Abstract
Photoreceptor cyclic nucleotide gated (CNG) channels are critical elements in phototransduction and light adaptation. Here we report that insulin receptor (IR), an integral membrane protein, directly phosphorylates the CNGA1 subunit of CNG channels that in turn affects the function of these channels negatively. The IR phosphorylates Tyr498 and Tyr503 residues on CNGA1 that are situated at the membrane-cytoplasmic interface. The IR tyrosine kinase activity is essential for the inhibition of CNG channel. To maintain the channels in an off state, it is necessary not only to have a precise balance of the cGMP levels but also to have a control on the cGMP sensitivity of the CNG channels itself. In this study, we observed that the channel opens at a lower concentration of cGMP in IR−/− mice. These studies suggest that IR regulates the modulation of CNG channel activity in vivo.
Keywords: cyclic nucleotide gated channels; insulin receptor; rod outer segments; phosphorylation; guanosine 3′,5′-cyclic monophosphate
cyclic nucleotide gated (CNG) channels play an important role in visual and olfactory transduction (1, 7, 15, 22). These are crucial for generating the light response in photoreceptors and are directly and cooperatively gated by cGMP. These are critical elements in the maintenance of accurate balance of pace and sensitivity required for phototransduction and play a vital role in photoreceptor homeostasis. CNG channels respond to slight alterations in cytosolic cGMP and have very swift gating kinetics. They transduce a decrease in cGMP into hyperpolarization during the light response and vice versa. These heteromeric, nonselective cation channels in rods are comprised of three α (CNGA1)- and one β (CNGB1)-subunits (39). The expressed homotetramers of α-subunits of CNG channels are also equally functional (27). They contain an NH2-terminal and a large COOH-terminal nucleotide binding cytoplasmic domain. The critical roles played by CNG channels necessitate comprehensive regulation of its function at several regulatory points. The sensitivity of CNG channel toward cGMP has been extensively studied and is shown to be regulated by several factors other than the cGMP availability like phosphorylation state (18, 26), divalent cations (10, 14), Ca2+/calmodulin (13, 17), diacylglycerol (9), and phospholipids (41). We have reported previously that Grb14, an insulin receptor (IR)-binding protein, directly binds to the COOH-terminal region (CTR) of CNGA1 subunit and inhibits channel activity in a light-dependent manner (11). The interaction is mediated through a Ras-associating domain of Grb14 binding to Ras-like domain in CNGA1 (11, 12, 36). Our studies also suggest that Grb14 is a competitive inhibitor of cGMP, and thereby it facilitates the closure of the channel to an off state (11). The interaction between Grb14 and CNGA1 is a protein-protein interaction (36).
Phosphorylation of CNG channels has been shown to control its function in several studies (18, 24, 26), but a detailed study about the residues involved and the responsible protein kinase(s) has not been done so far. In this study, we examined the effect of IR on CNG channels. IRs are widely present in photoreceptor outer segment membranes (30) where CNG channels are predominantly concentrated (15). The effect of IR on CNG channel regulation is convincingly demonstrated through the use of genetic, pharmacological treatments, coexpression studies, and bioinformatics tools. Our studies suggest that CNGA1 is a direct substrate of IR, and IR tyrosine kinase activity is essential for the modulation of CNG channel activity.
MATERIALS AND METHDOS
Cell lines and culture condition.
HEK 293T cells were maintained in DMEM medium containing 10% (vol/vol) FBS at 37°C. Approximately 2.5 × 105 cells were seeded in each 60-mm culture dish 12–18 h before transfection. Calcium phosphate-mediated DNA transfection was performed using each of the plasmids containing the cDNA of interest (40), and cells were harvested for experiments ∼48 h posttransfection.
Plasmids and DNA.
Myc-tagged bovine Grb14 construct was cloned as described previously (31). Myc-tagged CNGA1 (wild-type) construct was generated from the amplification of pCDNA3-CNGA1 as explained previously (11). Site-directed mutagenesis (SDM) was carried out according to the method described earlier (35). The primers used for SDM are CNGA1-Y498F (sense: CAA GTC TTC GCT CCT GGA GAT TAC ATT TGC; antisense GCA AAT GTA ATC TCC AGG AGC GAA GAC TTG ), CNGA1-Y503F (sense: CAA GTC TAC GCT CCT GGA GAT TTC ATT TGC; antisense: GCA AAT GAA ATC TCC AGG TGC GTA GAC TTG), and CNGA1-Y498/503F (sense: CAA GTC TTC GCT CCT GGA GAT TTC ATT TGC; antisense: GCA AAT GAA ATC TCC AGG AGC GAA GAC TTG). After sequencing, the mutant cDNAs were excised from the sequencing vector and cloned into pCDNA3 mammalian expression vector. Wild-type and mutant IR (Met-1153-Ile) constructs were kindly provided by Dr. Michael Quon, National Institute of Diabetes and Digestive and Kidney Diseases. The wild-type and mutant CTR of CNGA1 (residues 483–690) (Y498F; Y503F; Y498, 503F) were amplified from the respective full-length clones using the primers (sense: GAA TTC ACC ATG GAT TAC AAG GAT GAC GAC GAT AAG GCT TGG TCT GTT GGT GGAG; antisense: CTC GAG TCA GTC CTG TGT AGA GTC TGT GGG CCC ACT TTC) and cloned into PGEX-4T2 vector with 5′-ECoRI and 3′-XhoI sites for generation of glutathione S-transferase (GST)-tagged constructs. pCDNA3-CNGA1 clone and CNGA1 specific channel antibody were kind gifts from Dr. Robert Molday, University of British Columbia (Canada).
Animals.
All animal work was in strict accordance with the National Institutes of Health Guide for the Care Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology on the Use of Animals in Vision Research. All of the protocols were approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center and the Dean McGee Eye Institute. The generation of photoreceptor-specific conditional IR−/− mice has been reported previously (32). A breeding colony of albino Sprague-Dawley rats is maintained in our vivarium in cyclic light (12 h on/off; ∼300 lux). Experiments were carried out on both male and female rats (150–200 g).
Assessment of the channel activity by ratiometric measurement of intracellular Ca2+ concentration.
The fluorescent indicator indo 1-AM was used to monitor Ca2+ influx through the CNGA1 channels in cell suspensions. The assays were performed as described (11) using a spectrofluorometer (Fluostar Omega; BMG lab tech, Offenburg, Germany). This assay was designed to determine CNG channel activity in cell populations (2 × 106) in response to 8-para-chloro phenyl thio (pCPT)-cGMP stimulation. Briefly, cells (36–48 h posttransfection) were harvested with cell dissociation medium (Invitrogen, Carlsbad, CA), washed with the extracellular solution (ECS; 140 mM NaCl, 5 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, 10 mM glucose, and 15 mM HEPES, pH 7.4), and incubated with 2 μM indo 1-AM (Sigma-Aldrich) in ECS in the presence of 0.05% Pluronic F-127 (Invitrogen) for 40 min at room temperature. Next, the cells were washed three times with ECS and resuspended in ECS (1 × 106/ml). Ca2+ influx in response to 8-pCPT-cGMP was determined by ratiometric measurement, which represents the free intracellular Ca2+ concentration ([Ca2+]i). Changes of [Ca2+]i were expressed as a Δ405/485 ratio.
Assessment of the channel activity in reactive oxygen species vesicles.
Mouse rod outer segments (ROS) were prepared on a discontinuous sucrose gradient (31) and washed two times in buffer that removes all soluble proteins (13). The CNG channel activity in IR−/− mouse ROS was investigated using fluo 3, a fluorometric calcium ion assay (11). The ROS membranes were suspended in buffer containing 10 μM fluo 3, a fluorescent Ca2+ indicator, and sonicated. The sonicated ROS membranes were then extruded three times through a mini-Teflon and stainless steel lipid extruder (Avanti Polar Lipids) at room temperature, containing two layers of nucleopore polycarbonate membranes with pore sizes of 400 and 200 nm. This method is regularly used by us and others to prepare vesicles (23). The vesicles were dialyzed extensively at 4°C for 6 h to exclude the untrapped dye. The whole experiment was carried out in the dark to minimize the light bleaching effect on fluo 3. The sample was diluted, and the Ca2+ influx assay was performed as described previously (11). Stock CaCl2 was then added to achieve a final concentration of 100 μM. One minute after the addition of Ca2+, the Ca2+ influx assay was initiated by the addition of different cGMP concentrations. Free Ca2+ concentration was measured by emission at 520 nm using a fluorometer.
Ex vivo retinal organ culture preparation.
Retinas were removed from Sprague-Dawley albino rats that were born and raised in dim cyclic light (5 lux; 12 h on/12 h off) and incubated either in dark or light at 300 lux in Dulbecco's modified Eagle's medium (Invitrogen) in the presence or absence of DMSO, HNMPA-(AM)3 (Calbiochem), or insulin followed by snap-freezing in liquid nitrogen. The retinas were lysed in lysis buffer [1% Nonidet P-40, 20 mM HEPES (pH 7.4), and 2 mM EDTA] containing phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O7, 1 mM NaVO3, and 1 mM molybdate) and protease inhibitors [10 μM leupeptin, 10 μg/ml aprotinin, and 1 mM phenylmethylsulfonyl fluoride (PMSF)] and kept on ice for 10 min followed by centrifugation at 4°C for 20 min.
Immunoprecipitation.
Retinas were lysed in a lysis buffer [1% Nonidet P-40, 20 mM HEPES (pH 7.4), and 2 mM EDTA] containing phosphatase inhibitors (100 mM NaF, 10 mM Na4P2O7, 1 mM NaVO3, and 1 mM molybdate) and protease inhibitors (10 μM leupeptin, 10 μg/ml aprotinin, and 1 mM PMSF) and kept on ice for 10 min. Insoluble material was removed by centrifugation at 17,000 g for 20 min at 4°C. Retina lysates were precleared by incubation with 40 μl of protein A-Sepharose for 1 h at 4°C with mixing. The supernatant was incubated with anti-CNGA1 antibody overnight at 4°C and subsequently with 40 μl of protein A-Sepharose for 2 h at 4°C. Following centrifugation at 14,000 rpm for 1 min, immune complexes were washed three times with wash buffer [50 mM HEPES (pH 7.4) containing 118 mM NaCl, 100 mM NaF, 2 mM NaVO3, 0.1% (wt/vol) SDS, and 1% (vol/vol) Triton X-100]. Immunoprecipitates were subjected to immunoblot analysis with anti-CNGA1 and anti-PY-99 (1:1,000) antibodies.
SDS-PAGE and western blotting.
Proteins were resolved by 10% SDS-PAGE and transferred onto nitrocellulose membranes. The blots were washed two times for 10 min with 20 mM Tris·HCl (pH 7.4), 100 mM NaCl, and 0.1% Tween 20 (TTBS) and blocked with either 5% BSA or nonfat dry milk powder (Bio-Rad) in TTBS for 1 h at room temperature. Blots were then incubated with anti-IRβ (1:1,000), anti-phosphotyrosine (PY99, 1:1,000), anti-IR [Tyr(P)1158/1162/1163] (1:1,000), anti-IRβ (1:1,000), anti-Myc (1:1,000), or anti-GST (1:5,000) antibodies overnight at 4°C. Following primary antibody incubations, immunoblots were incubated with horseradish peroxidase-linked secondary antibodies (either anti-rabbit or anti-mouse) and developed by enhanced chemiluminescence according to the manufacturer's instructions.
IR kinase activity.
The GST fusion proteins were expressed and purified as described (35). The kinase reaction was performed at room temperature in kinase assay buffer (50 mM HEPES, pH 7.4, 12 mM MgCl2, and 5 mM MnCl2) containing 100 μM ATP, GST fusion proteins CTR-CNGA1 (WT, Y498F, Y503F, and Y498/503F), and 10 μCi/ml [γ-32P]ATP for 60 min. The reaction was briefly centrifuged at 14,000 rpm for 1 min, and 15 μl of supernatant were spotted on Whatman p81 phosphocellulose paper discs. Filter paper discs were washed three times for 5 min in 0.75% O-phosphoric acid and one time for 5 min in acetone before counting the radioactivity in a Beckman LS 6000SC Scintillation counter (Beckman Instruments, Fullerton, CA).
Primary structure analysis.
The amino acid sequences at the NH2 and COOH terminal of bovine CNGA1 were examined for the prediction of potential posttranslational modification motifs of Tyr residues by phosphorylation using the program NetPhos 2.0 (2). Residues located at the interface of membrane and cytoplasm were also analyzed by reversal of the primary amino acid sequence. Results of phosphorylation scoring were analyzed by NetPhosK 1.0 (3) to identify the specific kinase responsible for phosphorylation of the Tyr residues. The localization of the phosphorylated residues was ascertained by comparison with predicted topological domain and transmembrane regions at UniprotKB accession no. Q00194 (www.expasy.org).
Statistical methods.
Data were analyzed and graphed using Graphpad Prism software (GraphPad Software, San Diego, CA). The data were expressed as means ± SD and compared by Student's t-test for unpaired data. The critical level of significance was set at P < 0.05.
RESULTS
IR alters the CNG channel sensitivity toward cGMP in vivo.
Our laboratory has previously demonstrated a light-dependent tyrosine phosphorylation of IR in photoreceptor outer segments (34). The functional significance of this phosphorylation in outer segment membranes is not known. However, tyrosine phosphorylation has previously been shown to modulate the CNG channel (18, 24, 26). In this study, we have directly asked the question: Does light-dependent activated state of IR regulate the CNG channel activity? The CNG channel activity upon cGMP stimulation was measured in ROS membrane vesicles prepared from light-adapted wild-type and IR−/− mouse using a Ca2+-sensitive dye, fluo 3, as described in materials and methods. We observed that the increase in Ca2+ influx in response to cGMP was greater in IR−/− mouse ROS compared with wild-type ROS (Fig. 1, A and B). The similar fluorescence values at 0 s and upon saturation suggest that the differences in fluorescence upon Ca2+ influx are not an artifact due to differences in the amounts of fluo 3-AM dye trapped in ROS vesicles. These data suggest two important aspects of IR activation on CNG: 1) IR or its signaling might be a physiological modulator of CNG channel and 2) the channel opens at a lower concentration of cGMP in IR−/− mice. Therefore, we have characterized the mechanism of IR-mediated channel modulation in the subsequent experiments.
Fig. 1.
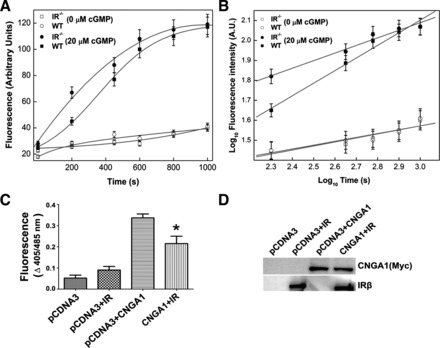
Insulin receptor (IR) regulates the cyclic nucleotide gated (CNG) channel function in vivo. Rod outer segment (ROS, 6 mg/ml) vesicles were prepared from light-adapted wild-type and insulin receptor-deficient (IR−/−) mice, and the fluo 3 dye was trapped inside them. Ca2+ influx was induced by the addition of cGMP (20 μM), and the increase in fluorescence triggered by Ca2+ influx into micelles was monitored from 0 to 1,000 s (A). WT, wild type. Unstimulated micelles were used as control. The data in A are replotted as log10 to distinguish the initial Ca2+ influx differences (B). AU, arbitrary units. The data are represented as means ± SD, n = 3 independent ROS micelles. IR modulates the CNG channel function in mammalian cells. CNGA1 was coexpressed with IR or pCDNA3 in HEK 293T cells, and channel activity in each case was measured as a function of Ca2+ influx in response to cGMP stimulation (C). The non-channel-mediated Ca2+ transport across the membrane was subtracted from HEK 293T cells transfected with either pCDNA3 or IR. The data are means ± SD, n = 3 independent transfections. Significance between CNGA1 plus pCDNA3 and CNGA1 plus IR: *P < 0.001. The expression of proteins in total cellular lysate 48 h posttransfection is shown in each case (30 μg protein) using immunoblot analysis with Myc (for CNGA1) and IR antibodies (D).
To test whether IR could modulate CNGA1 function, we measured the 8-pCPT-cGMP-induced increase in [Ca2+]i in HEK 293T cells overexpressing CNGA1 alone or in combination with wild-type IR. We measured this with the Ca2+-sensitive fluorescent dye indo 1-AM (11). Figure 1C shows that overexpression of CNGA1 alone in HEK 293T cells led to a fourfold increase in [Ca2+]i in response to 8-pCPT-cGMP stimulation compared with mock-transfected cells. Overexpression of IR suppressed the 8-pCPTcGMP-induced increase in [Ca2+]i seen in cells transfected with CNGA1 alone by ∼43% (Fig. 1C). To exclude the possibility that IR suppressed CNGA1 function by reducing the expression of CNGA1, transfected proteins were immunoblotted with Myc (for CNGA1) and IR antibodies. The results indicate equal amounts of CNGA1 in both conditions (Fig. 1D). These experiments suggest that IR modulates CNG channel function.
Light- or insulin-mediated IR activation results in CNG channel phosphorylation in vivo.
The in vivo phosphorylation status of mouse retinal CNGA1 was examined by immunoprecipitating CNGA1 with CNGA1-specific antibodies and examining the blot with PY99 specific antibodies in both dark- and light-adapted conditions. IgG was used as a control in both cases. The results indicate a light-dependent tyrosine phosphorylation of CNGA1 but absence of phosphorylation on CNGA1 in both dark-adapted and control immunoprecipitates (Fig. 2A). The blot was also probed with CNGA1, showing that an equal amount of CNGA1 was precipitated in each case, and the results suggest similar levels of CNGA1 in dark- and light-adapted conditions (Fig. 2A).
Fig. 2.
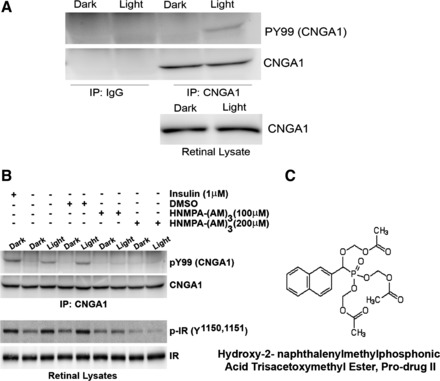
Light- and insulin-induced tyrosine phosphorylation of CNGA1. Retinal lysates from dark- and light-adapted rats were immunoprecipitated (IP) with either IgG or anti-CNGA1 antibody and immunoblotted with phosphotyrosine (PY99) and CNGA1 antibodies (A). Retinal lysates show an equal amount of CNGA1 in both conditions. Retinal ex vivo explants from dark-adapted rats were incubated with or without 1 μM insulin or dimethyl sulfoxide (DMSO) or IR kinase inhibitor HNMPA-(AM)3. One set of the explants was incubated in dark, and the other was exposed to light for 30 min at 300 lux. Anti-CNGA1 immunoprecipitates were immunoblotted with PY99 and CNGA1 antibodies. Lysates from the ex vivo explants were also immunoblotted with phosphospecific IR (pY1158, 1162, and 1163) and IR antibodies. Chemical structure of the IR kinase inhibitor HNMPA-(AM)3 is shown (C).
To examine the effect of insulin on CNGA1 phosphorylation, ex vivo retina explants prepared from dark-adapted rats were incubated with insulin and examined for the phosphorylation on CNGA1 as described in Fig. 2A. Similar to light, insulin also induced the tyrosine phosphorylation on CNGA1. To determine whether the light-induced tyrosine phosphorylation on CNGA1 is indeed mediated through IR activation, we have included the IR-specific inhibitor HNMPA-(AM3) (Fig. 2C) to ex vivo retinal explants prepared from dark-adapted rats that were either kept in dark for 30 min or exposed to light (300 lux) for 30 min. Control incubations were carried out with dimethyl sulfoxide (DMSO). Corresponding to in vivo findings (Fig. 2A), a light-dependent CNGA1 phosophorylation was observed in the ex vivo retinal explants (Fig. 2B). No Tyr phosphorylation was observed in the dark-adapted conditions. The use of HNMPA-(AM3) completely eliminated the light-dependent phosphorylation on CNGA1 (Fig. 2B). Similar results were also observed with ex vivo retinal explants treated with IR-specific inhibitor followed by insulin (data not shown). These experiments suggest that CNGA1 tyrosine phosphorylation is mediated through light-dependent activation of IR in vivo. The above-treated retinal lysates were also subjected to immunoblot analysis and probed with phospho-IR and IR specific antibodies. While IR was uniform in each case, indicating that equal amount of protein was loaded in each well (Fig. 2B), phospho-IR signal was much higher in samples where CNGA1 phosphorylation signal was high (Fig. 2B). The signal was much higher in light samples compared with dark- and the IR inhibitor-treated samples (Fig. 2B). To determine whether HNMPA-(AM3) directly inhibited the IR kinase activity, HEK 293T cells expressing Myc-tagged CNGA1 were subjected to in vitro phosphorylation by purified IR in the presence of either DMSO (control) or the IR kinase inhibitor HNMPA-(AM3). The reaction products were subjected to immunoblot analysis with anti-PY99 (Fig. 3A) antibody, and the blot was reprobed with anti-Myc antibody (Fig. 3A). The results indicate a significant decrease in the IR-mediated CNGA1 phosphorylation in the presence of IR kinase inhibitor HNMPA-(AM3) compared with IR-mediated CNGA1 phosphorylation in the presence of DMSO control (Fig. 3, A and B). To further demonstrate that CNGA1 is a direct in vivo substrate of IR, we examined the phosphorylation status of CNGA1 in IR-competent and IR-deficient mouse retinas. Consistent with the results reported in Fig. 2, A and B, on the light-dependent tyrosine phosphorylation of CNGA1 and its inhibition by IR kinase inhibitor ex vivo, we found a decrease in CNGA1 phosphorylation in IR knockout mouse retinas compared with IR-competent mouse retinas in vivo (Fig. 3, C and D). The residual CNGA1 phosphorylation in IR knockout mouse retinas could be due to the IR recombination efficiency (32). These results collectively suggest that CNGA1 is a direct substrate of IR in vivo.
Fig. 3.
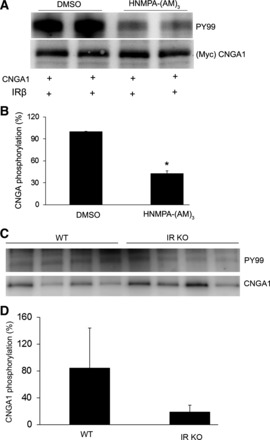
Inhibition of IR kinase activity by HNMPA-(AM)3 and CNGA1 phosphorylation by IR in vivo. HEK 293T cells were transiently transfected with Myc-CNGA1, and the protein was subjected to in vitro phosphorylation by purified IR in the presence of either DMSO (control) or HNMPA-(AM)3. The reaction products were subjected to immunoblot analysis with PY99 and anti-Myc (A) antibodies. Densitometric analysis of PY99 signal normalized to CNGA1 (B). Values are means ± SD, n = 2. The DMSO control was set as 100%. *P < 0.02, significance compared with DMSO control. Retinal lysates from IR knockout (KO) mice and wild-type mice were subjected to immunoprecipitation followed by immunoblot analysis with PY99 and CNGA1 (C) antibodies. Densitometric analysis of PY99 signal normalized to CNGA1 (D). Values are means ± SD, n = 4. The CNGA1 phosphorylation in wild-type mice was set as 100%.
IR tyrosine kinase activity is essential for CNG channel modulation.
HEK 293T cells were transiently cotransfected with either wild-type IR (two different clones a and b) or a naturally occurring tyrosine kinase-deficient mutant IR (Met1153→Ile) (29) and Myc-CNGA1 channel. pCDNA3 alone and coexpression of each of these constructs along with pCDNA3 were used as controls. Our results indicate that both wild-type clones of IR suppressed the 8-pCPTcGMP-induced increase in [Ca2+]i seen in cells transfected with CNGA1 alone, whereas the tyrosine kinase-deficient mutant IR was not able to do so (Fig. 4A). The lack of suppression of 8-pCPTcGMP-induced increase in [Ca2+]i by CNGA1 in the presence of mutant IR is not due to increased expression of either CNGA1 or IR, since we found a comparable level of expression of these proteins on immunoblots (Fig. 4B). These results demonstrate that IR tyrosine kinase activity is essential for IR-mediated modulation of CNG channel activity.
Fig. 4.
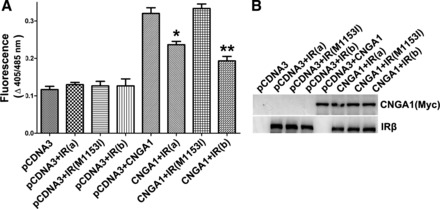
IR tyrosine kinase activity is essential for CNG channel modulation. HEK 293T cells were transiently cotransfected either with wild-type (two independent clones, a and b) or tyrosine kinase-deficient mutant of IR (M1153I). Expression of pCDNA3 alone or its coexpression with each of the IR or CNGA1 clones was used as the control. CNGA1 channel activity was measured as a function of channel-mediated Ca2+ transport across the plasma membrane in response to cGMP stimulation (A). Data are means ± SD, n = 3 independent transfections. Significance between either of the IR clones plus CNGA1 and mutant IR (M1153I) plus CNGA1: *P < 0.05 and **P < 0.001. The expression of proteins in total cellular lysate 48 h posttransfection is shown in each case (30 μg protein) using immunoblot analysis with Myc (for CNGA1) and IR antibodies to establish a comparable expression in each case (B).
Phosphorylation analysis of the cytoplasmic regions of CNGA1.
The propensity of the cytoplasmic regions of CNGA1 to become tyrosine phosphorylated was analyzed using the programs NetPhos 2.0 and NetPhosK 1.0 (2, 3). These programs analyze a given peptide sequence from the NH2- through the COOH-terminus. It was observed that Tyr503 in CNGA1 shows a high degree of phosphorylation potential by the IR kinase (Table 1). Previously, Tyr498 has been shown to undergo phosphorylation and has been implicated in the modulation of CNG channel activity (18). However, the analysis of cytoplasmic region of CNGA1 to predict potential phosphorylation sites revealed an extremely low scoring for Tyr498 residue (Table 1). This disparity could possibly be due to the fact that, in a tertiary structural configuration, the sequence is recognized from COOH- toward the NH2-terminus by the IR kinase. Upon reversal of the sequence, the predicted phosphorylation scoring of Tyr498 by IR kinase increases significantly (Table 1), in accordance with the experimental observations (18).
Table 1.
Phosphorylation analysis of cytoplasmic COOH-terminal region of CNGA1
| Sequence1 | Position | Score2 | Prediction |
|---|---|---|---|
| NH2-SGNTYYNWL-COOH | 163 | 0.563 | Y |
| NH2-QPQVYSPGD-COOH | 498 | 0.025 | |
| COOH-QPQVYSPGD-NH2 | 498 | 0.976 | Y |
| NH2-SPGDYICKK-COOH | 503 | 0.945 | Y |
| NH2-GREMYIIKE-COOH | 515 | 0.336 | |
| NH2-SDGSYFGEI-COOH | 541 | 0.219 | |
| NH2-KSIGYSDLF-COOH | 568 | 0.378 | |
| NH2-ALTEYPDAK-COOH | 586 | 0.495 | |
| NH2-ILAEYESMQ-COOH | 646 | 0.270 |
The analysis of the amino acid sequence of the COOH-terminal region of cyclic nucleotide gated (CNG) A1 was done using NetPhos 2.0. The Tyr residues were further analyzed by NetPhosK 1.0 for prediction of kinase-specific phosphorylation.
The amino acid sequence surrounding the Tyr (Y).
Phosphorylation scores were calculated based on the phosphorylation site prediction program. Scores above 0.5 are deemed to be possible phosphorylation sites, and the higher the score, the more likely a particular site will be phosphorylated. The known phosphorylation on the Tyr498 at the membrane-cytoplasm interface could be predicted by reversal of the amino acid sequence (bold).
IR-induced modulation of CNG channel activity is mediated through Tyr498 and Tyr503.
HEK 293T cells were transiently cotransfected with either wild-type or each of the Y498F, Y503F, or Y498/503F mutant Myc-CNGA1 and either pCDNA3 vector or IR. Transfection with IR alone was used as control. When Y498F and Y503F mutants of CNGA1 were coexpressed along with the IR, it resulted in a significant suppression of Ca2+ influx through the CNGA1 channel compared with wild-type CNGA1 (Fig. 5A). However, when the Y498/503F double mutant was coexpressed along with the IR, no suppression of Ca2+ influx was observed upon cGMP stimulation (Fig. 5A). The comparable expression of each of the CNGA1 channel mutants and IR was assessed by immunoblot analysis of the lysates with anti-CNGA1 and anti-IR specific antibodies (Fig. 5B). These results suggest that IR-mediated inhibition of CNG function is mediated jointly through the Y498 and Y503 residues.
Fig. 5.
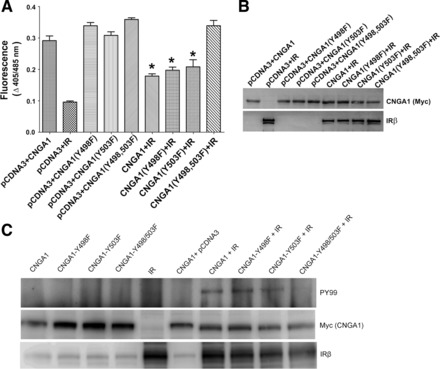
Role of tyrosine phosphorylation of residues Tyr498 and Tyr503 on CNG channel function. Wild-type or mutant Myc-CNGA1 (Y498F, Y503F, and Y498/503F) was either transiently coexpressed with pCDNA3 or along with IR in HEK 293T cells. CNGA1 channel activity was measured as a function of channel-mediated Ca2+ transport across the cell membrane in response to cGMP stimulation (A). Control experiments include the expression of IR alone to subtract the non-channel-mediated Ca2+ transport across the cell membranes. Data are means ± SD, n = 3 independent transfections. Significance between wild-type CNGA1 or mutant CNGA1 (Y498F, Y503F, and Y498/503F) vs. CNGA1 plus IR, CNGA1 (Y498F) plus IR, CNGA1 (Y503F) plus IR: *P < 0.001. To ensure the comparable expression of wild-type and mutant proteins of CNGA1 and IR, lysates from HEK 293T cells expressing these proteins were subjected to immunoblot analysis with Myc and IR antibodies, respectively (B). Myc immunoprecipitates from HEK 293T cell-expressed proteins were subjected to immunoblot analysis with PY99 and Myc (CNGA1) antibodies. The expression of IR was analyzed in each case using IRβ antibody (C).
To further demonstrate that lack of channel modulation in Y498/503F double mutant is due to the absence of phosphorylation by IR, the above samples were subjected to immunoprecipitation by anti-Myc (CNGA1) followed by immunoblot analysis with anti-PY99 and reprobed with anti-Myc (CNGA1) antibodies. Total levels of IR in the lysates were examined by anti-IRβ antibody. The results indicate an IR-mediated phosphorylation of wild-type CNGA1, Y498F, and Y503F mutant but not Y498/503F double mutant (Fig. 5C). In the absence of IR, there was no phosphorylation observed with either wild-type or each of the mutant CNGA1s (Fig. 5C). These results further confirm that IR phosphorylation on Y498 and Y503 mediates the inhibition of CNG channel function. To determine whether CNGA1 is a direct substrate of IR kinase, we further expressed wild-type CTR region of CNGA1 and their respective phosphorylation mutants (Y498F, Y503F, and Y498/503F) as GST fusion proteins in Escherichia coli BL21 cells and purified them by using GST-Sepharsoe-4B column chromatography (35). Fusion proteins were incubated with purified IR kinase domain (33) and radiolabeled ATP, and the IR kinase activity was carried out as described (37). The results indicate that, with each of the Tyr mutated CNGA1, the radioactive phosphate incorporation was reduced by ∼50–60% (Fig. 6A). However, the double mutant exhibited >90% reduction in the incorporation of the phosphate by IR (Fig. 6A). To further demonstrate that CNGA1 is a direct substrate of IR, GST fusion proteins of wild-type and tyrosine phosphorylation mutants of CTR-CNGA1 were incubated with purified IR and cold ATP, and the IR kinase activity assay was carried out for 30 min. The reaction products were subjected to immunoblot analysis with anti-PY99 antibody, and the blot was reprobed with anti-GST antibody to ensure an equal amount of fusion in each reaction. Similar to kinase assays (Fig. 6A), a significantly higher phosphorylation was observed in the wild-type CTR-CNGA1 incubated with IR compared with that of Y503F and Y498F mutants (Fig. 6B). In the Y498/503F double mutant, however, no phosphorylation was observed (Fig. 6B). These results collectively suggest that IR-mediated modulation of CNG channel function is mediated through Tyr498 and Tyr503 residues.
Fig. 6.
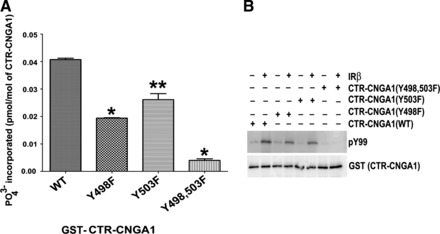
CNGA1 is a direct substrate of IR. Glutathione S-transferase (GST) purified wild-type (WT) or mutant (Y498F, Y503F, and Y498/503F) proteins were incubated with radioactive [γ-32P]ATP and purified IR kinase domain. The incorporation of radioactive phosphate in each of these samples was measured using a scintillation counter and plotted (A). Data are means ± SD, n = 6. **P < 0.05 and *P < 0.001. The experiment was carried out similar to A except the radiolabeled ATP was replaced with cold ATP, and the reaction products were subjected to immunoblot analysis with PY99 and GST antibodies (B).
IR-mediated inhibition of CNGA1 is independent of Grb14-mediated inhibition.
We have previously reported that Ras-associating domain of Grb14 binds to Ras-like domain on the CTR of CNGA1 and inhibits the channel activity (36). To confirm that the Grb14-binding site is different from that of Y498- and Y503-mediated IR modulation, the double-mutant Y498/503F was coexpressed along with Grb14 and analyzed for CNG channel activity. Grb14 coexpressed along with wild-type CNGA1, Grb14 alone, wild-type CNGA1, and CNGA1 mutant alone were used as controls. It was observed that Grb14 still inhibited the channel activity irrespective of these mutations (Fig. 7). These results suggest that IR-mediated modulation of channel activity is independent of Grb14-mediated modulation.
Fig. 7.
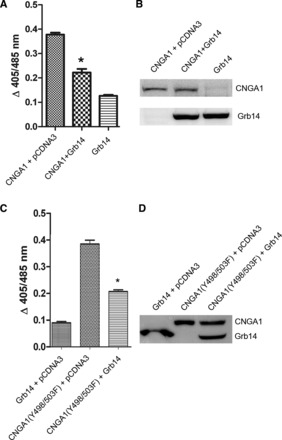
IR-induced inhibition of CNG channel is independent of Grb14-mediated regulation. HEK 293T cells were transiently cotransfected with Myc-Grb14 and Myc-CNGA1 or Myc-CNGA1 (Y498/503F) double mutant. The expression of Grb14, wild-type CNGA1, and mutant CNGA1 (Y498/503F) was used as control. CNGA1 channel activity was measured as a function of channel-mediated Ca2+ transport across the plasma membrane in response to cGMP stimulation (A and C). Data are means ± SD, n = 3 independent transfections. Significance between CNGA1 and CNGA1 plus Grb14, CNGA1 (Y498/503F) and CNGA1 (Y498/503F) plus Grb14: *P < 0.001. The comparable expression of proteins in total cellular lysate 48 h posttransfection is shown in each case (30 μg protein) using immunoblot analysis with Myc (for both CNGA1 and Grb14) antibody (B and D).
DISCUSSION
Protein-protein interactions and posttranslational modifications play an important role in defining protein function. Our results suggest that a cross talk exists between phototransduction and the IR-signaling pathway in photoreceptor neurons. This cross talk phenomenon has been shown for other G protein-coupled receptors (GPCRs), and many tyrosine kinase cascades are regulated by GPCRs (20, 21). We have reported earlier that rhodopsin signaling is essential for the activation of IR (30) through the translocation of an adapter protein, Grb14, to photoreceptor outer segment membranes (31). Both IR and Grb14 belong to the category of tyrosine kinase signaling proteins. We have reported earlier that Grb14 not only regulates IR kinase activity (31) but also modulates the CNGA1 channel function in vivo (11). In this study, we have demonstrated that CNGA1 is an in vivo IR substrate, and the channel activity is modulated through phosphorylation of Y498 and Y503 residues on CNGA1. These studies suggest a novel regulatory feedback mechanism that phototransduction regulates the IR signaling pathway, and, upon activation, the IR regulates the CNG channel, one of the components of the phototransduction. The hand-in-hand regulatory mechanism is important for photoreceptor functions, since IR and IR-signaling proteins are known neuron survival factors, and a defect in the phototransduction cascade always results in cell death.
Protein phosphorylation is one of the well-characterized and best-understood mechanisms of cellular regulation. Phosphorylation of CNG channels has been shown to control its function in several studies (18, 24, 26), but a detailed study about the residues involved and the responsible protein kinase(s) has not been done so far. Insulin-induced modulation of several ion channels has been reported earlier (8, 16, 28), but no studies are available on the direct involvement of IR in the regulation of ion channels. Tyrosine residue 498 has previously been shown to be tyrosine phosphorylated and is implicated in the modulation of CNG channel activity (18); however, the actual kinase that phosphorylates this residue has not been indentified. In this study, we showed that IR directly phosphorylates the CNGA1 on residues Y498 and Y503. Our studies clearly suggest that IR tyrosine kinase activity is essential for the tyrosine phosphorylation and channel modulation. Conversely, insulin-like growth factor-I (IGF-I) application has been shown to increase the cGMP sensitivity of the rod CNG channels two- to threefold by promoting dephosphorylation of rod CNG channel (38). IGF-I effect in homomeric CNGA1 channels has been shown to be mediated through the changes in Tyr dephosphorylation mediated by protein Tyr kinases and phosphatases (25).
The overexpression of IR along with CNGA1 in a heterologous system significantly decreased the CNG channel function. Conclusive results were obtained from studies in rod-specific IR−/− mice (Fig. 1). We could not perform the channel measurements in dark-adapted ROS from IR−/− mice. This is because of the complex genetic breeding to generate conditional rod photoreceptor-specific knockout mice and also require significantly more numbers of mice to prepare ROS. Phosphatidylinositol 3-kinase, the downstream effector of IR-generated inositol trisphosphate, can inhibit the action of olfactory (4, 6) and cone CNG (5) channels. The effect of activated IR downstream signaling proteins on CNG channel modulation cannot be ruled out.
Based on our studies, we propose that IR becomes activated in light conditions that, in turn, phosphorylate the Tyr residues located at the membrane-cytoplasm interface of CNGA1, in close proximity to the cytoplasmic kinase region of IR. This phosphorylation further promotes and maintains the channels in an off state, which is very important in the light conditions. The gating of CNG channels is governed by molecular interactions between and within subunits. Studies on CNGA1 channels show that their NH2-terminus can bind to the COOH-terminus of an adjacent subunit, which produces an excitatory effect on channel gating, allowing them to turn on at lower concentrations of cyclic nucleotides. The disruption of this interaction may negatively affect the on state of the channel. Our hypothesis is that a similar phosphorylation might be taking place at another set of Tyr residues located strategically at the NH2-terminal membrane-cytoplasmic interface of CNGA1. Consistent with this hypothesis, our bioinformatics search also identified a putative phosphorylation site of Y163 located at the NH2-terminal end of the CNGA1 (Table 1). However, we have not tested its effect on CNGA1 (either activity or phosphorylation). This phosphorylation will result in generation of negative charges in the vicinity of the membrane. This massive phosphorylation leads to not only a repulsion between the various subunits of CNG channel but also between the free cytoplasmic arms of channel subunits and phospholipid-rich OS membranes, thereby giving them a push into the cytoplasm away from the membranes. This push regulates the channel gating physically and alters the kinetics and sensitivity of the channel for the cGMP. This repulsion may either help closing the pore mechanically or through accompanying conformational changes. Furthermore, the OS membrane lipids have been shown to undergo massive phosphorylation upon illumination (19). A slight functional disruption of tertiary structure due to this repulsion too cannot be ruled out. Our hypothesis is strengthened by the fact that the CNGB1 subunit of CNG channel also becomes phosphorylated at Y1097 (18), which too may disrupt a similar interface between the COOH-terminus region of CNGB1 and the NH2-terminus region of CNGA1.
Our studies also suggest that the effects of IR-mediated phosphorylation on CNGA1 and the effect of Grb14 on CNGA1 appear to be additive and reinforce each other. These observations suggest that the relevant phosphorylation sites and the Grb14-binding site are located at different regions, and the inhibition caused by both processes does not share a common mechanism. We propose that the phosphorylation of these sites disrupts the interaction between the COOH terminus of CNGA1 and NH2 terminus of the CNGB1 or other CNGA1 subunits. It does not prevent Grb14 from having an additional effect. This model assumes that phosphorylation plays a role in gating. Although the relevant phosphorylation sites and the Grb14-binding sites are different, it is still possible that phosphorylation allosterically promotes Grb14 binding.
This phosphorylation-mediated inhibition of CNG channels is yet another way of regulating light adaptation. Light adaptation is a powerful mechanism for adjusting photoreceptor sensitivity through CNG channels over several orders of magnitude, in accordance with versatile light intensities encountered under various physical conditions. How tyrosine dephosphorylation takes place needs to be explored. These issues are currently being vigorously addressed and will expectantly continue to project light on ways to increase potential therapeutic uses. This study further shows that a cross talk exists between IR survival signaling and phototransduction machinery. In dark, the CNG channels are in an open state due to cGMP stimulation (Fig. 8A). In light, the declining cGMP levels promote channel closure. The kinase activity of IR phosphorylates CNG channel and brings about further changes essential for the maintenance of the channel closure (Fig. 8B). Therefore, this study not only reveals a previously unknown mechanism of rod CNG channel regulation that should direct future therapeutic approaches but also provides an explanation for observations reported in the literature with respect to the degenerative progression of photoreceptors in diabetic retinopathy and retinitis pigmentosa. Given that, in ROS, IR regulates the state of CNG channel phosphorylation, it is tempting to speculate that IR antagonists are potential therapeutic agents to manipulate CNG channel behavior in certain disease-causing mutations and other pathological conditions.
Fig. 8.
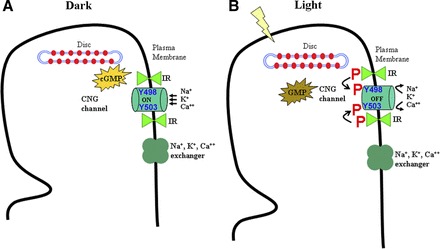
Working model of IR-induced modulation of CNG channel. In dark, the generation of cGMP through gunylate cyclase keeps the channels in an on state, thereby maintaining an inward flow of Na+, K+, and Ca2+ cations (A). In light, the activity of phosphodiesterase depletes the cGMP levels, thereby forcing the channels to be in an off or less permeability state. Our studies suggest that, in ROS, IR kinase becomes tyrosine phosphorylated in light, which further phosphorylates critical Tyr residues in CNGA1 subunit of CNG channel (B). This phosphorylation either promotes pore closure or upholds the channel in an off state by reinforcing this closure until a relevant phosphatase event relieves these Tyr residues from their phosphorylated state. P, phosphorylation.
GRANTS
This work was supported by National Institutes of Health Grants EY-016507, EY-00871, EY-12190, and NCRR COBRE (P20-RR-17703).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: V.K.G., A.R., and R.V.R. performed experiments; V.K.G., A.R., and R.V.R. analyzed data; V.K.G., A.R., and R.V.R. interpreted results of experiments; V.K.G., A.R., and R.V.R. prepared figures; V.K.G. and R.V.R. drafted manuscript; V.K.G. and R.V.R. edited and revised manuscript; V.K.G., A.R., and R.V.R. approved final version of manuscript; R.V.R. conception and design of research.
ACKNOWLEDGMENTS
We thank Dr. Robert S. Molday (University of British Columbia, Canada) for providing channel antibodies and mammalian expression constructs of CNG channel.
REFERENCES
- 1. Biel M, Zong X, Ludwig A, Sautter A, Hofmann F. Structure and function of cyclic nucleotide-gated channels. Rev Physiol Biochem Pharmacol 135: 151–171, 1999 [DOI] [PubMed] [Google Scholar]
- 2. Blom N, Gammeltoft S, Brunak S. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Blom N, Sicheritz-Ponten T, Gupta R, Gammeltoft S, Brunak S. Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence. Proteomics 4: 1633–1649, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Brady JD, Rich ED, Martens JR, Karpen JW, Varnum MD, Brown RL. Interplay between PIP3 and calmodulin regulation of olfactory cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 103: 15635–15640, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bright SR, Rich ED, Varnum MD. Regulation of human cone cyclic nucleotide-gated channels by endogenous phospholipids and exogenously applied phosphatidylinositol 3,4,5-trisphosphate. Mol Pharmacol 71: 176–183, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Brunert D, Klasen K, Corey EA, Ache BW. PI3Kgamma-dependent signaling in mouse olfactory receptor neurons. Chem Senses 35: 301–308, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finn JT, Grunwald ME, Yau KW. Cyclic nucleotide-gated ion channels: an extended family with diverse functions. Annu Rev Physiol 58: 395–426, 1996 [DOI] [PubMed] [Google Scholar]
- 8. Foutz RM, Grimm PR, Sansom SC. Insulin increases the activity of mesangial BK channels through MAPK signaling. Am J Physiol Renal Physiol 294: F1465–F1472, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Gordon SE, Downing-Park J, Tam B, Zimmerman AL. Diacylglycerol analogs inhibit the rod cGMP-gated channel by a phosphorylation-independent mechanism. Biophys J 69: 409–417, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gordon SE, Zagotta WN. Subunit interactions in coordination of Ni2+ in cyclic nucleotide-gated channels. Proc Natl Acad Sci USA 92: 10222–10226, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gupta VK, Rajala A, Daly RJ, Rajala RV. Growth factor receptor-bound protein 14: a new modulator of photoreceptor-specific cyclic-nucleotide-gated channel. EMBO Rep 11: 861–867, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta V, Rajala A, Rodgers K, Rajala RV. Mechanism involved in the modulation of photoreceptor-specific cyclic nucleotide-gated channel by the tyrosine kinase adapter protein Grb14. Protein Cell 2: 906–917, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hsu YT, Molday RS. Modulation of the cGMP-gated channel of rod photoreceptor cells by calmodulin. Nature 361: 76–79, 1993 [DOI] [PubMed] [Google Scholar]
- 14. Karpen JW, Brown RL, Stryer L, Baylor DA. Interactions between divalent cations and the gating machinery of cyclic GMP-activated channels in salamander retinal rods. J Gen Physiol 101: 1–25, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaupp UB, Seifert R. Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824, 2002 [DOI] [PubMed] [Google Scholar]
- 16. Khan FA, Goforth PB, Zhang M, Satin LS. Insulin activates ATP-sensitive K(+) channels in pancreatic beta-cells through a phosphatidylinositol 3-kinase-dependent pathway. Diabetes 50: 2192–2198, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Koutalos Y, Yau KW. Regulation of sensitivity in vertebrate rod photoreceptors by calcium. Trends Neurosci 19: 73–81, 1996 [DOI] [PubMed] [Google Scholar]
- 18. Krajewski JL, Luetje CW, Kramer RH. Tyrosine phosphorylation of rod cyclic nucleotide-gated channels switches off Ca2+/calmodulin inhibition. J Neurosci 23: 10100–10106, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li G, Rajala A, Wiechmann AF, Anderson RE, Rajala RV. Activation and membrane binding of retinal protein kinase Balpha/Akt1 is regulated through light-dependent generation of phosphoinositides. J Neurochem 107: 1382–1397, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Luttrell LM, Daaka Y, Lefkowitz RJ. Regulation of tyrosine kinase cascades by G-protein-coupled receptors. Curr Opin Cell Biol 11: 177–183, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Luttrell LM, van Biesen T, Hawes BE, Koch WJ, Krueger KM, Touhara K, Lefkowitz RJ. G-protein-coupled receptors and their regulation: activation of the MAP kinase signaling pathway by G-protein-coupled receptors. Adv Second Messenger Phosphoprotein Res 31: 263–277, 1997 [PubMed] [Google Scholar]
- 22. Matulef K, Zagotta WN. Cyclic nucleotide-gated ion channels. Annu Rev Cell Dev Biol 19: 23–44, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Mayer LD, Hope MJ, Cullis PR. Vesicles of variable sizes produced by a rapid extrusion procedure. Biochim Biophys Acta 858: 161–168, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Molokanova E, Krajewski JL, Satpaev D, Luetje CW, Kramer RH. Subunit contributions to phosphorylation-dependent modulation of bovine rod cyclic nucleotide-gated channels. J Physiol 552: 345–356, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molokanova E, Maddox F, Luetje CW, Kramer RH. Activity-dependent modulation of rod photoreceptor cyclic nucleotide-gated channels mediated by phosphorylation of a specific tyrosine residue. J Neurosci 19: 4786–4795, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Molokanova E, Trivedi B, Savchenko A, Kramer RH. Modulation of rod photoreceptor cyclic nucleotide-gated channels by tyrosine phosphorylation. J Neurosci 17: 9068–9076, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nache V, Schulz E, Zimmer T, Kusch J, Biskup C, Koopmann R, Hagen V, Benndorf K. Activation of olfactory-type cyclic nucleotide-gated channels is highly cooperative. J Physiol 569: 91–102, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. O'Malley D, Harvey J. Insulin activates native and recombinant large conductance Ca(2+)-activated potassium channels via a mitogen-activated protein kinase-dependent process. Mol Pharmacol 65: 1352–1363, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Quon MJ, Guerre-Millo M, Zarnowski MJ, Butte AJ, Em M, Cushman SW, Taylor SI. Tyrosine kinase-deficient mutant human insulin receptors (Met1153→Ile) overexpressed in transfected rat adipose cells fail to mediate translocation of epitope-tagged GLUT4. Proc Natl Acad Sci USA 91: 5587–5591, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rajala A, Anderson RE, Ma JX, Lem J, Al Ubaidi MR, Rajala RV. G-protein-coupled receptor rhodopsin regulates the phosphorylation of retinal insulin receptor. J Biol Chem 282: 9865–9873, 2007 [DOI] [PubMed] [Google Scholar]
- 31. Rajala A, Daly RJ, Tanito M, Allen DT, Holt LJ, Lobanova ES, Arshavsky VY, Rajala RV. Growth factor receptor-bound protein 14 undergoes light-dependent intracellular translocation in rod photoreceptors: functional role in retinal insulin receptor activation. Biochemistry 48: 5563–5572, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rajala A, Tanito M, Le YZ, Kahn CR, Rajala RV. Loss of neuroprotective survival signal in mice lacking insulin receptor gene in rod photoreceptor cells. J Biol Chem 283: 19781–19792, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rajala RV, Chan MD. Identification of a NPXY Motif in Growth Factor Receptor-Bound Protein 14 (Grb14) and Its Interaction with the Phosphotyrosine-Binding (PTB) Domain of IRS-1. Biochemistry 44: 7929–7935, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Rajala RV, McClellan ME, Ash JD, Anderson RE. In vivo regulation of phosphoinositide 3-kinase in retina through light-induced tyrosine phosphorylation of the insulin receptor beta-subunit. J Biol Chem 277: 43319–43326, 2002 [DOI] [PubMed] [Google Scholar]
- 35. Rajala RV, McClellan ME, Chan MD, Tsiokas L, Anderson RE. Interaction of the Retinal Insulin Receptor beta-Subunit with the P85 Subunit of Phosphoinositide 3-Kinase. Biochemistry 43: 5637–5650, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Rajala RV, Rajala A, Gupta VK. Conservation and divergence of Grb7 family of Ras-binding domains. Protein Cell 3: 60–70, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rajala RV, Wiskur B, Tanito M, Callegan M, Rajala A. Diabetes reduces autophosphorylation of retinal insulin receptor and increases protein-tyrosine phosphatase-1B activity. Invest Ophthalmol Vis Sci 50: 1033–1040, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Savchenko A, Kraft TW, Molokanova E, Kramer RH. Growth factors regulate phototransduction in retinal rods by modulating cyclic nucleotide-gated channels through dephosphorylation of a specific tyrosine residue. Proc Natl Acad Sci USA 98: 5880–5885, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Weitz D, Ficek N, Kremmer E, Bauer PJ, Kaupp UB. Subunit stoichiometry of the CNG channel of rod photoreceptors. Neuron 36: 881–889, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Wigler M, Pellicer A, Silverstein S, Axel R. Biochemical transfer of single-copy eucaryotic genes using total cellular DNA as donor. Cell 14: 725–731, 1978 [DOI] [PubMed] [Google Scholar]
- 41. Womack KB, Gordon SE, He F, Wensel TG, Lu CC, Hilgemann DW. Do phosphatidylinositides modulate vertebrate phototransduction? J Neurosci 20: 2792–2799, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]


