Abstract
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a lethal ventricular arrhythmia evoked by physical or emotional stress. Recessively inherited CPVT is caused by either missense or null-allele mutations in the cardiac calsequestrin (CASQ2) gene. It was suggested that defects in CASQ2 cause protein deficiency and impair Ca2+ uptake to the sarcoplasmic reticulum and Ca2+-dependent inhibition of ryanodine channels, leading to diastolic Ca2+ leak, after-depolarizations, and arrhythmia. To examine the effect of exercise training on left ventricular remodeling and arrhythmia, CASQ2 knockout (KO) mice and wild-type controls underwent echocardiography and heart rhythm telemetry before and after 6 wk of training by treadmill exercise. qRT-PCR and Western blotting were used to measure gene and protein expression. Left ventricular fractional shortening was impaired in KO (33 ± 5 vs. 51 ± 7% in controls, P < 0.05) and improved after training (43 ± 12 and 51 ± 9% in KO and control mice, respectively, P = nonsignificant). The exercise tolerance was low in KO mice (16 ± 1 vs. 29 ± 2 min in controls, P < 0.01), but improved in trained animals (26 ± 2 vs. 30 ± 3 min, P = nonsignificant). The hearts of KO mice had a higher basal expression of the brain natriuretic peptide gene. After training, the expression of natriuretic peptide genes markedly decreased, with no difference between KO and controls. Exercise training was not associated with a change in ventricular tachycardia prevalence, but appeared to reduce arrhythmia load, as manifested by a decrease in ventricular beats during stress. We conclude that, in KO mice, which recapitulate the phenotype of human CPVT2, exercise training is well tolerated and could offer a strategy for heart conditioning against stress-induced arrhythmia.
Keywords: arrhythmia, cardiomyopathy, sympathetic, calcium, calsequestrin
catecholaminergic polymorphic ventricular tachycardia (CPVT) is a malignant ventricular arrhythmia, evoked by emotional or physical stress, which can lead to syncope, convulsions, and sudden death. The arrhythmia appears to be mediated by abnormal calcium release from the sarcoplasmic reticulum (SR), evoking delayed after-depolarizations, and commencing in bidirectional or polymorphic ventricular tachycardia (VT) (8). Heterozygous mutations in the cardiac ryanodine receptor 2 and recessively inherited mutations in the calsequestrin gene (CASQ2) constitute the molecular cause in the majority of patients with CPVT and in their affected family members (14, 19, 24). The first-line therapies are β-adrenergic blockers, but they fail to prevent arrhythmia in a substantial minority of patients. These individuals require additional therapies, such as flecainide, calcium channel blockers, implantable defibrillator, and eventually sympathetic denervation (36, 46).
Exercise is discouraged for patients who suffer from CPVT or carry one of the causative gene mutations. Abstaining from physical activity in young individuals may lead to considerable functional, social, and medical disadvantages. Exercise rehabilitation is currently recommended for patients postmyocardial infarction and those suffering from congestive heart failure (9, 23). It was shown to improve the functional state and the general well-being and possibly has a positive effect on cardiac function and longevity (11, 23, 30). Yet the effect of long-term exercise training in primary arrhythmic disorders, in particular those manifested as exercise-induced arrhythmia, is unknown.
CPVT is associated with high risk of VT and ventricular fibrillation secondary to increased sympathetic drive and catecholamine release. One of the methods to antagonize these is to reduce sympathetic reflexes while increasing the vagal tone. Therefore, exercise could benefit CPVT patients through increased vagal tone, decreasing the sympathetic drive and/or the increased expression of calcium-handling proteins, as shown by Billman and colleagues (1, 2) and other investigators (4, 25). On the contrary, recurrent exposure to exercise-induced arrhythmia could lead to the development of tachycardia-induced cardiomyopathy and/or exacerbate the disease phenotype.
Our laboratory has previously described mice homozygous for CASQ2 null-allele that suffer from typical arrhythmia and develop mild cardiomyopathy attributed to abnormal calcium handling (40). In the present study, we evaluate the effect of exercise training on heart rate, arrhythmia severity, exercise capacity, and heart function in young animals with CPVT2.
MATERIALS AND METHODS
Experimental Protocol
Animal studies.
The animal experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no. 85-23, revised 1996) and was approved by the institutional animal care and use committee of Tel Aviv University (M-10-004). The murine model for the recessively inherited CPVT was previously described (40). Mutant and wild-type (WT) SvEv mice were maintained and bred in a pathogen-free facility on regular rodent chow, with free access to water and 12:12-h light and dark cycles.
Male WT and CASQ2Δ/Δ [knockout (KO)] mice were randomly assigned at 8 wk of age to four groups: WT sedentary, WT exercise training, KO sedentary, and KO training.
Mice from exercise groups underwent training on a motorized rodent treadmill (Exer-6M; Columbus Instruments, OH) with an adjustable speed and a slope of 5°. Our protocol included 2 wk of gradual habituation, followed by 4 wk of intense training. During the first week, the mice exercised 3 days/wk, 30 min/day, at a speed of 8, 10, and 12 m/min, respectively. During the second week, the mice exercised 3 days/wk, 60 min/day, at a speed of 10, 12, and 15 m/min, respectively. Thereafter, mice were forced to run for 60 min/day at 15 m/min, 5 days/week, for 4 wk (5, 38).
Following completion of the training protocol, all mice underwent echocardiography, assessment of maximal exercise capacity, and implantation of a telemetry device. After 24 h to recover from surgery, mice were tested for susceptibility to catecholamine-induced arrhythmia.
Procedures
Murine telemetry transducer.
Murine telemetry transducer (DSI, St. Paul, MN, device weight 3.8 g) was surgically implanted under the skin on the back of the animal, as previously described (36). Mice were anesthetized with ketamin 75–90 mg/kg and xylazine 5–8 mg/kg ip (Kepro).
Two-dimensional (2D) and M-mode echocardiography was performed using an echocardiogram (Visual, Sonix) equipped with a 30-MHz linear transducer. Animals were lightly anesthetized with inhalation of isoflurane (Terrell, TX). 2D parasternal short-axis imaging of the heart was performed. An M-mode cursor was positioned perpendicular to the interventricular septum and posterior wall of the left ventricle (LV) at the level of the papillary muscles. An M-mode image was obtained at a sweep speed of 100 mm/s. Diastolic and systolic LV wall thickness, LV end-diastolic dimensions, and LV end-systolic chamber dimensions were measured. Left ventricular end-diastolic and end-systolic area were obtained from the 2D parasternal short-axis view. The LV fractional shortening and LV fractional area change were calculated.
To determine the maximal exercise capacity, mice were placed on the treadmill at the speed of 7.5 m/min with a 5° slope. The speed was increased by 2.5 m/min every 3 min up to 25 m/min, followed by increasing the slope to 15°. The mouse was then left to run until the limit of its capacity to follow the track. The total exercise duration was measured.
Provocation testing for CPVT susceptibility.
Mice were subjected to exercise test and then to intraperitoneal injection of epinephrine, as previously described (20, 40). In brief, baseline telemetry was recorded at rest in caged sedentary animals with no stressful stimuli. Then mice were then moved to a rodent treadmill. The treadmill speed was gradually increased over 2 min from 5 to 8 m/min at a 5° slope. Heart rhythm recording was obtained during the last 30 s of this stage, at a speed of 8 min/s. The animal was then forced to run at 15 m/min. The ECG was continuously recorded during the last 10 s of this “sprint” and the subsequent 60 s of recovery. After 5 min of rest, mice were injected with epinephrine (0.5 mg/kg ip), followed by 5 min of continuous telemetric monitoring.
Quantitative real-time PCR.
Total RNA was purified from hearts using TRIzol method (Ambion, Austin, TX), according to the manufacturer's instructions. The quantity of total RNA was determined by OD260 measurements. cDNA was synthesized from total RNA using the TaqMan High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA), according to the manufacturer's protocol. Quantitative real-time PCR analysis for mouse atrial natriuretic peptide (ANP) [natriuretic peptide precursor A (NPPA)], brain natriuretic peptide (BNP) [natriuretic peptide precursor B (NPPB)], actin-assembly inducing protein (ACTA), and connexin 43 (CX43) was performed using the ABI 7000 Sequence Detection system (Applied Biosystems). The primers and TaqMan FAM probes were ordered from Applied Biosystems. A total of 2 μl of cDNA was amplified with 10 μl TaqMan Universal PCR MasterMIX, 1 μl TaqMan SNP genotyping assays, and 7 μl diethyl pyrocarbonate. PCR amplification was preformed in triplicate. The average cycle threshold was normalized to endogenous control gene, mouse TATA-box. Stability of TATA expression across the experimental groups was verified by assessing its own expression relative to another constitutively expressed gene, β-actin (ACTB).
Western blot analysis.
Whole ventricles were homogenized in RIPA buffer. The total homogenate was centrifuged at 8,000 RPM for 10 min at 4°C. The supernatant was collected, and the total protein levels were quantified by the Bradford reagent (Sigma, St. Louis, MO), with bovine serum albumin as a standard.
Protein (30 μg/lane) was separated on 10% SDS-polyacrylamide gel under denaturing conditions and was transferred to a nitrocellulose membrane. The membrane was blocked by incubation for 2 h in 5% nonfat milk in Tris buffer containing 0.05% Tween 20 and then was immunoblotted overnight at 4°C with rabbit polyclonal antibodies against β-adrenergic receptor (β-AR) (Santa Cruz Biotechnology), sodium calcium exchanger (NCX) (R&D Systems, Minneapolis, MN), L-type calcium channel (LTCC), Na-K-ATPase, CX43 (Santa Cruz Biotechnology), and CASQ2 (Affinity Bioreagents, Golden, CO). Proteins were detected using a horseradish peroxidase-conjugated secondary antibody with ECL detection kit (Santa Cruz Biotechnology) and quantitated by densitometry.
Statistical Analysis
Results are expressed as means ± SD for continuous variables and categorically for arrhythmia prevalence. Heart rate and ventricular arrhythmia were analyzed manually from computer electrogram recordings and defined as follows: nonsustained VT, four or more consequent ventricular complexes; sustained VT, a VT lasting ≥15 s; monomorphic VT, a continuous ventricular rhythm with one predominant morphology; bidirectional VT, a ventricular rhythm with two predominant alternating morphologies; and polymorphic VT, a ventricular rhythm having at least three alternating morphologies. For purposes of statistical comparison, an animal having nonsustained VT or sustained VT under any condition was accepted as positive for VT. All other ventricular arrhythmias were defined as premature beats [premature ventricular complexes (PVCs)], ventricular bigeminy, and complex PVCs (salvos, couplets, and triplets).
The results of real-time PCR were expressed per each sample as relative quantification calculated as 2−ΔCT. Protein levels were normalized for GAPDH or ACTB expression and expressed as a ratio of density values. A statistical difference between the groups was assessed using Student's t-test and the ANOVA using the multiple-comparison option of Duncan and χ2/Fisher exact test, as appropriate. Two-tailed P < 0.05 was accepted as statistically significant.
RESULTS
Comparison of Sedentary KO and WT Mice
Echocardiography of 15-wk-old animals showed a decrease in systolic function in KO mice and an increase in the LV size, as manifested by measurement of end-systolic and end-diastolic cross-sectional area in the short-axis view (Table 1). There were no significant differences in the LV wall thickness, but the heart-to-body weight ratio was higher in the KO mice (Fig. 1, P < 0.05).
Table 1.
Echocardiographic studies before and after 6-wk exercise training of WT and KO mice
| n | HR, beats/min | IVS, mm | PW, mm | LVESD, mm | LVEDD, mm | FS, % | 2D ESA, mm2 | 2D EDA, mm2 | LVFAC, % | |
|---|---|---|---|---|---|---|---|---|---|---|
| WT sedentary | 5 | 428 ± 36 | 1.1 ± 0.1 | 0.9 ± 0.1 | 1.6 ± 0.3 | 3.2 ± 0.3 | 50.9 ± 7.3 | 2.4 ± 1.3 | 7.9 ± 1.6 | 69.8 ± 12.0 |
| WT trained | 9 | 422 ± 36 | 1.0 ± 0.1 | 0.9 ± 0.1 | 1.6 ± 0.4 | 3.2 ± 0.4 | 50.7 ± 8.7 | 2.4 ± 1.2 | 8.4 ± 2.1 | 71.7 ± 8.8 |
| KO sedentary | 5 | 470 ± 84 | 1.0 ± 0.1 | 0.9 ± 0.1 | 2.5 ± 0.2 | 3.7 ± 0.3 | 33.1 ± 4.6*# | 4.6 ± 1.0*# | 10.9 ± 2.0* | 57.4 ± 8.9 |
| KO trained | 7 | 439 ± 59 | 1.0 ± 0.1 | 0.8 ± 0.1 | 1.8 ± 0.5 | 3.2 ± 0.5 | 43.2 ± 12.3 | 2.9 ± 1.4 | 7.4 ± 2.2 | 61.1 ± 13.1 |
Values are means ± SD; n, no. of mice. WT, wild type; KO, knockout; HR, heart rate during light anesthesia; IVS, interventricular septum; PW, posterior wall; LVESD, left ventricular end-systolic dimension; LVEDS, left ventricular end-diastolic dimension; FS, fractional shortening; 2D ESA, end-systolic area on two-dimensional echo; 2D EDA, end-diastolic area on two-dimensional echo; LVFAC, left ventricular fractional area change.
P < 0.05 vs. KO trained.
P < 0.01 vs. WT sedentary.
Fig. 1.
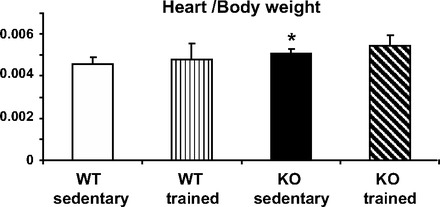
Heart-to-body weight ratio in wild-type (WT) and calsequestrin (CASQ2) knockout (KO) mice. There was a significant difference between the sedentary WT vs. KO animals, *P < 0.05. Physical training was associated with an increase in heart/body weight in both groups, making the difference between WT and KO nonsignificant. Values are means ± SD; WT sedentary n = 10, WT trained n = 10, KO sedentary n = 6, KO trained n = 9 mice in each group.
Under resting conditions, KO mice had a lower sinus heart rate compared with WT mice (562 ± 134 vs. 732 ± 59 beats/min, P < 0.05) and often exhibited complex ventricular arrhythmia, including VT (Table 2). The exercise capacity was impaired in KO mice, as manifested by significantly decreased duration of the maximal treadmill exercise test (Fig. 2).
Table 2.
Arrhythmia characteristics of the different groups following stress
| HR, beats/min | n | PVC, no. | Bigeminy, no. | Complex PVC, no. | NSVT, no. | SVT, no. | Monomorphic, no. | Polymorphic, no. | Bidirectional, no. | VT, % | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Rest | |||||||||||
| WT sedentary | 731 ± 59 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT trained | 663 ± 57# | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KO sedentary | 562 ± 134* | 6 | 3 | 3 | 3 | 4 | 4 | 1 | 6 | 4 | 67 |
| KO trained | 615 ± 105 | 9 | 3 | 7 | 3 | 2 | 1 | 1 | 0 | 1 | 22* |
| Stress | |||||||||||
| WT sedentary | 794 ± 20 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT trained | 685 ± 55 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KO sedentary | NA | 6 | 6 | 3 | 6 | 6 | 4 | 1 | 6 | 4 | 100 |
| KO trained | NA | 9 | 4 | 5 | 6 | 9 | 4 | 1 | 5 | 3 | 100 |
| Epinephrine | |||||||||||
| WT sedentary | 754 ± 64 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| WT trained | 677 ± 43 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| KO sedentary | NA | 6 | 6 | 3 | 6 | 6 | 1 | 1 | 3 | 2 | 100 |
| KO trained | NA | 9 | 3 | 4 | 4 | 8 | 1 | 1 | 1 | 0 | 89 |
n, No. of animals tested. Testing was performed after 6 wk of training. HR (means ± SD) is for animals resting in the cage. PVC, premature ventricular contractions; VT, ventricular tachycardia; NSVT, nonsustained VT; SVT, sustained VT (values represent no. of animals with each of the arrhythmias). SVT was subclassified as monomorphic, polymorphic, or bidirectional. VT (%) represents the percentage of animals showing VT under any condition. The sinus rate of WT mice decreased after training, #P < 0.05. Sedentary KO mice had a lower basal heart rate *P < 0.05), but no further decrease was found after training. Noteworthy, trained KO mice had a lower prevalence of VT under resting conditions (P < 0.05). Sinus heart rate could not be determined in stressed KO mice due to frequent ventricular arrhythmia [not applicable (NA)].
Fig. 2.
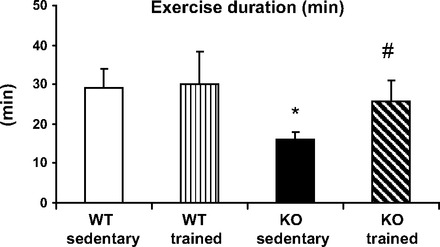
Maximal exercise capacity in WT and CASQ2 KO mice. Sedentary KO mice had a significant decrease in exercise capacity expressed as time (min) on treadmill. Values are means ± SD. *P < 0.05 compared with WT controls. Exercise capacity of trained KO mice had improved compared with the sedentary KO group, #P < 0.05.
Hearts from KO mice had a significant increase in the BNP gene expression associated with myocardial dysfunction (see Fig. 4). There was no significant difference in the expression of ANP, CX43, and α-skeletal actin.
Fig. 4.
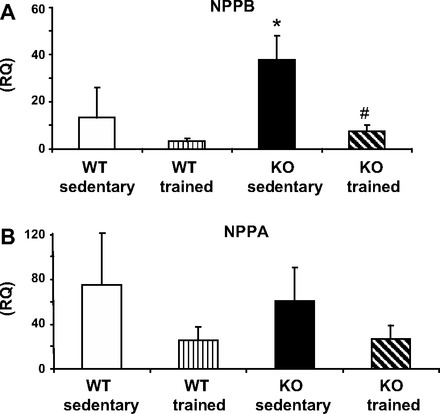
RNA expression of natriuretic peptides: natriuretic peptide precursor B (NPPB; A) and natriuretic peptide precursor A (NPPA; B) in WT and CASQ2 KO mouse hearts by qRT-PCR. Sedentary KO mice had higher expression of the brain natriuretic peptide (BNP) gene, *P < 0.05. In trained animals, there was a decrease in RNA expression levels in both peptides, statistically significant for BNP, #P < 0.05. Values are means ± SD; n = 5–6/group. RQ, respiratory quotient.
CASQ2 protein was absent from the homozygous KO mice studied. There were no differences between KO and control mice in the levels of CX43, NCX, Na-K-ATPase, LTCC, and β1-AR, proteins that were assumed to play a potential role in calcium handling and CPVT (see Fig. 5).
Fig. 5.
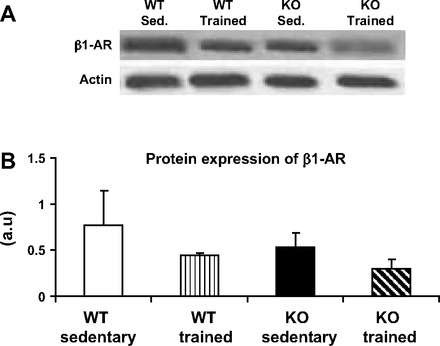
Protein levels of β1-adrenoceptor (β1-AR) in WT and CASQ2 KO mouse hearts. Protein levels showed a trend for decrease in trained compared with sedentary mice, P = 0.08. A: representative blot. B: bar graph presenting means ± SD; n = 5–6/group. au, Arbitrary units.
Effects of Exercise Training in KO Mice
Two mice from KO exercise group and three mice from the WT exercise group did not complete the training protocol. They were not able to continuously run for 1 h and were excluded. There was no mortality during the training period.
Exercise training led to a significant decrease in the basal heart rate in controls, but not CASQ2 KO mice (Table 2). There was no change in heart dimensions or function determined by echocardiography in WT mice. Exercise training improved the mean fractional shortening of KO mice from 33 to 43% (P < 0.05), while the LV cross-sectional end-systolic area and end-diastolic area became significantly lower than in sedentary KO mice (Table 1). Remarkably, after training, echocardiographic measures in mutant mice became comparable to that of the WT animals.
Concomitantly, trained KO mice, but not WT controls, exhibited a significant increase in their exercise endurance compared with sedentary mice (Fig. 2).
The arrhythmia severity in trained KO mice was lower than in sedentary KO under resting conditions (P < 0.05, Table 2). However, during exercise stress and epinephrine injections, i.e., provocation testing for CPVT, the arrhythmia severity was similar to that of sedentary KO (Table 2). We further assessed the arrhythmia load by counting the number of abnormal ventricular complexes during peak exercise. As can be seen in Fig. 3, the ventricular premature beat count was significantly lower in trained compared with sedentary KO mice (P < 0.05).
Fig. 3.
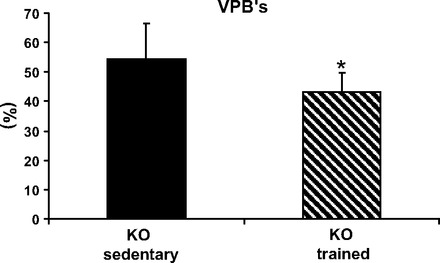
Effect of training on arrhythmia load during exercise stress expressed in sedentary and trained CASQ2 KO mice. The load, presented as percent ventricular premature beats (VPBs) of all beats, decreased in trained KO mice, *P < 0.05. Values are means ± SD; KO sedentary n = 6, KO trained n = 11 mice in each group.
Following exercise conditioning, there was a significant decrease in the expression of BNP mRNA in KO mice compared with the sedentary group. A similar trend was found in the expression of the gene of ANP (P = 0.07, Fig. 4). Training was also associated with a trend for a decrease in the β1-AR, which was, in particular, pronounced in the KO mice (Fig. 5, P = 0.08). The levels of other proteins studied, CX43, NCX, Na-K-ATPase, and LTCC, did not change in either group following exercise conditioning (data not shown).
DISCUSSION
CPVT is manifested by lethal ventricular arrhythmia evoked by physical or emotional stress. Recessively inherited CPVT2 is caused by either missense or null-allele mutations in the cardiac CASQ2 gene. It has been suggested that defects in CASQ2 cause protein deficiency, impairing Ca2+ uptake to the SR and Ca2+-dependent inhibition of ryanodine channels, thereby leading to diastolic Ca2+ leak, delayed after-depolarizations, and arrhythmia (19). CASQ2-deficient murine models were also reported to be associated with mild cardiomyopathy (40). While acute physical stress is known to be potentially lethal in CPVT patients (14), the effect of repeated mild-to-moderate exercise has not yet been studied in depth. In this study, we examined the effect of exercise training on LV remodeling and arrhythmia in CASQ2 KO mice compared with WT.
Exercise training improves symptoms in chronic heart failure and has numerous beneficial effects of cardiovascular and skeletal muscle function (9, 13, 29). An increase in calcium sensitivity has been associated with improvement in heart function (25, 35). In one study, running exercise protected transgenic mice from developing heart failure attributed to sympathetic hyperactivity, probably due to the upregulation of calcium-handling proteins and improving in SR calcium re-uptake (35). Exercise has been tested also in animal models of human arrhythmia. In 1978, McElroy et al. (28) demonstrated protection against ischemia-reperfusion damage. They found that regular swimming training causes a decrease in infarct size in rats. Exercise training in dogs subjected to myocardial infarction resulted in fewer episodes of VT, as well as in increased capability to withstand arrhythmic episodes, attributed to a decrease in β-ARs in the heart (1, 2). The effect of exercise training on the myocardium and the autonomic nervous system has been extensively investigated (4, 21, 31, 39). The findings of these studies have shown a decrease in the sympathetic activity and/or an increase in the parasympathetic tonus in laboratory animals and in humans, manifesting as a decrease in the resting heart rate and in blood pressure (2, 12, 32, 38). While the exact signaling pathway causing this phenomenon is not fully understood, exercise does affect myocardial gene expression and protein levels (18). An increase in the myosin α heavy chain, sarco(endo)plasmic reticulum Ca2+-ATPase, and phospholamban expression was demonstrated after 13 wk of intensive training in rats (18, 47). Others reported a decrease in the natriuretic factor A, skeletal actin (7), and β-ARs expression in trained animals (34, 42, 45). Because sympathetic activity is a major determinant of arrhythmia susceptibility, investigators have examined the effect of exercise training on the β-ARs using radioligand binding or protein level measurements. These measurements could not demonstrate a change in β-receptor expression following exercise training (3, 12, 16, 37). While differences between the studies could result from protocol duration and intensity, none of these studies found a change in the β-receptor affinity.
Our study is the first report documenting the effect of exercise training on the severity of arrhythmia and exercise capacity in a mouse model of CPVT. The in vivo outcomes comprised heart rhythm disturbances, endurance, and echocardiography measures after a 6-wk training protocol. Selected gene and protein expression has been examined in cardiac extracts. Sedentary KO mice had decreased systolic function, an increase in the LV size, and a higher heart/body weight ratio compared with WT controls (Fig. 1). The exercise capacity was impaired in KO mice (Fig. 2), and cardiac gene expression showed an increase in the BNP gene, which is associated with myocardial dysfunction (Fig. 4). BNP secretion is regulated by the cardiac chamber wall stretch and correlates with cardiac filling pressure (26, 41). High levels of BNP indicate cardiac dysfunction and are a useful prognostic indicator in postmyocardial infarction, cardiomyopathy, and heart failure patients.
As opposed to humans with CPVT, ventricular arrhythmia is rather prevalent in CASQ2 KO mice, even at rest (Table 2), possibly due to stressful stimuli in the external environment. Ventricular arrhythmia present at rest could contribute to development of contractile dysfunction through a mechanism resembling tachycardia-induced cardiomyopathy. Noteworthy, the phenotypic alterations in CASQ2 KO mice were not associated with altered expression of either a CX43 gene or its protein (10, 17). We postulate that these mice develop cardiomyopathy due to a detrimental combination of frequent ventricular arrhythmia and abnormal calcium handling.
Exercise training in KO mice was associated with neither mortality nor arrhythmia exacerbation. It led to decreased arrhythmia load at rest and during exercise. While we found no difference in stress-induced VT prevalence, trained KO had less VT at rest and fewer PVCs at peak exercise points compared with sedentary KO. At the end of the training protocol, they demonstrated a significant improvement in LV function and exercise capacity. The improvement in cardiac function was paralleled by a reduction in natriuretic gene expression (Fig. 4). We postulate that better cardiac function and less rhythm disturbances account for improved exercise capacity in trained KO mice. The impact of CASQ2 deficiency in fast skeletal muscle and the role of muscle conditioning deserve a further study (33). The WT mice were not significantly affected by exercise. Because the training protocol was adjusted according to the running speed of KO mice, the exercise intensity (at most 15 m/min) was rather mild for normal animals. Apparently, exercise adaptation was less pronounced in healthy animals due to inadequate training volume (3, 15).
The activity of the sympathetic system could constitute the link between the effects of exercise training and arrhythmia in a murine model of CPVT2. The expression of β-AR is a major determinant of cardiac function and ventricular arrhythmia in heart failure, cardiomyopathy, and arrhythmic disorders. Our results show a modest effect of exercise training on reducing β-AR expression, which was more pronounced in CASQ2-deficient mice. A decrease in β-AR has previously been described after dog training in association with attenuating malignant arrhythmia (34, 39, 45). We believe that decreased sensitivity to catecholamine stimulation in animals suffering from CPVT led to a decreased arrhythmia load and contributed to improvement in LV function. A training protocol longer than 6 wk and functional assessment of β1-AR activity could show even larger benefits.
Exercise training had a very pronounced differential effect on the resting heart rate. As could be expected, heart rate decreased in trained WT mice. Conversely, KO mice have a low resting heart rate, which is a part of the disease phenotype (19, 22, 40), but their heart rate increases after training (Table 2). These divergent responses suggest a profound difference in the activities of either calcium clock or autonomic nervous system, which are not adequately explained by β1-AR expression. Since we found no change in the expression of NCX, Na-K-ATPase, and LTCC, proteins that are involved in calcium handling and could mediate the effect of exercise training on cardiac rhythm and function, the putative mechanism remains to be identified (44, 48).
Limitations
Mice with CASQ2 KO develop cardiomyopathy, and their improved endurance may be attributed to improved LV function with exercise. Because human CPVT patients have no structural heart disease, their benefit from training might be more limited. Furthermore, mice with CPVT causing mutations recapitulate the arrhythmic phenotype, but do not suffer from sudden death. Humans with CPVT would necessarily receive β-blockers and require continuous monitoring during exercise. β-Blockers could blunt the effect of training by upregulating the level of β-receptors. Our study was performed without β-blockers, because those were found to be ineffective against CPVT in CASQ2 mice (20). Importantly, β-blockers should not be expected to abolish cardiac and autonomic adaptation to exercise training in humans and rodents (6, 27, 43).
Provocation testing for CPVT susceptibility by injecting epinephrine introduces an artificial element and blood pressure elevation, which might prevent correct assessment of adaptation to exercise. However, the results of testing with epinephrine paralleled the results of exercise stress testing (Table 2). It is still possible that we underestimated some of the benefits of exercise conditioning, either because of inadequate training, or because of using crude and categorical methods to define arrhythmia. Measurement of the sympathetic nervous activity and vagal tone could provide further insights on the effect of training on the autonomic system in CPVT mice.
In conclusion, while competitive sports and strenuous exercise are hazardous and shall be prohibited in CPVT patients, low- to moderate-level exercise training may, in fact, be beneficial. In mice, it improves cardiac function and exercise capacity and seems to decrease arrhythmia load without increasing arrhythmia severity. Drug and device therapy, combined with exercise training, may have a synergistic protective effect. Hence, supervised exercise rehabilitation programs should be carefully investigated for their potential to optimize the care of patients with CPVT and other arrhythmic disorders.
GRANTS
This work was supported by the Israel Science Foundation (ISF Grants 876/2005 and 763/10).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: E.K.J., G.K., and Y.C. performed experiments; E.K.J. analyzed data; E.K.J. drafted manuscript; E.K.J., E.H., J.G.S., C.E.S., A.S., M.E., and M.A. approved final version of manuscript; E.H., E.P., M.E., and M.A. conception and design of research; E.H., A.S., and M.A. interpreted results of experiments; M.A. edited and revised manuscript.
ACKNOWLEDGMENTS
We are indebted to Elaine Finkelstein for editorial assistance.
REFERENCES
- 1. Billman GE, Kukielka M. Effect of endurance exercise training on heart rate onset and heart rate recovery responses to submaximal exercise in animals susceptible to ventricular fibrillation. J Appl Physiol 102: 231–240, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Billman GE, Schwartz PJ, Stone HL. The effects of daily exercise on susceptibility to sudden cardiac death. Circulation 69: 1182–1189, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Carroll JF, Thaden JJ, Wright AM. A comparison of two exercise training programs on cardiac responsiveness to beta-stimulation in obesity. Exp Biol Med (Maywood) 230: 180–188, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Collins HL, DiCarlo SE. Daily exercise attenuates the sympathetic component of the arterial baroreflex control of heart rate. Am J Physiol Heart Circ Physiol 273: H2613–H2619, 1997 [DOI] [PubMed] [Google Scholar]
- 5. De Angelis K, Wichi RB, Jesus WR, Moreira ED, Morris M, Krieger EM, Irigoyen MC. Exercise training changes autonomic cardiovascular balance in mice. J Appl Physiol 96: 2174–2178, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Demopoulos L, Yeh M, Gentilucci M, Testa M, Bijou R, Katz SD, Mancini D, Jones M, LeJemtel TH. Nonselective beta-adrenergic blockade with carvedilol does not hinder the benefits of exercise training in patients with congestive heart failure. Circulation 95: 1764–1767, 1997 [DOI] [PubMed] [Google Scholar]
- 7. Diffee GM, Seversen EA, Stein TD, Johnson JA. Microarray expression analysis of effects of exercise training: increase in atrial MLC-1 in rat ventricles. Am J Physiol Heart Circ Physiol 284: H830–H837, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Eldar M, Pras E, Lahat H. A missense mutation in the CASQ2 gene is associated with autosomal-recessive catecholamine-induced polymorphic ventricular tachycardia. Trends Cardiovasc Med 13: 148–151, 2003 [DOI] [PubMed] [Google Scholar]
- 9. European Heart Failure Training Group Experience from controlled trials of physical training in chronic heart failure. Eur Heart J 19: 466–475, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Akar FG, Nass RD, Hahn S, Cingolani E, Shah M, Hesketh GG, DiSilvestre D, Tunin RS, Kass DA, Tomaselli GF. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 293: H1223–H1230, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Fagard RH. Exercise characteristics and the blood pressure response to dynamic physical training. Med Sci Sports Exerc 33: 1229–1233, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Favret F, Henderson KK, Clancy RL, Richalet JP, Gonzalez NC. Exercise training alters the effect of chronic hypoxia on myocardial adrenergic and muscarinic receptor number. J Appl Physiol 91: 1283–1288, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Hambrecht R, Niebauer J, Fiehn E, Kälberer B, Offner B, Hauer K, Riede U, Schlierf G, Kübler W, Schuler G. Physical training in patients with stable chronic heart failure: effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol 25: 1239–1249, 1995 [DOI] [PubMed] [Google Scholar]
- 14. Hayashi M, Denjoy I, Extramiana F, Maltret A, Buisson NR, Lupoglazoff JM, Klug D, Hayashi M, Takatsuki S, Villain E, Kamblock J, Messali A, Guicheney P, Lunardi J, Leenhardt A. Incidence and risk factors of arrhythmic events in catecholaminergic polymorphic ventricular tachycardia. Circulation 119: 2426–2434, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Høydal MA, Wisløff U, Kemi OJ, Ellingsen O. Running speed and maximal oxygen uptake in rats and mice: practical implications for exercise training. Eur J Cardiovasc Prev Rehabil 14: 753–760, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Hughson RL, Sutton JR, Fitzgerald JD, Jones NL. Reduction of intrinsic sinoatrial frequency and norepinephrine response of the exercised rat. Can J Physiol Pharmacol 55: 813–820, 1977 [DOI] [PubMed] [Google Scholar]
- 17. Zhong J, Zhang W, Gao H, Li Y, Zhong M, Li D, Zhang C, Zhang Y. Changes in connexin 43 metalloproteinase and tissue inhibitor of metalloproteinase during tachycardia-induced cardiomyopathy in dogs. Eur J Heart Fail 9: 23–29, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Van Peborgh J, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol 279: H2994–H3002, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Katz G, Arad M, Eldar M. Catecholaminergic polymorphic ventricular tachycardia: from bedside to bench and beyond. Curr Probl Cardiol 34: 9–43, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Katz G, Khoury A, Kurtzwald E, Hochhauser E, Porat E, Shainberg A, Seidman JG, Seidman CE, Lorber A, Eldar M, Arad M. Optimizing catecholaminergic polymorphic ventricular tachycardia therapy in calsequestrin-mutant mice. Heart Rhythm 7: 1676–1682, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Krieger EM, Brum PC, Negrão CE. Role of arterial baroreceptor function on cardiovascular adjustments to acute and chronic dynamic exercise. Biol Res 31: 273–279, 1998 [PubMed] [Google Scholar]
- 22. Lahat H, Eldar M, Levy-Nissenbaum E, Bahan T, Friedman E, Khoury A, Lorber A, Kastner DL, Goldman B, Pras E. Autosomal recessive catecholamine or exercise induced polymorphic ventricular tachycardia: clinical features and assignment of the disease gene to chromosome 1p13–21. Circulation 103: 2822–2827, 2000 [DOI] [PubMed] [Google Scholar]
- 23. La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation 106: 945–949, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children: 7 year follow up in 21 patients. Circulation 91: 1512–1519, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Lu L, Mei DF, Gu AG, Wang S, Lentzner B, Gutstein DE, Zwas D, Homma S, Yi GH, Wang J. Exercise training normalizes altered calcium handling proteins during development of heart failure. J Appl Physiol 92: 1524–1530, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Mair J, Hammerer-Lercher A, Puschendorf B. The impact of cardiac natriuretic peptide determination on the diagnosis and management of heart failure. Clin Chem Lab Med 39: 571–588, 2001 [DOI] [PubMed] [Google Scholar]
- 27. Martinez DG, Nicolau JC, Lage RL, Toschi-Dias E, de Matos LD, Alves MJ, Trombetta IC, Dias da Silva VJ, Middlekauff HR, Negrão CE, Rondon MU. Effects of long-term exercise training on autonomic control in myocardial infarction patients. Hypertension 58: 1049–1056, 2011 [DOI] [PubMed] [Google Scholar]
- 28. McElroy CL, Gissen SA, Fishbein MC. Exercise induced reduction in myocardial infarct size after coronary artery occlusion in the rat. Circulation 57: 958–962, 1978 [DOI] [PubMed] [Google Scholar]
- 29. McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, Hendrican K, Yusuf S. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). Am Heart J 144: 23–30, 2002 [DOI] [PubMed] [Google Scholar]
- 30. McKelvie RS. Exercise training in patients with heart failure: clinical outcomes, safety, and indications. Heart Fail Rev 13: 3–11, 2008 [DOI] [PubMed] [Google Scholar]
- 31. O'Sullivan SE, Bell C. The effects of exercise and training on human cardiovascular reflex control. J Auton Nerv Syst 81: 16–24, 2000 [DOI] [PubMed] [Google Scholar]
- 32. Paffenbarger RS, Jr, Hyde RT, Wing AL, Lee IM, Jung DL, Kampert JB. The association of changes in physical-activity level and other lifestyle characteristics with mortality among men. N Engl J Med 328: 538–545, 1993 [DOI] [PubMed] [Google Scholar]
- 33. Paolini C, Quarta M, D'Onofrio L, Reggian C, Protasi F. Differential effect of calsequestrin ablation on structure and function of fast and slow skeletal muscle fibers. J Biomed Biotechnol 2011: 634075, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Plourde G, Rousseau-Migneron S, Nadeau A. Beta-adrenoceptor adenylate cyclase adaptation to physical training in rat ventricular tissue. J Appl Physiol 70: 1633–1638, 1991 [DOI] [PubMed] [Google Scholar]
- 35. Rolim NP, Medeiros A, Rosa KT, Mattos KC, Irigoyen MC, Krieger EM, Krieger JE, Negrão CE, Brum PC. Exercise training improves the net balance of cardiac Ca2-handling protein expression in heart failure. Physiol Genomics 29: 246–252, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Rosso R, Kalman JM, Rogowski O, Diamant S, Birger A, Biner S, Belhassen B, Viskin S. Calcium channel blockers and beta-blockers versus beta-blockers alone for preventing exercise-induced arrhythmias in catecholaminergic polymorphic ventricular tachycardia. Heart Rhythm 4: 1149–1154, 2007 [DOI] [PubMed] [Google Scholar]
- 37. Schaefer ME, Allert JA, Adams HR, Laughlin MH. Adrenergic responsiveness and intrinsic sinoatrial automativity of exercise-trained rats. Med Sci Sports Exerc 24: 887–894, 1992 [PubMed] [Google Scholar]
- 38. Scheuer J, Tipton CM. Cardiovascular adaptations to physical training. Annu Rev Physiol 39: 221–251, 1977 [DOI] [PubMed] [Google Scholar]
- 39. Shi X, Stevens GH, Foresman BH, Stern SA, Raven PB. Autonomic nervous system control of the heart: endurance exercise training. Med Sci Sports Exerc 27: 1406–1413, 1995 [PubMed] [Google Scholar]
- 40. Song L, Alcalai R, Arad M, Wolf CM, Toka O, Conner DA, Berul CI, Eldar M, Seidman CE, Seidman JG. Increased calreticulin and ryanodine receptors by calsequestrin-2 (CASQ2) mutations cause catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 117: 1814–1823, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Struthers AD. Introducing a new role for BNP: as a general indicator of cardiac structural disease. Heart 87: 97–98, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sylvestre-Gervais L, Nadeau A, Nguyen MH, Tancrède G, Rousseau-Migneron S. Effects of physical training on beta-adrenergic receptors in rat myocardial tissue. Cardiovasc Res 16: 530–534, 1982 [DOI] [PubMed] [Google Scholar]
- 43. Vanzelli AS, Medeiros A, SirventeRde A, Salemi VM, Mady C, Brum PC. Association of physical training with beta-blockers in heart failure in mice. Arq Bras Cardiol 95: 373–380, 2010 [DOI] [PubMed] [Google Scholar]
- 44. Maltsev VA, Lakatta EG. Dynamic interactions of an intracellular Ca+2 clock and membrane ion channel clock underlie robust initiation and regulation of cardiac pacemaker function. Cardiovasc Res 77: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 45. Werle EO, Strobel G, Weicker H. Decrease in rat cardiac beta1- and beta2-adrenoceptors by training and endurance exercise. Life Sci 46: 9–17, 1990 [DOI] [PubMed] [Google Scholar]
- 46. Wilde AA, Bhuiyan ZA, Crotti L, Facchini M, De Ferrari GM, Paul T, Ferrandi C, Koolbergen DR, Odero A, Schwartz PJ. Left cardiac sympathetic denervation for catecholaminergic polymorphic ventricular tachycardia. N Engl J Med 358: 2024–2029, 2008 [DOI] [PubMed] [Google Scholar]
- 47. Wisløff U, Loennechen JP, Falck G, Beisvag V, Currie S, Smith G, Ellingsen O. Increased contractility and calcium sensitivity in cardiac myocytes isolated from endurance trained rats. Cardiovasc Res 50: 495–508, 2001 [DOI] [PubMed] [Google Scholar]
- 48. Gao Z, Chen B, Joiner MA, Wu Y, Guan X, Koval OM, Chaudhary AK, Cunha SR, Mohler PJ, Martins JB, Song LS, Anderson ME. If and SR Ca2+ release both contribute to pacemaker activity in canine sinoatrial node cells. J Mol Cell Cardiol 49: 33–40, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


