Abstract
Breast cells drive bone demineralization during lactation and metastatic cancers. A shared mechanism among these physiological and pathological states is endocrine secretion of parathyroid hormone-related protein (PTHrP), which acts through osteoblasts to stimulate osteoclastic bone demineralization. The regulation of PTHrP has not been accounted for fully by any conventional mammotropic stimuli or tumor growth factors. Serotonin (5-HT) synthesis within breast epithelial cells is induced during lactation and in advancing breast cancer. Here we report that serotonin deficiency (knockout of tryptophan hydroxylase-1) results in a reduction of mammary PTHrP expression during lactation, which is rescued by restoring 5-HT synthesis. 5-HT induced PTHrP expression in lactogen-primed mammary epithelial cells from either mouse or cow. In human breast cancer cells 5-HT induced both PTHrP and the metastasis-associated transcription factor Runx2/Cbfa1. Based on receptor expression and pharmacological evidence, the 5-HT2 receptor type was implicated as being critical for induction of PTHrP and Runx2. These results connect 5-HT synthesis to the induction of bone-regulating factors in the normal mammary gland and in breast cancer cells.
Keywords: 5-hydroxytryptamine, lactation, osteoblast, prolactin, RANK ligand, RUNX2/CBFA1
a key function of the mammary glands is to regulate the mobilization of calcium from bone. During lactation women and other mammals lose a significant portion of bone mass, which is restored after lactation ceases (2, 8, 26, 54). Failure to mobilize bone calcium extraction at the onset of lactation causes hypocalcemia in dairy cows, leading to a severe convulsive syndrome referred to as periparturient paresis or “milk fever” (17, 35). To drive calcium mobilization, the mammary glands become endocrine organs and secrete parathyroid hormone-related peptide (PTHrP) into the bloodstream (9, 27, 46, 50, 51, 53, 54). PTHrP was originally discovered as the factor responsible for humoral hypercalcemia of malignancy and is secreted from a variety of advanced soft-tissue cancers (5, 8, 28, 47). The NH2-terminal portion of PTHrP is similar to that of parathyroid hormone (PTH) and acts via the type 1 PTH receptors (PTH1R) to induce the receptor activator of NF-κB ligand (RANKL) (34).
PTHrP is undetectable in the circulation except during lactation, in advanced metastastic disease, or in patients with hyperprolactinemia (5, 6, 9, 24, 42, 48). Despite obvious correlations with states of elevated prolactin (PRL), PRL did not induce PTHrP in conventional cell cultures of mammary epithelium (29, 52), and our laboratory has done numerous experiments that confirmed that PRL does not induce PTHrP in mammary cells by a direct mechanism (unpublished results).
A previous study showed that serotonin (5-hydroxytryptamine; 5-HT) induced PTHrP expression in vascular smooth muscle cells (40). In the mammary glands, 5-HT regulates key aspects of epithelial homeostasis by autocrine-paracrine signaling. The processes regulated by 5-HT include not only specialized mammary gland functions such as milk protein and milk lipid biogenesis but also fundamental cell biological processes (i.e., apoptosis, barrier permeability, cell shedding, etc.) (16, 30, 31, 36, 38, 49). Epithelia lining other ductal/alveolar secretory organs also possess local 5-HT signaling systems, which have been implicated in various aspects of epithelial homeostasis (39).
Given the central role of 5-HT in mammary gland homeostasis and evidence that 5-HT regulates PTHrP, these studies were initiated to discover new pathways associated with the breast-bone axis. Our results implicate the 5-HT autocrine system as a critical component of the mechanisms by which breast cells regulate bone mobilization.
MATERIALS AND METHODS
Animal studies.
Mice with gene disruptions for tryptophan hydroxylase-1 [TPH1, the rate-limiting enzyme in 5-HT biosynthesis (31)], the type 7 serotonin receptor (5-HT7) (15), and corresponding wild-type control animals (C57BL/6J genetic background) were bred and maintained in our animal facility. The knockout strains were capable of lactating and rearing litters of offspring, although they were less efficient than the normal strain. All experiments were performed under protocols approved by the University of Cincinnati Institutional Animal Care and Use Committee. Plasma was collected for PTHrP assay from nonlactating wild-type mice and both wild-type and TPH1−/− animals on day 10 of lactation. Mammary gland tissues (no. 4 glands counted from most rostral) were collected for immunostaining from TPH1−/− and 5-HT7−/− animals and wild-type controls on day 10 of lactation and were fixed in 4% paraformaldehyde before being paraffin embedded and sectioned.
PTHrP immunoradiometric assay.
Plasma PTHrP levels were measured using a two-site immunoradiometric assay (IRMA) specific for PTHrP1–86 (Becton-Dickinson), following the manufacturer's instructions. The detection limit (blank serum + 1SD) was 0.3 pM under the conditions of this assay.
Cell culture.
Primary bovine mammary epithelial cells (pBMECs) grown in collagen gels were induced to differentiate by release of the gel from the substratum and treating them with lactogenic hormones (prolactin 1 μg/ml, insulin 5 μg/ml, cortisol, 1 μM) as described (16, 32). The mouse mammary epithelial cells (HC11) were maintained under growth medium conditions and lactogen induced by treatment of 3-day-confluent cultures with prolactin, insulin, and cortisol as previously described (18). MDA-MB-231, MCF7, and T47D breast cancer cell lines and MCF10A (normal human ductal mammary epithelial cell lines) were obtained from the American Type Culture Collection (ATCC) and cultured as previously described (31, 38, 49). pBMECs were obtained as a generous gift from Dr. Robert Collier, University of Arizona, and cultured as previously described (16, 32). Serotonin-HCl (5-HT, Sigma-Aldrich) was freshly prepared and diluted into the respective media at the concentrations described in the text. MC-3T3 clone E1 cells were obtained from ATCC and cultured as described (13, 14). MC3T3-E1 cells were stimulated to differentiate into osteoblasts (OB), using 50 μg/ml L-ascorbic acid.
Conditioned medium (CM) was collected following incubation of MDA-MB-231 cells for 72 h in growth medium or in growth medium plus 100 μg/ml ρ-chloro-phenylalanine (pCPA, Tocris), a specific and irreversible inhibitor of TPH (21). MC3T3-E1 cells were treated with 50% CM.
Quantitative real-time RT-PCR amplification.
Total RNA was isolated using Tri Reagent (Molecular Research) following the manufacturer's procedures. RNA quality was determined through spectrophometric methods on a Nanodrop 2000 (Thermo Scientific). A total of 1 μg was reverse transcribed using a QuantiTect reverse transcription kit (Qiagen). Quantitative real-time RT-PCR was performed using the Applied Biosystems Step One Plus system using fast SYBR Green Master Mix (Applied Biosystems). The following conditions were utilized: 95°C for 20 s followed by 40 cycles of 95°C for 3 s and 60°C for 30 s. Primers, designed with similar amplification efficiencies, are listed in Table 1.
Table 1.
Primers used for quantitative real-time PCR
| Primer | Forward (5′-3′) | Reverse (5′-3′) | Species |
|---|---|---|---|
| PTHrP | TTCCTGCTCAGCTACTCCGT | GATGGACTTGCCCTTGTCAT | Mouse |
| PTHrP | GGAGGCTAGTTCAGCAATGG | CCGAGGTAGCTCTGATTTCG | Bovine |
| PTHrP | CCTCCAGCACCATAGAGAGG | GCCCAGGTGTGAGAGTAAGG | Human |
| RANKL | GGCCACAGCGCTTCTCAG | GAGTGACTTTATGGGAACCCGAT | Mouse |
| RANK | TGCAGCTCAACAAGGATACG | TGGTCTCCTCAGTGTCATGG | Mouse |
| Runx2 | CGGAATGCCTCTGCTGTTAT | TTCCCGAGGTCCATCTACTG | Human |
| rpS15 | ACCTACCGTGGCGTAGACC | ATGTCCCTCAGGTGAGTCTTC | Mouse |
| rpS15 | CGCGACATGATCATTCTACC | TTACTTGAGGGGGATGAAGC | Bovine |
| rpS15 | CAACCAGGTGGAGATCAAGC | TCATGTGCGCCTTTATTAGC | Human |
Ribosomal protein S15 was used as the internal control.
Immunohistochemistry and Western blotting.
PTHrP fluorescent immunostaining was performed using a 1:50 dilution of goat anti-PTHrP (N-19; Santa Cruz Biotechnology) overnight at 4°C and 1:1,000 dilution of the rabbit anti-goat Alexa fluor 488 Fa(b) fragments (Invitrogen) for 30 min at room temperature on paraffin-embedded sections of mammary gland tissue collected from TPH1−/− and wild-type (TPH1+/+) mice collected at day 10 of lactation. Nuclei were visualized using a 1:1,000 dilution of TOPRO-3 (Invitrogen) for 20 min at room temperature. Fluorescence was visualized on a Zeiss LSM 10 confocal microscope.
Statistical analyses.
Statistical significance was determined in each experiment by analysis of variance, with Bonferroni's post hoc test for relevant differences among groups or two-tailed Student's t-test, on log10-transformed data. The cell culture data are reported with the number of replicate dishes in a single experiment, and each experiment was repeated independently at least twice with similar results. Significance was accepted at P < 0.05.
RESULTS
5-HT induces PTHrP during lactation.
We examined PTHrP levels in mammary glands of TPH1−/− mice and their corresponding normal controls (TPH1+/+) at midlactation by immunostaining (Fig. 1A). In the mammary tissue in control mice (TPH1+/+), PTHrP was detected both in the cytoplasm of the secretory cells and within the secretory deposits in the alveolar lumens (Fig. 1A left). Staining was observed in occasional cells that bordered the luminal epithelium, which were most likely to be myoepithelium. PTHrP immunoreactivity was markedly less in the glands of TPH1−/− lactating mice (Fig. 1A right).
Fig. 1.
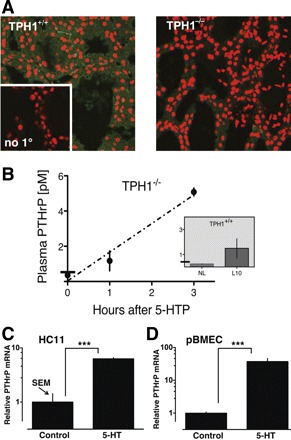
Serotonin (5-HT) induces expression of parathyroid hormone-related protein (PTHrP) in normal breast cells. A: PTHrP immunostaining (green fluorescence) in wild-type tryptophan hydroxylase-1 (TPH1+/+) and TPH knockout (TPH1−/−) day 10 lactating mammary glands; nuclei (red). Negative control (no primary antibody) is shown in the inset. B: plasma PTHrP levels in TPH1+/+ (inset) and (TPH1−/−) mice. Bar crossing the ordinate in each graph indicates the lower limit of PTHrP detection. Results are shown for TPH1+/+ mice that were nonlactating (NL) and day 10 lactating (L10). Experimental day 10 lactating TPH1−/− mice were treated with 100 mg/kg 5-hydroxytryptophan (5-HTP) to rescue 5-HT synthesis and killed at time 0, 1 h, and 3 h, as indicated (n = 3 per group). PTHrP mRNA expression was measured in mouse HC11 cells differentiated with lactogenic hormones and treated with control medium (CM) or medium containing 5-HT (2 × 10−4 M) for 48 h (C); and “3D lactogenic” cultures of primary bovine mammary epithelial cells (pBMECs) treated with CM or 5-HT as labeled (D). PCR data were quantified using the 2−ΔΔCT method, with ribosomal S15 used as internal control gene, and comparisons were normalized to control lactogenic cells. Data are represented as means ± SE of log-transformed values of relative expression (n = 4 or 6, ***P < 0.001).
Consistent with many published reports, PTHrP was below the level of detectability in the blood plasma of nonlactating females and was readily detectable during lactation (Fig. 1B, inset).
The conversion of l-tryptophan to 5-hydroxytryptophan (5-HTP) is the rate-limiting step in 5-HT synthesis; therefore, we injected 5-HTP to bypass the enzyme deficiency in TPH1−/− mice and measured PTHrP plasma levels (Fig. 1B). The mice rescued with 5-HTP showed a time-dependent increase in plasma PTHrP (P < 0.0001, R2 = 0.92), demonstrating that TPH1 activity was necessary for PTHrP secretion during lactation.
To determine whether PTHrP expression in mammary epithelial cells was directly responsive to 5-HT, two cell models of lactogenic mammary epithelium were studied: lactogen-treated rodent HC11 cells and pBMECs embedded in floating collagen gels. In these models, 5-HT induced PTHrP gene expression 8- and 20-fold, respectively (Fig. 1, C and D).
5-HT regulates PTHrP and Runx2 expression in breast cancer cells.
5-HT induced PTHrP gene expression at least 20-fold in each of three breast cancer cell lines (Fig. 2A), and the stimulation of PTHrP was time and concentration dependent in MDA-MB-231 cells (Fig. 2B). We also measured expression of Runx2 (mammalian runt-related transcription factor 2), which is associated with the metastatic phenotype of breast cancer cells (20, 45) and with controlling bone tissue tropism (11, 19, 25, 41). There was significant gene induction of both PTHrP and Runx2 within 4 h, and sustained stimulation (36–48 h) resulted in greater than 100-fold induction (Fig. 2B). Runx2 mRNA was stimulated by 5-HT in MDA-MB-231 and T47D but not in MCF7 cells (Fig. 2, A and B).
Fig. 2.
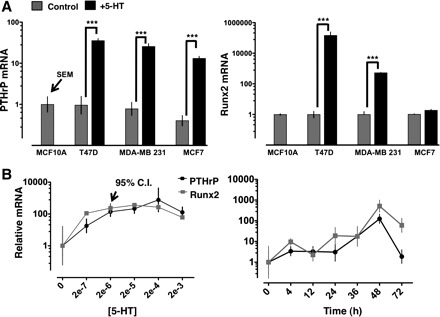
5-HT induces PTHrP and Runx2 mRNA in breast cancer cells. A: PTHrP and Runx2 mRNA measured by quantitative real-time RT-PCR. MCF10A, a nontransformed ductal mammary epithelial cell line, was used as a reference so that all of the breast cancer cell lines could be compared on a single scale. T47D, MDA-MB-231, and MCF7 breast cancer epithelial cell lines were treated with control medium or 5-HT (2 × 10−4 M, 48 h, n = 6, ***P < 0.001). B: concentration (left) and time dependence (right) of PTHrP and Runx2 mRNA induction by 5-HT in MDA-MB-231 cells. CI, confidence interval. Abscissa is labeled with medium 5-HT concentrations (2 × −7 to 2 × 10−3 M) or time (h after addition of 5-HT). Concentration-response was measured at 48 h, and the time course experiment was done with a concentration of 2 × 10−4 M (n = 4 at each point).
PTHrP and other factors that may be secreted from breast cancer cells induce osteoclastic bone resorption by stimulating RANKL expression in osteoblasts (34). We confirmed that CM from MDA-MB-231 cells induced RANKL gene expression (Fig. 3A). Inhibiting TPH activity in MDA-MB-231 by treating them with pCPA blocked RANKL stimulation by MDA-MB-231 CM. In contrast, MDA-MB-231 CM suppressed osteoprotegerin (OPG, a decoy receptor for RANKL), but the inhibition of OPG was not affected by treating MDA-MB-231 cells with pCPA (Fig. 3B).
Fig. 3.
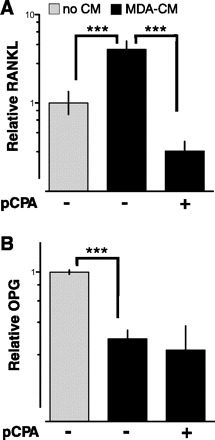
5-HT inhibition attenuates RANKL (receptor-activator of NF-κB ligand) gene induction in response to breast cancer CM. RANKL and osteoprotegerin (OPG) mRNA levels in differentiated osteoblasts (OBs) that were untreated or treated with CM collected from MDA-MB-231 cells. ρ-Chloro-phenylalanine (pCPA; 100 μg/ml) was added 72 h before collecting CM to inhibit 5-HT synthesis in MDA-MB-231 cells (+ on the abscissa). Data are represented as means ± SE of log-transformed values (n = 8, ***P < 0.001).
Induction of PTHrP is mediated by 5-HT2 but not 5-HT7.
Multiple 5-HT receptor isoforms are expressed in mammary epithelium and in breast cancer cells. Of these, previous studies have implicated 5-HT7 in several aspects of epithelial homeostasis (16, 37, 49); so we considered it possible that 5-HT7 also mediated induction of PTHrP. To address any potential role of 5-HT7, we stained glands from 5-HT7 knockout mice for PTHrP immunoreactivity during lactation. PTHrP expression was similar in glands from normal (5-HT7+/+) and knockout (5-HT7−/−) mice (Fig. 4A), implying that a receptor other than 5-HT7 was involved in PTHrP induction.
Fig. 4.
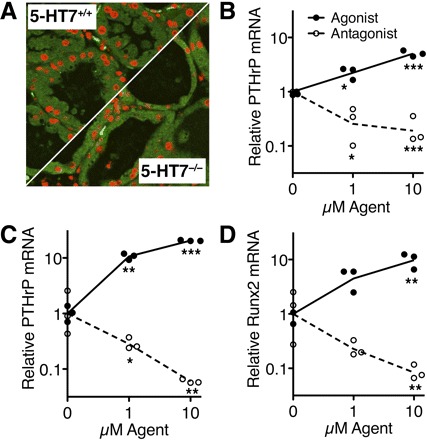
Induction of PTHrP expression depends on 5-HT2 but not 5-HT7. A: lactation levels of PTHrP were similar by immunostaining in wild-type (5-HT7+/+) and 5-HT7 knockout (5-HT7−/−) mammary gland epithelium (representative of 3 replicate glands of each genotype). B: PTHrP mRNA expression levels in HC11 cells (lactogen differentiated) treated with specified concentrations of 5-HT2 agonist (BW-723C86, closed symbols) or antagonist (SB-204741; open symbols). PTHrP (C) and Runx2 (D) mRNA expression levels in MDA-MB-231 cells treated with 5-HT2 agonist (closed symbols) or antagonist (open symbols). Data are represented as individual values of log-transformed data with lines drawn through the means (*P < 0.05, **P < 0.01, ***P < 0.001).
Of the various 5-HT receptor isoforms, 5-HT2 (specifically 5-HT2B) was expressed in all normal mammary cells and breast cancers that we have tested (mouse, cow, and human; Table 2). Consequently, we tested the hypothesis that 5-HT2 activity regulated PTHrP in untransformed mouse mammary cells (HC11; Fig. 4B) and in human breast cancer cells (MDA-MB-231; Fig. 4C). Activating 5-HT2 with BW-723C86 [α-methyl-5-(2-thienylmethoxy)-1H-indole-3-ethanamine; Tocris, http://www.iuphar-db.org/DATABASE/] stimulated PTHrP in a concentration-dependent manner. Correspondingly, the 5-HT2 antagonist SB-204741 [1-(1-methylindol-5-yl)-3-(3-methyl-1,2-thiazol-5-yl)urea; Tocris, http://www.iuphar-db.org/DATABASE/] suppressed PTHrP expression. Moreover, Runx2 was regulated similarly in MDA-MB-231 cells by activating or inhibiting 5-HT2 receptors (Fig. 4D). Because it inhibits not only ligand-induced but also basal receptor activity, SB-204741 is best described as an inverse agonist of 5-HT2.
Table 2.
5-HT receptor expression profiles of mammary gland and breast cancer cells
| Untransformed Mammary Cells |
Breast Cancer Cells |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Receptor Subtype | Primary Signal Modality | Primary Human (h) | Primary Mouse (m) | Primary Bovine (b) | MCF10A (h) | BME-UV (b) | 4T1 (m) | MCF7 (h) | T47D (h) | MDA-MB-231 (h) |
| 1A | Gi/o | |||||||||
| 1B | “ | + | + | |||||||
| 1D | “ | + | + | + | + | + | + | + | + | |
| 1E | “ | + | + | |||||||
| 1F | “ | + | + | + | ||||||
| 2A | Gq/11 | + | + | |||||||
| 2B | “ | + | + | + | + | + | + | + | + | + |
| 2C | “ | + | + | |||||||
| 3A | Channel | + | + | |||||||
| 4 | Gs | + | + | |||||||
| 5A/B | Gi/o | + | + | |||||||
| 6 | Gs | |||||||||
| 7 | Gs | + | + | + | + | + | + | |||
Each cell system was examined by RT-PCR for expression of genes encoding all receptors listed here (16, 37, 38, 49), and previously unpublished data. 5-HT, serotonin; + indicates that expression of the receptor subtype was detected in the designated cell system, and empty cells represent no detectable expression for that receptor. The species from which cell lines were derived is as indicated: human (h), mouse (m), bovine (b).
DISCUSSION
Herein, we report that 5-HT induces PTHrP secretion via an autocrine pathway in the mammary glands. A previous study demonstrated that 5-HT stimulated PTHrP expression in vascular smooth muscle (40); therefore, the signaling mechanisms employed by 5-HT to induce PTHrP may operate in a variety of cell types. Our results suggest that the 5-HT-PTHrP system may contribute to calcium mobilization associated with lactation and hyperprolactinemia. The presence of the autocrine 5-HT-PTHrP signaling system in breast cancer cells also indicates that this same system contributes to local bone loss and systemic hypercalcemia in metastatic cancers.
It has been evident that PTHrP is involved in bone mobilization during lactation and hyperprolactinemia and in hypercalcemia caused by cancers (5, 47, 48, 53). The transcription factor Runx2 is an important regulator of the metastatic and bone-homing phenotypes of breast cancer cells (41). In addition, Runx2 and PTHrP are linked in a reciprocal stimulatory network between breast cancer cells and osteoblasts (1, 11). The factors upstream of PTHrP are largely unknown (7, 10, 23, 54). One relevant hypothesis is that factors liberated from bone matrix drive a “vicious cycle” of PTHrP secretion from metastatic breast cancer cells (34). However, this vicious cycle hypothesis cannot account for the high level of PTHrP secretion from normal lactating mammary cells.
We propose that PTHrP secretion is driven by 5-HT signaling (Fig. 5). This autocrine-paracrine 5-HT hypothesis is able to account for secretion from normal mammary epithelial cells and for initiation of a vicious cycle in the bone microenvironment.
Fig. 5.
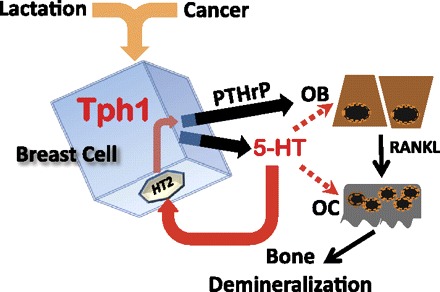
Schema depicting proposed relationship between breast and bone cells. TPH1 activity in lactating and cancer cells catalyzes 5-HT biosynthesis. Autocrine 5-HT (solid red arrow) drives PTHrP secretion via activation of type 2 receptors. For the sake of simplicity, potential roles of Runx2 are omitted. OB signaling activates OC functions (solid black line). Additional 5-HT actions via paracrine signaling to OBs and OCs may be important in cancer (broken lines).
Calcium secretion into milk can place extreme pressures on calcium homeostatic mechanisms. One consequence of the onset of mammary calcium demand is periparturient hypocalcemia (aka milk fever), which is observed frequently in high-producing dairy cows and less often in humans and other mammals (17, 35). Although the metabolic consequences of milk fever are well known, the pathophysiological mechanisms responsible for the breakdown of calcium homeostasis have remained obscure, and the potential involvement of 5-HT provides a new set of mechanisms to explore.
Bone mobilization and elevated 5-HT activity.
Epidemiological evidence in humans has consistently pointed to a relationship between 5-HT and bone mobilization. One common observation is that antidepressant use is associated with reduced bone mineral density (3). Another class of psychoactive drugs, the atypical antipsychotics, have well-documented negative effects on bone accrual (33). Enhanced 5-HT bioactivity leading to local PTHrP secretion may be one mechanism involved in bone loss caused by these drugs. In addition, antidepressant and antipsychotic drugs tend to cause hyperprolactinemia, and the degree of hyperprolactinemia can be substantial (4, 33). Demineralization of the bones is also commonly associated with hyperprolactinemia resulting from prolactinomas (12, 27, 44, 48). One explanation for bone loss in hyperprolactinemic patients is the antigonadal effect of PRL, which reduces estrogen secretion in females. In addition, hyperprolactinemic patients had elevated circulating PTHrP levels, and PTHrP concentrations were directly correlated with bone loss (48). Mammary gland 5-HT biosynthesis was discovered because TPH1 was highly induced by hyperprolactinemia (31). Consequently, we propose that induction of 5-HT upstream of PTHrP contributes to bone loss in patients with hyperprolactinemia.
Signal transduction from 5-HT to PTHrP and Runx2.
We considered it possible that 5-HT7 was involved in PTHrP regulation because it mediates several other important 5-HT effects in mammary epithelial cells of mice, cows, and humans (16, 37, 38, 49). However, PTHrP was expressed normally in 5-HT7 knockout glands.
The 5-HT receptor expression profile has been examined extensively in mammary epithelium from three species (mouse, cow, human), represented by isolates from tissues and nine specific primary or cell line systems (Table 2). Only type 2B receptors are expressed in all of these systems, and types 2A and 2D are additionally expressed in certain normal and cancer cells. The ubiquity of 5-HT2 expression (particularly 5-HT2B) led us to hypothesize that these receptors might be responsible for induction of PTHrP and/or Runx2. Correspondingly, PTHrP and Runx2 were sensitive to pharmacological activation and inhibition of 5-HT2. Other 5-HT receptor types are likely to be involved in regulating bone-related signals by modulating signaling downstream from 5-HT2.
Previous studies have implicated PKC in the regulation of both PTHrP and Runx2 (11, 22, 41, 43). Therefore, the apparent involvement of type 2 receptors, which signal through Gq and PKC, provides a basis for future hypotheses regarding mechanisms of regulation for PTHrP and Runx2 and for identifying other genes that may be involved in signaling from breast cells to bone cells.
Conclusions.
Autocrine-paracrine 5-HT stimulates PTHrP gene expression and secretion and the expression of the bone-related transcription factor Runx2. The 5-HT2 type receptors appear to play an important role in the induction of bone-relevant signals. The complexity of 5-HT signaling demands cautious interpretations and the testing of new hypotheses and additional model systems. With improved knowledge, serotonergic drugs may provide novel opportunities for therapeutic interventions.
GRANTS
This work was supported by NRI Grant no. 2007-35206-17898 from the USDA-NIFA (N. D. Horseman), a grant from the Marlene Harris-Ride Cincinnati Foundation (N. D. Horseman), DOD Grant no. BC095713 (K. A. Gregerson), and a CURE postdoctoral fellowship to L. L. Hernandez from the National Cancer Institute (CA059268).
DISCLOSURES
L. L. Hernandez and N. D. Horseman are coinventors on a provisional patent related to this subject matter.
AUTHOR CONTRIBUTIONS
Author contributions: L.L.H., K.A.G., and N.D.H. conception and design of research; L.L.H. and K.A.G. performed experiments; L.L.H., K.A.G., and N.D.H. analyzed data; L.L.H., K.A.G., and N.D.H. interpreted results of experiments; L.L.H. and N.D.H. prepared figures; L.L.H. and N.D.H. drafted manuscript; L.L.H., K.A.G., and N.D.H. approved final version of manuscript; K.A.G. and N.D.H. edited and revised manuscript.
ACKNOWLEDGMENTS
5-HT7 knockout mice were a generous gift of Peter Hedlund, Scripps Institute. We thank Ekta Yadav and Michael Murawsky for technical assistance.
Current address for L. L. Hernandez: University of Wisconsin-Madison, Dairy Science, 1675 Observatory Dr., Madison, WI 53706.
REFERENCES
- 1. Akech J, Wixted JJ, Bedard K, van der Deen M, Hussain S, Guise TA, van Wijnen AJ, Stein JL, Languino LR, Altieri DC, Pratap J, Keller E, Stein GS, Lian JB. Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29: 811–821, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ardeshirpour L, Dann P, Adams DJ, Nelson T, VanHouten J, Horowitz MC, Wysolmerski JJ. Weaning triggers a decrease in receptor activator of nuclear factor-kappaB ligand expression, widespread osteoclast apoptosis, and rapid recovery of bone mass after lactation in mice. Endocrinology 148: 3875–3886, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bliziotes M. Update in serotonin and bone. J Clin Endocrinol Metab 95: 4124–4132, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bostwick JR, Guthrie SK, Ellingrod VL. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy 29: 1: 64–73, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Broadus AE, Mangin M, Ikeda K, Insogna KL, Weir EC, Burtis WJ, Stewart AF. Humoral hypercalcemia of cancer. Identification of a novel parathyroid hormone-like peptide. N Engl J Med 319: 556–563, 1988 [DOI] [PubMed] [Google Scholar]
- 6. Caplan RH, Wickus GG, Sloane K, Silva PD. Serum parathyroid hormone-related protein levels during lactation. J Reprod Med 40: 216–218, 1995 [PubMed] [Google Scholar]
- 7. Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med 10: e7, 2008 [DOI] [PubMed] [Google Scholar]
- 8. DeMauro S, Wysolmerski J. Hypercalcemia in breast cancer: an echo of bone mobilization during lactation? J Mammary Gland Biol Neoplasia 10: 157–167, 2005 [DOI] [PubMed] [Google Scholar]
- 9. Dobnig H, Kainer F, Stepan V, Winter R, Lipp R, Schaffer M, Kahr A, Nocnik S, Patterer G, Leb G. Elevated parathyroid hormone-related peptide levels after human gestation: relationship to changes in bone and mineral metabolism. J Clin Endocrinol Metab 80: 3699–3707, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Fiaschi-Taesch NM, Stewart AF. Minireview: parathyroid hormone-related protein as an intracrine factor–trafficking mechanisms and functional consequences. Endocrinology 144: 407–411, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Franceschi RT, Xiao G, Jiang D, Gopalakrishnan R, Yang S, Reith E. Multiple signaling pathways converge on the Cbfa1/Runx2 transcription factor to regulate osteoblast differentiation. Connect Tissue Res 44, Suppl 1: 109–116, 2003 [PMC free article] [PubMed] [Google Scholar]
- 12. Galli-Tsinopoulou A, Nousia-Arvanitakis S, Mitsiakos G, Karamouzis M, Dimitriadis A. Osteopenia in children and adolescents with hyperprolactinemia. J Pediatr Endocrinol Metab 13: 439–441, 2000 [DOI] [PubMed] [Google Scholar]
- 13. Graham TR, Agrawal KC, Abdel-Mageed AB. Independent and cooperative roles of tumor necrosis factor-alpha, nuclear factor-kappaB, and bone morphogenetic protein-2 in regulation of metastasis and osteomimicry of prostate cancer cells and differentiation and mineralization of MC3T3–E1 osteoblast-like cells. Cancer Sci 101: 103–111, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guo Y, Tiedemann K, Khalil JA, Russo C, Siegel PM, Komarova SV. Osteoclast precursors acquire sensitivity to breast cancer derived factors early in differentiation. Bone 43: 386–393, 2008 [DOI] [PubMed] [Google Scholar]
- 15. Hedlund PB, Danielson PE, Thomas EA, Slanina K, Carson MJ, Sutcliffe JG. No hypothermic response to serotonin in 5-HT7 receptor knockout mice. Proc Natl Acad Sci USA 100: 1375–1380, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hernandez LL, Limesand SW, Collier JL, Horseman ND, Collier RJ. The bovine mammary gland expresses multiple functional isoforms of serotonin receptors. J Endocrinol 203: 123–131, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Horst RL, Goff JP, Reinhardt TA. Adapting to the transition between gestation and lactation: Differences between rat, human and dairy cow. J Mammary Gland Biol Neoplasia 10: 141–156, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Hou Z, Bailey JP, Vomachka AJ, Matsuda M, Lockefeer JA, Horseman ND. Glycosylation-dependent cell adhesion molecule 1 (GlyCAM 1) is induced by prolactin and suppressed by progesterone in mammary epithelium. 141: 4278–83, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Huang W, Yang S, Shao J, Li YP. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front Biosci 12: 3068–3092, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Inman CK, Shore P. The osteoblast transcription factor Runx2 is expressed in mammary epithelial cells and mediates osteopontin expression. J Biol Chem 278: 48684–48689, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Jackson JC, Cross RJ, Walker RF, Markesbery WR, Brooks WH, Roszman TL. Influence of serotonin on the immune response. Immunology 54: 505–512, 1985 [PMC free article] [PubMed] [Google Scholar]
- 22. Kim HJ, Kim JH, Bae SC, Choi JY, Kim HJ, Ryoo HM. The protein kinase C pathway plays a central role in the fibroblast growth factor-stimulated expression and transactivation activity of Runx2. J Biol Chem 278: 319–326, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther 6: 2609–2617, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Klein M, Weryha G, Dousset B, Aubert V, Kaminsky P, Leclere J. [Physiological role of PTHrP]. Ann Endocrinol (Paris) 56: 193–204, 1995 [PubMed] [Google Scholar]
- 25. Komori T. Regulation of bone development and maintenance by Runx2. Front Biosci 13: 898–903, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Kovacs CS. Calcium and bone metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia 10: 105–118, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Kovacs CS, Chik CL. Hyperprolactinemia caused by lactation and pituitary adenomas is associated with altered serum calcium, phosphate, parathyroid hormone (PTH), and PTH-related peptide levels. J Clin Endocrinol Metab 80: 3036–3042, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Liao J, McCauley LK. Skeletal metastasis: established and emerging roles of parathyroid hormone related protein (PTHrP). Cancer Metastasis Rev 25: 4: 559–571, 2006 [DOI] [PubMed] [Google Scholar]
- 29. Luparello C, Santamaria F, Schilling T. Regulation of PTHrP and PTH/PTHrP receptor by extracellular Ca2+ concentration and hormones in the breast cancer cell line 8701-BC. Biol Chem 381: 303–308, 2000 [DOI] [PubMed] [Google Scholar]
- 30. Marshall AM, Nommsen-Rivers LA, Hernandez LL, Dewey KG, Chantry CJ, Gregerson KA, Horseman ND. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J Clin Endocrinol Metab 95: 837–846, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, Bailey JP, Nieport KM, Walther DJ, Bader M, Horseman ND. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell 6: 193–203, 2004 [DOI] [PubMed] [Google Scholar]
- 32. McGrath MF. A novel system for mammary epithelial cell culture. J Dairy Sci 70: 1967–1980, 1987 [DOI] [PubMed] [Google Scholar]
- 33. Misra M, Papakostas GI, Klibanski A. Effects of psychiatric disorders and psychotropic medications on prolactin and bone metabolism. J Clin Psychiatry 65: 1607–18; quiz 1590, 1760–1761, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2: 584–593, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Murray RD, Horsfield JE, McCormick WD, Williams HJ, Ward D. Historical and current perspectives on the treatment, control and pathogenesis of milk fever in dairy cattle. Vet Rec 163: 561–565, 2008 [DOI] [PubMed] [Google Scholar]
- 36. Pai VP, Horseman ND. Regulation of epithelial turnover by serotonin through induction of cell loss. PLoS One: e17208, 2011. 21383847 [Google Scholar]
- 37. Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem 283: 30901–30910, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res 11: R81, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pai VP, Marshall AM. Intraluminal volume homeostasis: a common sertonergic mechanism among diverse epithelia. Commun Integr Biol 4: 532–537, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pirola CJ, Wang HM, Kamyar A, Wu S, Enomoto H, Sharifi B, Forrester JS, Clemens TL, Fagin JA. Angiotensin II regulates parathyroid hormone-related protein expression in cultured rat aortic smooth muscle cells through transcriptional and post-transcriptional mechanisms. J Biol Chem 268: 1987–1994, 1993 [PubMed] [Google Scholar]
- 41. Pratap J, Javed A, Languino LR, van Wijnen AJ, Stein JL, Stein GS, Lian JB. The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol Cell Biol 25: 8581–8591, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rankin W, Grill V, Martin TJ. Parathyroid hormone-related protein and hypercalcemia. Cancer 80 Suppl: 1564–1571, 1997 [DOI] [PubMed] [Google Scholar]
- 43. Richard V, Rosol TJ, Foley J. PTHrP gene expression in cancer: do all paths lead to Ets? Crit Rev Eukaryot Gene Expr 15: 115–132, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shibli-Rahhal A, Schlechte J. The effects of hyperprolactinemia on bone and fat. Pituitary 12: 96–104, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Shore P. A role for Runx2 in normal mammary gland and breast cancer bone metastasis. J Cell Biochem 96: 484–489, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney CA, Zhang D, Schork A, Crutchfield M, Stanczyk F, Russell-Aulet M. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 276: 549–554, 1996 [PubMed] [Google Scholar]
- 47. Stewart AF. Hyperparathyroidism, humoral hypercalcemia of malignancy, and the anabolic actions of parathyroid hormone and parathyroid hormone-related protein on the skeleton. J Bone Miner Res 17: 758–762, 2002 [DOI] [PubMed] [Google Scholar]
- 48. Stiegler C, Leb G, Kleinert R, Warnkross H, Ramschak-Schwarzer S, Lipp R, Clarici G, Krejs GJ, Dobnig H. Plasma levels of parathyroid hormone-related peptide are elevated in hyperprolactinemia and correlated to bone density status. J Bone Miner Res 10: 751–759, 1995 [DOI] [PubMed] [Google Scholar]
- 49. Stull MA, Pai V, Vomachka AJ, Marshall AM, Jacob GA, Horseman ND. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc Natl Acad Sci USA 104: 16708–16713, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thiede MA. Parathyroid hormone-related protein: a regulated calcium-mobilizing product of the mammary gland. J Dairy Sci 77: 1952–1963, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Thiede MA, Rodan GA. Expression of a calcium-mobilizing parathyroid hormone-like peptide in lactating mammary tissue. Science 242: 278–280, 1988 [DOI] [PubMed] [Google Scholar]
- 52. Uemura H, Yasui T, Umino Y, Yamada M, Kuwahara A, Matsuzaki T, Maegawa M, Irahara M. Regulatory factors on parathyroid hormone-related peptide production by primary culture of lactating rat mammary gland. Horm Metab Res 37: 463–467, 2005 [DOI] [PubMed] [Google Scholar]
- 53. VanHouten JN, Dann P, Stewart AF, Watson CJ, Pollak M, Karaplis AC, Wysolmerski JJ. Mammary-specific deletion of parathyroid hormone-related protein preserves bone mass during lactation. J Clin Invest 112: 1429–1436, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wysolmerski JJ. Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann NY Acad Sci 1192: 161–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]


