Abstract
Renal failure is associated with aortic valve calcification. Using our rat model of uremia-induced reversible aortic valve calcification, we assessed the role of apoptosis and survival pathways in that disease. We also explored the effects of raloxifene, an estrogen receptor modulator, on valvular calcification. Gene array analysis was performed in aortic valves obtained from three groups of rats (n = 7 rats/group): calcified valves obtained from rats fed with uremic diet, valves after calcification resolution following diet cessation, and control. In addition, four groups of rats (n = 10 rats/group) were used to evaluate the effect of raloxifene in aortic valve calcification: three groups as mentioned above and a fourth group fed with the uremic diet that also received daily raloxifene. Evaluation included imaging, histology, and antigen expression analysis. Gene array results showed that the majority of the altered expressed genes were in diet group valves. Most apoptosis-related genes were changed in a proapoptotic direction in calcified valves. Apoptosis and decreases in several survival pathways were confirmed in calcified valves. Resolution of aortic valve calcification was accompanied by decreased apoptosis and upregulation of survival pathways. Imaging and histology demonstrated that raloxifene significantly decreased aortic valve calcification. In conclusion, downregulation of several survival pathways and apoptosis are involved in the pathogenesis of aortic valve calcification. The beneficial effect of raloxifene in valve calcification is related to apoptosis modulation. This novel observation is important for developing remedies for aortic valve calcification in patients with renal failure.
Keywords: growth arrest-specific 6, uremia
cardiovascular calcification is one of the highest causes of morbidities and mortalities in patients with end-stage renal disease. These patients develop aortic valve calcification (AVC) and coronary calcification at an accelerated rate. Associated with this cardiovascular calcification are increased rates of myocardial infarctions and valvular heart disease. The pathogenesis of AVC in renal failure (RF) is not fully elucidated. It has been suggested that the process involves active osteoblast transformation of valve tissue, which results in increased formation of bone matrix (4, 25). Most of the data regarding the pathogenesis of AVC were obtained from animal models based on various components of the metabolic syndrome, emphasizing the role of atherogenesis in AVC (26, 37). In patients with RF, AVC and aortic stenosis are common and progress especially rapidly (18). The prevalence and extent of AVC in this population is poorly explained by traditional cardiovascular risk factors; abnormalities of mineral metabolism are likely to contribute to its development and progression. Current models of RF and accelerated calcification have demonstrated that calcium phosphate metabolism is critical for disease development (13), and inhibitors of calcification (fetuin A) are important in its prevention (24).
Previously, we (31) demonstrated that an adenine- and phosphate-enriched diet can induce AVC in rats. This unique model is based on the development of transient uremia-induced secondary hyperparathyroidism and represents the metabolic and hormonal changes occurring in calcification secondary to RF. Interestingly, AVC almost completely regressed after RF resolution and normalization of metabolic abnormalities, implying, for the first time, that AVC might be a reversible process (31). Similar results have been shown in a mouse model using a hypercholesterolemic animal model of aortic valve disease (21). We propose a novel model to study the effects of RF and uremia on the development of AVC. This model is unique and differs from the current models, which use lipid biology to manifest AVC; therefore, it will help to further understand the disease.
Raloxifene, a second-generation selective estrogen receptor modulator (SERM), is indicated for osteoporosis treatment in postmenopausal women. It is a mixed estrogen receptor agonist and antagonist, which has been shown to reduce cardiovascular events in women at high risk for coronary artery disease (5). Although the mechanism of the raloxifene effect on atherosclerosis is not completely understood, it has been shown to reduce both osteoblast cell apoptosis and oxidative stress damage (16, 36). However, its role in valvular calcification is still unknown. In the present study, using a model of reversible AVC, we further characterized the pathogenesis of RF-associated AVC using several modalities. We hypothesize that apoptosis and intracellular survival pathways are involved in the process; hence, modulating them by raloxifene may protect against AVC.
METHODS
Animals
Sixty-one Sprague-Dawley female rats (8 wk old, weight: ∼250 g) were used for the study. This protocol was approved by the Hebrew University Ethics Committee.
Animal Model
We developed a RF-induced animal model for AVC based on feeding rats with a high-adenine (0.75%) and high-phosphate (1.5%) diet (31). Adenine causes crystal precipitates in renal tubules and forms 2,8-dihydroxyadenine aggregates. Adenine alone causes renal failure due to interstitial nephritis (32). Clinically, chronic long-term RF in patients results in abnormal phosphate metabolism. To reproduce this state of abnormal phosphate metabolism acutely in our model, we added a high phosphate concentration to the adenine diet. This high phosphate concentration in the adenine diet causes two abnormalities: 1) interstitial nephritis, which results in polyuric renal failure; and 2) hyperphosphatemia and secondary hyperparathyroidism.
The experimental design consisted of exclusively administrating the uremia-induced diet to rats for 7 wk; this relatively long duration of diet allowed evaluation of the pathophysiological processes underlying the long-term effects of RF, such as ectopic calcification. Continuing the diet regimen for >7 wk may cause significant morbidity and mortality. As the metabolic and hormonal effects of RF last even after diet cessation, rats were kept alive for additional 2 wk on a regular chow diet to allow the full expression of long-term uremia effects. After 9 wk, rats were killed, and clinical and histological evaluation were performed.
Next, we demonstrated the reversibility of AVC in this model system. We terminated the diet after 7 wk and switched to a normal diet for an additional 12 wk, allowing full recovery from RF. Cessation of the uremic diet resulted in a gradual resolution of RF and normalization of phosphate levels. AVC was dramatically improved, with a statistical drop in calcium burden in the heart. Resolution was also associated with a downregulation of inflammatory markers and osteoblastic features in valve tissue.
Gene Array Analysis
Aortic valve samples were obtained from three groups: a diet group, a reversibility group, and a control group (n = 7 rats/group). Rats in the diet and reversibility groups were fed exclusively with the high-adenine diet for 7 wk. Rats in the diet group were switched to normal chow for 2 wk and then anaesthetized using ketamine-xylazine and killed. The reversibility group was switched to normal chow and kept alive for an additional 12 wk, allowing full recovery from RF, and then killed. The control group received normal rat chow with no treatment. Aortic valves were dissected from the hearts. In each group, aortic valve RNA was pooled together at equal RNA amounts from each sample.
RNA Labeling, Hybridization, and Array Scanning
One microgram of total RNA was amplified and labeled with a fluorescent dye (either Cy3 or Cy5) using the Low RNA Input Linear Amplification and Labeling kit (Agilent Technologies, Palo Alto, CA). The amount and quality of the resulting labeled cRNA were measured, and equal amounts of Cy3- or Cy5-labeled cRNA were hybridized to the 4 X 44K Agilent Whole Rat Genome Oligo-Microarray (Agilent Technologies). Arrays were later washed using a Gene Expression Wash Buffer Kit (Agilent Technologies). The microarray was scanned using the GenePix4000B Scanner (Axon), and data were extracted from the resulting images using GenePix Pro 4.1 software (Axon).
Array Design and Analysis
The three pooled rat samples were hybridized one against the other in four separate arrays (see Supplemental Material, Supplemental Fig. S1).1 The resultant files (gpr) produced by GenePix were analyzed using the LIMMA (33) software package. Cy5 and Cy3 intensities within each array were normalized using the print-tip locally weighted scatter plot smoothing (LOESS) function while no background correction was applied. To identify differentially expressed genes, a parametric empirical Bayesian approach implemented in LIMMA was used (20). A moderated t-test was performed in parallel, with the use of a false discovery rate (27) correction for multiple testing. LIMMA calculated an emission intensity A value {A = [log2(Cy5) × Cy3]/2}, where Cy5 and Cy3 are the normalized emission intensities of each feature. P value < 0.05 confidence levels were used to pinpoint those significantly differentiated genes. Genes had to have an A value (average expression level for the gene across all arrays and channels) of >8.5, thus avoiding faint emissions. The differentially expressed genes for each binary comparison retrieved by LIMMA were clustered using EXPANDER software (30).
Survival Pathways and Raloxifene in AVC
Forty other rats were divided into four groups (n = 10 rats/group): a diet group, a reversibility group, a raloxifene-treated group, and a control group. All rats except those in the control group were exclusively fed with the uremic diet for 7 wk, and all rats were then fed with normal chow for additional 2 wk as described in the model. Rats in the raloxifen-treated group were given raloxifene daily by subcutaneous injection (1 mg/kg), whereas rats in the diet and reversibility groups were treated with subcutaneous injections of the vehicle. The control group received normal rat chow with no treatment. After 9 wk, rats from diet, raloxifene-treated, and control groups were anaesthetized, and a multislice computed tomography (MSCT) scan was performed. After the procedure, rats were anesthetized and killed by exsanguination. Aortic valve tissue was excised, snap frozen in liquid nitrogen, and kept at −80°C. Rats in the reversibility group were kept alive for an additional 10 wk on normal rat chow and then underwent the same protocol as mentioned above.
Tissue Analysis
Aortic valves were dissected, fixed in formalin, and embedded in paraffin. Serial cross-sections of valve tissue were stained using hematoxylin and eosin as well as Von-Kossa staining to assess histology as well as calcium deposits.
Immunohistochemistry Experiments
Formalin-fixed aortic valve tissues at 5-mm cross-sections were used for immunohistochemistry experiments. Sections were incubated overnight with anti-osteocalcin, anti-collagen 1α, anti-CD68, and anti-α-smooth muscle actin (α-SMA). After a wash with PBS, sections were incubated with goat anti-rabbit (1:200) secondary antibody conjugated with Cy5 (Jackson Immunoresearch Laboratories).
Apoptosis Assessment Using TUNEL
Apoptotic cells were identified with the TUNEL assay carried out using the Promega DeadEnd Fluorometric TUNEL system, based on a previously described method (7). Slides were analyzed using a confocal laser scanning microscope (Zeiss 410, Carl Zeiss, Goettingen, Germany).
Computed Tomography Scan
Sixty-four-slice chest MSCT scans without contrast were performed on all rats (Brilliance, Philips Medical Systems, Groningen, The Netherlands). Study parameters were as follows: 120 kVp, 300 mAs, slice thickness 0.67 mm, and increment 0.3 mm. The scan was analyzed by an operator blinded to the study groups. Agatston calcium scores were calculated by multiplying the area of a calcified lesion restricted only to aortic valve area by a weighted computed tomography attenuation score dependent on the maximal computed tomography attenuation within a lesion as previously described (1).
Antibodies
Polyclonal antibodies to growth arrest-specific 6 (Gas6), Axl, osteopontin (osteoblast marker), and caspase 3 were purchased from Sigma (St. Louis, MO). MAPK and Akt pathways were assessed using ERK, JNK, p38, Akt, and their phosphorylated forms (Santa Cruz Biotechnology, Santa Cruz, CA). All antibodies were used according to standard procedures (7a).
Western Blot Analysis
Aortic valves were obtained from all groups; tissue was hydrolyzed, homogenized under ultrasound, and boiled for 5 min. After quantification of the protein concentration, 12 μg of extracts were separated on 5–15% SDS-polyacrylamide gradient gels and transferred to nitrocellulose membranes. Bands were detected after incubation with Western blotting Luminol reagent. Bands on the X-ray film were quantified by scanning densitometry (21a) and were expressed as a percentage of the control.
Statistical Analyses
Data are presented as means ± SE. Statistical differences between the groups were calculated using ANOVA followed by a Student-Neumann-Keuls test. P values of <0.05 were considered significant.
RESULTS
Gene Array
The objective of the gene array study was to use a focused gene discovery approach to identify genes whose expression is significantly altered during AVC. Furthermore, we may assume that genes that returned to their baseline level after regression of AVC are most likely related to calcification; hence, exploring these pathways is highly important for better understanding the pathogenesis of AVC.
More than 41,490 genes were represented on the array, and 959 transcripts were differentially expressed between the study groups. The majority of the altered genes were in the diet group (compared with the reversibility and control groups); 496 of these genes were upregulated in the diet group compared with the control group and then returned to baseline levels in the reversibility group. In contrast, 328 genes were downregulated in the diet group compared with the control group and then returned to baseline levels in the reversibility group. These patterns of gene expression indicate that calcification is an active process associated with multiple biological pathways. Furtheremore, regression of calcification is associated with inhibition of specific pathways, stressing the importace of these pathways in the pathogenesis of AVC. Entire array results with a description of the differentially expressed genes are available at http://www.hadassah.org.il/English/Eng_SubNavBar/Departments/Medical+departments/Cardiology/Research/Gene+Array+Results.htm.
Data from both array and gene expression analysis were deposited in the National Center for Biotechnology Information Gene Expression Omnibus and are accessible through series record GSE21771.
To evaluate the processes that are most likely related to AVC, we focused on genes that were upregulated or dowregulated in calcified valves compared with the control. These genes returned to their baseline levels in reversibility group valves. Using the Gene Ontology database, we categorized these differentially expressed genes into functional categories in three different issues: biological processes, cellular components, and molecular functions.
The differentially expressed genes associated with biological processes were mainly related to developmental processes such as responses to stimuli, interactions between cells, and proliferation, including death.
The majority of the differentially expressed genes involved in molecular functions were related to protein binding as well as ion and nucleic binding. The cellular components that were most differentially expressed were the membrane, nucleus, and macromolecular complex. A functional analysis of genes found to be differentially expressed in calcified valves is shown in Fig. 1.
Fig. 1.
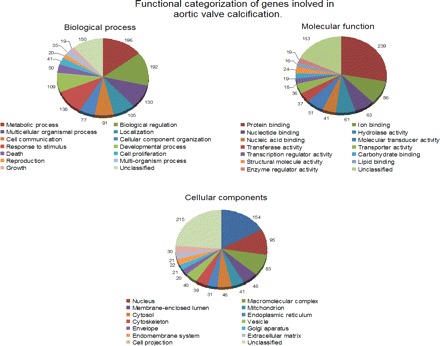
Distribuation of genes related to biological processes, molecular processes, and cellular components during aortic valve calcification (AVC). Shown is the functional categorization of genes that are involved in biological process, molecular function, and cellular components. Genes were upregulated or downregulated in calcified valves and then returned to their baseline levels after the regression of calcification.
These findings stress that AVC is a highly active and regulated process, involving inflammation, cell activation and proliferation, and eventually cell death.
As apoptosis may have a crucial role in AVC pathogenesis, in this study we focused on apoptosis-related genes. Seventeen genes were related to apoptosis; twelve proapoptotic genes were upregulated in diet group valves, whereas no proapoptotic genes were downregulated in this group. Two antiapoptotic genes, including Gas6, were downregulated in diet group valves, whereas three antiapoptotic genes were downregulated in this group. Differential expression levels (expressed in fold change) comparing the diet group with the control group and reversibility group ranged from 1.74 to 8.1 (upregulation) and from −1.61 to −2.94 (downregulation). In summary, 14 apoptosis-related genes were altered in a proapoptotic way during AVC, whereas only 3 apoptosis-related were altered in the antiapoptotic direction (Table 1).
Table 1.
Expression of apoptosis-related genes in aortic valves during aortic valve calcification
| Diet Group Versus Control Group |
Diet Group Versus Reversibility Group |
|||
|---|---|---|---|---|
| Gene Name | Fold change | Adjusted P value | Fold change | Adjusted P value |
| Increase in proapoptosis-related genes | ||||
| Protein tyrosine phosphatase, nonreceptor type 6 | 4.32 | 1.05 e−7 | 4.57 | 1.01 e−6 |
| Complement component 6 | 8.10 | 7.85 e−9 | 5.81 | 2.44 e−7 |
| IL-1β | 5.43 | 4.21 e−8 | 2.94 | 1.36 e−5 |
| JAK-2 | 2.19 | 1.29 e−5 | 2.13 | 0.000208 |
| IL-18 | 1.86 | 7.43 e−5 | 1.80 | 0.007695 |
| β2-Integrin | 5.12 | 1.95 e−6 | 5.17 | 2.38 e−5 |
| Lymphocyte-specific 1 | 3.05 | 7.68 e−7 | 3.45 | 4.07 e−6 |
| Procollagen type 18, α1 | 2.31 | 9.74 e−6 | 2.35 | 0.0001 |
| Receptor-interacting serine-threonine kinase 3 | 3.64 | 3.98E-07 | 3.35 | 8.44 e−6 |
| Sialophorin | 1.97 | 6.45 e−5 | 3.76 | 2.44 e−6 |
| Pycard | 1.74 | 0.000611 | 2.59 | 5.83 e−5 |
| Lymphocyte antigen 86 | 4.55 | 1.86 e−7 | 4.69 | 2.05 e−6 |
| Chemokine (C-C motif) ligand 2 | 6.77 | 1.70 e−8 | 7.80 | 1.34 e−7 |
| Chemokine (C-C motif) ligand 6 | 3.89 | 1.19 e−7 | 3.61 | 2.35 e−6 |
| Fc receptor, IgG, low-affinity 3 | 7.12 | 1.14 e−8 | 7.54 | 1.13 e−7 |
| Fc receptor, IgG, high-affinity 1 | 2.19 | 5.76 e−5 | 8.94 | 7.69 e−8 |
| Increase in antiapoptosis-related genes | ||||
| Tissue inhibitor of metallopeptidase 1 | 5.99 | 3.08 e−8 | 6.47 | 2.57 e−7 |
| Lipocalin 2 | 6.58 | 1.15 e−7 | 6.00 | 2.13 e−6 |
| Bcl-2-related protein | 2.38 | 1.44E-05 | 2.74 | 5.60 e−5 |
| Decrease in antiapoptosis-related genes | ||||
| Growth arrest-specific 6 | −2.94 | 1.43 e−5 | −2.94 | 0.000178 |
| Heat shock protein 1 (chaperonin) | −1.85 | 0.001848 | −1.95 | 0.000773 |
Summary of significant differentially expressed genes in calcified aortic valves compared with valves from the reversibility and control groups. All of the mentioned genes are involved in apoptosis in different tissues. Most of the changes in these genes support apoptotic pathways in calcified valves.
Survival Pathways and Raloxifene in AVC
Electrolytes and kidney function.
As we (31, 32) have previously reported, the diet induces reversible RF and hyperparathyroidism. A biochemical profile was obtained at three different time points during the diet protocol: after 5 wk of diet (using an additional 14 rats), at 9 wk (the diet group), and after 19 wk (the reversibility group). A biochemical profile was also obtained in the raloxifene-treated group (after 9 wk) and in the control group. The diet caused RF, as reflected by high urea and phosphate levels. After diet cassation, creatinine and phosphate levels were gradualy decreased, and, in the reversibility group, RF and metabolic abnormalities were resolved. There were no significant differences in total calcium levels between the study groups (Table 2).
Table 2.
Biochemical profiles of the study groups
| Control Group |
Diet Group |
Reversibility Group |
Raloxifene-Treated Group |
||
|---|---|---|---|---|---|
| Week 9 | Week 5 | Week 9 | Week 19 | Week 9 | |
| No. of rats/group | 6 | 15 | 7 | 7 | 10 |
| Urea, mmol/l | 6.1 ± 0.3 | 37.8 ± 3.6* | 23.3 ± 1.9 | 9 ± 0.8 | 16.4 ± 3.2 |
| Phosphate, mmol/l | 2.65 ± 0.3 | 6.6 ± 0.9* | 2 ± 0.2 | 1.93 ± 0.13 | 1.8 ± 0.15 |
| Calcium, mmol/l | 2.65 ± 0.1 | 1.8 ± 0.3 | 2.64 ± 0.2 | 2.35 ± 0.06 | 2.58 ± 0.06 |
Biochemical profiles were obtained from the study groups. The effects of a high-adenine diet were evaluated at three different time points: 5 wk (during the adenine diet), 9 wk (2 wk after the cessation of the diet), and at 19 wk (the reversibility group). At 5 wk, there was significant increase in urea and phosphate compared with the control group. After 9 wk, urea and phosphate levels in the diet group decreased and were similar to the raloxifene-treated group. In the reversibility group, urea and phosphate levels were normalized after 19 wk. There were no significant differences in calcium levels between the study groups.
P < 0.01 for the comparison of the diet group with the control group.
MSCT scans.
AVC was found in all diet group rats and was significantly higher compared with raloxifene-treated rats, with mean Agatston scores of 105 ± 15 versus 23 ± 7, respectively (P < 0.05). Only mild calcification was found in the reversibility group, and no calcification was found in the control group (Fig. 2A).
Fig. 2.
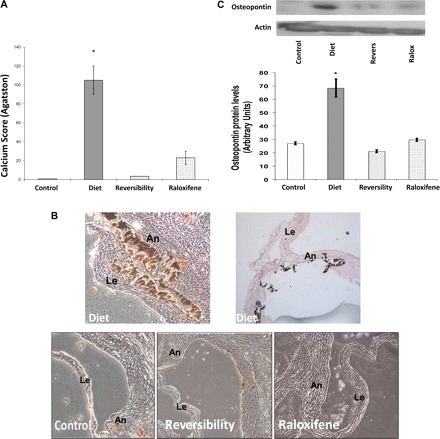
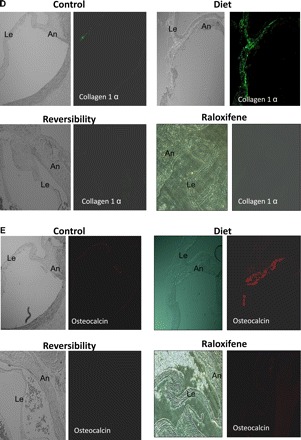
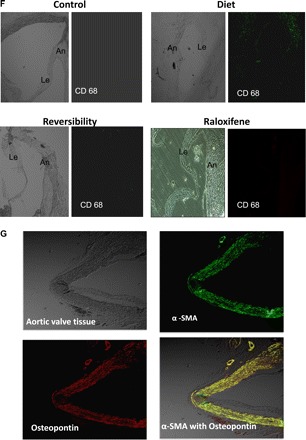
Calcification, osteoblast markers, and macrophages in aortic valves. A: the average calcium score was significantly lower in the raloxifene (Ralox)-treated group than in the diet group (23 ± 7 vs. 105 ± 15, respectively, P < 0.05), reflecting the beneficial effect of raloxifene in AVC. No significant calcification was found in the reversibility (Revers) and control groups. B: cross-section of valves obtained from the diet group through the aortic sinuses showing calcification of the annulus (An) and base of the leaflet (Le). No significant calcification was found in the other groups (Von-Kossa stain). Magnification: ×50. C: Western blot analyses of osteopontin protein levels demonstrated higher levels in the diet group, reflecting the reversibility of osteoblast markers and the effect of raloxifene on osteoblast transformation. D and E: aortic valve sections, including the annulus and a leaflet, from the different study groups are shown before and after immunohistochemistry staining. Stainings for collagen 1α (D; in green) and osteocalcin (E; in red) demonstrated increased expression of these osteoblast markers within the calcified valve and, in particular, in the calcified areas; both were decreased after diet cessation as well as in the raloxifene-treated group. F: staining using anti-CD68 antibody for macrophages (in green) was found only in the diet group. G: calcified valves from the diet group were stained for osteopontin (in red) and α-smooth muscle actin (α-SMA, in green) and with double staining for both markers, demonstrating that valvular cells in the calcified valve express both markers. Values in C are expressed as arbitrary numbers and are presented as means ± SE. *P < 0.05 for the comparison of study groups with the control group.
Aortic valve assessment using Von-Kossa staining and osteoblast, myofibroblast, and macrophage markers.
Von-Kossa staining of tissues obtained from diet group valves revealed diffuse calcium precipitates, which involved the valve annulus and leaflets. Only mild scattered calcification was noticed in reversibility and raloxifene-treated group valves, whereas no calcification was observed in control group valves (Fig. 2B). Osteopontin protein expression was significantly higher in valves obtained from diet group than in the control group. Osteopontin levels were lower in reversibility and raloxifene-treated groups than in the diet group (2.26- and 3.23-fold decrease, respectively; Fig. 2C). Similarly, immunohistochemistry evaluation of additional osteoblast markers, collagen 1α and osteocalcin, showed that both markers were increased in calcified valves and decreased after diet cessation as well as in raloxifene-treated rats. Furthermore, the calcified areas correlate with the valvular areas that expressed osteoblast markers (Fig. 2, D and E, respectively).
As AVC involves significant inflammatory process, we evaluated the presence of macrophages in the valves using staining for CD68. We showed that raloxifene significantly reduced macrophages accumulation in valves, suggesting that the beneficial effect of raloxifene is associated with decreased inflammation (Fig. 2F). To further analyze the cells that underwent osteoblast transformation in calcified valves, we performed double staining for α-SMA, which is a marker for myofibroblasts and for osteopontin. We showed that cells expressing osteoblast markers also express the specific myofibroblast marker (Fig. 2G).
Apoptosis assessment using TUNEL and caspase 3 protein levels.
Significant amounts of apoptotic cells were found in diet group valves (mean percentage of TUNEL-positive nuclei: 0.88 ± 0.02%). Interestingly, the apoptotic bodies were located in calcified areas, as demonstrated by Von-Kossa stain. This observation significantly stresses the hypothesis that the calcification process requires apoptotic changes in the valve. Raloxifene treatment significantly reduced the percentage of apoptotic cells to 0.009% (P < 0.05). No apoptosis was found in reversibility and control group valves (Fig. 3A). Similarly, apoptosis was confirmed using analysis of caspase 3 protein levels, which showed a significantly higher level in diet group calcified valves compared with the reversibility, raloxifene-treated, and control groups (1.58-, 1.68-, and 1.84-fold, respectively; Fig. 3B).
Fig. 3.
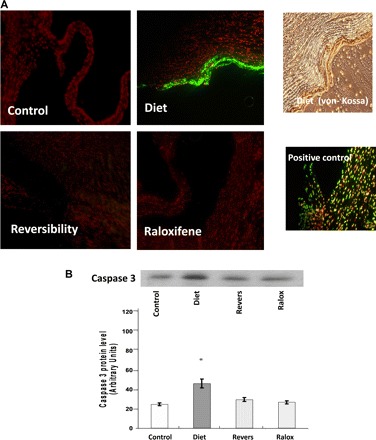
Apoptosis assessment in aortic valves. TUNEL staining (original magnification: ×50) demonstrated a significantly higher level of DNA fragmentation (in orange) in the diet group. Von-Kossa staining from the same area demonstrated the presence of apoptotic bodies in the calcified areas. Only scattered apoptotic cells were found in other groups (A). Apoptosis was also assessed using protein-level analysis. Caspase 3 demonstrated significantly higher levels of expression in valves from the diet group, suggesting that apoptosis plays an important part in AVC and that apoptotic features are decreased during reversibility and after raloxifene treatment (B). Western blot analysis for actin is shown in Fig. 2. Values are expressed as arbitrary numbers and are presented as means ± SE. *P < 0.05 for the comparison of study groups with the control group.
Gas6, Axl, MAPK, and Akt pathways in AVC.
Valves obtained from the diet group showed a highly significant 14-fold decrease of Gas6 levels compared with controls (Fig. 4, A and B). Furthermore, in the reversibility and raloxifene-treated groups, Gas6 protein levels were significantly higher compared with the diet group (5- and 3.57-fold increase, respectively). The pattern of changes in the Axl protein level was similar to Gas6 but did not reach statistical significance. AVC also resulted in a significant decrease in the ratio of the phosphorylated form of ERK to ERK-1 and the ratio of the phosphorylated form of Akt at Ser473 to Akt. In the reversibility and raloxifene-treated groups, both ERK and Akt pathways were upregulated (Fig. 4, A, C, and D). A graphic presentation of total ERK-1 and total Akt proteins levels is shown in Supplemental Fig. S2.
Fig. 4.
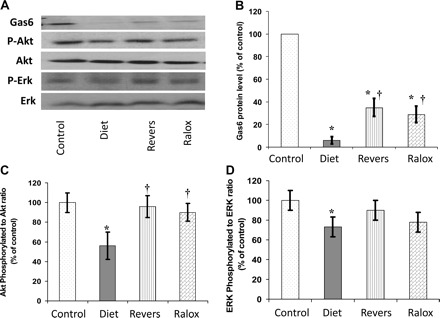
Akt, ERK, and growth arrest-specific 6 (Gas6)-Axl pathways in aortic valves. A–D: Western blot analysis of survival pathways (A) with the graphic presentation of Gas6 levels (B), the phosphorylated (P-)Akt-to-Akt ratio (C), and P-ERK-to-ERK ratio (D). The results reflect the downregulation of these pathways during AVC and upregulation (in particular the Akt pathway) after AVC resolution. Raloxifene treatment restored the Akt pathway and increased the P-ERK-to-ERK ratio. Western blot analysis for actin is shown in Fig. 2. *P < 0.05 for the comparison of study groups with the control group; †P < 0.05 for the comparison of study groups with the diet group.
There were no changes in the expression of JNK, p38, and their phosphorylated forms (data not shown).
DISCUSSION
AVC is a complex process, involving cellular changes that are modulated by active inflammatory processes. One of the important risk factors for AVC is RF. In AVC secondary to RF, as opposed to senile AVC, significant metabolic and electrolytes abnormalities are present. In this study, we used an animal model of AVC induced by RF to characterize the role of apoptosis and several related intracellular pathways. To evaluate the genes and pathways involved in AVC, we used gene array methods and evaluated gene expression in three groups: control, calcified valves, and valves after calfication regression. The differentially expressed genes were associated with several biological processes and molecular pathways including cell proliferation and death. Gene array results demonstrated a significant upregulation of proapoptotic genes in calcified valves, with parallel downregulation of important antiapoptotic pathways (e.g., Gas6). This expression pattern was reversed in the reversibility group.
We further evaluated the pathogenesis of AVC by several modalities and showed that cells expressing osteoblast markers also express a specific myofibroblast marker. This important observation stresses the importance of valvular myofibroblasts in ectopic calcification. Using TUNEL staining, we observed a significantly higher number of apoptotic cells in calcified valves. These apoptotic bodies were found in calcified areas, stressing the relationship between apoptosis and calcification. Western blot analysis of calcified valves established downregulation of the Gas6-Axl antiapoptotic pathway, with a significant increase in the level of caspase 3, a final common pathway and a primary executioner of apoptosis (17). We also demonstrated that two important survival pathways, ERK and Akt, are downregulated during calcification and are restored after its resolution.
A particularly important finding is the beneficial effect of raloxifene in preventing osteoblast transformation, macrophage accumulation, and AVC. This effect was associated with inhibition of valvular cell apoptosis and upregulation of Gas6-Axl and related pathways.
Apoptosis and Related Pathways in AVC
The role of apoptosis in vascular calcification is under investigation. Apoptosis in calcified aortic valves has been shown in vitro (8), with the assumption that apoptosis precedes calcification and that apoptotic bodies may serve as nucleating structures for calcium crystals (23). It has been shown that, due to metabolic abnormalities, particularly hyperphosphatemia, apoptosis may play an even more important role in RF-associated calcification. Phosphate induces osteoblast differentiation and apoptosis of vascular smooth muscle cells (VSMCs), resulting in calcification (13, 22). Furthermore, the proapoptotic effect of phosphate is mediated through the inhibition of survival pathways, including the Gas6-Axl pathway (34).
Gas6, structurally homologous to anticoagulant protein S, has a Gla domain and is the ligand for the tyrosine kinase receptor Axl (22). Tyrosine kinase receptors play an important role in transducing signals from the extracellular environment, resulting in a variety of cellular responses, including cell survival (22). This pathway has been shown to protect a variety of cell types from apoptotic death (28).
Our results indicate that both the resolution of AVC and the effect of raloxifene restore the Gas6-Axl anti apoptotic pathway, attesting to the importance of this pathway in the pathogenesis of AVC. These results further support previous observations in VSMCs suggesting that Gas6-Axl is downregulated during VSMC calcification (9, 38).
Gas6-Axl activation can also modulate a number of downstream signaling pathways, such as the Akt and ERK pathways (34). These pathways are involved in cardioprotection from ischemia-reperfusion injury and are also involved in vascular cell survival (12). The role of the ERK pathway is controversial in the pathogenesis of ectopic calcification. Several studies (11, 14) have shown that the ERK pathway is important in promoting osteoblastic differentiation and calcification, whereas others (19) have demonstrated its protective role in preventing calcification. The diversity of results may be explained by the complexity of the calcification process. A specific pathway may be activated in the course of the calcification process, whereas at a different phase the same pathway may be suppressed.
Our findings demonstrate that the Akt and ERK pathways are downregulated during calcification and upregulated through the resolving process, thus emphasizing their protective role in AVC and apoptosis inhibition.
The present study confirmed our hypothesis that the resolution of AVC is accompanied by a parallel decrease in apoptotic features. The decline in apoptosis might be explained by a reduction in the inflammatory process bringing up a new equilibrium in cell apoptosis and renewal. The reversibility of calcification was recently confirmed in an additional model of AVC based on high LDL-cholesterol levels (21, 31). The authors of that study demonstrated that rapid normalization of cholesterol levels in mice resulted in a regression of AVC. The lack of valvular osteoblast features and the reduction in macrophages infiltration after reversibility in both animal models suggest that the process is not permanent and requires continuous activation. In line with this hypothesis, AVC much resembles bone metabolism, which is dynamic and requires continuous maintenance to preserve calcification. The reversibility of the process and the ability to modify apoptosis give hope for several therapeutic options that might alleviate AVC.
Raloxifene Effects on AVC
The association of osteoporosis and vascular calcification has been widely reported, suggesting that osteoporosis may increase vascular calcification, although the specific mechanism is still unknown (15, 29). The epidemiological linkage between osteoporosis and vascular calcification as well as the common osteoblast genes expressed in both bone formation and pathological ectopic calcification emphasize the relationship between the pathogenesis of these conditions. The association between bone and vascular pathology is especially important in RF patients, as AVC and osteoporosis are frequently found and progress more rapidly in this condition.
The potential for therapeutic agents to treat osteoporosis and simultaneously prevent ectopic calcification is appealing; in this study, we show that raloxifene may reduce osteoblast transformation and lessen AVC.
Raloxifene is currently indicated for osteoporosis treatment in postmenopausal women, including those with chronic renal disease (2). The cardiovascular effects of raloxifene and other SERMs were evaluated in both basic and clinical studies. These drugs were shown to improve endothelial dysfunction, increase coronary dilatation, and improve lipid profiles (35). Although raloxifene decreased cardiovascular events in osteoporotic women with prior established atherosclerotic disease (5), major clinical studies, such as the Raloxifene Use for the Heart study, did not show a significant reduction in cardiovascular morbidity and mortality in the healthy population (6).
In this study, the beneficial effect of raloxifene was apparent in the modification of several pathways and in the prevention of valvular cell apoptosis. Although the protective effect of raloxifene on bone mass is known, less is known regarding its action mechanism. It has been suggested that raloxifene prevents osteoblast death and inhibits oxidative stress-induced endothelial cell apoptosis (16, 36). These effects may operate through a receptor-independent mechanism related to the antioxidant activity of SERMs (36). Our results lay the foundation for further studies to better understand the role of raloxifene in preventing ectopic calcification.
Summary
In this study, we show that RF-induced AVC is an active process involving the downregulation of several survival pathways and apoptosis. The beneficial role of raloxifene seen in this model might be related to the activation of these antiapoptotic pathways. Our findings indicate a potential role for bone-related therapies such as raloxifene in preventing cardiovascular calcification, especially in patients with advanced RF; this novel observation may have significant importance in developing efficient remedies for AVC.
GRANTS
This work was supported by The Chief Scientist Office of the Ministry of Health (Israel), The Berman Foundation for Cardiovascular Research of Hadassah Medical Center, and The Joint Research Fund of Hebrew University and Hadassah Medical Center.
DISCLOSURES
N. M. Rajamannan is the inventor of the use of medical therapy in aortic valve disease. This patent is owned by the Mayo Clinic and she does not receive any royalties for this patent.
Footnotes
Supplemental Material for this article is available at the American Journal of Physiology-Heart and Circulatory Physiology website.
REFERENCES
- 1.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 2.American Congress of Obstetricians and Gynecologists. ACOG practice bulletin. Selective estrogen receptor modulators. Number 39, October 2002 (replaces Committee Opinion Number 224, October 1999). Int J Gynaecol Obstet 79: 289–298, 2002 [DOI] [PubMed] [Google Scholar]
- 4.Aikawa E, Nahrendorf M, Sosnovik D, Lok VM, Jaffer FA, Aikawa M, Weissleder R. Multimodality molecular imaging identifies proteolytic and osteogenic activities in early aortic valve disease. Circulation 115: 377–386, 2007 [DOI] [PubMed] [Google Scholar]
- 5.Barrett-Connor E, Grady D, Sashegyi A, Anderson PW, Cox DA, Hoszowski K, Rautaharju P, Harper KD. Raloxifene and cardiovascular events in osteoporotic postmenopausal women: four-year results from the MORE (Multiple Outcomes of Raloxifene Evaluation) randomized trial. JAMA 287: 847–857, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med 355: 125–137, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Ben-Sasson SA, Sherman Y, Gavrieli Y. Identification of dying cells-in situ staining. Methods Cell Biol 46: 29–39, 1995 [PubMed] [Google Scholar]
- 7a.Catty D.Antibodies: a Practical Approach. Oxford, MA: IRL, 1988, vol. 1 [Google Scholar]
- 8.Clark-Greuel JNCJ, Sorichillo E, Narula NR, Rapoport HS, Mohler ER, 3rd, Gorman JH, 3rd, Gorman RC, Levy RJ. Transforming growth factor-β1 mechanisms in aortic valve calcification: increased alkaline phosphatase and related events. Ann Thorac Surg 83: 946–953, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Collett GDM, Sage AP, Kirton JP, Alexander MY, Gilmore AP, Canfield AE. Axl/phosphatidylinositol 3-kinase signaling inhibits mineral deposition by vascular smooth muscle cells. Circ Res 100: 502–509, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Ding HT, Wang CG, Zhang TL, Wang K. Fibronectin enhances in vitro vascular calcification by promoting osteoblastic differentiation of vascular smooth muscle cells via ERK pathway. J Cell Biochem 99: 1343–1352, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101: 660–667, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giachelli CM. Vascular calcification mechanisms. J Am Soc Nephrol 15: 2959–2964, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Gu X, Masters KS. Role of the MAPK/ERK pathway in valvular interstitial cell calcification. Am J Physiol Heart Circ Physiol 296: H1748–H1757, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hak AE, Pols HAP, van Hemert AM, Hofman A, Witteman JCM. Progression of aortic calcification is associated with metacarpal bone loss during menopause: a population-based longitudinal study. Arterioscler Thromb Vasc Biol 20: 1926–1931, 2000 [DOI] [PubMed] [Google Scholar]
- 16.Kallio A, Guo T, Lamminen E, Seppanen J, Kangas L, Vaananen HK, Harkonen P. Estrogen and the selective estrogen receptor modulator (SERM) protection against cell death in estrogen receptor alpha and beta expressing U2OS cells. Mol Cell Endocrinol 289: 38–48, 2008 [DOI] [PubMed] [Google Scholar]
- 17.Kothakota S, Azuma T, Reinhard C, Klippel A, Tang J, Chu K, McGarry TJ, Kirschner MW, Koths K, Kwiatkowski DJ, Williams LT. Caspase-3-generated fragment of gelsolin: effector of morphological change in apoptosis. Science 278: 294–298, 1997 [DOI] [PubMed] [Google Scholar]
- 18.Kume T, Kawamoto T, Akasaka T, Watanabe N, Toyota E, Neishi Y, Wada N, Okahashi N, Yoshida K. Rate of progression of valvular aortic stenosis in patients undergoing dialysis. J Am Soc Echocardiogr 19: 914–918, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Liao XB, Zhou XM, Li JM, Yang JF, Tan ZP, Hu ZW, Liu W, Lu Y, Yuan LQ. Taurine inhibits osteoblastic differentiation of vascular smooth muscle cells via the ERK pathway. Amino Acids 34: 525–530, 2008 [DOI] [PubMed] [Google Scholar]
- 20.Lönnstedt IS, T Replicated microarray data. Stat Sinica 12: 31–46, 2002 [Google Scholar]
- 21.Miller JD, Weiss RM, Serrano KM, Brooks RM, Berry CJ, 2nd, Zimmerman K, Young SG, Heistad DD. Lowering plasma cholesterol levels halts progression of aortic valve disease in mice. Circulation 119: 2693–2701, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.National Institutes of Health ImageJ: Image Processing and Analysis in Java (1.29 ed.) (online). http://rsb.info.nih.gov/ij/ [28 February 2011]
- 22.Nagata K, Ohashi K, Nakano T, Arita H, Zong C, Hanafusa H, Mizuno K. Identification of the product of growth arrest-specific gene 6 as a common ligand for Axl, Sky, and Mer receptor tyrosine kinases. J Biol Chem 271: 30022–30027, 1996 [DOI] [PubMed] [Google Scholar]
- 23.Proudfoot D, Skepper JN, Hegyi L, Bennett MR, Shanahan CM, Weissberg PL. Apoptosis regulates human vascular calcification in vitro: evidence for initiation of vascular calcification by apoptotic bodies. Circ Res 87: 1055–1062, 2000 [DOI] [PubMed] [Google Scholar]
- 24.Qiu P, Moeschberger ML, Cooke GE, Goldschmidt-Clermont PJ. Sample size to test for interaction between a specific exposure and a second risk factor in a pair-matched case-control study. Stat Med 19: 923–935, 2000 [DOI] [PubMed] [Google Scholar]
- 25.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation 107: 2181–2184, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation 105: 2660–2665, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics 19: 368–375, 2003 [DOI] [PubMed] [Google Scholar]
- 28.Sawabu T, Seno H, Kawashima T, Fukuda A, Uenoyama Y, Kawada M, Kanda N, Sekikawa A, Fukui H, Yanagita M, Yoshibayashi H, Satoh S, Sakai Y, Nakano T, Chiba T. Growth arrest-specific gene 6 and Axl signaling enhances gastric cancer cell survival via Akt pathway. Mol Carcinog 46: 155–164, 2007 [DOI] [PubMed] [Google Scholar]
- 29.Schulz E, Arfai K, Liu X, Sayre J, Gilsanz V. Aortic calcification and the risk of osteoporosis and fractures. J Clin Endocrinol Metab 89: 4246–4253, 2004 [DOI] [PubMed] [Google Scholar]
- 30.Sharan R, Maron-Katz A, Shamir R. CLICK and EXPANDER: a system for clustering and visualizing gene expression data. Bioinformatics 19: 1787–1799, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Shuvy M, Abedat S, Beeri R, Danenberg HD, Planer D, Ben-Dov IZ, Meir K, Sosna J, Lotan C. Uraemic hyperparathyroidism causes a reversible inflammatory process of aortic valve calcification in rats. Cardiovasc Res 79: 492–499, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shuvy M, Nyska A, Beeri R, Abedat S, Gal-Moscovici A, Rajamannan NM, Lotan C. Histopathology and apoptosis in an animal model of reversible renal injury. Exp Toxicol Pathol; doi:10.1016.j.etp.2010.02.002. [DOI] [PMC free article] [PubMed]
- 33.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol 3: Article3, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Son BK, Kozaki K, Iijima K, Eto M, Kojima T, Ota H, Senda Y, Maemura K, Nakano T, Akishita M, Ouchi Y. Statins protect human aortic smooth muscle cells from inorganic phosphate-induced calcification by restoring Gas6-Axl survival pathway. Circ Res 98: 1024–1031, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Stamatelopoulos KS, Lekakis JP, Poulakaki NA, Papamichael CM, Venetsanou K, Aznaouridis K, Protogerou AD, Papaioannou TG, Kumar S, Stamatelopoulos SF. Tamoxifen improves endothelial function and reduces carotid intima-media thickness in postmenopausal women. Am Heart J 147: 1093–1099, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Val M, Christene H, Giolanta K, Frances C, Brendon N. The antioxidant effect of estrogen and selective estrogen receptor modulators in the inhibition of osteocyte apoptosis in vitro. Bone 40: 674–684, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Weiss RM, Ohashi M, Miller JD, Young SG, Heistad DD. Calcific aortic valve stenosis in old hypercholesterolemic mice. Circulation 114: 2065–2069, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Xue W, Wallin R, Olmsted-Davis EA, Borras T. Matrix GLA protein function in human trabecular meshwork cells: inhibition of BMP2-induced calcification process. Invest Ophthalmol Vis Sci 47: 997–1007, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]


