Abstract
Neurokinin B (NKB) and its cognate receptor neurokinin 3 (NK3R) play a critical role in reproduction. NKB and NK3R are coexpressed with dynorphin (Dyn) and kisspeptin (Kiss1) genes in neurons of the arcuate nucleus (Arc). However, the mechanisms of action of NKB as a cotransmitter with kisspeptin and dynorphin remain poorly understood. We explored the role of NKB in the control of LH secretion in the female rat as follows. 1) We examined the effect of an NKB agonist (senktide, 600 pmol, administered into the lateral cerebral ventricle) on luteinizing hormone (LH) secretion. In the presence of physiological levels of estradiol (E2), senktide induced a profound increase in serum levels of LH and a 10-fold increase in the number of Kiss1 neurons expressing c-fos in the Arc (P < 0.01 for both). 2) We mapped the distribution of NKB and NK3R mRNAs in the central forebrain and found that both are widely expressed, with intense expression in several hypothalamic nuclei that control reproduction, including the Arc. 3) We studied the effect of E2 on the expression of NKB and NK3R mRNAs in the Arc and found that E2 inhibits the expression of both genes (P < 0.01) and that the expression of NKB and NK3R reaches its nadir on the afternoon of proestrus (when circulating levels of E2 are high). These observations suggest that NKB/NK3R signaling in Kiss1/NKB/Dyn-producing neurons in the Arc has a pivotal role in the control of gonadotropin-releasing hormone (GnRH)/LH secretion and its regulation by E2-dependent negative feedback in the rat.
Keywords: estradiol, hypothalamus
neurokinin B (NKB) is a member of the tachykinin family that has recently emerged as a key neuropeptide in the control of reproductive function. Humans bearing loss-of-function mutations of TAC3 or TAC3R, which are homologous to NKB and its cognate receptor neurokinin 3 (NK3R, aka Tac2 and Tac3r), respectively, in rodents, exhibit hypogonadotropic hypogonadism and infertility (34, 38). Clues regarding NKB's specific role in the regulation of reproduction come from studies in animals. In rodents, the compensatory rise of luteinizing hormone (LH) following ovariectomy (OVX) is ablated by treatment with the NKB agonist senktide (24, 30). In addition, the expression of NKB and its receptor NK3R in the hypothalamic arcuate nucleus (Arc) is inhibited by estradiol (E2) via estrogen receptor-α (9, 24). Together, these observations suggest that NKB/NK3R signaling plays an important role in the E2-dependent negative-feedback control of gonadotropin-releasing hormone (GnRH) and LH in mammals.
In the Arc of sheep and rodents, NKB is coexpressed with kisspeptin and dynorphin, which are encoded by the Kiss1 and preprodynorphin (Dyn) genes, respectively (4, 15, 24). Kisspeptin is a potent GnRH secretagogue (25), and GnRH neurons express the kisspeptin receptor (Kiss1r, aka GPR54) (17), which plays a critical role in the neuroendocrine regulation of GnRH and LH secretion (8, 25, 31). Kisspeptin neurons in the Arc express estrogen receptor-α and are thought to be direct targets for the negative-feedback action of E2 on GnRH secretion (32). Kiss1/NKB cells in the Arc are surrounded by a dense network of NKB-containing fibers (4), and they express NK3R, at least in the mouse (24), perhaps reflecting autosynaptic processes that control the rhythmic activity of this cellular network. Thus, from different species, fragments of a model have begun to emerge that would directly link a NKB/NK3R signaling pathway in Kiss1 neurons to the regulation of GnRH secretion; however, critical elements of the model are missing.
The purpose of this study was to investigate the proposition that NKB induces the release of GnRH by activating Kiss1 neurons, which serve as a conduit for the E2-dependent negative-feedback control of GnRH/LH secretion. 1) We tested the hypothesis that NKB regulates GnRH secretion by assessing the effect of the NKB agonist senktide on LH release in the female rat. 2) To determine whether kisspeptin/NKB neurons are possible targets for the action of senktide on GnRH/LH secretion, we assessed whether senktide could activate c-fos expression in Kiss1 neurons. 3) To elucidate the anatomic circuitry that links NKB-NK3R signaling to E2-dependent regulation of GnRH/LH secretion, we mapped the distribution of NKB and NK3R mRNAs in the brain and evaluated whether their expression is regulated by E2.
MATERIALS AND METHODS
Animals and Drugs
Female Wistar rats were bred in the vivarium at the University of Córdoba. The animals were maintained under constant conditions of light (14 h of light, lights on at 0700) and temperature (22°C) and were weaned at 21 days postpartum: they were housed in groups of five rats per cage with free access to standard rat chow and tap water. For hormone tests involving intracerebroventricular cannulation, the rats were caged individually from the day before cannula implantation until termination of experiments. Correct positioning of the cannulas was checked by visual inspection (to exclude animals showing obvious displacement or detachment) and confirmed at necropsy. Experimental procedures were approved by the University of Córdoba Ethical Committee for animal experimentation and were conducted in accordance with the European Union normative for care and use of experimental animals. Rat/mouse kisspeptin-10 (Kp-10) was obtained from Phoenix Pharmaceuticals (Belmont, CA). The NK3R agonist senktide was purchased from Sigma-Aldrich. The dose of senktide was selected on the basis of previous references as maximally effective in inducing gonadotropin responses in the rat (4).
OVX and Steroid Replacement
OVX was performed on adult female rats. Briefly, bilateral lumbar incisions were made while the animals were under fluorourethane anesthesia. Vasculature to the ovary was sutured, the ovary was removed, and wound clips were used to close the incision. Immediately after OVX, capsules filled with oil (sham) or with E2 + oil were implanted subcutaneously via a small midscapular incision at the base of the neck; wound clips were used to close the incision. For E2 implantation, Silastic tubing (20 mm long, 0.062 cm ID, 0.125 cm OD; Dow Corning) was used. Crystalline E2 (Sigma) at a low (physiological) dose of 100 μg/ml was dissolved in olive oil, as previously described (35). After capsules were filled with E2 in oil, the end of the capsule was sealed with silicone cement and allowed to cure overnight. On the day before surgery, implants were washed twice for 10 min in changes of 100% ethanol and then placed in sterile physiological saline overnight.
Tissue Preparation
Blood was centrifuged to isolate the serum, which was stored at −20°C until hormone measurements. Uteri were removed and weighed to provide an additional marker of plasma E2 levels (and their biological effect). Brains were removed for in situ hybridization (ISH), frozen on dry ice, and then stored at −80°C until sectioned. Five sets of 20-μm sections in the coronal plane were cut on a cryostat (from the diagonal band of Broca to the mammillary bodies), thaw-mounted onto SuperFrost Plus slides (VWR Scientific), and stored at −80°C. A single set of slides was used for ISH (adjacent sections 100 μm apart).
RIA for LH and E2
Serum LH levels were measured in 25- to 50-μl samples using a double-antibody method and RIA kits (supplied by Dr. A. F. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases National Hormone and Peptide Program, Torrance, CA). Rat LH-I-10 was labeled with 125I with the use of Iodo-gen tubes (Pierce, Rockford, IL) according to the manufacturer's instructions. Hormone concentrations were expressed, with the reference preparation LH-RP-3 used as a standard. Intra- and interassay coefficients of variation were <8 and 10%, respectively. The sensitivity of the LH assay was 5 pg/tube. E2 levels were measured in 100-μl aliquots of serum samples with the use of a commercial, ultrasensitive E2 RIA kit (Immunotech-Beckman Coulter, Prague, Czech Republic) according to the manufacturer's instructions. The sensitivity of the E2 assay was 0.6 pg/tube.
Detection of Kiss1, NKB, NK3R, and c-fos mRNAs
The probes used for detection of Kiss1, NKB, NK3R, and c-fos mRNA are described elsewhere (11, 24). Sense probes for every transcript were used as controls for the specificity of the ISH procedures, which are outlined below.
Single-Labeled ISH of NKB and NK3R mRNAs
NKB and NK3R mRNA sense and antisense probes were transcribed with T7 or T3 polymerase (Fermentas), as described previously (24). Briefly, radiolabeled probes were synthesized in vitro by inclusion of the following ingredients in a volume of 20 μl: 250 Ci of [33P]UTP (Perkin Elmer Life and Analytical Sciences), 1 μg of PCR product, 0.5 mM each ATP, CTP, and GTP, and 40 U of polymerase. Residual DNA was digested with 4 U of DNase (Ambion), and the DNase reaction was terminated by addition of 2 μl of 0.5 M EDTA, pH 8.0. The riboprobes were separated from unincorporated nucleotides with NucAway spin columns (Ambion). Slides with mouse hypothalamic sections from the different experimental groups were processed as reported previously (7, 16).
Double-Labeled ISH
Antisense rat Kiss1 and c-fos probes were transcribed from linearized pAMP1 plasmid containing the mouse Kiss1 or the rat c-fos insert with T7 polymerase (Fermentas) (11, 16). Radiolabeled riboprobe for c-fos was synthesized as described above for the NKB and NK3R riboprobes. Digoxigenin-labeled Kiss1 antisense riboprobe was synthesized with T7 RNA polymerase and digoxigenin labeling mix (Roche) according to the manufacturer's instructions. Slides were processed for double-labeled ISH, as described previously (17). Slides were stored at 4°C and developed 8 days later.
Quantification and Analysis of NKB, NK3R, Kiss1, and c-fos mRNAs
The brain sections were analyzed unilaterally. Slides from all the animals were assigned a random three-letter code, arranged alphabetically, and read under dark-field illumination with custom-designed software designed to count the total number of cells and the number of silver grains (corresponding to radiolabeled NKB, NK3R, or c-fos mRNA) over each cell (5). Kiss1 mRNA-containing cells were visualized under fluorescent illumination, and custom-designed software was used to count the number of silver grains over each Kiss1 cell. The number of cells reported for each experiment represents the number of cells within the coronal sections containing the hypothalamic nucleus studied for each set, not the total number of cells in this specific nucleus. The starting and ending points of quantification were determined according to the atlas of Paxinos and Franklin (26a). Signal-to-background ratios for individual cells were calculated; an individual cell with a signal-to-background ratio of ≥3 was considered to be double-labeled. For each animal, the number of double-labeled cells was calculated as a percentage of the total number of particular mRNA-positive cells and then averaged across animals to produce a mean ± SE.
Statistical Analysis
Values are means ± SE for each group. In addition, when appropriate, integrated LH secretory responses are expressed as the area under the curve (AUC), calculated following the trapezoidal rule (27), over a 120-min period after administration of senktide. Two-way ANOVA, followed by Bonferroni's post hoc comparison and t-tests, was used to assess variation among experimental groups. Significance level was set at P ≤ 0.05. All analyses were performed with Statview 5.0.1 for Macintosh (SAS Institute).
Experimental Designs
Experiments 1a and 1b: LH response to NKB agonist in intact and OVX animals treated with E2.
Although it has been demonstrated in the rat and mouse that the NKB agonist senktide inhibits LH secretion in untreated OVX animals and has no discernable effect on LH in OVX animals treated with exceedingly high doses of E2 (24), the effect of senktide has not been assessed in intact or OVX animals (rodents) treated with physiological levels of E2. In experiments 1a and 1b, we assessed the ability of senktide to modulate LH secretion in vivo under two experimental conditions: animals (killed on diestrus 1 and proestrus) were left intact, and a capsule containing a low physiological dose of E2 was immediately implanted subcutaneously into OVX animals. We selected the dose of senktide for these experiments (600 pmol of senktide in 10 μl of vehicle) on the basis of a previous report showing its efficacy (30).
In experiment 1a, the effect of central administration of senktide on LH release during two different phases of the estrous cycle was determined. Estrous cyclicity was monitored by daily vaginal cytology in adult female rats, and only animals showing at least two consecutive 4-day estrous cycles were used. On the day prior to the experiment, intracerebroventricular cannulas were implanted into groups of female rats (n = 10), as described in detail elsewhere (12). On the morning of the day of the experiment, the phase of the estrous cycle was confirmed, and only animals in proestrus or diestrus 1 were selected (n = 10 per group). Then senktide or vehicle alone (physiological saline, 0.9% NaCl) was injected intracerebroventricularly into each animal, and blood samples (300 μl) were obtained by jugular venipuncture, while the animals were maintained under fluorourethane anesthesia [following a process described elsewhere (28)]. Blood was collected before (0 min) and 20, 60, and 120 min after the injection and stored as described above.
In experiment 1b, we analyzed the effect of centrally administered senktide in OVX animals that were treated with sustained, low physiological level of E2. For this purpose, sham (OVX) or E2-containing capsules (OVX + E2) were implanted subcutaneously into adult female OVX rats, as described above (n = 20 per group). After 1 wk, each group of animals was divided into two subgroups and subjected to the protocol described in experiment 1a. Plasma levels of LH and E2, as well as uterine weight, were measured to determine the efficacy of the hormone replacement.
Experiment 2: marking Kiss1 neurons as possible targets for NKB.
Having observed that centrally administered senktide stimulated LH secretion in intact animals and OVX animals treated with physiological levels of E2, we sought to determine whether kisspeptin neurons were activated by NKB and could plausibly mediate its effect on GnRH/LH secretion. Thus, in experiment 2, we used double-labeled ISH to measure the cellular content of c-fos mRNA as a marker for activation in Kiss1 mRNA-expressing cells following treatment with the NKB agonist senktide (vs. controls). Adult OVX + E2 animals were divided into two groups (n = 7 per group): one was injected intracerebroventricularly with senktide, and the other was treated with vehicle. After 30 min, the animals were decapitated, and the brains and trunk blood were collected for ISH and hormone measurements, respectively.
Experiments 3 and 4: mapping distribution of NKB and NK3R mRNA and assessing their regulation by E2.
To learn more about the cellular origin of NKB and NK3R in the brain and their possible role in feedback control of gonadotropin secretion, we had three objectives. 1) We mapped the distribution of NKB and NK3R mRNAs in the brain of OVX + sham and OVX + E2 rats. 2) We measured the expression of NKB and NK3R in two stages of the estrous cycle representing different levels of circulating E2 in intact animals: diestrus 1 and proestrus (morning and afternoon). 3) We evaluated the effect of E2 on the expression of NKB and NK3R in OVX animals.
In experiment 3, intact animals were studied. Estrous cycles were monitored daily, as described in experiment 1. Groups of animals were killed on the morning of diestrus 1, the morning of proestrus, and the afternoon of proestrus (n = 5 per group). Brains were immediately removed, frozen on dry ice, and stored at −80°C until they were sectioned on a cryostat, thaw-mounted, and stored at −80°C until they were used for ISH. Trunk blood samples for hormone measurements were also collected at the time of decapitation.
In experiment 4, OVX animals were divided into two groups (n = 10 per group): sham capsules were implanted into one group (OVX), and E2-containing capsules were implanted into the other group (OVX + E2), as described in experiment 1b. At 1 wk after the surgery and capsule implantation, brains were collected in the morning (1000) and stored as described above for ISH.
RESULTS
Action of the NKB Agonist Senktide on LH Secretion in Intact Female Rats
In normal intact animals, we observed that senktide induced a robust increase in serum levels of LH on diestrus 1 and proestrus. At diestrus, the values were as follows: 1.1 ± 0.2 and 3.6 ± 0.4 ng/ml for vehicle and senktide, respectively, at 20 min (P < 0.01), 0.6 ± 0.2 and 1.4 ± 0.4 ng/ml for vehicle and senktide, respectively, at 60 min (P < 0.01), and 0.4 ± 0.1 and 0.9 ± 0.2 ng/ml for vehicle and senktide, respectively, at 120 min (P < 0.01; Fig. 1A). At proestrus, the values were as follows: 1.8 ± 0.2 and 3.6 ± 0.4 ng/ml for vehicle and senktide, respectively, at 20 min (P < 0.01), 1.4 ± 0.3 and 1.9 ± 0.6 ng/ml for vehicle and senktide, respectively, at 60 min, and 0.9 ± 0.1 and 0.8 ± 0.2 ng/ml for vehicle and senktide, respectively, at 120 min (Fig. 1B). The overall integrated secretory response (calculated as AUC over a 2-h study period) showed a significant induction in both cycle phases: 80 ± 13 and 213 ± 39 AUC for vehicle alone and senktide, respectively, at diestrus 1 (P < 0.01) and 159 ± 13 and 232 ± 45 AUC for vehicle alone and senktide, respectively, at proestrus (P < 0.01; Fig. 1, A and B).
Fig. 1.
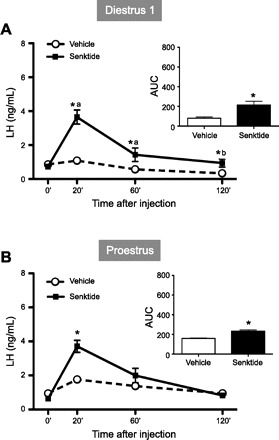
Effects of acute administration of senktide on luteinizing hormone (LH) release in intact female rats at diestrus 1 (A) and proestrus (B). Senktide (600 pmol) or vehicle was acutely injected intracerebroventricularly. Blood samples for LH determination were obtained before [0 min (0′)] and 20, 60, and 120 min after injection. In addition to time course profiles, integrated secretory responses to central administration of senktide [calculated as area under the curve (AUC) over the 120-min study period] are shown. Hormone values are means ± SE. Statistical significance was determined by 2-way ANOVA: *P < 0.01 vs. corresponding control values; aP < 0.01 vs. senktide basal (0′); bP < 0.05 vs. senktide basal.
Effect of NKB Agonist in OVX Animals With and Without E2 Replacement
Adult OVX rats injected with 600 pmol of senktide showed a significant decrease in LH release 120 min after treatment: 3.7 ± 0.4 and 2.1 ± 0.2 ng/ml for vehicle and senktide, respectively (P < 0.01; Fig. 2A). The overall release of LH, as represented by the AUC, was significantly different between groups: 464 ± 39 and 388 ± 33 AUC for vehicle and senktide, respectively (P < 0.01; Fig. 2A). In OVX animals treated with a low dose of E2, senktide evoked a significant increase in LH release at all time points: 1.9 ± 0.4 and 6.6 ± 0.8 ng/ml for vehicle and senktide, respectively, at 20 min (P < 0.01), 2.9 ± 0.5 and 5.3 ± 1.2 ng/ml for vehicle and senktide, respectively, at 60 min (P < 0.01), and 1.0 ± 0.2 and 3.1 ± 1.2 ng/ml for vehicle and senktide, respectively, at 120 min (P < 0.01; Fig. 2B). The overall release of LH, as represented by the AUC, was significantly different between groups: 252 ± 49 and 584 ± 99 AUC for vehicle and senktide, respectively (P < 0.01; Fig. 2B). Circulating E2 levels in OVX + E2 animals matched those of proestrus, whereas OVX + sham animals showed E2 levels equivalent to the diestrus phase. Similarly, uterine weight showed a significant increase in OVX + E2 animals compared with the OVX + sham group (Table 1).
Fig. 2.
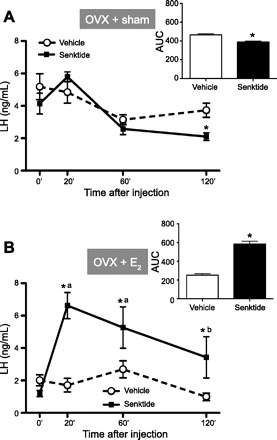
Effects of acute administration of senktide on LH release in ovariectomized (OVX) rats implanted subcutaneously with a sham (oil) capsule (A) and with an estradiol (E2)-filled capsule (OVX + E2, B). Senktide (600 pmol) or vehicle was acutely injected intracerebroventricularly. Blood samples for LH determination were obtained before (0 min) and 20, 60, and 120 min after injection. In addition to time course profiles, integrated secretory responses to central administration of senktide (calculated as AUC over the 120-min study period) are shown. Hormone values are means ± SE. *P < 0.01 vs. corresponding control values; aP < 0.01 vs. senktide basal (2-way ANOVA).
Table 1.
LH, E2 levels, and uterine weights in experimental groups
| Proestrus | Diestrus | OVX | OVX + E2 | |
|---|---|---|---|---|
| LH, ng/ml | 0.9 ± 0.1 (11) | 0.9 ± 0.2 (10) | 5.2 ± 0.8* (9) | 2.0 ± 0.4 (10) |
| E2, pg/ml | 12 ± 2a (14) | <6b (11) | <6b (9) | 12 ± 3a (6) |
| Uterine wt, mg/100 g body wt | 46.3 ± 1.7 (10) | 153.6 ± 4.2* (10) |
Values are means ± SE of number of rats in parentheses. LH, luteinizing hormone; E2, estradiol; OVX, ovariectomized.
P < 0.01 (1-way ANOVA). Values represented with different letters (a, b) are statistically significant (P < 0.05).
Coexpression of c-fos and Kiss1 mRNA in the Arc After Senktide Treatment
The aim of this experiment was to determine whether Kiss1 neurons are activated by NKB. The percentage of Kiss1 neurons expressing c-fos in OVX + E2 rats after senktide injection was assessed by double-labeled ISH. In the Arc, after the senktide injection, ∼40% of the Kiss1 neurons were found to coexpress c-fos, reflecting a 10-fold increase in the coexpression of c-fos and Kiss1 compared with animals treated with vehicle alone: 39.4 ± 8.1% vs. 4.5 ± 1.7% (P < 0.01; Fig. 3). No expression of c-fos was detected in Kiss1 neurons in the anteroventral paraventricular nucleus following any of the treatments (data not shown). As anticipated, senktide stimulated LH secretion, as previously shown: 0.9 ± 0.3 and 3.0 ± 0.3 ng/ml for vehicle and senktide, respectively (P < 0.01).
Fig. 3.
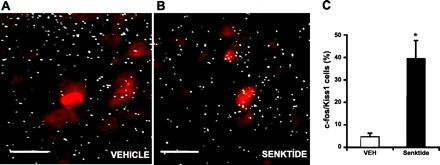
A and B: photomicrographs of kisspeptin 1 (Kiss1) and c-fos mRNA in situ hybridization in OVX + E2 rats 30 min after vehicle or senktide treatment. Red stain indicates cells expressing Kiss1 mRNA; white grains indicate cells expressing c-fos mRNA. Scale bars, 25 μm. C: percentage of Kiss1 cells expressing c-fos mRNA in the arcuate nucleus (Arc). Values are means ± SE. *P < 0.01 (Student's t-test).
Distribution of NKB mRNA in the Hypothalamus and Its Regulation by E2
Figure 4 (top) shows areas of the brain where NKB mRNA was clearly detectable in OVX + sham and OVX + E2 animals. NKB mRNA was expressed in the cerebral cortex, the bed nucleus of the stria terminalis, the anterocortical amygdaloid nucleus, the supraoptic nucleus (SON), the basolateral nucleus of the amygdala, the medial habenular nucleus (MH), the lateral hypothalamus (LHA), the zona incerta, and the Arc. Within the hypothalamus, expression was found to be concentrated in two regions: the Arc and the LHA. As a function of cycle stage, we found significantly reduced levels of NKB mRNA in the Arc during the afternoon of proestrus (when E2 levels are highest) relative to the morning of diestrus 1 (P < 0.05) and the morning of proestrus (P < 0.05; Fig. 5A), whereas no differences were detected in levels of NKB mRNA in the LHA as a function of cycle stage. To test whether E2 might be responsible for the differences in NKB expression we had observed in different cycle stages, we compared the expression of NKB in OVX animals with subcutaneously implanted empty (sham) capsules or E2-filled capsules (OVX vs. OVX + E2) and found that levels of NKB mRNA in the Arc were significantly reduced by E2 treatment (P < 0.05; Fig. 5B). Despite the equivalent levels of plasma E2 in animals in proestrus and OVX + E2, the expression of NKB was significantly reduced only in the OVX + E2 group compared with diestrous and OVX animals, suggesting the presence of additional, steroid-independent factors that stimulate NKB expression.
Fig. 4.
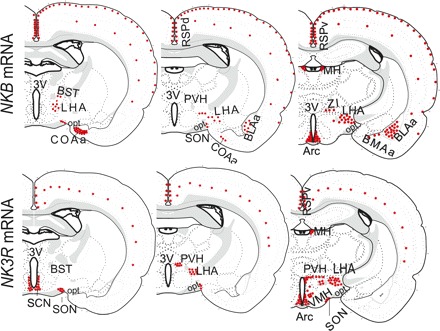
Schematic representation of distribution of neurokinin B (NKB) mRNA (top) and NKB receptor (NK3R) mRNA (bottom) in brain of adult female rat. No differences in general distribution of transcripts were observed between treatments (OVX + sham vs. OVX + E2). Both transcripts were widely distributed throughout the brain but concentrated in discrete areas. BST, bed nucleus of the stria terminalis; COAa, anterocortical amygdaloid nucleus; SON, supraoptic nucleus; BLAa, basolateral nucleus of amygdala; MH, medial habenular nucleus; LHA, lateral hypothalamus; ZI, zona incerta; SCN, suprachiasmatic nucleus; VMH, ventromedial hypothalamic nucleus; PVH, paraventricular hypothalamic nucleus.
Fig. 5.
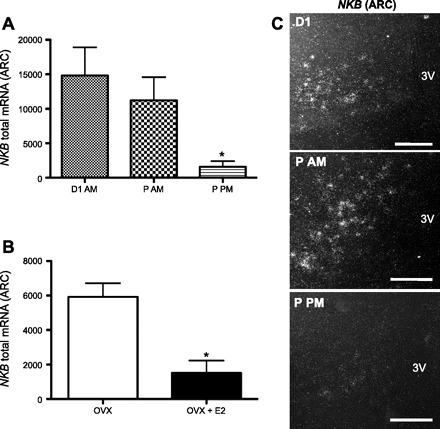
Total NKB mRNA in the Arc of adult female rats (no. of cells × grains per cell). A: total mRNA in intact cycling females in different phases of estrous cycle [morning of diestrus (D1 AM), morning of proestrus (P AM), and afternoon of proestrus (P PM)]. B: total mRNA in OVX + sham or OVX + E2 rats. Values are means ± SE. *P < 0.01 (1-way ANOVA). C: representative photomicrographs of NKB mRNA in situ hybridization in different phases of the estrous cycle. 3V, 3rd ventricle. Scale bars, 50 μm.
Distribution of NK3R mRNA in the Rat Hypothalamus and Its Regulation by E2
Cells expressing NK3R mRNA were observed in discrete regions of the brain, including the cerebral cortex, the suprachiasmatic nucleus, ventromedial hypothalamic nucleus (VMH), paraventricular hypothalamic nucleus, medial habenular nucleus, LHA, SON, and Arc (Fig. 4, bottom). No difference in the general distribution of the transcript was found between the range of E2 treatments in OVX animals (OVX + sham vs. OVX + E2). In intact animals, the expression of NK3R in the Arc was at its maximum on the morning of diestrus 1 (when E2 levels are low), as was the case for NKB. Moreover, levels of NK3R were markedly reduced on proestrus but, in this case, in the morning and afternoon (compared with diestrus 1; Fig. 6). Similarly, the expression of NK3R in the Arc was significantly reduced by E2 (Fig. 6). The expression of NK3R in most other hypothalamic regions (e.g., LHA, SON, and VMH) was unaffected by E2, with one notable exception: the paraventricular hypothalamic nucleus, where there appeared to be a modest induction of NK3R by E2 (P < 0.05; data not shown).
Fig. 6.
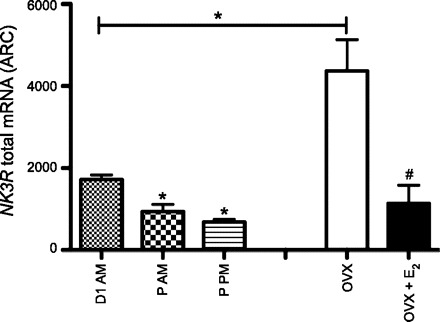
Total NK3R mRNA in the Arc of adult female rats (no. of cells × grains per cell). Left: total mRNA in intact cycling females in different phases of the estrous cycle. Right: total mRNA in OVX + sham or OVX + E2 rats. Values are means ± SE. *P < 0.01 (1-way ANOVA). #P < 0.01 (Student's t-test).
DISCUSSION
Previous studies have demonstrated that NKB agonists inhibit LH secretion in OVX rats but have no effect on LH secretion in OVX mice treated with supraphysiological levels of E2 (24, 30). However, when administered to intact ewes during the follicular phase, NKB stimulates LH secretion (3). Thus, under extremes in circulating levels of E2 (i.e., very high or low), NKB/senktide inhibits LH secretion, at least in rats and mice. Neurotransmitters having the opposite effect on LH secretion in OVX and intact animals (physiological levels of E2) have long been recognized [e.g., neuropeptide Y (37)]. We puzzled whether this paradox might be reconciled by differential effects of E2 on NK3R signaling. We have demonstrated in the rat that, in the presence of physiological levels of E2 (intact or OVX with physiological E2 restoration), the NKB agonist senktide stimulates LH secretion. We have also confirmed that, in the absence of sex steroids in the rat (sham-treated OVX animals), senktide exerts an inhibitory effect on LH release, as previously reported (30).
It would appear that the facilitatory effect of NKB requires a relatively narrow physiological range of circulating E2, which somehow alters the NKB/NK3R signaling pathway. At first glance, the fact that E2 levels in the diestrous females were as low as those in the OVX group seems to argue against a role for E2 in this process. However, LH levels are very low during diestrus, indicating that they are still under the inhibitory influence of higher E2 levels that occurred earlier in the cycle. In fact, LH levels in female rats remain under the inhibitory influence of E2 for ∼1 wk after removal of their ovaries (14); therefore, it is plausible that the same E2-dependent mechanism that restrains GnRH/LH secretion during diestrus also sustains the ability of NKB (senktide) to induce GnRH/LH release.
Within the hypothalamus, E2 has profound effects on the expression of NKB and NK3R. In mice, the expression of NK3R mRNA in the Arc appears to be particularly sensitive to the inhibitory effects of E2, as documented also here in the rat (Fig. 6), which correlates with the relative insensitivity of the LH response to senktide in animals treated with high doses of E2 (24). However, additional regulation at the level of translation or the rate of elimination of the protein cannot be ruled out. This may also account for the lack of LH stimulation by NKB in male rodents (6), in which circulating testosterone may act through the androgen receptor or may be aromatized to E2 in sufficient quantities to inhibit NK3R expression in Kiss1 neurons and, thus, reduce responsiveness to exogenous NKB (24, 32). How could this explanation account for the paradoxical inhibitory effect of NKB on LH release observed in the absence of E2, i.e., OVX animals, which have high levels of NK3R mRNA and should thus be responsive to exogenous NKB? We note that, in the absence of E2 (OVX animals), the expression of NKB mRNA is extraordinarily high (24). We postulate that, under these circumstances, elevated levels of endogenous ligand flooding the receptor may destabilize the Kiss1(NKB/NK3R)-GnRH pulse generator, so that additional exogenous ligand inhibits GnRH/LH secretion; however, this hypothesis remains to be tested.
We and others have argued that Kiss1 neurons in the Arc serve as the nodal point for the negative-feedback regulation of GnRH secretion by sex steroids (25). However, it has been recently recognized that Kiss1 neurons in this region have several cotransmitters. Indeed, NKB is coexpressed with kisspeptin and dynorphin in the Arc in many species, including the rat, sheep, and mouse (4, 15, 24). We have suggested that NKB acts autosynaptically on these kisspeptin/NKB/dynorphin cells to feed back and stimulate kisspeptin release (24, 36), and there is accumulating evidence to support this theory. 1) Kisspeptin/NKB/dynorphin neurons coexpress NK3R (4, 24). 2) There is a dense network of NKB-containing fibers that surround and make direct contact with the NKB-containing cell bodies in the Arc (4). 3) As reported for the first time here, central administration of senktide induces c-fos expression in Kiss1 neurons in the Arc (but not the anteroventral paraventricular nucleus), demonstrating that NKB-induced activation of Kiss1 neurons is restricted to those Kiss1 cells that express NK3R and likely reflects a direct action of NKB on them. These observations provide additional evidence that NKB/NK3R signaling serves an important role in the regulation of GnRH/LH secretion, bolstered by the fact that, in humans, disabling mutation of homologous genes coding for NKB and NK3R (TAC3 and TAC3R) produces hypogonadotropic hypogonadism (34, 38). In view of these new discoveries, we must expand the original model of kisspeptin in the Arc serving as the sole mediator of the sex steroid-dependent negative-feedback control of GnRH/LH secretion to include NKB as an accomplice. In addition, we have shown that, during the estrous cycle, the expression of NKB and NK3R in the Arc is dramatically reduced at the time of the preovulatory LH surge (on the afternoon of proestrus), when circulating levels of E2 reach their zenith, which mirrors the profile of Kiss1 expression in the Arc (23, 33) and, thus, reinforces the concept that kisspeptin and NKB in the Arc mediate the negative-feedback control of GnRH/LH secretion.
In addition to its possible role in the E2-dependent negative-feedback control of gonadotropin secretion, NKB, along with kisspeptin and dynorphin in the Arc, plays a central role in generating the pulsatile secretion of GnRH/LH. 1) Keen and colleagues (18) observed that kisspeptin secretion in the vicinity of the stalk median eminence of the monkey is pulsatile and that kisspeptin release is synchronized with pulsatile GnRH secretion. 2) A kisspeptin antagonist blocks the pulsatile release of GnRH/LH in the sheep and rat (21, 29). 3) Multiunit activity (MUA) in the Arc of the goat (recorded near kisspeptin cell bodies) is tightly synchronized with pulses of LH; moreover, exogenous administration of kisspeptin modifies pulsatile LH secretion but has no effect on MUA volleys, suggesting that the signal driving pulsatile GnRH/LH secretion originates outside the GnRH network itself (26). 4) Wakabayashi et al. (36) demonstrated that NKB initiates MUA volleys in the Arc of the goat. 5) NKB induces c-fos in Kiss1/NKB neurons, which coexpress NK3R and, thus, could plausibly complete an activational feedback loop to trigger kisspeptin and GnRH secretion (24, 36). It is conceivable that GnRH neurons themselves are targets for NKB, since a small fraction of GnRH neurons in the rat seems to express NK3R (20), although apparently not in the goat (1). However, this explanation seems less plausible than a direct action of NKB on kisspeptin neurons; they clearly express NK3R, and their input drives GnRH secretion (22). Thus, compelling evidence suggests that Kiss1/NKB neurons themselves are direct targets for the action of NKB, which could stimulate kisspeptin secretion and, thereby, participate in driving the pulsatile release of GnRH (19).
The patterns of expression of NKB and NK3R we observed in sections through the central forebrain corroborate previous studies, documenting their widespread distribution and notable regional concentrations (10). Although the functional significance of NKB/NK3R signaling in reproductive physiology is beginning to emerge (34, 36, 38), its relevance to other brain functions remains largely a mystery. There is some evidence to suggest that NKB/NK3R circuits in the frontal cortex and amygdala play a role in learning and memory (2), and other studies link NKB-expressing neurons in the striatum with their NK3R-expressing targets in the basal forebrain, which loop back to modulate the motor cortex (13). Thus, NKB/NK3R signaling is likely to serve highly diverse functions in the brain, providing the rationale for the development of new technologies that will permit cell-specific targeting of particular NKB/NK3R circuits.
GRANTS
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Cooperative Agreements U54 HD-12629 and R01 HD-049651, the Marie Curie Outgoing International Fellowship within the 7th Framework Programme of the European Union, Ministerio de Ciencia e Innovación (Spain) Grant BFU 2008-00984, and Junta de Andalucía (Spain) Grant P08-CVI-00603.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We are grateful to David García-Galiano and Miguel Ángel Sánchez-Garrido for assistance with the animal treatments, Dr. Enrique Aguilar for support, and Drs. Michelle Gottsch and Amy Oakley for assistance and comments on the final manuscript.
REFERENCES
- 1. Amstalden M, Coolen LM, Hemmerle AM, Billings HJ, Connors JM, Goodman RL, Lehman MN. Neurokinin 3 receptor immunoreactivity in the septal region, preoptic area and hypothalamus of the female sheep: colocalisation in neurokinin B cells of the arcuate nucleus but not in gonadotrophin-releasing hormone neurones. J Neuroendocrinol 22: 1–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beaujouan JC, Torrens Y, Saffroy M, Kemel ML, Glowinski J. A 25 year adventure in the field of tachykinins. Peptides 25: 339–357, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology 151: 3836–3846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burke MC, Letts PA, Krajewski SJ, Rance NE. Coexpression of dynorphin and neurokinin B immunoreactivity in the rat hypothalamus: morphologic evidence of interrelated function within the arcuate nucleus. J Comp Neurol 498: 712–726, 2006 [DOI] [PubMed] [Google Scholar]
- 5. Chowen JA, Argente J, Vician L, Clifton DK, Steiner RA. Pro-opiomelanocortin messenger RNA in hypothalamic neurons is increased by testosterone through aromatization to estradiol. Neuroendocrinology 52: 581–588, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Corander MP, Challis BG, Thompson EL, Jovanovic Z, Loraine Tung YC, Rimmington D, Huhtaniemi IT, Murphy KG, Kemal Topaloglu A, Yeo GS, O'Rahilly S, Dhillo WS, Semple RK, Coll AP. A study of the effects of neurokinin B (NKB) upon gonadotrophin release in male rodents. J Neuroendocrinol 22: 181–187, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Cunningham MJ, Scarlett JM, Steiner RA. Cloning and distribution of galanin-like peptide mRNA in the hypothalamus and pituitary of the macaque. Endocrinology 143: 755–763, 2002 [DOI] [PubMed] [Google Scholar]
- 8. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA 100: 10972–10976, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dellovade TL, Merchenthaler I. Estrogen regulation of neurokinin B gene expression in the mouse arcuate nucleus is mediated by estrogen receptor-α. Endocrinology 145: 736–742, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Duarte CR, Schutz B, Zimmer A. Incongruent pattern of neurokinin B expression in rat and mouse brains. Cell Tissue Res 323: 43–51, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Dungan Lemko HM, Naderi R, Adjan V, Jennes L, Navarro VM, Clifton D, Steiner RA. Interactions between neurotensin and GnRH neurons in the positive feedback control of GnRH/LH secretion in the mouse. Am J Physiol Endocrinol Metab 298: E80–E88, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fernandez-Fernandez R, Tena-Sempere M, Aguilar E, Pinilla L. Ghrelin effects on gonadotropin secretion in male and female rats. Neurosci Lett 362: 103–107, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Furuta T, Kaneko T. Third pathway in the cortico-basal ganglia loop: neurokinin B-producing striatal neurons modulate cortical activity via striato-innominato-cortical projection. Neurosci Res 54: 1–10, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Gay VL, Midgley ARJ. Response of the adult rat to orchidectomy and ovariectomy as determined by LH radioimmunoassay. Endocrinology 84: 1359–1364, 1969 [DOI] [PubMed] [Google Scholar]
- 15. Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology 148: 5752–5760, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Gottsch ML, Cunningham MJ, Smith JT, Popa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology 145: 4073–4077, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Irwig MS, Fraley GS, Smith JT, Acohido BV, Popa SM, Cunningham MJ, Gottsch ML, Clifton DK, Steiner RA. Kisspeptin activation of gonadotropin releasing hormone neurons and regulation of KiSS-1 mRNA in the male rat. Neuroendocrinology 80: 264–272, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149: 4151–4157, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Khawaja AM, Rogers DF. Tachykinins: receptor to effector. Int J Biochem Cell Biol 28: 721–738, 1996 [DOI] [PubMed] [Google Scholar]
- 20. Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE. Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. J Comp Neurol 489: 372–386, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Li XF, Kinsey-Jones JS, Cheng Y, Knox AM, Lin Y, Petrou NA, Roseweir A, Lightman SL, Milligan SR, Millar RP, O'Byrne KT. Kisspeptin signalling in the hypothalamic arcuate nucleus regulates GnRH pulse generator frequency in the rat. PLoS One 4: e8334, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Acad Sci USA 102: 1761–1766, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology 145: 4565–4574, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci 29: 11859–11866, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Oakley AE, Clifton DK, Steiner RA. Kisspeptin signaling in the brain. Endocr Rev 30: 713–743, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol 21: 813–821, 2009 [DOI] [PubMed] [Google Scholar]
- 26a. Paxinos G, Franklin KBT. The Rat Brain in Stereotaxic Coordinates. New York: Academic, 2003 [Google Scholar]
- 27. Protter MH, Morrey CBJ. College Calculus With Analytical Geometry. 1964, p. 525–526 [Google Scholar]
- 28. Roa J, Vigo E, Castellano JM, Gaytan F, Navarro VM, Aguilar E, Dijcks FA, Ederveen AG, Pinilla L, van Noort PI, Tena-Sempere M. Opposite roles of estrogen receptor (ER)-α and ERβ in the modulation of luteinizing hormone responses to kisspeptin in the female rat: implications for the generation of the preovulatory surge. Endocrinology 149: 1627–1637, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci 29: 3920–3929, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sandoval-Guzman T, Rance NE. Central injection of senktide, an NK3 receptor agonist, or neuropeptide Y inhibits LH secretion and induces different patterns of Fos expression in the rat hypothalamus. Brain Res 1026: 307–312, 2004 [DOI] [PubMed] [Google Scholar]
- 31. Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JSJ, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WFJ, Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med 349: 1614–1627, 2003 [DOI] [PubMed] [Google Scholar]
- 32. Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146: 3686–3692, 2005 [DOI] [PubMed] [Google Scholar]
- 33. Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26: 6687–6694, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Ozbek MN, Imamoglu S, Akalin NS, Yuksel B, O'Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet 41: 354–358, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsukahara S, Tsukamura H, Foster DL, Maeda KI. Effect of corticotropin-releasing hormone antagonist on oestrogen-dependent glucoprivic suppression of luteinizing hormone secretion in female rats. J Neuroendocrinol 11: 101–105, 1999 [DOI] [PubMed] [Google Scholar]
- 36. Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci 30: 3124–3132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wojcik-Gladysz A, Polkowska J. Neuropeptide Y—a neuromodulatory link between nutrition and reproduction at the central nervous system level. Reprod Biol 6 Suppl 2: 21–28, 2006 [PubMed] [Google Scholar]
- 38. Young J, Bouligand J, Francou B, Raffin-Sanson ML, Gaillez S, Jeanpierre M, Grynberg M, Kamenicky P, Chanson P, Brailly-Tabard S, Guiochon-Mantel A. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab 95: 2287–2295, 2010 [DOI] [PubMed] [Google Scholar]


