Abstract
Purpose of review
This review highlights the role of phosphorylation in the trafficking and targeting of aquaporin 2. Current knowledge will be put into the context of modulating the cell surface expression of aquaporin 2 by vasopressin in renal epithelial cells, which is critical for regulation of urinary concentration and control of fluid and electrolyte homeostasis.
Recent findings
In addition to previously identified phosphorylation sites on aquaporin 2, new data have revealed three other serine residues in the C-terminus whose phosphorylation is altered by vasopressin. Several steps in aquaporin 2 recycling, including exocytosis and endocytosis, are coordinated by phosphorylation and dephosphorylation to regulate cell surface accumulation. Aquaporin 2 phosphorylation on serine 256 regulates aquaporin 2 association with proteins that are involved in trafficking, including hsc/hsp70 and myelin and lymphocyte-associated protein.
Summary
Aquaporin 2 trafficking is regulated by phosphorylation of serine 256 and other amino acid residues in its cytoplasmic domain. These events increase or decrease interaction of aquaporin 2 with key regulatory proteins to determine the cellular distribution and fate of aquaporin 2, both after vasopressin addition and under baseline conditions. Better understanding of these mechanisms may provide new therapeutic avenues for patients with X-linked nephrogenic diabetes insipidus, as well as providing basic cell biological information relevant to membrane trafficking processes in general.
Keywords: aquaporin 2 water channel, endocytosis, exocytosis, vasopressin
Introduction
Aquaporin 2 (AQP2) contains many putative kinase recognition sequences, which are presented in Table 1. The presence of these actual or theoretical targets of different kinases including protein kinase A (PKA), protein kinase G (PKG), casein kinase II, mitogen activated protein (MAP) kinases, glycogen synthase kinase 3 (GSK-3) and protein kinase C (PKC) suggests a complex role for reversible phosphorylation in modulating the cell surface accumulation and intracellular distribution of AQP2. Phosphorylation of AQP2 on serine residues, including S256, S264 and S269, is increased by vasopressin stimulation, while phosphorylation of S261 is decreased. Phosphorylation of S256 regulates the interaction of AQP2 with key ‘trafficking’ proteins, resulting in an increased rate of exocytosis and an inhibition of endocytosis as summarized in Fig. 1.
Table 1.
Predicted phosphorylation sites on aquaporin 2
| Putative phosphorylation site | Kinase | Kinase family | Species |
|---|---|---|---|
| N-terminus | |||
| S6 | GSK-3 kinaseb | Acidophilic serine/threonine | h,r,m,sh |
| S10 | Clk2 kinasea | Basophilic serine/threonine | r,m,sh |
| Second intracellular loop | |||
| S148 | Casein kinase 2b, c | Acidophilic serine/threonine | h,r,m,sh |
| T159 | GSK-3 kinasea, b | Acidophilic serine/threonine | h,r,m |
| T159 | Cdk5 kinasea | Proline-dependent serine/threonine | h, |
| T159 | Cdc2 kinasea | Proline-dependent serine/threonine | h, |
| S/T*159 | P38 MAPKa | Proline-dependent serine/threonine | h,r,m |
| S/T*159 | Erk1 kinasea, b | Proline-dependent serine/threonine | h,r,m |
| S163 | GSK-3 kinasea, b | Acidophilic serine/threonine | h,r,m |
| C-terminus | |||
| S216 | PKC-zetaa, h | Basophilic serine/threonine | h,r,m |
| S229 | Casein kinase 2b, c | Acidophilic serine/threonine | h,r,m,sh |
| S231 | PKCc | Basophilic serine/threonine | h,sh |
| T244 | Casein kinase 2a, b, c | Acidophilic serine/threonine | h,r,m,sh |
| S256 | Protein kinase Aa, b, c, d, e | Basophilic serine/threonine | h,r,m,sh |
| S256 | Protein kinase Gg | Basophilic serine/threonine | h,r,m,sh |
| S256 | Calmodulin dependent kinase 2a | Basophilic serine/threonine | h,r,m,sh |
| S256 | Akt kinasea | Basophilic serine/threonine | h,r,m,sh |
| S256 | Clk2 kinasea | Basophilic serine/threonine | h,r,m,sh |
| S256 | Casein kinase 2f | Acidophilic serine/threonine | h,r,m,sh |
| S261 | Cdk5 kinasea | Proline-dependent serine/threonine | h,r,m,sh |
| S261 | Cdc2 kinasea | Proline-dependent serine/threonine | h,r,m,sh |
| S261 | Erk1 kinasea, b | Proline-dependent serine/threonine | h,r,m,sh |
| S261 | p38 kinased | Proline-dependent serine/threonine | h,r,m,sh |
| S264 | Casein kinase 1b | Acidophilic serine/threonine | h,r,m,sh |
| S264 | PKCd | Basophilic serine/threonine | h,r,m,sh |
| S/T*269 | Protein kinase A ??d | Basophilic serine/threonine | h,r,m,sh |
Using Scansite [scansite.mit.edu] algorithms, 10 of 14 cytoplasmic serine/threonine residues of aquaporin 2 (AQP2) were designated as potential phosphorylation sites.
Elm [elm.eu.org] algorithms, 10 of 14 cytoplasmic serine/threonine residues of aquaporin 2 (AQP2) were designated as potential phosphorylation sites.
In addition, Van Balkom et al. [3] proposed and investigated several putative sites by site-directed mutagenesis.
Hoffert et al. [4] identified several AQP2 phosphorylation sites by phosphoproteomic analysis.
Kuwahara et al. showed that the AQP2 C-terminus is a subtrate for cAMP-sensitive phosphorylation kinase.
Using a synthetic peptide, Brunati et al. [2] showed that S256 can be phosphorylated by casein kinase II.
We recently showed that an AQP2 C-terminal peptide can be phosphorylated by PKG. Interestingly, some serine/threonine residues are predicted to be phosphorylated by several kinases. For example, S256 is hypothetically phosphorylated by seven different kinases.
It should be noted that S216, a PKC-zeta phosphorylation motif, may be part of the sixth transmembrane domain and consequently cytoplasmic PKC-zeta may be unable to target this residue.
Serine in mouse and rat while threonine in human and sheep. GSK, glycogen synthase kinase; h, human; m, mouse; MAP, mitogen activated protein; PKA, protein kinase A; PKC, protein kinase C; PKG, protein kinase G; r, rat; sh, sheep.
Figure 1.
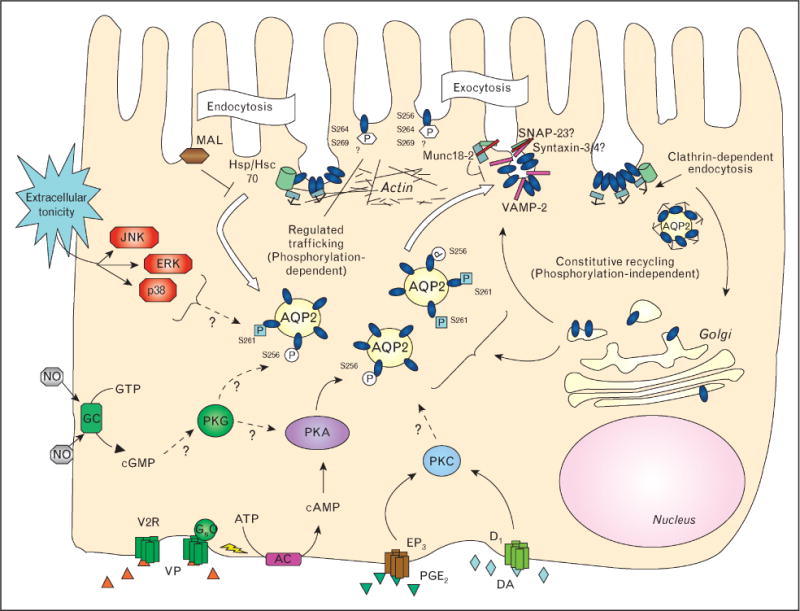
Aquaporin 2 recycling, including endocytosis and both constitutive and regulated exocytosisConstitutive exocytosis occurs independently of aquaporin 2 (AQP2) phosphorylation and involves recycling of AQP2 through a trans-Golgi or recycling endosome compartment. AQP2 membrane accumulation can be increased simply by inhibiting clathrin-mediated endocytosis. The regulated pathway occurs upon vasopressin (VP) interaction with its basolateral receptor (V2R), which increases cAMP formation after Gas stimulation of the adenylyl cyclase (AC). Protein kinase A (PKA) activation results in AQP2 phosphorylation initially on residue S256. At some point, S261 is dephosphorylated and S264 and S269 phosphorylation is increased. AQP2 phosphorylation can also be increased by the cGMP/protein kinase G (PKG) pathways, upon increased activity of the soluble guanylyl cyclase (GC) by, for example, nitric oxide (NO). Extracellular hypertonicity activates the mitogen activated protein (MAP) kinase pathway and JNK, ERK and p38 activities are all required for AQP2 surface accumulation after an acute hypertonic shock. During exocytosis, AQP2 interacts with SNARE proteins and their regulatory proteins such as Munc 18-2, and these interactions may be regulated by phosphorylation. Once at the cell surface, phosphorylated AQP2 resides in endocytosis-resistant domains, and its interaction with hsc70, a protein required for clathrin-mediated endocytosis, is inhibited. AQP2 that is not phosphorylated at S256 interacts strongly with hsc70 and this may be one of several protein–protein interactions that leads to AQP2 accumulation in clathrin-coated pits, followed by endocytosis. The myeloid and lymphocyte-associated protein (MAL) also is involved in AQP2 endocytosis by an as yet unknown mechanism. Endocytosis of AQP2 is also facilitated by protein kinase C (PKC) activation (but possibly not by direct phosphorylation of AQP2), as well as by activation of dopamine (DA, D1) and prostaglandin receptors (EP3, PGE2). Finally, the actin cytoskeleton is centrally involved in AQP2 trafficking: actin depolymerization alone results in cell surface accumulation of AQP2.
Phosphorylation and aquaporin 2 trafficking: general considerations
The function and distribution of a host of cellular proteins, including membrane channels, transporters and receptors, is modified by reversible phosphorylation. As for many of these phospho-proteins, dissecting the functional effects of AQP2 phosphorylation is complicated by the presence of a large number of putative and actual phosphorylation sites on AQP2 (Table 1). These sites could be phosphorylated by a variety of kinases, including PKA, PKG, PKC, casein kinase II and ERK [1–4], and they are dephosphorylated by several phosphatases that are expressed in renal epithelial cells [5–7]. The presence of these actual or theoretical kinase targets suggests a complex role for reversible phosphorylation in modulating the cell surface accumulation and intracellular distribution of AQP2. Understanding the role of AQP2 phosphorylation/dephosphorylation is also rendered more complex by data showing that, as predicted by an early, preaquaporin, modeling paper by Knepper and Nielsen [8], modulation of both the exocytotic and endocytotic pathways involved in AQP2 recycling can affect its cell surface accumulation (Fig. 1). AQP2 recycles constitutively between an intracellular vesicular pool and the cell surface in the absence of vasopressin, and without phosphorylation [9]. Membrane accumulation of AQP2 (even in its nonphosphorylated state) can be induced by inhibiting clathrin-mediated endocytosis [9–11]. Furthermore, actin depolymerization is sufficient to cause membrane accumulation of AQP2 [12], via the involvement of actin-associated proteins including myosin Vb [13•] and ERM proteins [15], although whether exocytosis or endocytosis is modified under these conditions remains unknown and needs to be addressed in future studies. The Sasaki group have also proposed that a multiprotein motor complex regulates AQP2 trafficking [15, 16, 17••]. The involvement of both SPA-1/Rap1 and RhoA/RhoGAP activity must now be considered along with many other potential interactions when modeling the complexities of upstream signaling cascades.
While phosphatases must play an important role in AQP2 trafficking, and specific anchoring proteins are also required to bring some kinases close to their site of action [20–23], we will focus our attention here on kinase activity, protein interactions, and AQP2 membrane accumulation.
Role of S256 phosphorylation in aquaporin 2 trafficking
Vasopressin is the major antidiuretic hormone involved in the regulation of water reabsorption by the mammalian kidney. Vasopressin causes the steady state distribution of the AQP2 water channel to shift from cytoplasmic vesicles to the plasma membrane of collecting duct principal cells [24–26]. This translocation occurs in parallel with the vasopressin-stimulated phosphorylation of S256 on the cytoplasmic C-terminus of AQP2 that occurs upon cAMP elevation and PKA activation. The importance of this event was first shown in vitro[27, 28], and subsequently in vivo[29]. Preventing dephosphorylation of AQP2 with okadeic acid increases cell surface accumulation of AQP2 [5], while a constitutively ‘phosphorylated’ S256D AQP2 mutation accumulates at the plasma membrane even in the absence of vasopressin [3].
S256 lies within a canonical PKA phosphorylation motif (RRxS), but is also predicted to be part of other phosphorylation motifs (see Table 1). In support of this, we were able to show in vitro that S256 is phosphorylated by PKG [1, 30] and Brunati et al. [2] produced similar results for casein kinase II. Therefore, the physiological role of S256 phosphorylation by multiple kinases requires further investigation.
Perhaps surprisingly, S256 phosphorylation is not required for AQP2 membrane insertion, which occurs through a constitutive exocytosis pathway even when the S256 site is mutated to an alanine residue [9]. An S256A point mutation does not prevent the rapid membrane accumulation of AQP2 when endocytosis is inhibited either by expression of dominant negative (K44A) dynamin [10], or by treatment of cells with methyl-β-cyclodextrin (MBCD) [9, 11], which depletes membrane cholesterol and inhibits endocytosis. This indicates that even the nonphosphorylated form of AQP2 is constitutively and rapidly recycling through the plasma membrane, and that blockade of the retrieval pathway is sufficient to cause membrane accumulation, within 10 min in the case of MBCD treatment.
While S256 phosphorylation is required for the vasopressin-induced cell surface accumulation of AQP2, dephosphorylation of AQP2 may not be absolutely necessary for its re-internalization. Prostaglandin E2 and dopamine stimulate removal of AQP2 from the cell surface when added after vasopressin or forskolin treatment, but do not seem to alter the phosphorylated state of AQP2 [31]. These data imply that forskolin or vasopressin-induced phosphorylation of as yet unidentified cellular components is required for the PGE2 and dopamine effects on endocytosis. In support of this, it was shown that PKC-mediated endocytosis of AQP2 can also occur with no apparently detectable change in the S256 phosphorylation state of AQP2 [3]. While the PKC site on AQP2 was proposed to be S231 in the latter study, no direct studies have, to our knowledge, shown directly that this site is indeed phosphorylated by PKC and the involvement of this kinase may, therefore, be indirect or at another site (see below).
While S256 dephosphorylation may not be essential for internalization, however, it may affect the kinetics of AQP2 endocytosis. In addition, consideration must be given to the state of AQP2 within specific membrane microdomains and trafficking vesicles. An earlier study by Deen et al. [32] concluded that S256 phosphorylation of three out of four monomers within the AQP2 tetramer is required for AQP2 steady state membrane localization in Xenopus oocyte plasma membranes. This statistical value may not, however, apply to all tetramers within a given vesicle or membrane microdomain. It is also possible that the exocytotic and endocytotic pathways may have different ‘phosphorylation’ requirements for their modulation. For example, totally unphosphorylated tetramers might be trafficked to the cell surface after vasopressin treatment if other tetramers in the same vesicle have an ‘appropriate’ level of phosphorylation to interact with the trafficking machinery. Once at the cell surface, however, AQP2 tetramers may behave as independent entities with regard to engaging the endocytotic complex.
Phosphorylation modulates aquaporin 2 interaction with ‘endocytotic’ machinery proteins
It is well known that clathrin-mediated endocytosis involves several interacting proteins (including hsc70, clathrin, endophilin, RME1, dynamin, amphiphysin, synaptojanin 1, epsins, AP2), in addition to cytoskeletal proteins [33, 34]. The endocytotic complex can be regulated by reversible phosphorylation of many of the components involved in coated pit formation and fission including amphiphysins, dynamin and synaptojanin [35–38]. One key member of the endocytotic protein complex is heat shock protein 70 (hsp70) or its cognate protein hsc70. This protein is critically involved in the clathrin-mediated internalization pathway [39], which is responsible for AQP2 internalization [10]. Having demonstrated that AQP2 is present in ‘endocytosis-resistant’ membrane domains after vasopressin treatment [40], we went on to show that AQP2 interacts with hsc/hsp70 both in vitro and in vivo, and that this interaction is greatly reduced by phosphorylation of AQP2 at residue S256 [39••]. Functionally, AQP2 membrane accumulation was increased in cells expressing a dominant negative mutation of hsc70, because of a decrease in clathrin-mediated endocytosis. Interestingly, interaction of the GABA receptor with the AP2 adaptor complex is also negatively regulated by tyrosine phosphorylation of GABA to increase cell surface accumulation of the receptor [42].
Other protein interactions are also involved in modification of AQP2 membrane accumulation. The myelin and lymphocyte-associated protein (MAL), is an AQP2 interacting protein that enhances AQP2 cell surface expression by reducing AQP2 internalization [41••]. It remains unclear, however, whether this effect is due to AQP2 phosphorylation at S256 itself, since while MAL was shown to associate less with AQP2 S256A, the association of MAL with both WT-AQP2 and S256D-AQP2 was identical in co-immunoprecipitation experiments.
Modulation of the exocytotic pathway of aquaporin 2
While most in the AQP2 field agree that the literature points to an increase in exocytosis induced by vasopressin as proposed in the original ‘shuttle hypothesis’ of vasopressin action [44], much of the evidence does not clearly distinguish between endocytotic and exocytotic mechanisms of AQP2 membrane accumulation as originally proposed by Knepper and Nielsen [8]. With this in mind, we developed a novel fluorescence-based assay that relies on expression of secreted soluble YFP (ssYFP) that passively labels biosynthetic/post-Golgi vesicles. Our initial data indeed confirm that AQP2 exocytosis is increased upon vasopressin stimulation [45]. In addition, cells treated with the PKA inhibitor H89 and cells expressing the S256A mutation both showed a blunted exocytotic response to vasopressin using this assay, indicating that increased AQP2 exocytosis is at least partly dependent on PKA-dependent phosphorylation of S256.
So how does phosphorylation of AQP2 stimulate the exocytosis of AQP2 beyond the level that already occurs via the constitutive pathway? As suggested initially by Hays and colleagues [46], interaction with various components of the exocytotic machinery is called for. While the presence of SNARE proteins (which are involved in membrane docking and fusion in most if not all exocytotic events) in AQP2-containing principal cells was demonstrated several years ago [47–50], the critical involvement of proteins of the SNARE complex in this process has now been clearly shown [25, 51]. In addition, Munc18, a protein that inhibits SNARE proteinmediated membrane fusion, also inhibits the vasopressin effect on AQP2 trafficking [52] and knocking down Munc 18 levels with siRNA increases constitutive AQP2 membrane accumulation [53]. In inner medullary collecting duct cells, Ross et al. [50] have found, critically, that Munc18-2 complexes with both VAMP2 and AQP2 and that this complex dissociates following stimulation with vasopressin, facilitating SNARE-mediated exocytic insertion of AQP2 into the plasma membrane. Whether this dissociation is a direct result of AQP2 phosphorylation remains to be determined.
Environmental tonicity influences aquaporin 2 cell surface expression
In addition to vasopressin, environmental changes also contribute to AQP2 cell surface expression dynamics. An obvious candidate is environmental tonicity, which is extremely variable in different regions of the kidney. Recent data from the group of Valenti [52] show that exposure of cultured renal CD8 cells to hypotonic medium decreases AQP2 S256 phosphorylation and AQP2 expression at the cell surface. Ongoing work from our laboratory shows that hypertonicity enhances AQP2 accumulation at the cell surface and that while this effect depends on S256 phosphorylation, it occurs independently of a rise of intracellular cAMP [53]. Our data additionally show that hypertonicity increases p38, ERK1/2 and JNK1/2 MAP kinase activity and that pharmacological inhibition of any one of these kinases abolishes the effect of hypertonicity, but not that of vasopressin. Hypertonicity decreased both exocytotic and endocytotic activity, indicating that AQP2 accumulation at the cell surface results from decreased AQP2 internalization. Thus, in addition to cAMP and cGMP pathways, extracellular tonicity plays an important role in regulating AQP2 cell surface expression. Potential MAP kinase phosphorylation sites on AQP2 (Table 1) could be involved in this tonicity-induced cell surface accumulation of AQP2.
Phosphorylation of aquaporin 2 on residues other than S256
In addition to PKA-stimulated phosphorylation of S256 in the AQP2 C-terminus, other kinases are capable of phosphorylating S256, including PKG upon cGMP elevation [1, 28], and casein kinase II [2]. Several other sites, most located in the C-terminus, have now been identified and the effect of vasopressin on phosphorylation status examined. These include S261, S264 and S269. A phosphoproteomics screen of rat kidney medullary collecting ducts initially identified these sites [4, 54•], and their role in AQP2 trafficking is under investigation in several laboratories. S261 phosphorylation is actually decreased by vasopressin treatment, whereas the abundance of S264 phosphorylation is greatly increased [55•]. Interestingly, S264 is suggested to be a casein kinase type 1 phosphorylation site [S(p)XXS] [58]. Using phospho-specific antibodies, the abundance of p-S264 AQP2 increased, and it appeared on the plasma membrane of collecting duct principal cells in response to vasopressin [57•]. A S269D mutant, mimicking phosphorylation at this site, is constitutively located at the cell surface (much like the S256D mutation) [60]. A time course study [60] showed that phosphorylation of S256 is the earliest detectable event, while phosphorylation of S264 and S269 occur later, possibly even after AQP2 is already at the cell surface. Thus, S256 phosphorylation may be required for the subsequent phosphorylation of S264 and S269 [60]. Interestingly, both Scansite and Elm algorithms do not predict S269 as a putative phosphorylation site. On the other hand, Hoffert et al. [4] suggested that S269 is within a putative PKA motif, RxS. The nature of this motif and its cognate kinase are still unclear, therefore. Of note, S269 is located in the middle of the PDZ binding motif GSKA through which AQP2 associates with SPA-1 [17]. It will be interesting to determine whether S269 phosphorylation modulates this interaction.
To begin to dissect the roles of S256 and S261 phosphorylation in AQP2 trafficking, we made a variety of single and double mutations of these residues to mimic constitutive phosphorylation (S256D and S261D) and dephosphorylation (S256A and S261A). The single and double serine AQP2 mutations, in all combinations, were transfected into LLC-PK1 cells and in this way we determined that the phosphorylation state of S256 is always dominant over that of S261 with regard to cell surface expression, both constitutive and vasopressin-stimulated [59•].
The roles of several other putative phosphorylation sites on AQP2 trafficking have been investigated in the past.
Van Balkom et al. [3] mutated several stress-induced kinase and casein kinase (CKII) motifs ([S/T]XX[D/E]): mutation of S148, S229 and T244 had no effect on cAMP-induced AQP2 membrane insertion. CKII, however, was found to phosphorylate the synthetic peptide AQP2(251–269) at position S256 [2]. Interestingly, Procino et al. [62] reported that a transient increase in S256 phosphorylation that was independent of PKA activity occurred during transit through the Golgi. CKII-dependent phosphorylation is also involved in the trafficking of proteins through the trans-Golgi network in other systems [61], and plays a role in the trafficking of AQP4 toward the lysosomal compartment by phosphorylating S276 in this aquaporin [62].
In order to understand the effect of PKC inhibition on AQP2 trafficking, Van Balkom et al. [3] mutated S231, a suspected PKC motif phosphorylation site, to alanine. This mutation did not affect the trafficking of AQP2. Importantly, this serine residue is not conserved in rat and mouse. Hoffert et al. [4] suggested that S261 is a putative PKC phosphorylation site, but, as mentioned above, this has not been confirmed directly. Instead, we found a PKC-zeta phosphorylation site (S216) that is conserved in AQP2 across species (Table 1), but this serine seems to be localized within the last putative transmembrane domain and may not be accessible to cytosolic kinases. A different membrane topology analysis, however, shows a shorter transmembrane domain where S216 may be available for phosphorylation. Finally, putative GSK-3 sites were identified within AQP2 (Table 1) but their role in the trafficking or expression of AQP2 remains unknown.
Conclusion
Recent data have revealed an extensive series of phosphorylation sites for various kinases within the AQP2 sequence. Some of these sites are substrates for more than one kinase. The roles of these multiple sites are under current investigation in several laboratories, and their impact on protein–protein interactions that lead to AQP2 membrane accumulation and removal (Fig. 1) is an important research area. It is likely that different combinations and sequential phosphorylations of these sites occur under different physiological conditions and with different stimuli, and that together with the action of local phosphatases determines the cellular itinerary and location of AQP2. Dissecting the functional protein interactions that govern these processes will provide important insights that are relevant to nephrogenic diabetes insipidus therapy in particular, and to membrane protein trafficking in general.
Acknowledgments
This work was supported by NIH grant DK38452. R. Bouley received a Young Investigator Award from the National Kidney Foundation. U. Hasler is supported by a Swiss FSBMB Fellowship and an ECOR Fellowship from MGH. H. A. J. Lu is supported by an NIH KO8 grant DK075940-01, and a Doctoral Level Postgraduate Scholarship from NSERC supports P. Nunes. The Microscopy Core facility of the MGH Program in Membrane Biology receives additional support from the Boston Area Diabetes and Endocrinology Research Center (DK57521) and the Center for the Study of Inflammatory Bowel Disease (DK43341).
Footnotes
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Additional references related to this topic can also be found in the Current World Literature section in this issue (pp. 549–550).
References and recommended reading
- 1.Bouley R, Breton S, Sun T, et al. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J Clin Invest. 2000;106:1115–1126. doi: 10.1172/JCI9594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brunati AM, Marin O, Bisinella A, et al. Novel consensus sequence for the Golgi apparatus casein kinase, revealed using proline-rich protein-1 (PRP1)-derived peptide substrates. Biochem J. 2000;351(Pt 3):765–768. [PMC free article] [PubMed] [Google Scholar]
- 3.van Balkom BW, Savelkoul PJ, Markovich D, et al. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J Biol Chem. 2002;277:41473–41479. doi: 10.1074/jbc.M207525200. [DOI] [PubMed] [Google Scholar]
- 4.Hoffert JD, Pisitkun T, Wang G, et al. Quantitative phosphoproteomics of vasopressin-sensitive renal cells: Regulation of aquaporin-2 phosphorylation at two sites. Proc Natl Acad Sci USA. 2006;103:7159–7164. doi: 10.1073/pnas.0600895103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valenti G, Procino G, Carmosino M, et al. The phosphatase inhibitor okadaic acid induces AQP2 translocation independently from AQP2 phosphorylation in renal collecting duct cells. J Cell Sci. 2000;113:1985–1992. doi: 10.1242/jcs.113.11.1985. [DOI] [PubMed] [Google Scholar]
- 6.Jo I, Ward DT, Baum MA, et al. AQP2 is a substrate for endogenous PP2B activity within an inner medullary AKAP-signaling complex. Am J Physiol Renal Physiol. 2001;281:F958–F965. doi: 10.1152/ajprenal.2001.281.5.F958. [DOI] [PubMed] [Google Scholar]
- 7.Gooch JL, Guler RL, Barnes JL, Toro JJ. Loss of calcineurin Aalpha results in altered trafficking of AQP2 and in nephrogenic diabetes insipidus. J Cell Sci. 2006;119:2468–2476. doi: 10.1242/jcs.02971. [DOI] [PubMed] [Google Scholar]
- 8.Knepper MA, Nielsen S. Kinetic model of water and urea permeability regulation by vasopressin in collecting duct. Am J Physiol Renal Physiol. 1993;265:F214–F224. doi: 10.1152/ajprenal.1993.265.2.F214. [DOI] [PubMed] [Google Scholar]
- 9.Lu H, Sun TX, Bouley R, et al. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am J Physiol Renal Physiol. 2004;286:F233–F243. doi: 10.1152/ajprenal.00179.2003. [DOI] [PubMed] [Google Scholar]
- 10.Sun TX, Van Hoek A, Huang Y, et al. Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin. Am J Physiol Renal Physiol. 2002;282:F998–F1011. doi: 10.1152/ajprenal.00257.2001. [DOI] [PubMed] [Google Scholar]
- 11.Russo LM, McKee M, Brown D. Methyl-β-cyclodextrin induces vasopressin-independent apical accumulation of aquaporin-2 in the isolated perfused kidney. Am J Physiol Renal Physiol. 2006;291:F246–F253. doi: 10.1152/ajprenal.00437.2005. [DOI] [PubMed] [Google Scholar]
- 12.Klussmann E, Tamma G, Lorenz D, et al. An inhibitory role of Rho in the vasopressin-mediated translocation of aquaporin-2 into cell membranes of renal principal cells. J Biol Chem. 2001;276:20451–20457. doi: 10.1074/jbc.M010270200. [DOI] [PubMed] [Google Scholar]
- 13•.Nedvetsky PI, Stefan E, Frische S, et al. A role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic. 2007;8:110–123. doi: 10.1111/j.1600-0854.2006.00508.x. Cytoskeletal proteins play a critical role on AQP2 trafficking, This study elegantly demonstrates a critical interaction between myosin Vb and its partner rab11 in the recycling process. [DOI] [PubMed] [Google Scholar]
- 14.Tamma G, Klussmann E, Oehlke J, et al. Actin remodeling requires ERM function to facilitate AQP2 apical targeting. J Cell Sci. 2005;118:3623–3630. doi: 10.1242/jcs.02495. [DOI] [PubMed] [Google Scholar]
- 15.Noda Y, Horikawa S, Katayama Y, Sasaki S. Identification of a multiprotein, ‘motor’, complex binding to water channel aquaporin-2. Biochem Biophys Res Commun. 2005;330:1041–1047. doi: 10.1016/j.bbrc.2005.03.079. [DOI] [PubMed] [Google Scholar]
- 16.Noda Y, Sasaki S. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim Biophys Acta. 2006;1758:1117–1125. doi: 10.1016/j.bbamem.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17••.Noda Y, Sasaki S. The role of actin remodeling in the trafficking of intracellular vesicles, transporters, and channels: focusing on aquaporin-2. Pflugers Arch. 2007;456:737–745. doi: 10.1007/s00424-007-0404-2. This is a very nice and timely review that addresses the role of the actin cytoskeleton in various stages of the AQP2 recycling process. [DOI] [PubMed] [Google Scholar]
- 18.Henn V, Edemir B, Stefan E, et al. Identification of a novel A-kinase anchoring protein 18 isoform and evidence for its role in the vasopressin-induced aquaporin-2 shuttle in renal principal cells. J Biol Chem. 2004;279:26654–26665. doi: 10.1074/jbc.M312835200. [DOI] [PubMed] [Google Scholar]
- 19.Henn V, Stefan E, Baillie GS, et al. Compartmentalized cAMP signalling regulates vasopressin-mediated water reabsorption by controlling aquaporin-2. Biochem Soc Trans. 2005;33:1316–1318. doi: 10.1042/BST0331316. [DOI] [PubMed] [Google Scholar]
- 20.Klussmann E, Edemir B, Pepperle B, et al. Ht31: the first protein kinase A anchoring protein to integrate protein kinase A and Rho signaling. FEBS Lett. 2001;507:264–268. doi: 10.1016/s0014-5793(01)02995-7. [DOI] [PubMed] [Google Scholar]
- 21.McSorley T, Stefan E, Henn V, et al. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. Eur J Cell Biol. 2006;85:673–678. doi: 10.1016/j.ejcb.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Brown D. The ins and outs of aquaporin-2 trafficking. Am J Physiol Renal Physiol. 2003;284:F893–F901. doi: 10.1152/ajprenal.00387.2002. [DOI] [PubMed] [Google Scholar]
- 23.Valenti G, Procino G, Tamma G, et al. Minireview: aquaporin 2 trafficking. Endocrinology. 2005;146:5063–5070. doi: 10.1210/en.2005-0868. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki S, Noda Y. Aquaporin-2 protein dynamics within the cell. Curr Opin Nephrol Hypertens. 2007;16:348–352. doi: 10.1097/MNH.0b013e32818b27bf. [DOI] [PubMed] [Google Scholar]
- 25.Katsura T, Gustafson CE, Ausiello DA, Brown D. Protein kinase A phosphorylation is involved in regulated exocytosis of aquaporin-2 in transfected LLC-PK1 cells. Am J Physiol Renal Physiol. 1997;272:F817–F822. [PubMed] [Google Scholar]
- 26.Fushimi K, Sasaki S, Marumo F. Phosphorylation of serine 256 is required for cAMP-dependent regulatory exocytosis of the aquaporin-2 water channel. J Biol Chem. 1997;272:14800–14804. doi: 10.1074/jbc.272.23.14800. [DOI] [PubMed] [Google Scholar]
- 27.Nishimoto G, Zelenina M, Li D, et al. Arginine vasopressin stimulates phosphorylation of aquaporin-2 in rat renal tissue. Am J Physiol Renal Physiol. 1999;276:F254–F259. doi: 10.1152/ajprenal.1999.276.2.F254. [DOI] [PubMed] [Google Scholar]
- 28.Bouley R, Pastor-Soler N, Cohen O, et al. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra) Am J Physiol Renal Physiol. 2005;288:F1103–F1112. doi: 10.1152/ajprenal.00337.2004. [DOI] [PubMed] [Google Scholar]
- 29.Nejsum LN, Zelenina M, Aperia A, et al. Bidirectional regulation of AQP2 trafficking and recycling: involvement of AQP2-S256 phosphorylation. Am J Physiol Renal Physiol. 2005;288:F930–F938. doi: 10.1152/ajprenal.00291.2004. [DOI] [PubMed] [Google Scholar]
- 30.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol. 2000;151:919–930. doi: 10.1083/jcb.151.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodriguez-Boulan E, Kreitzer G, Musch A. Organization of vesicular trafficking in epithelia. Nat Rev Mol Cell Biol. 2005;6:233–247. doi: 10.1038/nrm1593. [DOI] [PubMed] [Google Scholar]
- 32.Ungewickell EJ, Hinrichsen L. Endocytosis: clathrin-mediated membrane budding. Curr Opin Cell Biol. 2007;19:417–425. doi: 10.1016/j.ceb.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 33.Korolchuk V, Banting G. Kinases in clathrin-mediated endocytosis. Biochem Soc Trans. 2003;31:857–860. doi: 10.1042/bst0310857. [DOI] [PubMed] [Google Scholar]
- 34.Gaidarov I, Keen JH. Membrane targeting of endocytic adaptors: cargo and lipid do it together. Dev Cell. 2005;8:801–802. doi: 10.1016/j.devcel.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Lakkaraju A, Rodriguez-Boulan E. Cell biology: caught in the traffic. Nature. 2007;448:266–267. doi: 10.1038/448266a. [DOI] [PubMed] [Google Scholar]
- 36.Mills IG. The interplay between clathrin-coated vesicles and cell signalling. Semin Cell Dev Biol. 2007;18:459–470. doi: 10.1016/j.semcdb.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Newmyer SL, Schmid SL. Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J Cell Biol. 2001;152:607–620. doi: 10.1083/jcb.152.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bouley R, Hawthorn G, Russo LM, et al. Aquaporin 2 (AQP2) and vasopressin type 2 receptor (V2R) endocytosis in kidney epithelial cells: AQP2 is located in ‘endocytosis-resistant’ membrane domains after vasopressin treatment. Biol Cell. 2006;98:215–232. doi: 10.1042/BC20040054. [DOI] [PubMed] [Google Scholar]
- 39••.Lu HA, Sun TX, Matsuzaki T, et al. Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J Biol Chem. 2007;282:28721–28732. doi: 10.1074/jbc.M611101200. The search for proteins whose interaction with AQP2 is regulated by phosphorylation is crucial to our understanding the mechanism by which vasopressin regulates AQP2 cell surface accumulation. This study identified hsc/hsp70 as such a protein and demonstrated a functional role for hsc70 in AQP2 endocytosis. [DOI] [PubMed] [Google Scholar]
- 40.Kittler JT, Chen G, Honing S, et al. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci USA. 2005;102:14871–14876. doi: 10.1073/pnas.0506653102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41••.Kamsteeg EJ, Duffield AS, Konings IB, et al. MAL decreases the internalization of the aquaporin-2 water channel. Proc Natl Acad Sci USA. 2007;104:16696–16701. doi: 10.1073/pnas.0708023104. AQP2 must interact with a number of proteins during its recycling itinerary in renal cells. This study identified one of them as the myelin and lymphocyte-associated protein (MAL), which is involved in the endocytosis of AQP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wade JB, Stetson DL, Lewis SA. ADH action: evidence for a membrane shuttle mechanism. Ann N Y Acad Sci. 1981;372:106–117. doi: 10.1111/j.1749-6632.1981.tb15464.x. [DOI] [PubMed] [Google Scholar]
- 43.Nunes P, Hasler U, McKee M, et al. A novel fluorimetry-based secretion assay to monitor AQP2 exocytosis and trafficking [abstract]; Fifth International Conference of Aquaporin; 13–16 July 7 2007; Nara. Tokyo: Congress Corporation; 2007. Abstract FC46. [Google Scholar]
- 44.Hays RM, Franki N, Simon H, Gao Y. Antidiuretic hormone and exocytosis: lessons from neurosecretion. Am J Physiol Cell Physiol. 1994;267:C1507–C1524. doi: 10.1152/ajpcell.1994.267.6.C1507. [DOI] [PubMed] [Google Scholar]
- 45.Mandon B, Nielsen S, Kishore BK, Knepper MA. Expression of syntaxins in rat kidney. Am J Physiol Renal Physiol. 1997;273:F718–F730. doi: 10.1152/ajprenal.1997.273.5.F718. [DOI] [PubMed] [Google Scholar]
- 46.InoueT, Nielsen S, Mandon B, et al. SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles. Am J Physiol Renal Physiol. 1998;275:F752–F760. doi: 10.1152/ajprenal.1998.275.5.F752. [DOI] [PubMed] [Google Scholar]
- 47.Nielsen S, Marples D, Birn H, et al. Expression of VAMP-2-like protein in kidney collecting duct intracellular vesicles. Colocalization with Aquaporin-2 water channels. J Clin Invest. 1995;96:1834–1844. doi: 10.1172/JCI118229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Breton S, Inoue T, Knepper MA, Brown D. Antigen retrieval reveals widespread basolateral expression of syntaxin 3 in renal epithelia. Am J Physiol Renal Physiol. 2002;282:F523–F529. doi: 10.1152/ajprenal.00128.2001. [DOI] [PubMed] [Google Scholar]
- 49.Gouraud S, Laera A, Calamita G, et al. Functional involvement of VAMP/synaptobrevin-2 in cAMP-stimulated aquaporin 2 translocation in renal collecting duct cells. J Cell Sci. 2002;115:3667–3674. doi: 10.1242/jcs.00053. [DOI] [PubMed] [Google Scholar]
- 50.Ross J, Alexander EA, Schwartz J. A novel role for Munc 18-2 in the regulated aquaporin trafficking cycle. J Am Soc Nephrol. 2004;15:300A. [Google Scholar]
- 51.Procino G, Barbieri C, Tamma G, et al. SNARE proteins and SNARE regulators mediate aquaporin 2 exocytosis in renal cells. Exp Biol. 2008;E138:312. [Google Scholar]
- 52.Tamma G, Procino G, Strafino A, et al. Hypotonicity induces aquaporin-2 internalization and cytosol-to-membrane translocation of ICln in renal cells. Endocrinology. 2007(148):111, 8–1130. doi: 10.1210/en.2006-1277. [DOI] [PubMed] [Google Scholar]
- 53.Hasler U, Nunes P, Bouley R, et al. Acute hypertonicity alters AQP2 trafficking independently of the cAMP/PKA pathway. J Am Soc Nephrol. 2007;18:103A. [Google Scholar]
- 54•.Hoffert JD, Wang G, Pisitkun T, et al. An automated platform for analysis of phosphoproteomic datasets: application to kidney collecting duct phospho-proteins. J Proteome Res. 2007;6:3501–3508. doi: 10.1021/PR0701153. This paper describes a powerful proteomic approach that was used to discover phospho-proteins in the kidney collecting duct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55•.Hoffert JD, Nielsen J, Yu MJ, et al. Dynamics of aquaporin-2 serine-261 phosphorylation in response to short-term vasopressin treatment in collecting duct. Am J Physiol Renal Physiol. 2007;292:F691–F700. doi: 10.1152/ajprenal.00284.2006. This manuscript shows that the newly discovered S261 phosphorylation site is also regulated by VP: in this case phosphorylation is reduced by VP. [DOI] [PubMed] [Google Scholar]
- 56.Watts AD, Hunt NH, Wanigasekara Y, et al. A casein kinase I motif present in the cytoplasmic domain of members of the tumour necrosis factor ligand family is implicated in ‘reverse signalling’. EMBO J. 1999;18:2119–2126. doi: 10.1093/emboj/18.8.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57•.Fenton RA, Moeller HB, Hoffert JD, et al. Acute regulation of aquaporin-2 phosphorylation at Ser-264 by vasopressin. Proc Natl Acad Sci USA. 2008;105:3134–3139. doi: 10.1073/pnas.0712338105. This paper describes the phosphorylation of AQP2 on the newly discovered S264 site, and uses specific antiphospho-serine 264 antibodies to show that VP induced membrane accumulation of S264-phosphorylated AQP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moeller HB, Hoffert JD, Ruetzler M, et al. Phosphorylation of serine-256 is essential for downstream polyphosphorylation of AQP2. Exp Biol. 2008;20:934. 312. [Google Scholar]
- 59•.Lu HA, Matsuzaki T, Bouley R, et al. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol. 2008 Apr 23; doi: 10.1152/ajprenal.00072.2008. [Epub ahead of print] The phosphorylation of S256 and S261 residues is modified by vasopressin. This study showed that the phosphorylation state of S256 is always dominant over that of S261 in controlling the cell surface accumulation of AQP2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Procino G, Carmosino M, Marin O, et al. Ser-256 phosphorylation dynamics of aquaporin 2 during maturation from the ER to the vesicular compartment in renal cells. FASEB J. 2003;17:1886–1888. doi: 10.1096/fj.02-0870fje. [DOI] [PubMed] [Google Scholar]
- 61.Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- 62.Madrid R, Le Maout S, Barrault MB, et al. Polarized trafficking and surface expression of the AQP4 water channel are coordinated by serial and regulated interactions with different clathrin-adaptor complexes. EMBO J. 2001;20:7008–7021. doi: 10.1093/emboj/20.24.7008. [DOI] [PMC free article] [PubMed] [Google Scholar]


