Abstract
Citrulline is an amino acid synthesized in the gut and utilized for the synthesis of the conditionally essential amino acid arginine. Recently, the origin of the ornithine utilized for citrulline synthesis has become a matter of discussion. Multiple physiological factors may have contributed to the differences found among different researchers; one of these is the developmental stage of the subjects studied. To test the hypothesis that during the neonatal period de novo synthesis is the main source of ornithine for citrulline synthesis, neonatal piglets were infused intravenously or intragastrically with [U-13C6]arginine, [U-13C5]glutamine, or [U-13C5]proline during the fasted and fed periods. [ureido-15N]citrulline and [2H2]ornithine were infused intravenously for the entire infusion protocol. During fasting, plasma proline (13%) and ornithine (19%) were the main precursors for citrulline synthesis, whereas plasma arginine (62%) was the main precursor for plasma ornithine. During feeding, enteral (27%) and plasma (12%) proline were the main precursors for the ornithine utilized in the synthesis of citrulline, together with plasma ornithine (27%). Enteral proline and glutamine were utilized directly by the gut to produce ornithine utilized for citrulline synthesis. Arginine was not utilized by the gut, which is consistent with the lack of arginase activity in the neonate. Arginine, however, was the main source (47%) of plasma ornithine and in this way contributed to citrulline synthesis. In conclusion, during the neonatal period, the de novo pathway is the predominant source for the ornithine utilized in the synthesis of citrulline, and proline is the preferred precursor.
Keywords: citrulline, arginine, neonate, stable isotope
citrulline is a nonessential amino acid synthesized in hepatocytes and enterocytes by condensation of ornithine and carbamoyl phosphate. In the liver, citrulline functions as part of the urea cycle in the detoxification of ammonia and, because of the channeling of urea cycle intermediates (6), little or no citrulline escapes the liver. The citrulline produced in the small intestine, however, enters the portal vein and appears in the peripheral circulation serving as precursor for arginine synthesis. Recently, the origin of the ornithine utilized for citrulline synthesis has become a matter of discussion (16, 17, 21). Multiple physiological factors may have contributed to the differences found among the different research groups. Feeding vs. fasting, luminal vs. arterial precursors together with species differences are just a few of the physiological factors that may affect the utilization of different precursors for citrulline synthesis. Other factors that add to the differences found by the different research groups are the choice of tracer to determine precursor-product relationships (21) and model employed to interpret the isotopic data (18).
A variable that has not been fully considered when determining the utilization of different precursors for citrulline and arginine synthesis is age. Developmental changes in enteral metabolism take place toward the end of the second week of life in mice, rats, and piglets concurrent with the cortisol surge, the initiation of solid feed ingestion, and gut colonization (25, 26). Among these changes, there are important differences in citrulline and arginine metabolism (for a review, see Ref. 19). The rapid increase in arginase expression (9, 12, 14) that coincides with a reduction in the expression and activity of argininosuccinate synthase and lyase (9, 14) results in the loss of the ability of the gut to make arginine. Changes in proline oxidase and pyrrolidine-5-carboxylate synthase activity during the preweaning period seem to reduce the contribution of proline and glutamine to the ornithine utilized for citrulline production (11, 12, 28, 40, 42). Furthermore, not only ornithine amino transferase (OAT) activity declines toward weaning (12, 28), but the direction of this bidirectional enzyme changes with age from synthesis to disposal of ornithine (36, 37). These enzymatic changes seem to suggest that there is a shift in the precursors utilized for the synthesis of ornithine, and thus citrulline, in the gut. During the neonatal period, the “de novo” route of ornithine production from glutamine and proline seems to be favored, whereas later in life the “preformed” route from arginine and extracellular ornithine may become predominant (Fig. 1). We have shown in adult mice that, in fact, arginine and plasma ornithine are the main precursors for citrulline synthesis (18, 21–23), indicating that the preformed pathway predominates in adulthood. The present experiments were conducted to test the hypothesis that the de novo pathway for ornithine synthesis is the predominant source of ornithine for citrulline synthesis during the neonatal period.
Fig. 1.
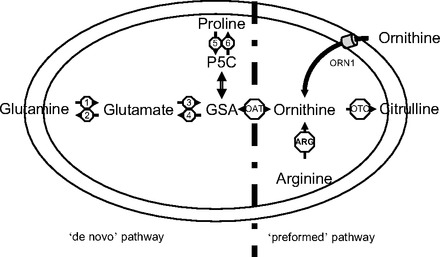
Pathways for the provision of ornithine for citrulline synthesis. The de novo pathway synthesizes glutamate semialdehyde (GSA) from glutamine/glutamate and proline by means of glutaminase (1), pyrroline-5-carboxylate (P5C) synthase (3), and proline oxidase (5). GSA can also be utilized for the synthesis of proline (P5C reductase, 6) and glutamate (P5C dehydrogenase, 4). In the preformed pathway, ornithine originates from the hydrolysis of arginine (ARG arginase) or is from extracellular sources [ornithine transporter 1 (ORN1)]. Ornithine aminotransferase (OAT) is bidirectional and can synthesize or dispose of ornithine. Finally, ornithine is utilized by ornithine transcarbamylase (OTC) for the synthesis of citrulline.
MATERIALS AND METHODS
Animals and Treatments
General.
Newborn (≤1-day-old), crossbred female pigs (n = 6), obtained from the Texas Department of Criminal Justice (Huntsville, TX), were transported to the animal facility of the Children's Nutrition Research Center (Houston, TX). Upon arrival (day 1), piglets were implanted with Silastic catheters in the jugular vein and stomach and a Tygon catheter in the carotid artery as previously described (30). After surgery, piglets were placed in individual cages in a heated room (∼30°C) and fed every 3 h at 50% of their requirement for 24 h. Their respective dietary intake was increased gradually to 100% within the next 3 days. Piglets were fed a cow's milk-based formula for baby pigs (Litter Life; Merrick, Middleton, WI) at 50 g·kg−1·day−1, suspended in 240 ml water, providing fat, protein, and lactose at 5, 12.5, and 25 g·kg−1·day−1, respectively, and including minerals and vitamins. All animal procedures were approved by the Baylor College of Medicine Institutional Animal Care and Use Committee.
Infusion and sampling.
Piglets were infused in three different occasions (days 6, 8, and 10) to investigate the contribution of arginine, glutamine, and proline to the synthesis of citrulline. The order of these three precursors was randomly assigned to each piglet, and there was not carryover isotopic enrichment as determined by the enrichment of background blood samples (time 0 h). On the day of the infusion, piglets were feed deprived for 8 h. After an arterial blood sample (0 h) for isotopic background determinations was collected, a primed-continuous infusion was started. The infusion schedule consisted of a fasted (0–3 h) and fed (3–11 h) periods (Fig. 2). Feeding was accomplished by an oral bolus of 20 ml/kg followed by an 8-h, intragastric continuous infusion of formula at 10 ml·kg−1·h−1. During the fasted phase and the first 4 h of the fed period, [U-13C]arginine, -glutamine, or -proline were infused intravenously to determine the contribution of plasma precursors. During the second part of the fed period (7–11 h), the labeled precursors were infused intragastrically to determine the contribution of enteral precursors to the synthesis of citrulline (Fig. 2). The rate of infusion of these precursors were as follows (iv prime, iv continuous, ig prime, ig continuous): [U-13C6]arginine (20 μmol/kg; 20 μmol·kg−1·h−1; 40 μmol/kg; 40 μmol·kg−1·h−1), [U-13C5]glutamine (27 μmol/kg; 27 μmol·kg−1·h−1; 135 μmol/kg; 135 μmol·kg−1·day−1), and [U-13C5]proline (22.5 μmol/kg; 22.5 μmol·kg−1·h−1; 30 μmol/kg; 30 μmol·kg−1·h−1). Additional tracers were infused intravenously throughout the infusion protocol to determine rates of appearance and conversion. These intravenous tracers (prime and continuous infusion rates) were as follows: [ureido-15N]citrulline (4.8 μmol/kg; 4.8 μmol·kg−1·h−1), [5,5-2H2]ornithine (5.6 μmol/kg; 5.6 μmol·kg−1·h−1), [ring-2H5]phenylalanine (10 μmol/kg; 10 μmol·kg−1·h−1), and [3,5-2H2]tyrosine (3.8 μmol/kg; 3.8 μmol·kg−1·h−1). The infused diet provided 82, 262, 300, 75, and 101 μmol·kg−1·h−1 of arginine, glutamine, proline, tyrosine, and phenylalanine, respectively. All tracers were obtained from Cambridge Isotope Laboratories (Andover, MA).
Fig. 2.
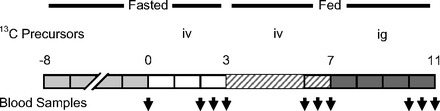
Infusion schedule. Piglets were feed deprived for 8 h before the beginning of the infusion. The infusion schedule consisted of a fasted and fed period. During the fasted period (0–3 h), [U-13Cn]arginine, -glutamine, or -proline were infused iv. During the fed period, the U-13Cn tracers were infused iv (3–7 h) and ig (7–11 h). In addition, citrulline, ornithine, phenylalanine, and tyrosine tracers were infused iv for the entire infusion. Blood samples were collected for isotopic enrichment determination at the beginning of the infusion and at 2, 2.5, 3, 6, 6.5, 7, 10, 10.5, and 11 h.
Arterial blood samples were collected at the end of the fasted period (2, 2.5, and 3 h) and during the fed phase (6, 6.5, 7, 10, 10.5, and 11 h).
Sample analysis.
Plasma amino acid isotopic enrichments were determined as their dansyl derivatives by LC-MS/MS (20) utilizing a TSQ Quantum Ultra System (Thermo Finnigan, San Jose, CA). The parent-daughter ion transitions monitored have been described elsewhere (18).
Calculations.
The calculations have been described in great detail in a previous publication (18). In brief, the rate of appearance and conversion of the different amino acids were determined by isotopic dilution and transfer of the label between precursors and products, respectively. First-pass splanchnic extraction of amino acids was calculated based on the disappearance of the intragastric tracer with respect to the intravenously infused tracer. The contribution of the different enteral and parenteral precursors for citrulline synthesis was determined utilizing a multifactorial approach that takes into account the contribution of all the precursors simultaneously. This is accomplished by solving a set of simultaneous equations in which the fractional contribution of each precursor is multiplied by the observed enrichment of the precursors to yield the observed enrichment of citrulline.
Data analysis.
Rate of appearance and conversion of the different amino acids were analyzed statistically as a complete randomized design utilizing the proc mixed procedure of SAS (version 9.2; SAS Institute, Cary, NC), with fasting or fed as fixed effects and piglet and infusion day (age) as random effects of the model. The multiple equations used for the determination of the fractional contributions of the different precursors to citrulline synthesis were solved for each individual pig, and the data generated were analyzed by ANOVA. Values presented in the text are least square means ± SE and were tested for significance at the 5% level.
RESULTS
All piglets recovered well from the surgery and reached full feed by the third day. All animals gained weight, and the averaged daily weight gain over the whole experiment was 122 ± 18 g/day (60.4 ± 3.4 g·kg−1·day−1). The infused tracers and their products reached isotopic pseudo-plateau enrichment by the end of each sampling period (Figs. 3–5), which allowed us to apply steady-state models.
Fig. 3.
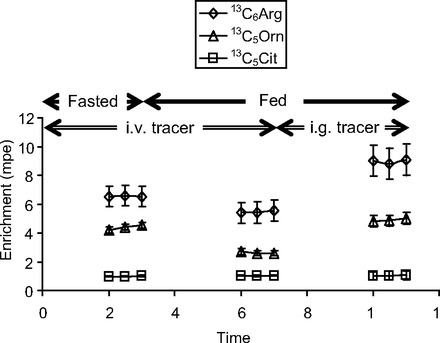
Plasma isotopic enrichment time course of infused [13C6]arginine (Arg) and its products {[13C5]ornithine (Orn) and [13C5]citrulline (Cit)} in neonatal piglets during fasting (0–3 h) and feeding (3–11 h). During feeding, tracers were infused iv (3–7 h) and ig (7–11 h). mpe, Mole %excess.
Fig. 4.
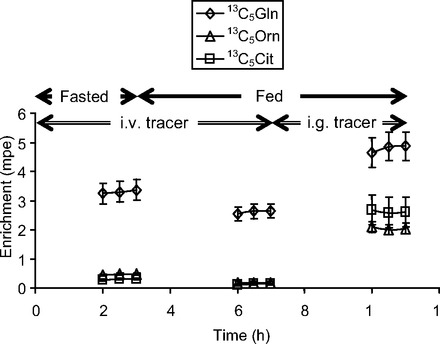
Plasma isotopic enrichment time course of infused [13C5]glutamine (Gln) and its products ([13C5]ornithine and [13C5]citrulline) in neonatal piglets during fasting (0–3 h) and feeding (3–11 h). During feeding, tracers were infused iv (3–7 h) and ig (7–11 h).
Fig. 5.
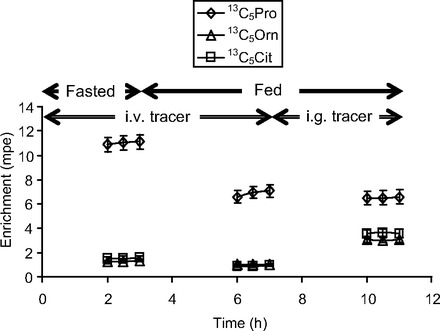
Plasma isotopic enrichment time course of infused [13C5]proline (Pro) and its products ([13C5]ornithine and [13C5]citrulline) in neonatal piglets during fasting (0–3 h) and feeding (3–11 h). During feeding, tracers were infused iv (3–7 h) and ig (7–11 h).
Rate of Amino Acid Appearance and Conversion
The rate of appearance of the amino acids studied increased (P < 0.0001) during the feeding period with the exception of citrulline, which remained unchanged (P = 0.432; Table 1). Of the three precursors studied, glutamine had the highest first-pass splanchnic extraction rate (64.3%), followed by proline (28.1%) and arginine (12.9%; Table 1).
Table 1.
Rate of appearance and first pass extraction of selected amino acids in piglets during fasting and fed conditions
| Fasted, μmol·kg−1·h−1 | Fed, μmol·kg−1·h−1 | P | FPE, % | |
|---|---|---|---|---|
| RaPhe* | 132 ± 7.0 | 173 ± 9.2 | <0.0001 | |
| RaTyr | 121 ± 7.7 | 209 ± 11.5 | <0.0001 | |
| RaCit | 75 ± 6.9 | 74 ± 6.7 | <0.432 | |
| RaOrn | 73 ± 1.9 | 91 ± 6.9 | <0.0001 | |
| RaArg | 293 ± 32.9 | 366 ± 56.1 | <0.0001 | 12.9 ± 2.0 |
| RaPro | 184 ± 10.1 | 313 ± 21.5 | <0.0001 | 28.1 ± 5.0 |
| RaGln | 810 ± 85.9 | 1,028 ± 94.2 | <0.0001 | 64.3 ± 3.7 |
Values are means ± SE; n = 6 experiments, except for ornithine (Orn), citrulline (Cit), phenylalanine (Phe), and tyrosine (Tyr) rate of appearance (Ra) where n = 18. FPE, first pass splanchnic extraction, calculated from the disappearance of the ig tracer with respect to the iv infused tracer. Arg, arginine; Pro, proline; Gln, glutamine.
Rate of appearance, calculated from the isotopic dilution of the iv infused tracer.
During fasting, the rate of conversion of phenylalanine into tyrosine (phenylalanine hydroxylation) accounted for a small percentage (2.9 ± 0.2%) of the rate of appearance of phenylalanine. During feeding, the rate of phenylalanine hydroxylation increased both in absolute and relative terms (P < 0.001) but still accounted for a small faction (<4%) of the phenylalanine rate of appearance (Table 2). Plasma ornithine contributed ∼14 and 20 μmol·kg−1·h−1 to the synthesis of citrulline in the fasted and fed state, respectively; this contribution represented almost 19 and 27% of the rate of appearance of citrulline and accounted for ∼19 and 22% of the fate of circulating ornithine. The rate of conversion of citrulline to arginine (de novo arginine synthesis) increased (P < 0.0001) during feeding but contributed a similar percentage (∼15%; P = 0.915) of the rate of appearance of arginine during the fasted and fed periods. Plasma arginine was the fate of 55 and 73% of the rate of appearance of citrulline during fasting and feeding, respectively (P < 0.0001; Table 2). Plasma arginine to plasma arginine recycling accounted for ∼8 and 14 μmol·kg−·h−1 during fasting and feeding, respectively, a small fraction (<4%) of the rate of appearance of arginine.
Table 2.
Rate of conversion of selected amino acids in piglets during fasting and fed conditions
| Fasted, μmol·kg−1·h−1 | Fed, μmol·kg−1·h−1 | P | |
|---|---|---|---|
| RcPhe to Tyr | |||
| μmol·kg−1·h−1 | 3.8 ± 0.4 | 6.9 ± 0.8 | <0.0001 |
| As%RaPhe | 2.9 ± 0.2 | 4.0 ± 0.3 | <0.0001 |
| As%RaTyr | |||
| RcOrn to Cit | |||
| μmol·kg−1·h−1 | 13.6 ± 0.7 | 19.4 ± 2.0 | <0.0001 |
| %RaCit | 18.6 ± 1.2 | 26.7 ± 1.8 | <0.0001 |
| %RaOrn | 18.8 ± 0.9 | 21.5 ± 1.0 | <0.0001 |
| RcCit to Arg | |||
| μmol·kg−1·h−1 | 44.5 ± 9.9 | 62.1 ± 13.6 | <0.0001 |
| %RaArg | 15.6 ± 2.6 | 15.6 ± 1.3 | <0.915 |
| %RaCit | 55.4 ± 7.0 | 73.9 ± 6.3 | <0.0001 |
| RcArg to Arg | |||
| μmol·kg−1·h−1 | 8.3 ± 1.5 | 13.7 ± 5.6 | <0.0003 |
| %Ra | 2.8 ± 0.4 | 3.5 ± 0.8 | <0.0126 |
Values are means ± SE; n = 18, except for RcArg to Arg where n = 6. Rc, rate of conversion.
Citrulline Precursors
During the fasting period, the contribution of the three arterial precursors studied accounted for ∼28% of the rate of appearance of citrulline (Table 3). Proline was the main precursor for citrulline synthesis (P < 0.001), followed by glutamine and arginine. Whereas proline and glutamine made most of their contribution at the site of citrulline synthesis, arginine contribution was made through plasma ornithine. In fact, plasma arginine was the main precursor for circulating ornithine, accounting for ∼62% of the rate of appearance of this amino acid.
Table 3.
Precursor contribution to the synthesis of ornithine and citrulline in fasted and fed piglets
| Precursor Contribution to the Synthesis of |
||||
|---|---|---|---|---|
| Citrulline as %RaCitrulline |
||||
| Ornithine as %RaOrnithine | From Plasma Ornithine | In Gut | Total | |
| Fasting | ||||
| Arterial | ||||
| Arginine | 61.6 ± 3.72 | 11.5 ± 0.71 | −4.8 ± 0.40 | 6.7 ± 0.63 |
| Glutamine | 20.1 ± 3.55 | 3.7 ± 0.62 | 4.8 ± 0.80 | 8.5 ± 0.28 |
| Proline | 10.4 ± 1.35 | 2.0 ± 0.30 | 10.9 ± 2.0 | 12.9 ± 1.92 |
| Ornithine | 18.6 ± 0.56 | |||
| Sum fasting | 92.1 ± 5.32 | 28.0 ± 2.04 | ||
| Fed | ||||
| Arterial | ||||
| Arginine | 47.0 ± 6.99 | 12.6 ± 1.93 | −5.5 ± 1.30 | 7.1 ± 0.67 |
| Glutamine | 16.4 ± 2.55 | 4.4 ± 0.70 | −0.1 ± 0.79* | 4.3 ± 0.62 |
| Proline | 14.8 ± 1.12 | 3.9 ± 0.33 | 8.3 ± 1.31 | 12.2 ± 1.10 |
| Ornithine | 26.7 ± 0.72 | |||
| Enteral | ||||
| Arginine | 0.4 ± 0.67* | 0.1 ± 0.18* | 1.1 ± 0.27 | 1.2 ± 0.34 |
| Glutamine | 2.4 ± 0.27 | 0.6 ± 0.07 | 4.1 ± 0.87 | 4.7 ± 0.88 |
| Proline | 18.6 ± 2.62 | 5.0 ± 0.81 | 22.8 ± 2.87 | 27.8 ± 2.44 |
| Sum fed | 99.5 ± 8.00 | 57.3 ± 2.98 | ||
Values are means ± SE.
Not different from zero.
During feeding, the contribution of the three precursors studied accounted for ∼57% of the rate of appearance of citrulline. The contribution of arterial and enteral proline accounted for ∼40% of the rate of appearance of citrulline (Table 3). Whereas the contribution of plasma glutamine was through plasma ornithine, enteral glutamine made its contribution directly at the site of citrulline synthesis. Arginine contributed to the synthesis of citrulline, but mainly through plasma ornithine (Table 3). Plasma arginine was the main precursor for circulating ornithine, accounting for ∼47% of the rate of appearance of this amino acid, whereas the contribution of enteral arginine was not different from zero (P = 0.541). Plasma and enteral proline contributed to a similar extent to plasma ornithine; the contribution of glutamine to the synthesis of ornithine, however, was mainly from plasma sources (Table 3).
DISCUSSION
The differences in the contribution of the different precursors utilized for citrulline and arginine synthesis reported by different research groups has been due, at least in part, to the different tracers (21) and models (18) used to interpret the tracer data. Another overlooked factor in the utilization of the different precursors for citrulline synthesis is developmental stage. There are major enzymatic changes between the neonatal and postweaning periods in humans, piglets, and rodents (12, 15, 28) that could translate in the utilization of different precursors in the synthesis of ornithine for citrulline production.
In the present work, all possible dietary and plasma precursors for citrulline synthesis were investigated during fasting and fed periods in neonatal pigs. This allowed for a direct comparison of the contribution of the different precursors to the synthesis of citrulline.
Rate of Amino Acid Appearance and Conversion
As expected, the rate of appearance of the amino acids studied increased during feeding. The only exception was citrulline, which remained unchanged. In humans, citrulline production has been shown to be rather constant and independent from feeding or arginine content of the diet (5, 31). In mice, however, we have shown that feeding increased the rate of appearance of citrulline (18).
The rates of appearance of phenylalanine and tyrosine were similar to the ones reported in conventionally and parenterally fed piglets of similar age (7, 13). Neonatal metabolism is characterized by high rates of protein synthesis (8) and dietary nitrogen retention (>85%, see Ref. 13). The low rate of plasma phenylalanine hydroxylation observed (∼3 and 4% for the fasted and fed period, respectively) demonstrates the high efficiency of amino acid recycling, utilization, and deposition during the neonatal period.
To the best of our knowledge, there have been no reports on the rate of appearance of citrulline and ornithine in neonatal pigs. Urschel et al. (33), however, infused labeled citrulline and ornithine intragastrically and calculated “enteral” fluxes. Because of the first-pass splanchnic disappearance of the tracers infused, these fluxes overestimate the real rate of appearance of these amino acids, which is consistent with our results.
The rate of appearance of arginine was within the wide range published (32, 34, 35); the first-pass extraction measured in the current communication (12%), however, was lower than the 50% reported by others (35). Urschel et al. (35) reported no changes in first splanchnic extraction despite a ninefold difference in arginine intake, which is surprising because the liver is the main site for (excess) arginine disposal (27).
The rate of appearance of glutamine and proline was similar to previous published observations (29, 34). Our data on the first-pass splanchnic extraction of glutamine and proline agrees with the reduced extraction seen by others (for a review, see Ref. 2). Whereas the first-pass extraction of dietary glutamine and proline is mostly by the gut (2), a reduced intestinal utilization of arginine by the gut is expected due to the lack of arginase during the neonatal period (9).
The contribution of plasma citrulline to de novo arginine production was ∼16% of the rate of appearance of arginine, a value similar to the one reported in humans (11%; see Ref. 4) and mice (16–26%; see Ref. 18). Not all the circulating citrulline, however, was accounted for as plasma arginine, indicating that a fraction of the citrulline flux is used by different cell types to meet local arginine needs (10). The recycling of plasma arginine for the synthesis of arginine was lower than the one reported in piglets fed a high-arginine (20%) or deficient arginine (35%; see Ref. 34) diets.
Citrulline Precursors
The multifactorial model utilized to integrate the tracer data takes simultaneously into account all the precursors for citrulline synthesis, thus avoiding some overaccounting seen by other models (18). We were able to account for 28 and 57% of the precursors for citrulline synthesis during fasting and feeding, respectively. The small contribution of other citrulline sources (nitric oxide synthesis, recycling of citrullinated proteins, and catabolism of methylarginines) is not included in these calculations. The failure to account for 100% of the citrulline produced was due to the utilization of unlabeled precursors released from protein breakdown or synthesized within the enterocyte; as expected, this contribution was greater during fasting than in the fed state.
Arginine contribution to the rate of appearance of citrulline (∼7–8%) was done through plasma ornithine, with very little, if any, direct arginine utilization by the gut in both fasting and fed conditions. This is consistent with the lack of arginase activity during the neonatal period (9, 39), and with our previous observations in arginase II knockout mice (23). Thus ornithine generated from arginine in other organs can enter the circulation, be taken up by the enterocytes, and contribute to the synthesis of citrulline. The utilization of extracellular ornithine by the gut has also been shown by the 40 and 75% recovery of intragastrically infused labeled ornithine as circulating citrulline in piglets fed an adequate and low-arginine diet, respectively (33).
The contribution of glutamine for citrulline synthesis was also modest (∼8–10%); glutamine provided roughly the same amount directly in the gut and through plasma ornithine during feeding. No reports on in vivo glutamine utilization for the synthesis of citrulline in the neonatal piglet are available, but glutamate has been shown to be a poor precursor (38). In vitro studies, however, seem to suggest that glutamine is in fact an important precursor (39, 41). This disagreement between the in vivo and in vitro data seems to indicate the challenge of in vitro systems to mimic complex multiprecursor interorgan processes.
Proline has been considered the main precursor for citrulline synthesis in the piglet (1, 3, 33). Whereas the incorporation of the proline tracer into citrulline and arginine in these reports is incontrovertible, the actual quantification of this contribution is confounded, due to the model used to integrate the tracer data (18). The utilization of this incorrect model, which ignores the first-pass utilization of enteral precursors to the synthesis of citrulline, not only results in a greater contribution of proline but also of glutamine (54 and 59% of circulating citrulline, respectively). In the present communication, we have demonstrated that dietary and plasma proline are in fact the main precursors for citrulline synthesis in the neonatal piglet during fasting and fed conditions. Interestingly, it has been shown that enteral proline is a better precursor than arterial proline (3, 32), which we have also confirmed in this report. The contribution of the different enteral amino acids to the rate of appearance of ornithine indicates that not only arginase is absent in the neonatal gut but that proline was the main substrate for enteral OAT.
The predominance of the de novo pathway of ornithine synthesis for citrulline production seems not only due to the lack of arginase activity in the neonatal gut but probably to the activity of OAT, which during this period works toward the synthesis of ornithine. Clear evidence of the change of direction of OAT, from synthesis during the neonatal period to disposal of ornithine during adulthood, can be found in the “paradoxical” neonatal hypo-ornithemia and hyperornithemia after weaning in OAT knockout mice, which mimics similar findings reported in humans with gyrate atrophy (37). In addition, the overexpression of OAT results in a decrease in the plasma concentration of ornithine in adult mice (36). The importance of OAT for the provision of ornithine during the neonatal period is evident in mice lacking OAT, which require arginine supplementation for their survival during the first two weeks of life, but not thereafter (37).
Although enterocytes readily utilize extracellular ornithine for citrulline synthesis when available (33), ornithine generated from the disposal of arginine during this period is probably limited. The high arginine demand for protein synthesis, together with the reduced amount of arginine present in the diet fed compared with other studies, likely resulted in a small fraction of arginine being disposed through arginase with the concomitant reduction in the production of ornithine by this route. For these reasons, it is likely that feeding diets with generous amounts of arginine may result in an increased arginine contribution to citrulline synthesis through plasma ornithine.
In conclusion, during the neonatal period, the de novo pathway is the predominant source for the ornithine utilized in the synthesis of citrulline, and proline is the preferred precursor. The lack of enteral arginase precludes the direct utilization of arginine by the gut; plasma arginine, however, contributes to the synthesis of citrulline through plasma ornithine.
GRANTS
This work was supported by federal funds from the United States Department of Agriculture, Agricultural Research Service under Cooperative Agreement No. 58-6250-6-001 and the National Institutes of Research Resources (K01 RR-024173).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: J.C.M. and D.G.B. conception and design of research; J.C.M., B.S., and I.C.D. performed experiments; J.C.M., B.S., and D.G.B. interpreted results of experiments; J.C.M. prepared figures; J.C.M. drafted manuscript; J.C.M., B.S., and D.G.B. edited and revised manuscript; J.C.M., B.S., I.C.D., and D.G.B. approved final version of manuscript; J.C.M. analyzed data.
REFERENCES
- 1. Bertolo RF, Brunton JA, Pencharz PB, Ball RO. Arginine, ornithine, and proline interconversion is dependent on small intestinal metabolism in neonatal pigs. Am J Physiol Endocrinol Metab 284: E915–E922, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Bertolo RF, Burrin DG. Comparative aspects of tissue glutamine and proline metabolism. J Nutr 138: 2032S–2039S, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Brunton JA, Bertolo RF, Pencharz PB, Ball RO. Proline ameliorates arginine deficiency during enteral but not parenteral feeding in neonatal piglets. Am J Physiol Endocrinol Metab 277: E223–E231, 1999 [DOI] [PubMed] [Google Scholar]
- 4. Castillo L, Beaumier L, Ajami AM, Young VR. Whole body nitric oxide synthesis in healthy men determined from [15N]arginine-to-[15N]citrulline labeling. Proc Nat Acad Sci USA 93: 11460–11465, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Castillo L, Sanchez M, Vogt J, Chapman TE, Derojas-Walker TC, Tannenbaum SR, Ajami AM, Young VR. Plasma arginine, citrulline, and ornithine kinetics in adults, with observations on nitric oxide synthesis. Am J Physiol Endocrinol Metab 268: E360–E367, 1995 [DOI] [PubMed] [Google Scholar]
- 6. Cheung CW, Cohen NS, Raijman L. Channeling of urea cycle intermediates insitu in permeabilized hepatocytes. J Biol Chem 264: 4038–4044, 1989 [PubMed] [Google Scholar]
- 7. Cvitkovic S, Bertolo RFP, Brunton JA, Pencharz PB, Ball RO. Enteral tryptophan requirement determined by oxidation of gastrically or intravenously infused phenylalanine is not different from the parenteral requirement in neonatal piglets. Pediatr Res 55: 630–636, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Davis TA, Fiorotto ML. Regulation of muscle growth in neonates. Curr Opin Clin Nutr 12: 78–85, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. De Jonge WJ, Dingemanse MA, De Boer PAJ, Lamers WH, Moorman AFM. Arginine-metabolizing enzymes in the developing rat small intestine. Pediatr Res 43: 442–451, 1998 [DOI] [PubMed] [Google Scholar]
- 10. Featherston WR, Rogers QR, Freedland RA. Relative importance of kidney and liver in synthesis of arginine by the rat. Am J Physiol 224: 127–129, 1973 [DOI] [PubMed] [Google Scholar]
- 11. Herzfeld A, Mezl VA, Knox WE. Enzymes metabolizing delta1-pyrroline-5-carboxylate in rat tissues. Biochem J 166: 95–103, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Herzfeld A, Raper SM. Enzymes of ornithine metabolism in adult and developing rat intestine. Biochim Biophys Acta 428: 600–610, 1976 [DOI] [PubMed] [Google Scholar]
- 13. House JD, Pencharz PB, Ball RO. Phenylalanine requirements determined by using l-[1–14C] phenylalanine in neonatal piglets receiving total parenteral nutrition supplemented with tyrosine. Am J Clin Nutr 65: 984–993, 1997 [DOI] [PubMed] [Google Scholar]
- 14. Hurwitz R, Kretchmer N. Development of arginine-synthesizing enzymes in mouse intestine. Am J Physiol Gastrointest Liver Physiol 251: G103–G110, 1986 [DOI] [PubMed] [Google Scholar]
- 15. Kohler ES, Sankaranarayanan S, Van Ginneken CJ, Van Dijk P, Vermeulen JLM, Ruijter JM, Lamers WH, Bruder E. The human neonatal small intestine has the potential for arginine synthesis; developmental changes in the expression of arginine-synthesizing and -catabolizing enzymes (Abstract). BMC Dev Biol 8: 107, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ligthart-Melis GC, Deutz NEP. Is glutamine still an important precursor of citrulline? Am J Physiol Endocrinol Metab 301: E264–E266, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ligthart-Melis GC, Vermeulen MAR, Van Leeuwen PAM, Deutz NEP. Glutamine: Precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 299: E683–E695, 2010 [DOI] [PubMed] [Google Scholar]
- 18. Marini JC. Arginine and ornithine are the main precursors for citrulline synthesis in mice. J Nutr 142: 572–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marini JC. Citrulline and urea metabolism in the gut. EAAP Scientific Series 127: 87–98, 2010 [Google Scholar]
- 20. Marini JC. Quantitative analysis of 15N-labeled positional isomers of glutamine and citrulline via electrospray ionization tandem mass spectrometry of their dansyl derivatives. Rapid Comm Mass Spectrom 25: 1291–1296, 2011 [DOI] [PubMed] [Google Scholar]
- 21. Marini JC, Didelija IC, Castillo L, Lee B. Glutamine: precursor or nitrogen donor for citrulline synthesis? Am J Physiol Endocrinol Metab 299: E69–E79, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Marini JC, Didelija IC, Castillo L, Lee B. Plasma arginine and ornithine are the main citrulline precursors in mice infused with arginine-free diets. J Nutr 140: 1432–1437, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marini JC, Keller B, Didelija IC, Castillo L, Lee B. Enteral arginase II provides ornithine for citrulline synthesis. Am J Physiol Endocrinol Metab 300: E188–E194, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nanthakumar NN, Dai D, Meng D, Chaudry N, Newburg DS, Walker WA. Regulation of intestinal ontogeny: effect of glucocorticoids and luminal microbes on galactosyltransferase and trehalase induction in mice. Glycobiology 15: 221–232, 2005 [DOI] [PubMed] [Google Scholar]
- 26. Nanthakumar NN, Henning SJ. Ontogeny of sucrase-isomaltase gene expression in rat intestine: Responsiveness to glucocorticoids. Am J Physiol Gastrointest Liver Physiol 264: G306–G311, 1993 [DOI] [PubMed] [Google Scholar]
- 27. Pan M, Choudry HA, Epler MJ, Meng QH, Karinch A, Lin CM, Souba W. Arginine transport in catabolic disease states. J Nutr 134: 2826S–2829S, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Riby JE, Hurwitz RE, Kretchmer N. Development of ornithine metabolism in the mouse intestine. Pediatr Res 28: 261–265, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Stoll B, Burrin DG, Henry J, Yu H, Jahoor F, Reeds PJ. Substrate oxidation by the portal drained viscera of fed piglets. Am J Physiol Endocrinol Metab 277: E168–E175, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Stoll B, Puiman PJ, Cui L, Chang X, Benight NM, Bauchart-Thevret C, Hartmann B, Holst JJ, Burrin DG. Continuous parenteral and enteral nutrition induces metabolic dysfunction in neonatal pigs. J Parenter Enteral Nutr 36: 538–550, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tharakan JF, Yu YM, Zurakowski D, Roth RM, Young VR, Castillo L. Adaptation to a long term (4 weeks) arginine- and precursor (glutamate, proline and aspartate)-free diet. Clin Nutr 27: 513–522, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Urschel KL, Evans AR, Wilkinson CW, Pencharz PB, Ball RO. Parenterally fed neonatal piglets have a low rate of endogenous arginine synthesis from circulating proline. J Nutr 137: 601–606, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Urschel KL, Rafii M, Pencharz PB, Ball RO. A multitracer stable isotope quantification of the effects of arginine intake on whole body arginine metabolism in neonatal piglets. Am J Physiol Endocrinol Metab 293: E811–E818, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Urschel KL, Shoveller AK, Pencharz PB, Ball RO. Arginine synthesis does not occur during first-pass hepatic metabolism in the neonatal piglet. Am J Physiol Endocrinol Metab 288: E1244–E1251, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Urschel KL, Shoveller AK, Uwiera RRE, Pencharz PB, Ball RO. Citrulline is an effective arginine precursor in enterally fed neonatal piglets. J Nutr 136: 1806–1813, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Ventura G, De Bandt JP, Segaud F, Perret C, Robic D, Levillain O, Le Plenier S, Godard C, Cynober L, Moinard C. Overexpression of ornithine aminotransferase: consequences on amino acid homeostasis. Br J Nutr 101: 843–851, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Wang T, Lawler AM, Steel G, Sipila I, Milam AH, Valle D. Mice lacking ornithine-delta-amino-transferase have paradoxical neonatal hypoornithinaemia and retinal degeneration. Nat Genet 11: 185–190, 1995 [DOI] [PubMed] [Google Scholar]
- 38. Wilkinson DL, Bertolo RFP, Brunton JA, Shoveller AK, Pencharz PB, Ball RO. Arginine synthesis is regulated by dietary arginine intake in the enterally fed neonatal piglet. Am J Physiol Endocrinol Metab 287: E454–E462, 2004 [DOI] [PubMed] [Google Scholar]
- 39. Wu G, Knabe DA. Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol Endocrinol Metab 269: E621–E629, 1995 [DOI] [PubMed] [Google Scholar]
- 40. Wu GY. Synthesis of citrulline and arginine from proline in enterocytes of postnatal pigs. Am J Physiol Gastrointest Liver Physiol 273: G1382–G1390, 1997 [DOI] [PubMed] [Google Scholar]
- 41. Wu GY, Knabe DA, Flynn NE. Synthesis of citrulline from glutamine in pig enterocytes. Biochem J 299: 115–121, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yamada E, Wakabayashi Y. Development of pyrroline-5-carboxylate synthase and n-acetylglutamate synthase and their changes in lactation and aging. Arch Biochem Biophys 291: 15–23, 1991 [DOI] [PubMed] [Google Scholar]


