2 delineates synaptic pathways of the autonomic nervous system. J Neurosci. 1996;16:7649–7660. doi: 10.1523/JNEUROSCI.16-23-07649.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi H, Kominami K, Waseda M, Komura H, Miyoshi J, Takeichi M, Takai Y. Nectins establish a checkerboard-like cellular pattern in the auditory epithelium. Science. 2011;333:1144–1147. doi: 10.1126/science.1208467. [DOI] [PubMed] [Google Scholar]
- Togashi H, Miyoshi J, Honda T, Sakisaka T, Takai Y, Takeichi M. Interneurite affinity is regulated by heterophilic nectin interactions in concert with the cadherin machinery. J Cell Biol. 2006;174:141–151. doi: 10.1083/jcb.200601089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Qiu R, Tsark W, Lu Q. Rapid promoter analysis in developing mouse brain and genetic labeling of young neurons by doublecortin-DsRed-express. J Neurosci Res. 2007;85:3567–3573. doi: 10.1002/jnr.21440. [DOI] [PubMed] [Google Scholar]
- Weisenhorn DM, Prieto EW, Celio MR. Localization of calretinin in cells of layer I (Cajal-Retzius cells) of the developing cortex of the rat. Brain Res Dev Brain Res. 1994;82:293–297. doi: 10.1016/0165-3806(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, La Cruz, De E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki H, Sekiguchi M, Takamatsu M, Tanabe Y, Nakanishi S. Distinct ontogenic and regional expressions of newly identified Cajal-Retzius cell-specific genes during neocorticogenesis. Proc Natl Acad Sci USa. 2004;101:14509–14514. doi: 10.1073/pnas.0406295101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Assimacopoulos S, Jones KR, Grove EA. Massive loss of Cajal-Retzius cells does not disrupt neocortical layer order. Development. 2006;133:537–545. doi: 10.1242/dev.02209. [DOI] [PubMed] [Google Scholar]
- Zhao C, Guan W, Pleasure SJ. A transgenic marker mouse line labels Cajal-Retzius cells from the cortical hem and thalamocortical axons. Brain Res. 2006;1077:48–53. doi: 10.1016/j.brainres.2006.01.042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.