Abstract
One of the most fundamental adaptive physiological events is the response of skeletal muscle to high-intensity resistance exercise, resulting in increased protein synthesis and ultimately larger muscle mass. However, muscle growth in response to contraction is attenuated in older humans. Impaired contractile-induced muscle growth may contribute to sarcopenia: the age-associated loss of muscle mass and function that is manifested by loss of strength, contractile capacity, and endurance. We hypothesized that the storage of ceramide would be increased in older individuals and this would be associated with increases in NFκB signaling and a decreased anabolic response to exercise. To test this hypothesis we measured ceramides at rest and anabolic and NFκB signaling after an acute bout of high-intensity resistance exercise in young and older males. Using lipidomics analysis we show there was a 156% increase in the accumulation of C16:0-ceramide (P < 0.05) and a 30% increase in C20:0-ceramide (P < 0.05) in skeletal muscle with aging, although there was no observable difference in total ceramide. C16:0-ceramide content was negatively correlated (P = 0.008) with lower leg lean mass. Aging was associated with a ∼60% increase in the phosphorylation of the proinflammatory transcription factor NFκB in the total and nuclear cell fractions (P < 0.05). Furthermore, there was an attenuated activation of anabolic signaling molecules such as Akt (P < 0.05), FOXO1 (P < 0.05), and S6K1 (P < 0.05) after an acute bout of high-intensity resistance exercise in older males. We conclude that ceramide may have a significant role in the attenuation of contractile-induced skeletal muscle adaptations and atrophy that is observed with aging.
Keywords: skeletal muscle growth, anabolic signaling, intramyocellular lipids, inflammation, ceramide, resistance exercise, aging
one of the most fundamental adaptive physiological events is the response of skeletal muscle to high-intensity resistance exercise, resulting in increased protein synthesis and ultimately larger muscle mass. However, contraction-induced muscle growth is attenuated in older rodents (6, 13, 16, 67) and humans (26, 32, 33, 70) and may be more manifest in the fasted state (19, 21, 55). Impaired muscle growth in response to contraction may contribute to sarcopenia: the age-associated loss of muscle mass and function that is accompanied by loss of strength, contractile capacity, and endurance (4, 14, 25, 38, 46). Yet the primary molecular defects that lead to impaired skeletal muscle growth with aging are poorly understood. Elucidating the molecular mechanisms underlying the dissimilar hypertrophic responses of skeletal muscle from young and older individuals in response to high-intensity resistance exercise is an important step in determining the cause of and potential treatments for sarcopenia.
Lipid accumulation in non-adipose tissue sites with advancing age has recently been implicated in the impairment of anabolic action either by its direct inhibition (lipotoxicity) of anabolic pathways and/or through the activation of proinflammatory cytokines and pathways such as interleukin 1 (IL1), interleukin 6 (IL6), and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) (36, 40, 65). Markers of inflammation generally increase with age, as indicated by higher concentrations of circulating cytokines and acute-phase proteins (12, 54) and greater basal cytokine production (62). Recent evidence presents a role for lipid accumulation in the promotion of proinflammatory macrophage infiltration in muscle tissue and/or intermuscular adipose tissue (49, 69).
Abundant evidence suggests that with increases in intramyocellular lipids (as found in obesity), there is a concomitant increase in specific “bioactive” lipids such as ceramide, a second messenger in the sphingomyelin signaling pathway (66). Ceramide has emerged as an important mechanistic modulator for the pathogenesis of metabolic diseases associated with lipotoxicity, such as diabetes, cardiomyopathy, and insulin resistance (66). Additionally, studies in insulin responsive cell types suggest that ceramide inhibits anabolic signaling, induces oxidative stress, inhibits muscle growth, and thus may initiate many of the molecular defects that underlie anabolic resistance (23, 39, 50). Of note, we and others have reported increases in intramyocellular lipid occurring with advancing age (15, 30, 60).
For muscle growth to occur, net protein turnover must be positive, which happens when the rate of protein synthesis surpasses the rate of protein breakdown. Protein synthesis is regulated in part by the activity of initiation and elongation factors that control the rate of mRNA translation. The Akt/mammalian target of rapamycin (mTOR) kinases make up a critical signaling pathway controlling protein synthesis during contraction-induced skeletal muscle growth (7, 53, 58). Akt serves as a branch point in the PI3K/mTOR/FOXO signaling pathway that regulates both protein synthesis and degradation. mTOR is downstream of Akt in the insulin signaling pathway, but can also be regulated through a variety of other hormonal, nutritional, and metabolic signals (59). When active, mTOR promotes an increase in the efficiency and capacity for translation of mRNA to protein by increasing the activity of proteins such as 70 kDa ribosomal protein S6 kinase (S6K1) (41, 53). Therefore, understanding the activation of these pathways in exercise-induced muscle hypertrophy is critical for the determination of molecular targets that may be potentially affected by increased intracellular lipid species.
The present study was undertaken to determine the relationship between intramyocellular ceramide content and the activation of anabolic, catabolic, and inflammatory regulators in young and older humans in response to acute resistance exercise. We hypothesized that the storage of ceramide would be increased in older individuals and this would be associated with increases in proinflammatory signaling and decreases in the anabolic signaling response to exercise.
METHODS
Subjects
Nine young (mean ± SD age 22 ± 0.6 yr) and 10 older (mean ± SD age 74 ± 1.5 yr) male subjects were recruited for this study. All subjects were healthy and were not currently participating in any regular endurance or resistance exercise training during the previous 6 mo. Each subject completed a medical history questionnaire and was examined by a physician before entry into the study. Subjects were excluded if they had any acute or chronic illness or injury, had been diagnosed with neuromuscular or cardiovascular disease, had uncontrolled hypertension (>150/90 mmHg), had a body mass index (BMI) higher than 32.5 or below 19 kg/m2, had experienced an upper or lower extremity fracture in the past 6 mo, or had any other unstable medical condition. The Tufts University Health Sciences Campus Institutional Review Board approved the study, and written informed consent was obtained from each participant.
Body Composition Measures
Total body mass and composition were measured at screening using dual energy x-ray absorptiometry (DXA). Whole body DXA scans were performed using a Hologic Discovery A densitometer and were analyzed locally using Hologic QDR software version 12.3 in array mode (Hologic, Bedford, MA). Scan acquisition and analysis were performed according to manufacturer guidelines, with three passes over the subject to acquire the full DXA image (right side, center, and left side). Total body measurements of fat and lean mass were reported.
Study Design
A schematic of the study design is shown in Fig. 1. On day 1 (D1), each subject was tested for bilateral leg muscle strength by measuring their 1 repetition maximum (1RM) on a knee extension and leg press machine (Cybex-VR2, Medway, MA). On day 7 (D7), each subject was admitted to the Metabolic Research Unit the day before the experimental exercise session and a baseline (BL) biopsy was performed after an overnight fast. The subjects were then provided a standard breakfast, lunch, and dinner (60% carbohydrate, 20% fat, and 20% protein) and a snack at 2200. However, caloric intake was not controlled and the subjects had ad libitum access for each meal. The morning of day 8 (D8), an acute bout of resistance exercise was performed that included bilateral leg press and bilateral knee extension consisting of three sets of 10 repetitions done at 80% of the individual subject's 1RM. Muscle biopsies were performed immediately (0H) postexercise and 6 h (6H) postexercise as described below. All subjects were studied during the same time of day, after an overnight fast, and remained fasted until following the final muscle biopsy.
Fig. 1.
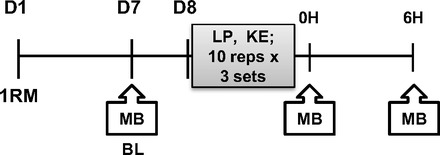
Schematic of the study design. D, day; 1RM, 1 repetition maximum test; MB, muscle biopsy; BL, baseline; LP, leg press; KE, knee extension; OH, immediately postexercise; 6H, 6 h postexercise.
Muscle Biopsies
Baseline (BL), immediately postexercise (0H), and 6 h (6H) postexercise muscle biopsies were obtained from the vastus lateralis at the level of the midthigh under local anesthesia (lidocaine 1%) with a 5-mm Duchenne biopsy needle and suction (3). Incisions were made immediately prior to the three biopsies and the procedure was alternated between each leg using three separate incision sites. After removal, the muscle samples were blotted on gauze dampened with ice-cold (4°C) normal saline to remove any excess blood and immediately placed in liquid N2 and subsequently stored at −195°C for later analysis.
Sphingolipid Analysis
Ceramide concentrations were analyzed using high-performance liquid chromatography/tandem mass spectrometry (LC/MS2) as previously described (5), with modifications. Briefly, 20–30 mg of flash-frozen muscle was homogenized in 1 ml of a Tris/sucrose buffer, then deuterated internal standards (cat. no. 860657, 868516; Avanti Polar Lipids; Alabaster, AL) were added to the homogenate. Four hundred microliters of the homogenate was extracted using two solvent extraction solutions: 1) iPrOH:H2O:EtOAc (30:10:60: vol/vol/vol) and 2) CHCl3:MeOH (2:1; vol/vol). All organic layers were combined, evaporated under N2 gas and then resuspended in 1,000 μl of mobile phase (1 mM NH4HCO2:0.2% HCO2H:MeOH). Separation by HPLC was carried out using a C8 X-Bridge (150 mm) column (Waters; Milford, MA). Deuterated (cat. no. 860657, 868516; Avanti Polar Lipids) and nondeuterated (cat. no. 860490, 860501, 860514, 860516, 860518, 860519, 860520, 860524, 860525, 860527, 860534; Avanti Polar Lipids) standards were analyzed in a separate run to establish retention time of the compounds under the specific assay conditions. Mass spectrometry was carried out in an AB QTRAP 5500 mass spectrometer (Agilent Technologies; Lexington, MA) following the expected fragmentation pattern of each of the lipids under specific conditions.
Serum Cytokine Analysis
Serum concentrations of IL-1β and IL-6 were measured according to the manufacturer's (Human High Sensitivity ELISA, eBiosciences, San Diego, CA) instructions, using solid-phase sandwich enzyme-linked immunosorbent assay kits with sensitivities of 0.16 and 0.08 pg/ml, respectively. Optical density was read on an automated microplate photometer (EL312e, BIO-TEK Instruments, Winooski, VT).
Western Blotting Analysis
The phosphorylation and concentration of signaling proteins were quantified by Western blot analyses as previously described (61). Muscle samples were cut and weighed frozen and homogenized in an ice-cold homogenization buffer (1:10 wt/vol) containing 50 mM Tris-HCl (pH 7.5), 5 mM Na-pyrophosphate, 50 mM NaF, 1 mM EDTA, 1 mM EGTA, 10% glycerol (vol/vol), 1% Triton-X, 1 mM DTT, 1 mM benzamidine, 1 mM PMSF, 10 μg/ml trypsin inhibitor, and 2 μg/ml aprotinin. Following centrifugation (21,000 g, 4°C) for 15 min the supernatant was collected and assayed for protein content. Nuclear fractionations were carried out using NE-PER Nuclear and Cytoplasmic Extraction Kit as per manufacturer's instructions (Thermo Scientific, Rockford, IL). The lysates (30 μg) were solubilized in Laemmli buffer, separated by SDS-PAGE, and transferred to PVDF membranes. The membranes were then blocked (5% NFDM), and incubated overnight at 4°C with primary antibodies specific for either Akt (4685; Cell Signaling Technology, Danvers, MA), phospho-Akt Ser473 (4058; Cell Signaling), mTOR (2983; Cell Signaling), phospho-mTOR Ser2448 (2971; Cell Signaling), S6K1 (2708; Cell Signaling), phospho-S6K1 Thr389 (9234, Cell Signaling), FOXO1 (2757; Cell Signaling), phospho-FOXO1 Thr24 (2599; Cell Signaling), IL6 (ab6672, Abcam, Cambridge, MA), IL1β (2022; Cell Signaling), phospho-NFκB Ser468(3039; Cell Signaling), NFκB p65 (3034; Cell Signaling), phospho-IκBα (9246; Cell Signaling), IκBα (9242; Cell Signaling), PP2A (2259; Cell Signaling), PP2C (3549; Cell Signaling), GAPDH (5174; Cell Signaling), and histone H1 (N-16) (sc-34464; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were probed with α/β-tubulin (2148; Cell Signaling) antibody to monitor protein loading. The immunoreactive proteins were detected with Supersignal Chemiluminescent Substrate (Thermo Scientific), and intensities were quantified by densitometry (Bio-Rad Chemidoc XRS+ system, San Leandro, CA).
Statistical Analysis
Descriptive characteristics.
Differences between groups were identified using a Student's t-test performed with GraphPad Prism version 5.00 for Windows (GraphPad Software, CA, http://www.graphpad.com). Results are expressed as means ± SD, and statistical significance was accepted at P < 0.05.
Ceramide data.
Baseline differences between OLD and YNG were presented as fold change compared with YNG. Differences between groups were identified using a Student's t-test performed with GraphPad Prism version 5.00 for Windows (GraphPad Software). Results are expressed as means ± SD and statistical significance was accepted at P < 0.05.
Associations between variables of interest were performed using Pearson's correlation and normal distribution of the data was determined using the D'Agostino-Pearson normality test with GraphPad Prism version 5.00 for Windows (GraphPad Software). Statistical significance was accepted at P < 0.05.
Signaling data.
All measured proteins were normalized to the loading control, except nuclear proteins, and presented as fold change after exercise. Differences between groups were identified using a two-way analysis of variance (ANOVA) with Bonferroni posttest performed with GraphPad Prism version 5.00 for Windows (GraphPad Software). Results are expressed as means ± SD and statistical significance was accepted at P < 0.05.
RESULTS
Study Participants, Body Composition, and Strength
The study subject's descriptive characteristics appear in Table 1. Older participants had a higher BMI compared with young (P < 0.05). There were no differences in whole body lean mass and appendicular lean skeletal muscle mass (ASM) between age groups. However, the older subjects had significantly lower ASM normalized to height squared (ASM/h2) compared with young (P < 0.05). The older subjects also had 7% higher whole body fat mass (P < 0.05). There was no difference in bilateral leg press strength between OLD and YNG. However, the older subjects tended to have significantly lower bilateral knee extension strength (P < 0.10).
Table 1.
Study subject characteristics
| YNG | OLD | |
|---|---|---|
| Subject number, n | 9 | 10 |
| Age, yr | 22 ± 1 | 74 ± 2* |
| Height, m | 1.75 ± 0.7 | 1.76 ± 0.7 |
| BMI | 22 ± 0.8 | 26 ± 0.7* |
| Lean mass, kg | 61.9 ± 7.1 | 59.1 ± 8.1 |
| ASM, kg | 28.9 ± 2.9 | 26.7 ± 4.0 |
| ASM/h2, kg/m2 | 9.4 ± 0.7 | 8.6 ± 0.7* |
| Fat mass, kg | 11.3 ± 4.8 | 17.0 ± 3.7* |
| Leg press 1RM/kg lean mass | 2.4 | 2.0 |
| Knee extension 1RM/kg lean mass | 1.7 | 1.3* |
Values are means ± SD; n = 9–10/group. BMI, body mass index; 1RM, 1 repetition maximum; ASM, appendicular lean skeletal muscle mass. Significant differences between groups (*P < 0.05 vs. younger (YNG).
Increased Ceramide Content in Aged Skeletal Muscle
To determine the baseline metabolic phenotype of young and aged skeletal muscle we first measured the concentration of long-chain ceramides, including C16-C24, which represent the most abundant ceramide subspecies in skeletal muscle (23). OLD had a 156% higher concentration of C16:0-ceramide compared with YNG at baseline (P < 0.05 vs. YNG; Fig. 2). C20:0-ceramide was increased 30% in OLD (P < 0.05 vs. YNG, Fig. 2). There also tended to be a higher concentration of C18:0-dihydroceramide in OLD compared with YNG (P = 0.07 vs. YNG; Fig. 2). Despite the elevation in these specific ceramide species there were no differences in total or unsaturated ceramide between age groups. However, there tended to be a greater amount of saturated ceramide in OLD (P = 0.09 vs. YNG; Fig. 2).
Fig. 2.
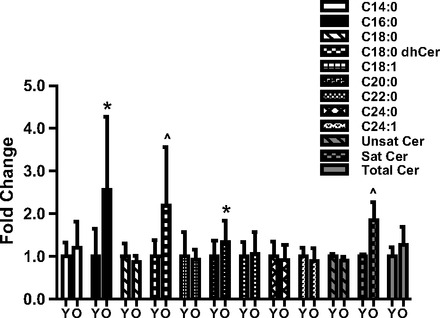
Lipidomic analysis of skeletal muscle from younger (YNG) and older (OLD) males at BL. Intramuscular ceramide content was determined in the vastus lateralis via liquid chromatography/tandem mass spectrometry. dhCer, dihydroceramide; Y, YNG; O, OLD (*P < 0.05 vs. YNG, ∧P < 0.10; n = 9–10/group).
We observed a significant negative association between intramuscular C16:0-ceramide content from the vastus lateralis and lower leg lean mass (P = 0.008, Fig. 3A) in the younger and older subjects combined. However, there was no association between intramuscular C16:0-ceramide concentrations and leg press (Fig. 3B), knee extensor strength (data not shown), or whole body fat mass (Fig. 3C).
Fig. 3.
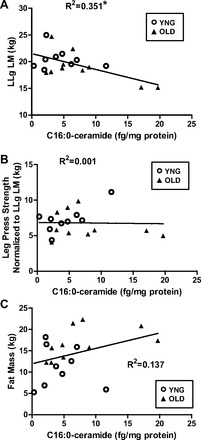
Associations of C16:0-ceramide and lower leg lean mass (LLg LM) (A), leg press strength normalized to LLg LM (B), and whole body fat mass (C) (*P < 0.05 vs. YNG; n = 9–10/group).
No Differences in Circulating Cytokine Levels at BL, 0H, and 6H Time Points
There were no differences between young and older in circulating IL1β and IL6 levels at BL (5.82 ± 0.76 vs. 7.26 ± 1.84 and 9.63 ± 1.04 vs. 10.58 ± 0.75 pg/ml, respectively), 0H (5.48 ± 0.75 vs. 7.30 ± 1.24 and 9.29 ± 1.31 vs. 11.18 ± 0.95 pg/ml, respectively), and 6H (5.18 ± 0.84 vs. 6.74 ± 1.15 and 10.08 ± 1.07 vs. 11.10 ± 1.08 pg/ml, respectively).
Increased NFκB Signaling in Aged Skeletal Muscle
We observed in OLD a significantly higher total protein concentration of the NFκB subunit p65 compared with YNG (P < 0.001 vs. YNG; Fig. 4A). This translated to a significant main effect of higher phosphorylation of NFκB in OLD (P = 0.01 vs. YNG; Fig. 4B). In addition, there was a significant interaction between age and time (P = 0.02; Fig. 4B) with a 30% decrease at 0H and 50% decrease at 6H in YNG and a 60% increase in OLD at 6H. There was a significantly higher total protein concentration (P < 0.02; Fig. 4C) of the NFκB p65 subunit and its phosphorylation at Ser 468 (P = 0.03; Fig. 4D) in the nuclear fraction of OLD at all time points. To confirm proper separation between cytosolic and nuclear fractions we tested and observed a difference in the amount of GAPDH (cytosolic) and histone 1 (nuclear) in each fraction (Fig. 4E). The phosphorylation of IκBα mirrored the NFκB results, where we observed an increase in IκBα phosphorylation with aging (P = 0.01; Fig. 5A) but no change in total protein expression (Fig. 5B). The protein expression of IL6 and IL1β was not different between age groups and after exercise (Fig. 5, C and D).
Fig. 4.
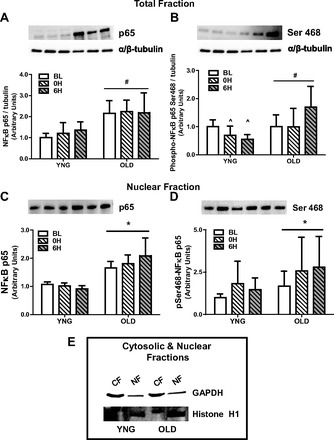
Phosphorylation and total protein content of NFκB in total and nuclear cell fractions and IκBα at BL and after resistance exercise (RE) in YNG and OLD. Relative protein levels of total NFκB p65 (A) and phospho-NFκB p65 (B) in the total fraction and total NFκB p65 (C) and phospho-NFκB p65 (D) in nuclear fraction were quantified using Western blot analysis in skeletal muscle. The cytosolic protein GAPDH and the nuclear protein histone H1 (E) were used to determine proper separation. CF, cytosolic fraction; NF, nuclear fraction (*P < 0.05 vs. BL, #P < 0.05 vs. YNG, ∧P < 0.05 vs. OLD 0H, 6H; n = 6–10/group).
Fig. 5.
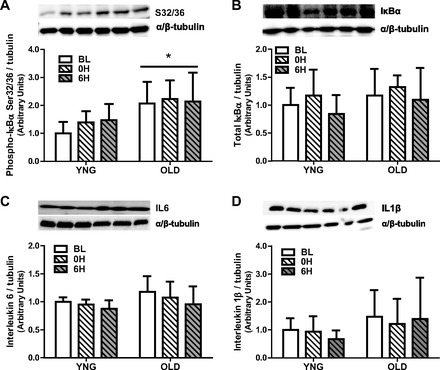
Phosphorylation and total protein content of the inhibitor of NFκB and the total protein content of cytokines at BL and after RE in YNG and OLD in skeletal muscle. Relative protein content of total IκBα (A), phospho-IκBα (B), total IL6 (C), and total IL1β (D) were quantified using Western blot analysis in skeletal muscle. (n = 8–10/group). (*P < 0.05 vs. YNG, n = 8–10/group).
Protein Phosphatase Content is Unaffected by Age or Resistance Exercise
We next measured the concentration of the ceramide-modulated protein phosphatases PP2A and PP2C. There were no differences in the concentration of PP2A or PP2C between age groups or after resistance exercise (Fig. 6, A and B).
Fig. 6.
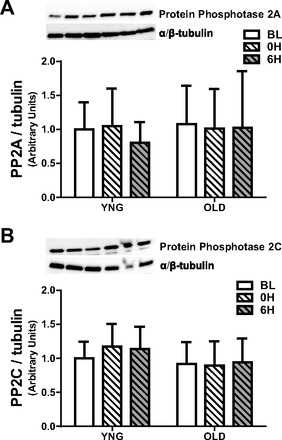
Total protein content of the serine-threonine protein phosphatases PP2A and PP2C at BL and after RE in YNG and OLD. Relative protein levels of PP2A (A) and PP2C (B) were quantified using Western blot analysis in skeletal muscle; n = 8–10/group.
Akt/FOXO/S6K1 Signaling in Young and Aged Skeletal Muscle After Resistance Exercise
As a result of the differences in ceramide content and inflammatory signaling, we next determined the phosphorylation of the activation site, Ser473, and the total protein concentration of the anabolic regulator, Akt. There was a significant age effect of the phosphorylation of Akt on Ser473 (P = 0.02 vs. YNG; Fig. 7A). In addition, there was a significant interaction between age and time (P = 0.01; Fig. 7A) with a 50% increase in YNG and a 20% increase in OLD after resistance exercise. There was no difference in the total protein expression of Akt between YNG and OLD (Fig. 7A).
Fig. 7.
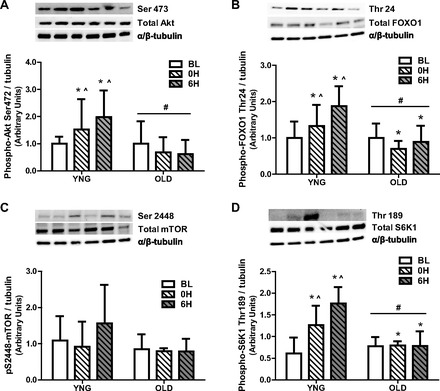
Phosphorylation and total protein content of anabolic and catabolic regulators at BL and after RE in YNG and OLD. Relative protein levels of phospho- and total Akt (A), phospho- and total FOXO1 (B), phospho- and total mammalian target of rapamycin (mTOR) (C), and phospho- and total S6K1 (D) were quantified using Western blot analysis in skeletal muscle. Significant differences between groups (*P < 0.05 vs. BL, #P < 0.05 vs. YNG, ∧P < 0.05 vs. OLD 0H, 6H; n = 8–10/group).
We next determined the total protein content and phosphorylation of the catabolic regulator FOXO1 on Thr24, its Akt regulated inhibitory site. There was a significant age and time effect of the phosphorylation of FOXO1 on Thr24 (P = 0.001 and P = 0.02, respectively vs. YNG; Fig. 7B). In addition there was a significant interaction between age and time (P = 0.001; Fig. 7B) with a 30% and 90% increase in the phosphorylation of FOXO1 on Thr24 immediately after and 6 h after resistance exercise, respectively, in YNG and a 20% and 10% decrease in old at 0H and 6H after resistance exercise in OLD. There were no differences in the total protein content of FOXO1 between YNG and OLD.
Subsequently we measured the total protein content and phosphorylation of the anabolic regulators mTOR and S6K1. There were no changes in mTOR phosphorylation on Ser2448 or total protein concentration between YNG and OLD at BL or after resistance exercise (Fig. 7C). In contrast, there was a significant age and time effect of the phosphorylation of S6K1 on Thr389 (P = 0.045 and P = 0.003, respectively, vs. YNG; Fig. 7D) and a significant interaction between age and time (P = 0.004; Fig. 7D) with a 40% and 90% increase in the phosphorylation of S6K1 on Thr389 immediately after and 6 h after resistance exercise, respectively, in YNG and no change in OLD at 0H and 6H after resistance exercise in OLD. There were no differences in the total protein expression of S6K1 between YNG and OLD (Fig. 7D).
DISCUSSION
In the current study, we first assessed age-related differences in the accumulation of the bioactive lipid metabolite, ceramide, between age groups. Recently, it has been observed that ceramide accumulation is required for proinflammatory cytokines to induce anabolic resistance in myotubes (17, 65). Furthermore, ceramide can induce anabolic resistance through the activation of protein phosphatases such as PP2A and PP2C (11, 52, 56). We now show for the first time there was a significantly higher accumulation of specific ceramide subspecies (Fig. 2) in healthy older males compared with young, although there was no observable difference in total ceramide. In addition, C16:0-ceramide content was negatively correlated with lower leg lean mass in both age groups (Fig. 3A). Higher ceramide content was associated with an increased phosphorylation and concentration of the proinflammatory transcription factor NFκB in both the total and nuclear fraction (Fig. 4, A–D). Furthermore, there was an attenuated anabolic signaling response after an acute bout of high-intensity resistance exercise in older compared with younger males (Fig. 7).
Aging is not only associated with a loss of skeletal muscle mass but also an increase in intramuscular lipid concentrations. More specifically, we and others have previously reported an increase in intramuscular lipid triglyceride concentration with aging (15, 60). We have now, for the first time, determined the concentration of the bioactive lipid species, ceramide, in the skeletal muscle of healthy older compared with healthy younger males. Ceramide, sphingolipids that are made up of a sphingosine backbone and a fatty acid chain, are ubiquitous regulators of cellular stress. Hyde et al. (39) recently observed in cell culture that ceramide reduces protein synthesis by inhibiting Akt/mTOR signaling and amino acid transport (39). Ceramides have fatty acid chain lengths that vary from 2 to 28 carbon atoms, with longer-chain fatty acids being the most abundant and the most prevalent of these being C16:0-ceramide (40–45% of total ceramide) (20, 23, 34). In the present study, we developed the ability to detect nine separate ceramide subspecies, or metabolites with fatty acid residues ranging from C14:0 to C24:1. We now report no observable significant differences in total or unsaturated ceramides between groups, although there tended to be higher saturated ceramides in OLD (Fig. 2). We did observe and now report significant differences in the concentration of specific ceramide carbon chains between age groups with both palmitic (C16:0) and arachidic (C20:0) ceramides being significantly higher and the ceramide metabolite stearic (18:0) dihydroceramide (dhCer) tending to be higher in older compared with younger males (Fig. 2). The result of higher saturated ceramide subspecies in OLD is interesting since there is a greater overall abundance of saturated ceramides within human skeletal muscle (20) and saturated fatty acids are more poorly oxidized than unsaturated, and thus are more likely to accumulate in insulin resistant tissues (28). Furthermore, saturated fatty-acids have been shown to be more associated with metabolic dysfunction than their unsaturated counterparts (22, 63).
The role of ceramide in age-associated skeletal muscle metabolic abnormalities and atrophy is still largely unexplored. Ceramide is shown to be elevated in the muscle of obese, insulin-resistant humans (1, 64) and rodents (44, 45, 68), and its accumulation affects downstream insulin signaling (63, 66). It is also well recognized that ceramide has a significant role in the induction of proinflammatory cytokines and activation of their regulators such as NFκB (27, 43). NFκB directly regulates the transcription of multiple genes, including those encoding cytokines and other mediators of immunity, regulators of redox status, cachexia and disuse atrophy, and sarcopenia (42). We now show significantly higher protein concentrations of the NFκB subunit p65 and its phosphorylation in older skeletal muscle in both total and nuclear fractions (Fig. 4, A–D). This is in agreement with Buford et al. (8) who reported higher activation of NFκB in older, sedentary individuals. However, the higher activation of NFκB in this study did not translate into higher concentrations of IL1β or IL6 (Fig. 5, C and D). These results are not unexpected since it has been previously reported that transgenic mice possessing constitutively active IκB kinase β (MIKK), thus allowing the liberation of NFκB, did not have increased cytokine production (9). This was despite a 15-fold higher NFκB activation and profound sarcopenia exhibited in this model. In agreement with the NFκB results, we also observed a significant increase in IκBα phosphorylation in OLD at all time points (Fig. 5B). We further observed no difference in circulating serum cytokines with age or after resistance. Although we cannot totally discount the role of cytokines in the activation of NFκB signaling, it has been previously reported that ceramides can activate NFκB independently (17). Taken together these data show that the negative association of C16:0-ceramide concentrations with lower leg lean mass may be mediated by the effect of ceramides on NFκB signaling.
Protein phosphatases are signal transduction enzymes that dephosphorylate cellular phosphoproteins (47). Altered expression and activation levels of protein phosphatases have been observed in models of obesity, insulin resistance, diabetes, and increased lipid concentration (e.g., ceramide, free fatty acids, etc.) and are believed to have a role in decreased Akt and S6K1 activation (35, 51, 56). However, we observed no differences in the concentration of the protein phosphatases PP2A (Fig. 6A) or PP2C (Fig. 6B) between groups or after exercise. This is likely a result of the concentration of the phosphatases not being as important as their activation which has been reported previously (11). Further work should be attempted to determine the role in the activation of the protein phosphatases during aging.
In healthy, young humans high-intensity resistance exercise is a robust activator of anabolic pathways such as Akt/mTOR (10, 18). In contrast, previous studies have shown a significant attenuation of anabolic stimulation with aging (21, 26, 31). We now report a significant stepwise increase in the phosphorylation of Akt on Ser473 (Fig. 7A) and S6K1 on Thr189 (Fig. 7D) in young at 0H and 6H after acute exercise; however, this response is attenuated in older males (Fig. 7, A and D). This is in line with previous studies that have observed a blunted effect of anabolic signaling after an acute bout of resistance exercise in elderly humans (26). The Akt/mTOR pathway plays a central role in the regulation of translation initiation and subsequent increase in muscle hypertrophy. In agreement with the present study, there is a known inability to activate this pathway after resistance exercise in aging skeletal muscle. A limitation of this study is that we only had the ability to measure signaling in a limited number of time points and have extrapolated these signaling measures with changes in skeletal muscle protein synthesis. Future studies are needed to determine fractional synthetic rates using isotopic tracers, a more robust measure of protein synthesis, to compare older and younger humans after exercise.
One potential mechanism for the inhibition of anabolic signaling is an increase in ceramide accumulation. Ceramide has been shown to inhibit both Akt and S6K1 activation in vitro (39) and the inhibition of its synthesis in vivo has been revealed to reverse these effects (37). Of interest, Ferreira et al. (23) recently observed that increasing ceramide concentrations with a sphingomyelinase enzyme repressed skeletal muscle contractile force in vitro. We now show that decreases in contraction-induced anabolic signaling in older humans may be associated with increased ceramide content in skeletal muscle.
The Akt target and catabolic regulator FOXO1 has a recognized role in the transcriptional control of ubiquitin ligases (29). We demonstrate for the first time that the phosphorylation and subsequent inhibition of FOXO1 on its Thr24 site is significantly increased after resistance exercise at 0H and 6H in young but remains unchanged in aged males (Fig. 7B). This is important because FOXO1 has an essential function regulating components of the ubiquitin proteasomal pathway such as muscle-RING-finger protein 1 (MuRF1) and Atrogin1/MAFbx (29, 48, 71). These muscle-specific (E3) ubiquitin ligases are responsible for the majority of intracellular protein degradation associated with muscle atrophy (24). Recently it has been shown that elevated gene expression of these two E3 ligases is highly associated with aging and multiple atrophy models, supporting the possibility of a common proteolytic program (2, 24). Raue et al. (57) recently reported a divergence in the regulation of ubiquitin proteasome-related genes (MuRF and MAFbx) at rest and after resistance exercise in younger and older humans. Therefore, the impairment of aging skeletal muscle to inhibit FOXO1 and the E3 ligases after resistance exercise may explain the decreased ability of skeletal muscle to hypertrophy in older humans.
In summary, the present results demonstrate that anabolic signaling after an acute bout of resistance exercise is blunted in aging skeletal muscle. This appears to be associated with an increased concentration of specific ceramide subspecies and the subsequent activation of the NFκB kinase. We were able to quantify nine different ceramide metabolites and observed three saturated fatty acid subspecies higher in older males. We conclude that ceramide may play a significant role in the attenuation of contractile-induced skeletal muscle adaptations and atrophy that is observed with aging.
GRANTS
This material is based upon the work supported by the USDA, under agreement No. 58-1950-0-014. The study was also supported by the Boston Claude D. Pepper Center OAIC (1P30AG031679).
DISCLAIMER
Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the authors and do not necessarily reflect the view of the U.S. Department of Agriculture.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.A.R. and R.A.F. conception and design of research; D.A.R., E.P.M., P.H.H., E.P.P., M.d.S.M., G.G.D., E.M.P., and R.A.F. performed experiments; D.A.R., E.P.M., P.H.H., M.d.S.M., and G.G.D. analyzed data; D.A.R., P.H.H., M.d.S.M., G.G.D., and R.A.F. interpreted results of experiments; D.A.R. and P.H.H. prepared figures; D.A.R. and R.A.F. drafted manuscript; D.A.R., E.P.M., P.H.H., E.P.P., M.d.S.M., G.G.D., E.M.P., and R.A.F. edited and revised manuscript; D.A.R., E.P.M., P.H.H., E.P.P., M.d.S.M., G.G.D., E.M.P., and R.A.F. approved final version of manuscript.
REFERENCES
- 1. Adams JM, 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Altun M, Besche HC, Overkleeft HS, Piccirillo R, Edelmann MJ, Kessler BM, Goldberg AL, Ulfhake B. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J Biol Chem 285: 39597–39608, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest 35: 609–616, 1975 [PubMed] [Google Scholar]
- 4. Betik AC, Thomas MM, Wright KJ, Riel CD, Hepple RT. Exercise training from late middle age until senescence does not attenuate the declines in skeletal muscle aerobic function. Am J Physiol Regul Integr Comp Physiol 297: R744–R755, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39: 82–91, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Blough ER, Linderman JK. Lack of skeletal muscle hypertrophy in very aged male Fischer 344 × Brown Norway rats. J Appl Physiol 88: 1265–1270, 2000 [DOI] [PubMed] [Google Scholar]
- 7. Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol 3: 1014–1019, 2001 [DOI] [PubMed] [Google Scholar]
- 8. Buford TW, Cooke MB, Manini TM, Leeuwenburgh C, Willoughby DS. Effects of age and sedentary lifestyle on skeletal muscle NF-kappaB signaling in men. J Gerontol A Biol Sci Med Sci 65: 532–537, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKbeta/NF-kappaB activation causes severe muscle wasting in mice. Cell 119: 285–298, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc 42: 1843–1852, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Cazzolli R, Carpenter L, Biden TJ, Schmitz-Peiffer C. A role for protein phosphatase 2A-like activity, but not atypical protein kinase Czeta, in the inhibition of protein kinase B/Akt and glycogen synthesis by palmitate. Diabetes 50: 2210–2218, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, Palla SL, Ambrosius WT, Tracy RP, Pahor M. Sarcopenia, obesity, and inflammation—results from the Trial of Angiotensin Converting Enzyme Inhibition and Novel Cardiovascular Risk Factors study. Am J Clin Nutr 82: 428–434, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Chale-Rush A, Morris EP, Kendall TL, Brooks NE, Fielding RA. Effects of chronic overload on muscle hypertrophy and mTOR signaling in young adult and aged rats. J Gerontol A Biol Sci Med Sci 64: 1232–1239, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Clark DJ, Patten C, Reid KF, Carabello RJ, Phillips EM, Fielding RA. Impaired voluntary neuromuscular activation limits muscle power in mobility-limited older adults. J Gerontol A Biol Sci Med Sci 65: 495–502, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cree MG, Newcomer BR, Katsanos CS, Sheffield-Moore M, Chinkes D, Aarsland A, Urban R, Wolfe RR. Intramuscular and liver triglycerides are increased in the elderly. J Clin Endocrinol Metab 89: 3864–3871, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Degens H, Alway SE. Skeletal muscle function and hypertrophy are diminished in old age. Muscle Nerve 27: 339–347, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Demarchi F, Bertoli C, Greer PA, Schneider C. Ceramide triggers an NF-kappaB-dependent survival pathway through calpain. Cell Death Differ 12: 512–522, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Dickinson JM, Fry CS, Drummond MJ, Gundermann DM, Walker DK, Glynn EL, Timmerman KL, Dhanani S, Volpi E, Rasmussen BB. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr 141: 856–862, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dillon EL, Casperson SL, Durham WJ, Randolph KM, Urban RJ, Volpi E, Ahmad M, Kinsky MP, Sheffield-Moore M. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO-induced hyperemia. Am J Physiol Regul Integr Comp Physiol 301: R1408–R1417, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dobrzyn A, Gorski J. Ceramides and sphingomyelins in skeletal muscles of the rat: content and composition. Effect of prolonged exercise. Am J Physiol Endocrinol Metab 282: E277–E285, 2002 [DOI] [PubMed] [Google Scholar]
- 21. Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol 104: 1452–1461, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, Yagi N, Ohto U, Kimoto M, Miyake K, Tobe K, Arai H, Kadowaki T, Nagai R. Saturated fatty acid and TLR signaling link beta cell dysfunction and islet inflammation. Cell Metab 15: 518–533, 2012 [DOI] [PubMed] [Google Scholar]
- 23. Ferreira LF, Moylan JS, Gilliam LA, Smith JD, Nikolova-Karakashian M, Reid MB. Sphingomyelinase stimulates oxidant signaling to weaken skeletal muscle and promote fatigue. Am J Physiol Cell Physiol 299: C552–C560, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foletta VC, White LJ, Larsen AE, Leger B, Russell AP. The role and regulation of MAFbx/atrogin-1 and MuRF1 in skeletal muscle atrophy. Pflügers Arch 461: 325–335, 2011 [DOI] [PubMed] [Google Scholar]
- 25. Frontera WR, Reid KF, Phillips EM, Krivickas LS, Hughes VA, Roubenoff R, Fielding RA. Muscle fiber size and function in elderly humans: a longitudinal study. J Appl Physiol 105: 637–642, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fry CS, Drummond MJ, Glynn EL, Dickinson JM, Gundermann DM, Timmerman KL, Walker DK, Dhanani S, Volpi E, Rasmussen BB. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle 1: 11, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gamard CJ, Dbaibo GS, Liu B, Obeid LM, Hannun YA. Selective involvement of ceramide in cytokine-induced apoptosis. Ceramide inhibits phorbol ester activation of nuclear factor kappaB. J Biol Chem 272: 16474–16481, 1997 [DOI] [PubMed] [Google Scholar]
- 28. Gaster M, Rustan AC, Beck-Nielsen H. Differential utilization of saturated palmitate and unsaturated oleate: evidence from cultured myotubes. Diabetes 54: 648–656, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Glass DJ. Signaling pathways perturbing muscle mass. Curr Opin Clin Nutr Metab Care 13: 225–229, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Goodpaster BH, Park SW, Harris TB, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newman AB. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 61: 1059–1064, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Greig CA, Gray C, Rankin D, Young A, Mann V, Noble B, Atherton PJ. Blunting of adaptive responses to resistance exercise training in women over 75y. Exp Gerontol 46: 884–890, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Hakkinen K, Kraemer WJ, Newton RU, Alen M. Changes in electromyographic activity, muscle fibre and force production characteristics during heavy resistance/power strength training in middle-aged and older men and women. Acta Physiol Scand 171: 51–62, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Hakkinen K, Newton RU, Gordon SE, McCormick M, Volek JS, Nindl BC, Gotshalk LA, Campbell WW, Evans WJ, Hakkinen A, Humphries BJ, Kraemer WJ. Changes in muscle morphology, electromyographic activity, and force production characteristics during progressive strength training in young and older men. J Gerontol A Biol Sci Med Sci 53: B415–B423, 1998 [DOI] [PubMed] [Google Scholar]
- 34. Helge JW, Dobrzyn A, Saltin B, Gorski J. Exercise and training effects on ceramide metabolism in human skeletal muscle. Exp Physiol 89: 119–127, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Hojlund K, Poulsen M, Staehr P, Brusgaard K, Beck-Nielsen H. Effect of insulin on protein phosphatase 2A expression in muscle in type 2 diabetes. Eur J Clin Invest 32: 918–923, 2002 [DOI] [PubMed] [Google Scholar]
- 36. Holland WL, Bikman BT, Wang LP, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest 121: 1858–1870, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab 5: 167–179, 2007 [DOI] [PubMed] [Google Scholar]
- 38. Hughes VA, Frontera WR, Wood M, Evans WJ, Dallal GE, Roubenoff R, Fiatarone Singh MA. Longitudinal muscle strength changes in older adults: influence of muscle mass, physical activity, and health. J Gerontol A Biol Sci Med Sci 56: B209–B217, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Hyde R, Hajduch E, Powell DJ, Taylor PM, Hundal HS. Ceramide down-regulates System A amino acid transport and protein synthesis in rat skeletal muscle cells. FASEB J 19: 461–463, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Jove M, Planavila A, Laguna JC, Vazquez-Carrera M. Palmitate-induced interleukin 6 production is mediated by protein kinase C and nuclear-factor kappaB activation and leads to glucose transporter 4 down-regulation in skeletal muscle cells. Endocrinology 146: 3087–3095, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Kimball SR, Horetsky RL, Jefferson LS. Signal transduction pathways involved in the regulation of protein synthesis by insulin in L6 myoblasts. Am J Physiol Cell Physiol 274: C221–C228, 1998 [DOI] [PubMed] [Google Scholar]
- 42. Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol 103: 388–395, 2007 [DOI] [PubMed] [Google Scholar]
- 43. Lam YY, Hatzinikolas G, Weir JM, Janovska A, McAinch AJ, Game P, Meikle PJ, Wittert GA. Insulin-stimulated glucose uptake and pathways regulating energy metabolism in skeletal muscle cells: the effects of subcutaneous and visceral fat, and long-chain saturated, n-3 and n-6 polyunsaturated fatty acids. Biochim Biophys Acta 1811: 468–475, 2011 [DOI] [PubMed] [Google Scholar]
- 44. Lessard SJ, Lo Giudice SL, Lau W, Reid JJ, Turner N, Febbraio MA, Hawley JA, Watt MJ. Rosiglitazone enhances glucose tolerance by mechanisms other than reduction of fatty acid accumulation within skeletal muscle. Endocrinology 145: 5665–5670, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB, 3rd, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes 56: 1856–1864, 2007 [DOI] [PubMed] [Google Scholar]
- 46. Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci 84: 275–294, 1988 [DOI] [PubMed] [Google Scholar]
- 47. Liu L, Chen L, Luo Y, Chen W, Zhou H, Xu B, Han X, Shen T, Huang S. Rapamycin inhibits IGF-1 stimulated cell motility through PP2A pathway. PLoS One 5: e10578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100: 11285–11290, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Maury E, Brichard SM. Adipokine dysregulation, adipose tissue inflammation and metabolic syndrome. Mol Cell Endocrinol 314: 1–16, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Meadows KA, Holly JM, Stewart CE. Tumor necrosis factor-alpha-induced apoptosis is associated with suppression of insulin-like growth factor binding protein-5 secretion in differentiating murine skeletal myoblasts. J Cell Physiol 183: 330–337, 2000 [DOI] [PubMed] [Google Scholar]
- 51. Mott DM, Stone K, Gessel MC, Bunt JC, Bogardus C. Palmitate action to inhibit glycogen synthase and stimulate protein phosphatase 2A increases with risk factors for type 2 diabetes. Am J Physiol Endocrinol Metab 294: E444–E450, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mukhopadhyay A, Saddoughi SA, Song P, Sultan I, Ponnusamy S, Senkal CE, Snook CF, Arnold HK, Sears RC, Hannun YA, Ogretmen B. Direct interaction between the inhibitor 2 and ceramide via sphingolipid-protein binding is involved in the regulation of protein phosphatase 2A activity and signaling. FASEB J 23: 751–763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Nader GA, Esser KA. Intracellular signaling specificity in skeletal muscle in response to different modes of exercise. J Appl Physiol 90: 1936–1942, 2001 [DOI] [PubMed] [Google Scholar]
- 54. Paolisso G, Rizzo MR, Mazziotti G, Tagliamonte MR, Gambardella A, Rotondi M, Carella C, Giugliano D, Varricchio M, D'Onofrio F. Advancing age and insulin resistance: role of plasma tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 275: E294–E299, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Pennings B, Koopman R, Beelen M, Senden JM, Saris WH, van Loon LJ. Exercising before protein intake allows for greater use of dietary protein-derived amino acids for de novo muscle protein synthesis in both young and elderly men. Am J Clin Nutr 93: 322–331, 2011 [DOI] [PubMed] [Google Scholar]
- 56. Peterson RT, Desai BN, Hardwick JS, Schreiber SL. Protein phosphatase 2A interacts with the 70-kDa S6 kinase and is activated by inhibition of FKBP12-rapamycin associated protein. Proc Natl Acad Sci USA 96: 4438–4442, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raue U, Slivka D, Jemiolo B, Hollon C, Trappe S. Proteolytic gene expression differs at rest and after resistance exercise between young and old women. J Gerontol A Biol Sci Med Sci 62: 1407–1412, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Reynolds THt, Bodine SC, Lawrence JC., Jr Control of Ser2448 phosphorylation in the mammalian target of rapamycin by insulin and skeletal muscle load. J Biol Chem 277: 17657–17662, 2002 [DOI] [PubMed] [Google Scholar]
- 59. Rivas DA, Lessard SJ, Coffey VG. mTOR function in skeletal muscle: a focal point for overnutrition and exercise. Appl Physiol Nutr Metab 34: 807–816, 2009 [DOI] [PubMed] [Google Scholar]
- 60. Rivas DA, Morris EP, Fielding RA. Lipogenic regulators are elevated with age and chronic overload in rat skeletal muscle. Acta Physiol (Oxf) 202: 691–701, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rivas DA, Yaspelkis BB, 3rd, Hawley JA, Lessard SJ. Lipid-induced mTOR activation in rat skeletal muscle reversed by exercise and 5′-aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside. J Endocrinol 202: 441–451, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roubenoff R, Harris TB, Abad LW, Wilson PW, Dallal GE, Dinarello CA. Monocyte cytokine production in an elderly population: effect of age and inflammation. J Gerontol A Biol Sci Med Sci 53: M20–M26, 1998 [DOI] [PubMed] [Google Scholar]
- 63. Schmitz-Peiffer C, Craig DL, Biden TJ. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J Biol Chem 274: 24202–24210, 1999 [DOI] [PubMed] [Google Scholar]
- 64. Straczkowski M, Kowalska I, Nikolajuk A, Dzienis-Straczkowska S, Kinalska I, Baranowski M, Zendzian-Piotrowska M, Brzezinska Z, Gorski J. Relationship between insulin sensitivity and sphingomyelin signaling pathway in human skeletal muscle. Diabetes 53: 1215–1221, 2004 [DOI] [PubMed] [Google Scholar]
- 65. Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145: 4592–4602, 2004 [DOI] [PubMed] [Google Scholar]
- 66. Summers SA, Nelson DH. A role for sphingolipids in producing the common features of type 2 diabetes, metabolic syndrome X, and Cushing's syndrome. Diabetes 54: 591–602, 2005 [DOI] [PubMed] [Google Scholar]
- 67. Thomson DM, Gordon SE. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J Physiol 574: 291–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Turinsky J, O'Sullivan DM, Bayly BP. 1,2-Diacylglycerol and ceramide levels in insulin-resistant tissues of the rat in vivo. J Biol Chem 265: 16880–16885, 1990 [PubMed] [Google Scholar]
- 69. Varma V, Yao-Borengasser A, Rasouli N, Nolen GT, Phanavanh B, Starks T, Gurley C, Simpson P, McGehee RE, Jr, Kern PA, Peterson CA. Muscle inflammatory response and insulin resistance: synergistic interaction between macrophages and fatty acids leads to impaired insulin action. Am J Physiol Endocrinol Metab 296: E1300–E1310, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Welle S, Thornton C, Statt M, McHenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab 81: 3239–3243, 1996 [DOI] [PubMed] [Google Scholar]
- 71. Zhao J, Brault JJ, Schild A, Cao P, Sandri M, Schiaffino S, Lecker SH, Goldberg AL. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab 6: 472–483, 2007 [DOI] [PubMed] [Google Scholar]


